Abstract
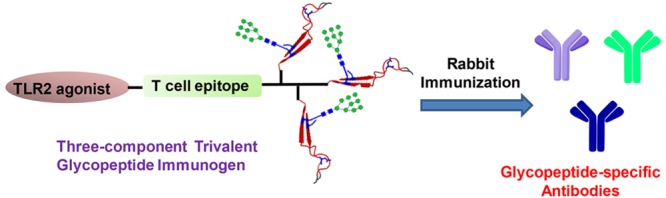
HIV-1 envelope glycoproteins gp120 and gp41 are presented on the virus surface as a trimer of heterodimer and are the targets of broadly neutralizing antibodies (bNAbs). We describe here the synthesis and preliminary immunological evaluation of a three-component trivalent HIV-1 V3 glycopeptide immunogen aiming to raise glycopeptide epitope-specific antibodies. Click chemistry confers efficient synthesis of the lipopeptide–glycopeptide conjugate that carries three copies of HIV-1 JR-FL gp120 V3 glycopeptide with a high-mannose glycan at the N332 glycosylation site. We found that the multivalent presentation substantially enhanced the immunogenicity of the V3 glycopeptide. The antisera induced by the three-component multivalent glycopeptide immunogen exhibited stronger binding to heterologous HIV-1 gp120s and the trimeric gp140s than that from the monovalent glycopeptide immunogen. The antisera generated from this preliminary rabbit immunization did not show virus neutralization activity, probably due to the lack of somatic maturation. The ability to elicit substantial glycopeptide epitope-specific antibodies by the three-component trivalent glycopeptide immunogen suggests that it could serve as a valuable vaccine component in combination with other vaccine candidates for further immunization studies.
Short abstract
The designed trivalent immunogen showed enhanced immunogenicity over the monovalent construct to raise glycopeptide epitope-specific antibodies cross-reactive to gp120s of different HIV-1 strains.
Introduction
The HIV-1 envelope glycoprotein (Env) trimer is responsible for viral entry into host cells and is the primary target for vaccine design.1,2 The Env comprises three gp120 and three gp41 subunits and is covered by a dense N-glycan coat.3 Glycan shielding helps the virus to evade the host immune response.3−5 Nevertheless, the glycosylation defense is not impermeable, as glycan-dependent broadly neutralizing antibodies (bNAbs) have been isolated from infected individuals, which can recognize and penetrate the glycan shield of HIV-1.6−10 Among the most potent neutralizers of these bNAbs is the PGT121–130 group that targets and penetrates the glycan shield to recognize both glycans and the protein surface of gp120 V3 region.7,11,12 Structural studies have revealed that the PGT-series bNAbs recognize the high-mannose patch centered around the N332 high-mannose N-glycan on V3 loop, suggesting the N332 high-mannose N-glycan is highly accessible and vulnerable to the human immune system.1,7,11−14 Designing appropriate HIV-1 immunogen using the glycopeptides recognized by these bNAbs is an alternative strategy for HIV-1 vaccine development, as the designed immunogen may elicit similar antibody responses to target the precise structure on Env.13,15,16 Recently, Haynes and co-workers mimicked this bNAb epitope using a synthetic V3 glycopeptide.17 The monomeric V3 glycopeptide was formulated in the Toll-like receptor 4 agonist GLA-SE adjuvant and administered to rhesus macaques. Glycan-dependent binding activity was observed using isolated monoclonal antibodies. Our group has also studied the immunogenicity of V3 glycopeptide by rational immunogen design. We first revealed the minimal epitope of several bNAbs by antibody–antigen binding study using synthetic V3 glycopeptides.18 It was found that antibody PGT128 exhibited specificity for high-mannose N-glycan with glycosylation site promiscuity; PGT121 showed binding specificity for glycopeptide carrying a sialylated N-glycan at N301 site, and 10-1074 was specific for glycopeptide carrying a high-mannose N-glycan at N332 site. We further designed a three-component glycopeptide immunogen, including a JR-FL strain 33-mer V3 glycopeptide carrying a high-mannose glycan at N332, a T-helper epitope peptide derived from tetanus toxoid, and a Toll-like receptor 2 ligand lipopeptide Pam3CSK4.19 Rabbit immunization revealed that the synthetic self-adjuvant glycopeptide immunogen could elicit substantial glycan-dependent antibodies with broad recognition to several gp120s across clades. These results suggest that the synthetic V3 glycopeptide immunogens may be a viable approach to elicit V3-glycan-specific bNAbs. However, new immunogen design requires further optimization to enhance the immunogenicity.
HIV-1 gp120/gp41 subunits are presented on the viral surface as a trimer,3,20 and some of the bNAbs, including the V3-glycan-specific antibodies, preferentially or specifically recognized the native Env trimer compared to monomeric gp120, suggesting their elicitation likely requires the presentation of trimeric immunogens.21,22 Indeed, our recent binding study disclosed that trivalent V3 glycopeptide were more efficient in recapitulating the epitope of some bNAbs.23 We designed and synthesized mono-, bi-, and trivalent gp120 V3 glycopeptides to mimic the V3 glycopeptide domains presented in Env trimer. Binding studies showed that 10-1074 antibody exhibits significantly enhanced binding to the bi- and trivalent V3 glycopeptide over the monomer, suggesting that the trivalent V3 glycopeptide could better mimic the actual epitope of bNAb 10-1074. However, design and synthesis of multivalent antigens to mimic the Env trimer is challenging due to the lack of structure details and the difficulties in chemical synthesis.5,24−26 We report here the synthesis of a novel three-component multivalent glycopeptide immunogen including a T-helper epitope peptide, a lipopeptide Pam3CSK4, and trivalent V3 glycopeptides, carrying high-mannose glycans at the N332 site. To evaluate immunogenicity, the trivalent glycopeptides were formulated into liposomes and administered to rabbits without additional adjuvants. Compared to the previous three-component monovalent glycopeptide immunogen, the immunogenicity of V3 glycopeptide was significantly enhanced by multivalent presentation. Similar to the case of the monovalent immunogen, the antisera generated from the preliminary immunization with the trivalent glycopeptide immunogen did not show viral neutralization activity, probably still due to the lack of somatic maturation. Nevertheless, the antisera induced by the three-component multivalent glycopeptide immunogen exhibited stronger binding to both gp120s and trimeric gp140s than those raised by our previously reported monovalent glycopeptide immunogen.19 We also found that substantial glycopeptide epitope-specific antibodies were elicited by the three-component multivalent glycopeptide immunogen. Glycopeptide binding analysis indicated that the antibodies induced by the multivalent glycopeptide immunogen preferentially recognized the tri- and bivalent glycopeptide antigens.
