The exploration of polyelectrolyte complexation commenced nearly a century ago1 but is receiving renewed attention in recent years owing to several very different lines of work that demand a deeper understanding of this process. One such line is the invention by Decher2 of layer-by-layer deposition of oppositely charged polyelectrolytes, leading to many potential applications in catalysis, membranes, and biomedicine. Spurring interest from an entirely different direction is the growing attention biologists are giving to the structures and functions of the membrane-less subcellular structures that are ubiquitous within cells.3
Mixing dilute solutions of a polyanion and a polycation will generally result in a new phase, formed by the spontaneous complexation of the two types of macromolecules. However, certain facts about this process are not very obvious. One is that the free energy of complex formation is dominated by the entropy gain of released counterions, not the enthalpy of the direct electrostatic interactions.4 The second unpredictable fact is whether the complex phase formed will be fluid or solid. The paper by Lutkenhaus et al.5 greatly illuminates this question. They provide strong, detailed evidence that solid phases are glassy and that the glass transition in polyelectrolyte complexes is controlled by the number of water molecules surrounding an oppositely charged polyelectrolyte–polyelectrolyte intrinsic ion pair. The distinction between intrinsic and extrinsic ion pairs is illustrated in Figure 1. The authors were able to deduce a formula for the variation of the glass transition temperature with the amount of water per intrinsic ion pair.
Figure 1.
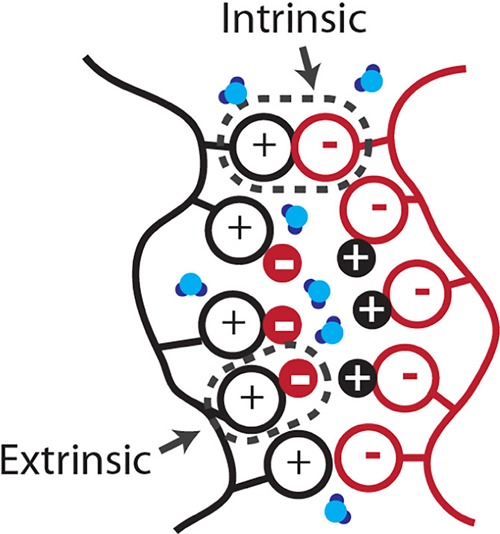
Schematic of a charged polyelectrolyte assembly. The charged polyelectrolyte assembly contains two different types of ion pairs: intrinsic and extrinsic. Intrinsic ion pairs are polycation–polyanion pairs, and extrinsic ion pairs are polyelectrolyte–counterion pairs. Reprinted with permission from ref (5). Copyright 2018 American Chemical Society.
The question of whether a polyelectrolyte complex material is fluid or solid is a very important matter for many useful properties and applications, including modulus, conductivity, wetting, healing, and others, and can be influenced in various ways. Schlenoff and co-workers6 have found that removing the salt in a polyelectrolyte complex encourages intrinsic ion pairing, drastically increasing the viscosity of the material until it becomes a rigid, glassy solid. Increasing the salt concentration dissolves the complex phase back into a homogeneous solution (Figure 2).
Figure 2.

Stoichiometric polyelectrolyte complexes of the strong polyelectrolytes at various concentrations of KBr. The vertical axis is the ratio of salt to polymer concentration. The color of the data points indicates the state of the polyelectrolyte-containing phase at that salt concentration. Reprinted with permission from ref (6). Copyright 2014 American Chemical Society.
Studying viscosity and elasticity of polymer complexes revealed that the glass transition in polyelectrolyte complexes depends on salt concentration, similarly to time–temperature dependency in neutral polymers.7 Salt influences both the thermodynamic8 and rheological properties of polyelectrolyte complexes in a manner analogous to temperature effects on melts of neutral polymers.
Solid phase formation is also affected by the “strength” of the polyelectrolyte. A strong polyelectrolyte is charged at all pHs in solution, whereas a weak polyelectrolyte can be varied from fully charged to uncharged by varying pH. Strong polyelectrolyte pairs are prone to form solid complexes.
The work by Lutkenhaus et al.5 is a significant advance in our understanding of the physical state of materials formed by polyelectrolyte complexation. The meticulous accounting for all of the mass in these systems is a model for how rigorous experimental work on polyelectrolyte complex materials should be done.
The author declares no competing financial interest.
References
- Bungenberg de Jong H. G.; Kruyt H. R. Coacervation (Partial Miscibility in Colloid Systems). Proc. K. Ned. Akad. Wet. 1929, 32, 849–856. [Google Scholar]
- Decher G. Fuzzy Nanoassemblies: Toward Layered Polymeric Multicomposites. Science 1997, 277, 1232–1237. 10.1126/science.277.5330.1232. [DOI] [Google Scholar]
- Brangwynne C. P.; Tompa P.; Pappu R. V. Polymer physics of intracellular phase transitions. Nat. Phys. 2015, 11, 899–904. 10.1038/nphys3532. [DOI] [Google Scholar]
- Priftis D.; Laugel N.; Tirrell M. Thermodynamic characterization of polypeptide complex coacervation. Langmuir 2012, 28, 15947–15957. 10.1021/la302729r. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Batys P.; O’Neal J. T.; Li F.; Sammalkorpi M.; Lutkenhaus J. L.. Molecular origin of the glass transition in polyelectrolyte assemblies. ACS Cent. Sci., 2018, DOI: 10.1021/acscentsci.8b00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q.; Schlenoff J. B. The polyelectrolyte complex/coacervate continuum. Macromolecules 2014, 47, 3108–3116. 10.1021/ma500500q. [DOI] [Google Scholar]
- Marciel A. B.; Srivastava S.; Tirrell M. V. Structure and rheology of polyelectrolyte complex coacervates. Soft Matter 2018, 14, 2454–2464. 10.1039/C7SM02041D. [DOI] [PubMed] [Google Scholar]
- Li L.; Srivastava S.; Andreev M.; Marciel A. B.; de Pablo J. J.; Tirrell M. Phase Behavior and Salt Partitioning in Polyelectrolyte Complex Coacervates. Macromolecules 2018, 51, 2988–2995. 10.1021/acs.macromol.8b00238. [DOI] [Google Scholar]