Results
Synthesis of the Three-Component Multivalent Glycopeptide Immunogen
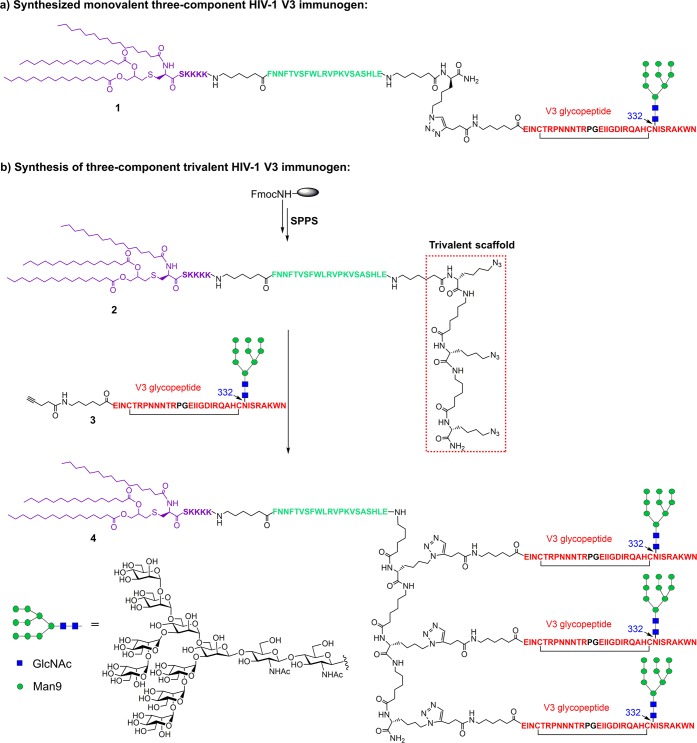
We focused our research on the 33-mer mini-V3 domain with a high-mannose glycan attached at N332, which is recognized by 10-1074 and a majority of the PGT-series bNAbs.7,13 We previously applied the copper(I)-catalyzed alkyne–azide 3 + 2 cycloaddition (click chemistry) to synthesize the monovalent, three-component HIV-1 V3 glycopeptide immunogen (1), which consists of a 33-mer V3 glycopeptide epitope, a universal T-helper epitope P30, and a lipopeptide (Pam3CSK4) that serves as a ligand of Toll-like receptor 2 (Scheme 1a).19 The synthesis of the trivalent, three-component HIV-1 V3 glycopeptide immunogen followed a similar strategy (Scheme 1b).
Scheme 1. (a) Structure of the Three-Component Monovalent Glycopeptide Immunogen, and (b) Synthesis of Three-Component Trivalent Glycopeptide Immunogen.
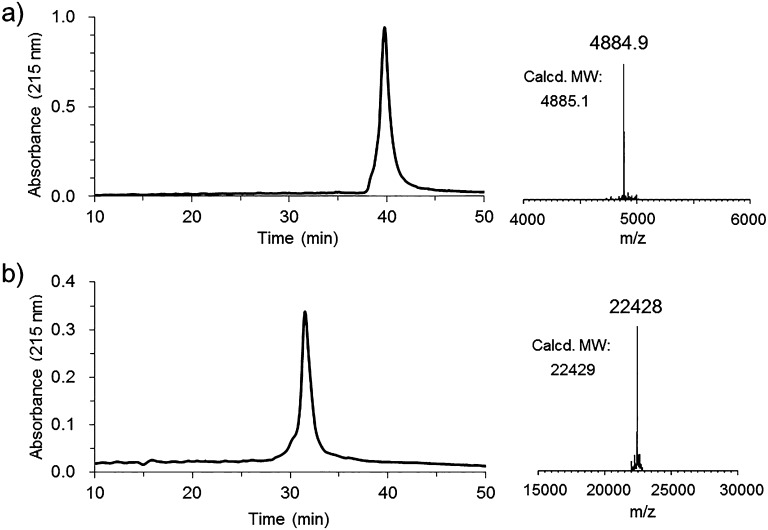
First, three Fmoc-Lys(N3)–OH moieties were installed at the C-terminus during solid phase peptide synthesis (SPPS), followed by the installation of the P30 T-cell epitope FNNFTVSFWLRVPKVSASHLE and the attachment of lipopeptide Pam3CSK4. The 6-aminohexanoic acid residues were placed to function as a flexible spacer. The crude peptide was cleaved from the resin by cocktail R (trifluoroacetic acid/thioanisole/ethanedithiol/anisole = 90/5/3/2) and then purified on a polar-CN column to afford trivalent lipopeptide scaffold 2 in excellent yield, and the identity was confirmed by analytical HPLC and ESI-MS analysis (Figure 1a). Finally, a cyclic 33-mer V3 high-mannose glycopeptide 3 carrying an alkyne on the N-terminus, which was previously synthesized by a chemoenzymatic method to construct the monovalent glycopeptide immunogen,19 was conjugated to lipopeptide scaffold 2 by the copper(I)-catalyzed alkyne–azide 3 + 2 cycloaddition reaction to give the desired three-component trivalent glycopeptide 4 after HPLC purification. The structure and purity of three-component trivalent glycopeptide 4 was confirmed by analytical HPLC and ESI-MS analysis (Figure 1b).
Figure 1.
HPLC and ESI-MS analysis of the synthetic three-component trivalent glycopeptide immunogen. (a) Trivalent lipopeptide scaffold 2. (b) Three-component trivalent glycopeptide immunogen 4. Left panel, analytical HPLC profile; right panel, the deconvoluted ESI-MS spectra. Analytical HPLC were run on a CN column using a linear gradient of 20–70% MeCN containing 0.1% TFA over 50 min. The LC-ESI-MS analysis was performed on an Exactive Plus Orbitrap mass spectrometer.
Immunization
To better mimic the Env trimer on the HIV virus surface, we formulated the synthetic three-component trivalent glycopeptide immunogen into liposomes following a reported procedure.19,27 The obtained liposomes were administered to rabbits (3 per group) at a relative low dose (50 μg of synthetic immunogen per immunization) without additional adjuvants via subcutaneous and intramuscular injections. After priming, three boosters were applied at intervals of 21 days. Bleeds were taken 7 days after the last injection, and antisera were used for immunological analysis.
Binding of Antisera to gp120s and gp140s
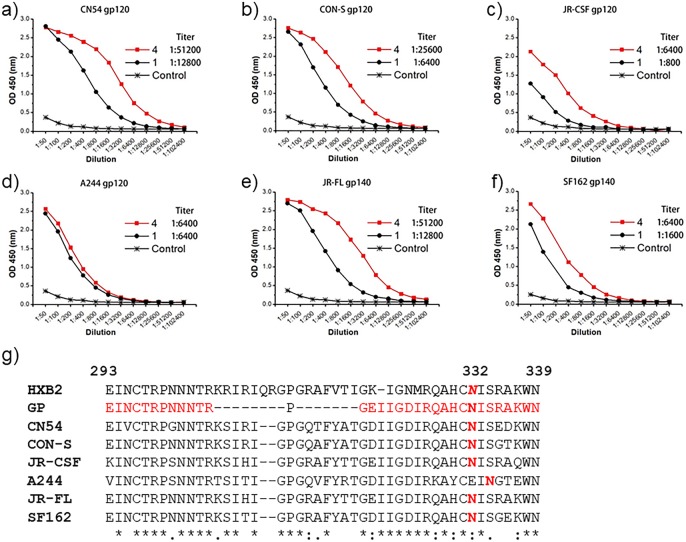
We combined the antisera from the three immunized rabbits and evaluated the binding to gp120s from HIV-1 strains CN54, CON-S, A244, and JR-CSF, as well as to gp140s from JR-FL and SF162 stains. In a previous study, the antisera induced by the three-component monovalent glycopeptide immunogen 1 have shown broad binding to the gp120s derived from some of the above strains with similar titers.19 In the present study, the antisera induced by the three-component trivalent glycopeptide immunogen 4 showed stronger binding to the CN54, CON-S, and JR-CSF gp120s than the antisera induced by the monovalent immunogen 1 (Figure 2a–c), with the observed titers being up to 4-fold higher. Interestingly, the trivalent immunogen 4 induced also higher immune responses toward the trimeric JR-FL and SF162 gp140s than the monovalent immunogen 1 (Figure 2e,f). In contrast, the titers of the antisera from both the mono- and trivalent immunogens (1 and 4) to the gp120 of HIV-1 A244 strain were relatively weak, and they did not show apparent differences for the two types of immunogens (Figure 2d). As shown by the alignment of the V3 domain sequences of the HIV-1 gp120s and gp140s derived from different HIV-1 strains (Figure 2g), the CN54, CON-S, JR-CSF, JR-FL, and SF162 all have a conserved N332 glycosylation site, while the A244 strain has this conserved N-glycosylation site shifted from N332 to the N334 position (Figure 2g). The presence of the N332 high-mannose glycan on the envelope glycoproteins gp120s and gp140s of the CN54, CON-S, JR-CSF, JR-FL, and SF162 strains was confirmed by ELISA analysis of their binding to the broadly neutralizing antibody (bNAb) 10-1074 (Figure S1), which specifically recognizes gp120/gp140 with a high-mannose glycan attached at the N332 site.12 The A244 gp120 did not show binding to bNAb 10-1074 due to the shift of the N-glycan from N332 to N334 (Figure S1). The synthetic glycopeptide incorporated in the trivalent immunogen was derived from JR-FL strain with the deletion of the highly variable tip sequence (Figure 2g) to avoid potentially strong strain-specific immune responses. The ability of the trivalent immunogen carrying the JR-FL V3 glycopeptide sequence to raise antibody responses that were broadly reactive to the envelope glycoproteins gp120/gp140 suggests that the antibodies raised by this immunogen targeted the common conserved epitopes on the envelope. In addition, the relatively weak binding to the A244 gp120 in which the conserved N-glycan shifted from N332 to N334 site indicated that the antibodies raised by this glycopeptide immunogen were glycan-dependent and preferably recognized the envelope glycoproteins carrying a conserved N-glycan at the N332 site.
Figure 2.
Comparison of the antisera binding to the envelope glycoprotein gp120s and gp140s derived from different HIV-1 strains. (a) HIV-1 CN54, (b) HIV-1 CON-S, (c) HIV-1 JR-CSF, (d) HIV-1 A244, (e) HIV-1 JR-FL, (f) HIV-1 SF162, and (g) the alignment of the V3 domain sequences derived from different HIV-1 strains. The numbering is based on the HBX2 strain; the gp is the sequence of the designed synthetic glycopeptide where the highly variable tip was deleted to avoid dominant strain-specific immune response. The CN54, CON-S, and JR-CSF gp120s and JR-FL and SF162 gp140s all have a conserved N-glycan at the N332 site, while the N332 glycosylation site was shifted to N334 in the A244 gp120.
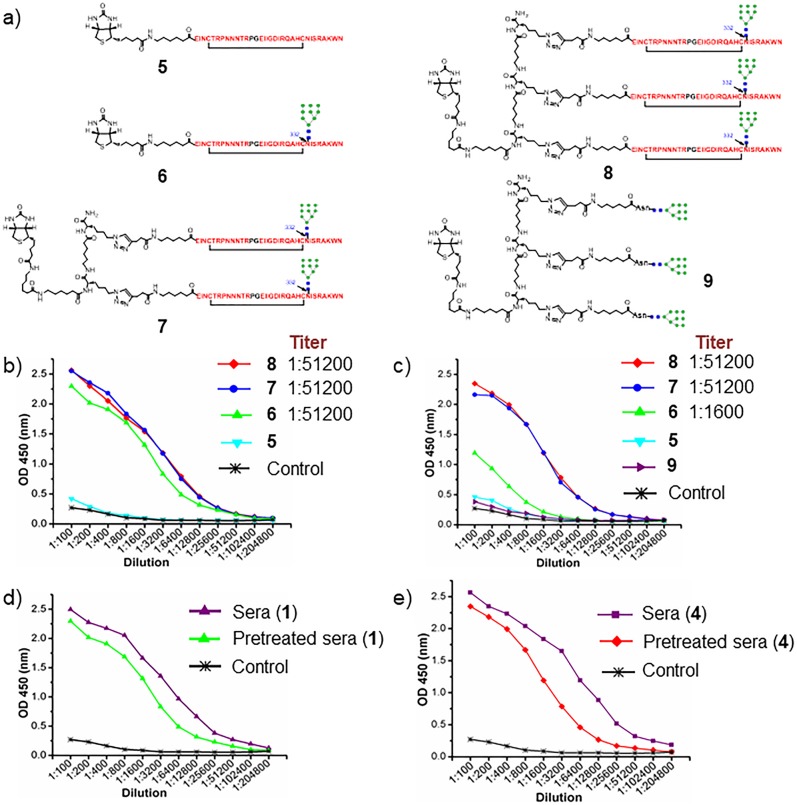
Binding of Antisera to Synthetic Glycopeptides
We next evaluated binding of the antisera to synthetic mono-, bi-, and trivalent V3 glycopeptides with a biotin tag (Figure 3a), which were used in our previous studies to recapitulate the neutralizing epitope of PGT bNAbs.23 To focus on the glycan-dependent antibodies, we pretreated the antisera by incubation with the aglycone V3 peptide 5 loaded on magnetic beads to remove the peptide binding antibodies.19 Then we analyzed the pretreated sera binding to mono-, bi-, and trivalent JR-FL V3 glycopeptide (6, 7, and 8) carrying a high-mannose glycan at the N332 site. For the pretreated antisera induced by the monovalent immunogen 1, the antibodies showed almost equal binding to the mono-, bi-, and trivalent glycopeptides (Figure 3b). However, the pretreated antisera induced by the three-component trivalent immunogen showed significant binding preference to the multivalent glycopeptides (Figure 3c). The antisera binding titers by the bi- and trivalent glycopeptides (1:25600) were 16-fold higher than the titers induced by the monovalent glycopeptide (1:1600). Although many antibodies, including partially glycopeptide binding antibodies, were removed by the aglycone peptide incubation, substantial glycan-dependent antibodies were still left in the sera (Figure 3d,e), and these antibodies preferentially bind to the multivalent glycopeptides. To evaluate whether the antisera bind to high-mannose glycans alone, we also performed ELISA binding to the corresponding trivalent high-mannose glycan conjugate (9), which has the same triazole linker as found in the mono-, bi-, and trivalent synthetic glycopeptides (6–8) (Figure 3a). It was found that the pretreated antisera induced by trivalent immunogen 4 showed only very weak binding to the trivalent conjugate (9) carrying three Man9GlcNAc2 glycans, without the V3 peptide context (Figure 3c). Surprisingly, the nontreated antisera induced by immunogen 1 and 4 also showed only weak binding to the trivalent glycan construct (9) (Figure S2). These results indicated that the linker used in the trivalent immunogen 4 was only weakly immunogenic compared to the glycopeptide antigen, and that the antibodies raised by the trivalent glycopeptide immunogen (4) were V3 glycopeptide-specific, as the antisera interacted only weakly with the respective peptide, linker, or the glycan portions alone. It should be noted that a majority of the antibodies raised by the immunization were glycopeptide-specific, as the antisera after depletion by pretreatment with the nonglycosylated V3 peptide, i.e., the pretreated antisera, did not show substantial loss of the responses to the respective glycopeptide antigens in the ELISAs (Figure 3d,e). To evaluate whether the antisera could distinguish between different N-glycans attached to the N332 site in the glycopeptides, we also compared the binding of the antisera to the biotinylated glycopeptide (6) carrying a Man9GlcNAc2 glycan at N332 and the corresponding glycopeptide carrying a Man5GlcNAc2 glycan at the N332 site (Figure S3). It was found that the pretreated sera induced by immunogen 1 showed substantially decreased binding to a JR-FL V3 glycopeptide with a Man5 glycan at the N332, in comparison with glycopeptide 6 (Figure S3). This result suggested that the antibodies induced also recognized the nature of the N-glycan at N332. The binding behavior of the induced antibodies was very similar to bNAb 10-1074, which also showed significant binding preference to the multivalent glycopeptides and HIV-1 gp120s carrying a high-mannose N-glycan (supposedly Man8/Man9GlcNAc2) at the conserved N332 glycosylation site, but did not demonstrate apparent binding to free high-mannose and other N-glycans in glycan array analysis.12,23
Figure 3.
ELISA binding to the synthetic V3 glycopeptides. (a) Structure of the V3 peptide and glycopeptides used for ELISA analysis. (b) Binding of the pretreated antisera induced by the three-component immunogen 1 to the V3 peptides and glycopeptides. (c) Binding of pretreated antisera induced by three-component trivalent immunogen 4 to the V3 peptide (5), glycopeptides (6–8), and trivalent glycan cluster (9). (d) Binding of the antisera and pretreated antisera induced by immunogen 1 to monovalent glycopeptide 6. (e) Antisera and pretreated antisera induced by immunogen 4 to trivalent glycopeptide 8.
Viral Neutralization Assays
HIV-1 bNAbs typically have unusual traits including long heavy-chain third complementarity-determining regions, high levels of somatic mutations, and high frequency of insertions and deletions.28,29 So far, there is only one example showing broad and potent neutralizing serum responses targeting the CD4 binding site by immunization with a well-ordered Env trimer in cow.30 Most bNAb evolution during HIV-1-infection has been observed after extensive virus Env diversification.31 To examine if the antisera generated in the short-term preliminary immunization possess any neutralizing activity, we performed a TZM-bl cell-based neutralization assay of the antisera against tier 1 and tier 2 HIV-1 viruses.32,33 The preliminary results indicated that the antisera induced by the trivalent glycopeptide immunogen (4) did not show neutralizing activities (data not shown). This result was similar to recent immunization studies with the monovalent immunogen in rabbits19 and with a synthetic mini-V3 glycopeptide mixed with an adjuvant in rhesus macaque.17 In another related study, repetitive vaccination with a high-mannose glycan form of HIV-1 Env over a 4 year period resulted in induction of V3-glycan-specific antibodies.34 The antibodies showed neutralizing activities, but they were neutralizing only pseudoviruses carrying high density of high-mannose N-glycans.
Discussion
HIV-1 envelope is presented on the virus surface as a trimer and is the sole target for HIV-1-specific antibodies. The envelope trimer is more immunogenic than monomeric gp120.35 Stable and soluble SOSIP Env trimers that mimic the Env spike on virus surface is a promising strategy for HIV-1 vaccine.36 However, except one recent immunization in cows that showed broadly neutralizing antibody responses targeting the CD4 binding site,30 immunization with SOSIP trimers has not been able to induce bNAbs, indicating that additional strategies are required. New immunogen designs need to be optimized to display the precise neutralizing epitopes. One potential method is to design well-defined synthetic vaccines to elicit an immune response to target the neutralizing epitope more reliably and precisely.37 In the present study, we designed a three-component trivalent HIV glycopeptide immunogen that contains a universal T-helper epitope, a TLR2 ligand, and a trivalent HIV-1 glycopeptide antigen in a single molecule aiming to mimic the trimeric antigen presentation on the Env. Preliminary rabbit immunization indicated that the three-component trivalent glycopeptide immunogen could elicit stronger antibody responses than the corresponding monovalent glycopeptide immunogen. Interestingly, the induced glycopeptide-specific antibodies showed binding preference to the bi- and trivalent glycopeptide antigens, although it is to be demonstrated whether any of the antibodies raised by the trivalent glycopeptide immunogen also showed preference to Env trimer. Similar to previous monovalent glycopeptide immunogens,17,19 no neutralizing activity against tier 1 and tier 2 HIV-1 viruses was detected for the antisera from the preliminary immunization study, possibly due to the lack of somatic mutation of the antibodies in a short-term immunization. These results are not unexpected because in natural infection it can take 1–2 years to develop potent broadly neutralizing antibodies. Nevertheless, the synthetic three-component trivalent glycopeptide immunogen showed substantially enhanced immunogenicity over the monovalent glycopeptide immunogen to elicit HIV-1 glycopeptide epitope-dependent antibody responses. As for HIV-1 vaccine design, future studies should be directed to further characterization of the glycopeptide-specific antibodies elicited, and the assessment of immunogenicity of the three-component trivalent glycopeptide immunogen in nonhuman primate models, in combination with other HIV-1 vaccine candidates, such as the SOSIP trimer, aiming to boost glycopeptide epitope-specific, broadly neutralizing antibody responses.
Conclusion
We describe in this paper a synthesis and preliminary immunization analysis of a three-component glycopeptide immunogen carrying a trivalent HIV-1 V3 glycopeptide antigen. The antisera induced by the three-component trivalent glycopeptide immunogen show stronger binding to gp120s and trimeric gp140s from several major HIV-1 strains than the antisera induced by the monovalent glycopeptide immunogen. Binding analysis also indicated that the synthetic immunogen induced substantial glycopeptide-specific antibodies that recognize the glycopeptide epitope as an integrated moiety. The ability to induce substantial glycopeptide epitope-specific antibodies suggests that this glycopeptide immunogen construct may serve as an important component for a prime-boost regimen in combination with other vaccine candidates to eventually elicit glycopeptide neutralizing epitope-specific, broadly neutralizing antibodies.
Acknowledgments
We thank other members of the Wang lab for technical assistance and discussions. The HIV-1 gp120s and gp140s were obtained from the NIH AIDS reagent program. This work was supported by the National Institutes of Health (Grant R01AI113896).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acscentsci.8b00060.
General procedures and methods; synthesis of lipopeptide 2; synthesis of three-component trivalent immunogen 4; rabbits immunization; ELISA analysis with HIV-1 gp120s and trimeric gp140s; ELISA binding analysis with synthetic V3 peptide, glycopeptides, and the trivalent glycan construct (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Julien J. P.; Cupo A.; Sok D.; Stanfield R. L.; Lyumkis D.; Deller M. C.; Klasse P. J.; Burton D. R.; Sanders R. W.; Moore J. P.; Ward A. B.; Wilson I. A. Crystal structure of a soluble cleaved HIV-1 envelope trimer. Science 2013, 342, 1477–1483. 10.1126/science.1245625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes B. F.; Burton D. R. Developing an HIV vaccine. Science 2017, 355, 1129–1130. 10.1126/science.aan0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart-Jones G. B.; Soto C.; Lemmin T.; Chuang G. Y.; Druz A.; Kong R.; Thomas P. V.; Wagh K.; Zhou T.; Behrens A. J.; Bylund T.; Choi C. W.; Davison J. R.; Georgiev I. S.; Joyce M. G.; Kwon Y. D.; Pancera M.; Taft J.; Yang Y.; Zhang B.; Shivatare S. S.; Shivatare V. S.; Lee C. C.; Wu C. Y.; Bewley C. A.; Burton D. R.; Koff W. C.; Connors M.; Crispin M.; Baxa U.; Korber B. T.; Wong C. H.; Mascola J. R.; Kwong P. D. Trimeric HIV-1-Env Structures Define Glycan Shields from Clades A, B, and G. Cell 2016, 165, 813–826. 10.1016/j.cell.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pancera M.; Zhou T.; Druz A.; Georgiev I. S.; Soto C.; Gorman J.; Huang J.; Acharya P.; Chuang G. Y.; Ofek G.; Stewart-Jones G. B.; Stuckey J.; Bailer R. T.; Joyce M. G.; Louder M. K.; Tumba N.; Yang Y.; Zhang B.; Cohen M. S.; Haynes B. F.; Mascola J. R.; Morris L.; Munro J. B.; Blanchard S. C.; Mothes W.; Connors M.; Kwong P. D. Structure and immune recognition of trimeric pre-fusion HIV-1 Env. Nature 2014, 514, 455–461. 10.1038/nature13808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiya S.; MacPherson I. S.; Krauss I. J. Recent strategies targeting HIV glycans in vaccine design. Nat. Chem. Biol. 2014, 10, 990–999. 10.1038/nchembio.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J.; Ofek G.; Laub L.; Louder M. K.; Doria-Rose N. A.; Longo N. S.; Imamichi H.; Bailer R. T.; Chakrabarti B.; Sharma S. K.; Alam S. M.; Wang T.; Yang Y.; Zhang B.; Migueles S. A.; Wyatt R.; Haynes B. F.; Kwong P. D.; Mascola J. R.; Connors M. Broad and potent neutralization of HIV-1 by a gp41-specific human antibody. Nature 2012, 491, 406–412. 10.1038/nature11544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker L. M.; Huber M.; Doores K. J.; Falkowska E.; Pejchal R.; Julien J. P.; Wang S. K.; Ramos A.; Chan-Hui P. Y.; Moyle M.; Mitcham J. L.; Hammond P. W.; Olsen O. A.; Phung P.; Fling S.; Wong C. H.; Phogat S.; Wrin T.; Simek M. D.; Koff W. C.; Wilson I. A.; Burton D. R.; Poignard P. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature 2011, 477, 466–470. 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker L. M.; Phogat S. K.; Chan-Hui P. Y.; Wagner D.; Phung P.; Goss J. L.; Wrin T.; Simek M. D.; Fling S.; Mitcham J. L.; Lehrman J. K.; Priddy F. H.; Olsen O. A.; Frey S. M.; Hammond P. W.; Kaminsky S.; Zamb T.; Moyle M.; Koff W. C.; Poignard P.; Burton D. R. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science 2009, 326, 285–289. 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corti D.; Lanzavecchia A. Broadly neutralizing antiviral antibodies. Annu. Rev. Immunol. 2013, 31, 705–742. 10.1146/annurev-immunol-032712-095916. [DOI] [PubMed] [Google Scholar]
- Kwong P. D.; Mascola J. R. Human antibodies that neutralize HIV-1: identification, structures, and B cell ontogenies. Immunity 2012, 37, 412–425. 10.1016/j.immuni.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong L.; Lee J. H.; Doores K. J.; Murin C. D.; Julien J. P.; McBride R.; Liu Y.; Marozsan A.; Cupo A.; Klasse P. J.; Hoffenberg S.; Caulfield M.; King C. R.; Hua Y.; Le K. M.; Khayat R.; Deller M. C.; Clayton T.; Tien H.; Feizi T.; Sanders R. W.; Paulson J. C.; Moore J. P.; Stanfield R. L.; Burton D. R.; Ward A. B.; Wilson I. A. Supersite of immune vulnerability on the glycosylated face of HIV-1 envelope glycoprotein gp120. Nat. Struct. Mol. Biol. 2013, 20, 796–803. 10.1038/nsmb.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouquet H.; Scharf L.; Euler Z.; Liu Y.; Eden C.; Scheid J. F.; Halper-Stromberg A.; Gnanapragasam P. N.; Spencer D. I.; Seaman M. S.; Schuitemaker H.; Feizi T.; Nussenzweig M. C.; Bjorkman P. J. Complex-type N-glycan recognition by potent broadly neutralizing HIV antibodies. Proc. Natl. Acad. Sci. U. S. A. 2012, 109, E3268–3277. 10.1073/pnas.1217207109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pejchal R.; Doores K. J.; Walker L. M.; Khayat R.; Huang P. S.; Wang S. K.; Stanfield R. L.; Julien J. P.; Ramos A.; Crispin M.; Depetris R.; Katpally U.; Marozsan A.; Cupo A.; Maloveste S.; Liu Y.; McBride R.; Ito Y.; Sanders R. W.; Ogohara C.; Paulson J. C.; Feizi T.; Scanlan C. N.; Wong C. H.; Moore J. P.; Olson W. C.; Ward A. B.; Poignard P.; Schief W. R.; Burton D. R.; Wilson I. A. A potent and broad neutralizing antibody recognizes and penetrates the HIV glycan shield. Science 2011, 334, 1097–1103. 10.1126/science.1213256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sok D.; Doores K. J.; Briney B.; Le K. M.; Saye-Francisco K. L.; Ramos A.; Kulp D. W.; Julien J. P.; Menis S.; Wickramasinghe L.; Seaman M. S.; Schief W. R.; Wilson I. A.; Poignard P.; Burton D. R. Promiscuous Glycan Site Recognition by Antibodies to the High-Mannose Patch of gp120 Broadens Neutralization of HIV. Sci. Transl. Med. 2014, 6, 236ra63. 10.1126/scitranslmed.3008104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam S. M.; Dennison S. M.; Aussedat B.; Vohra Y.; Park P. K.; Fernandez-Tejada A.; Stewart S.; Jaeger F. H.; Anasti K.; Blinn J. H.; Kepler T. B.; Bonsignori M.; Liao H. X.; Sodroski J. G.; Danishefsky S. J.; Haynes B. F. Recognition of synthetic glycopeptides by HIV-1 broadly neutralizing antibodies and their unmutated ancestors. Proc. Natl. Acad. Sci. U. S. A. 2013, 110, 18214–18219. 10.1073/pnas.1317855110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Tejada A.; Haynes B. F.; Danishefsky S. J. Designing synthetic vaccines for HIV. Expert Rev. Vaccines 2015, 14, 815–831. 10.1586/14760584.2015.1027690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam S. M.; Aussedat B.; Vohra Y.; Ryan Meyerhoff R.; Cale E. M.; Walkowicz W. E.; Radakovich N. A.; Anasti K.; Armand L.; Parks R.; Sutherland L.; Scearce R.; Joyce M. G.; Pancera M.; Druz A.; Georgiev I. S.; Von Holle T.; Eaton A.; Fox C.; Reed S. G.; Louder M.; Bailer R. T.; Morris L.; Abdool-Karim S. S.; Cohen M.; Liao H. X.; Montefiori D. C.; Park P. K.; Fernandez-Tejada A.; Wiehe K.; Santra S.; Kepler T. B.; Saunders K. O.; Sodroski J.; Kwong P. D.; Mascola J. R.; Bonsignori M.; Moody M. A.; Danishefsky S.; Haynes B. F. Mimicry of an HIV broadly neutralizing antibody epitope with a synthetic glycopeptide. Sci. Transl. Med. 2017, 9, eaai7521. 10.1126/scitranslmed.aai7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orwenyo J.; Cai H.; Giddens J.; Amin M. N.; Toonstra C.; Wang L. X. Systematic Synthesis and Binding Study of HIV V3 Glycopeptides Reveal the Fine Epitopes of Several Broadly Neutralizing Antibodies. ACS Chem. Biol. 2017, 12, 1566–1575. 10.1021/acschembio.7b00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H.; Orwenyo J.; Giddens J. P.; Yang Q.; Zhang R.; LaBranche C. C.; Montefiori D. C.; Wang L. X. Synthetic Three-Component HIV-1 V3 Glycopeptide Immunogens Induce Glycan-Dependent Antibody Responses. Cell Chem. Biol. 2017, 24, 1513–1522. 10.1016/j.chembiol.2017.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gristick H. B.; von Boehmer L.; West A. P. Jr.; Schamber M.; Gazumyan A.; Golijanin J.; Seaman M. S.; Fatkenheuer G.; Klein F.; Nussenzweig M. C.; Bjorkman P. J. Natively glycosylated HIV-1 Env structure reveals new mode for antibody recognition of the CD4-binding site. Nat. Struct. Mol. Biol. 2016, 23, 906–915. 10.1038/nsmb.3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo N. S.; Sutton M. S.; Shiakolas A. R.; Guenaga J.; Jarosinski M. C.; Georgiev I. S.; McKee K.; Bailer R. T.; Louder M. K.; O’Dell S.; Connors M.; Wyatt R. T.; Mascola J. R.; Doria-Rose N. A. Multiple Antibody Lineages in One Donor Target the Glycan-V3 Supersite of the HIV-1 Envelope Glycoprotein and Display a Preference for Quaternary Binding. J. Virol. 2016, 90, 10574–10586. 10.1128/JVI.01012-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. H.; Andrabi R.; Su C. Y.; Yasmeen A.; Julien J. P.; Kong L.; Wu N. C.; McBride R.; Sok D.; Pauthner M.; Cottrell C. A.; Nieusma T.; Blattner C.; Paulson J. C.; Klasse P. J.; Wilson I. A.; Burton D. R.; Ward A. B. A Broadly Neutralizing Antibody Targets the Dynamic HIV Envelope Trimer Apex via a Long, Rigidified, and Anionic beta-Hairpin Structure. Immunity 2017, 46, 690–702. 10.1016/j.immuni.2017.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H.; Orwenyo J.; Guenaga J.; Giddens J.; Toonstra C.; Wyatt R. T.; Wang L. X. Synthetic multivalent V3 glycopeptides display enhanced recognition by glycan-dependent HIV-1 broadly neutralizing antibodies. Chem. Commun. (Cambridge, U. K.) 2017, 53, 5453–5456. 10.1039/C7CC02059G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce J. G.; Krauss I. J.; Song H. C.; Opalka D. W.; Grimm K. M.; Nahas D. D.; Esser M. T.; Hrin R.; Feng M.; Dudkin V. Y.; Chastain M.; Shiver J. W.; Danishefsky S. J. An oligosaccharide-based HIV-1 2G12 mimotope vaccine induces carbohydrate-specific antibodies that fail to neutralize HIV-1 virions. Proc. Natl. Acad. Sci. U. S. A. 2008, 105, 15684–15689. 10.1073/pnas.0807837105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiya S.; Bailey J. K.; Temme J. S.; Guillen Schlippe Y. V.; Krauss I. J. Directed evolution of multivalent glycopeptides tightly recognized by HIV antibody 2G12. J. Am. Chem. Soc. 2014, 136, 5407–5415. 10.1021/ja500678v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivatare S. S.; Chang S. H.; Tsai T. I.; Tseng S. Y.; Shivatare V. S.; Lin Y. S.; Cheng Y. Y.; Ren C. T.; Lee C. C.; Pawar S.; Tsai C. S.; Shih H. W.; Zeng Y. F.; Liang C. H.; Kwong P. D.; Burton D. R.; Wu C. Y.; Wong C. H. Modular synthesis of N-glycans and arrays for the hetero-ligand binding analysis of HIV antibodies. Nat. Chem. 2016, 8, 338–346. 10.1038/nchem.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingale S.; Wolfert M. A.; Gaekwad J.; Buskas T.; Boons G. J. Robust immune responses elicited by a fully synthetic three-component vaccine. Nat. Chem. Biol. 2007, 3, 663–667. 10.1038/nchembio.2007.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton D. R.; Hangartner L. Broadly Neutralizing Antibodies to HIV and Their Role in Vaccine Design. Annu. Rev. Immunol. 2016, 34, 635–659. 10.1146/annurev-immunol-041015-055515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsoe G.; Haynes B. F. Host controls of HIV broadly neutralizing antibody development. Immunol. Rev. 2017, 275, 79–88. 10.1111/imr.12508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sok D.; Le K. M.; Vadnais M.; Saye-Francisco K. L.; Jardine J. G.; Torres J. L.; Berndsen Z. T.; Kong L.; Stanfield R.; Ruiz J.; Ramos A.; Liang C. H.; Chen P. L.; Criscitiello M. F.; Mwangi W.; Wilson I. A.; Ward A. B.; Smider V. V.; Burton D. R. Rapid elicitation of broadly neutralizing antibodies to HIV by immunization in cows. Nature 2017, 548, 108–111. 10.1038/nature23301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonsignori M.; Liao H. X.; Gao F.; Williams W. B.; Alam S. M.; Montefiori D. C.; Haynes B. F. Antibody-virus co-evolution in HIV infection: paths for HIV vaccine development. Immunol. Rev. 2017, 275, 145–160. 10.1111/imr.12509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonsignori M.; Kreider E. F.; Fera D.; Meyerhoff R. R.; Bradley T.; Wiehe K.; Alam S. M.; Aussedat B.; Walkowicz W. E.; Hwang K. K.; Saunders K. O.; Zhang R.; Gladden M. A.; Monroe A.; Kumar A.; Xia S. M.; Cooper M.; Louder M. K.; McKee K.; Bailer R. T.; Pier B. W.; Jette C. A.; Kelsoe G.; Williams W. B.; Morris L.; Kappes J.; Wagh K.; Kamanga G.; Cohen M. S.; Hraber P. T.; Montefiori D. C.; Trama A.; Liao H. X.; Kepler T. B.; Moody M. A.; Gao F.; Danishefsky S. J.; Mascola J. R.; Shaw G. M.; Hahn B. H.; Harrison S. C.; Korber B. T.; Haynes B. F. Staged induction of HIV-1 glycan-dependent broadly neutralizing antibodies. Sci. Transl. Med. 2017, 9, eaai7514. 10.1126/scitranslmed.aai7514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarzotti-Kelsoe M.; Bailer R. T.; Turk E.; Lin C. L.; Bilska M.; Greene K. M.; Gao H.; Todd C. A.; Ozaki D. A.; Seaman M. S.; Mascola J. R.; Montefiori D. C. Optimization and validation of the TZM-bl assay for standardized assessments of neutralizing antibodies against HIV-1. J. Immunol. Methods 2014, 409, 131–146. 10.1016/j.jim.2013.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders K. O.; Nicely N. I.; Wiehe K.; Bonsignori M.; Meyerhoff R. R.; Parks R.; Walkowicz W. E.; Aussedat B.; Wu N. R.; Cai F.; Vohra Y.; Park P. K.; Eaton A.; Go E. P.; Sutherland L. L.; Scearce R. M.; Barouch D. H.; Zhang R.; Von Holle T.; Overman R. G.; Anasti K.; Sanders R. W.; Moody M. A.; Kepler T. B.; Korber B.; Desaire H.; Santra S.; Letvin N. L.; Nabel G. J.; Montefiori D. C.; Tomaras G. D.; Liao H. X.; Alam S. M.; Danishefsky S. J.; Haynes B. F. Vaccine Elicitation of High Mannose-Dependent Neutralizing Antibodies against the V3-Glycan Broadly Neutralizing Epitope in Nonhuman Primates. Cell Rep. 2017, 18, 2175–2188. 10.1016/j.celrep.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs J. M.; Nkolola J. P.; Peng H.; Cheung A.; Perry J.; Miller C. A.; Seaman M. S.; Barouch D. H.; Chen B. HIV-1 envelope trimer elicits more potent neutralizing antibody responses than monomeric gp120. Proc. Natl. Acad. Sci. U. S. A. 2012, 109, 12111–12116. 10.1073/pnas.1204533109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Taeye S. W.; Ozorowski G.; Torrents de la Pena A.; Guttman M.; Julien J. P.; van den Kerkhof T. L.; Burger J. A.; Pritchard L. K.; Pugach P.; Yasmeen A.; Crampton J.; Hu J.; Bontjer I.; Torres J. L.; Arendt H.; DeStefano J.; Koff W. C.; Schuitemaker H.; Eggink D.; Berkhout B.; Dean H.; LaBranche C.; Crotty S.; Crispin M.; Montefiori D. C.; Klasse P. J.; Lee K. K.; Moore J. P.; Wilson I. A.; Ward A. B.; Sanders R. W. Immunogenicity of Stabilized HIV-1 Envelope Trimers with Reduced Exposure of Non-neutralizing Epitopes. Cell 2015, 163, 1702–1715. 10.1016/j.cell.2015.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L. H. Recent advances in the molecular design of synthetic vaccines. Nat. Chem. 2015, 7, 952–960. 10.1038/nchem.2396. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.