Abstract
Sunlight can initiate photochemical reactions of organic molecules though direct photolysis, photosensitization, and indirect processes, often leading to complex radical chemistry that can increase molecular complexity in the environment. α-Keto acids act as photoinitiators for organic species that are not themselves photoactive. Here, we demonstrate this capability through the reaction of two α-keto acids, pyruvic acid and 2-oxooctanoic acid, with a series of fatty acids and fatty alcohols. We show for five different cases that a cross-product between the photoinitiated α-keto acid and non-photoactive species is formed during photolysis in aqueous solution. Fatty acids and alcohols are relatively unreactive species, which suggests that α-keto acids are able to act as radical initiators for many atmospherically relevant molecules found in the sea surface microlayer and on atmospheric aerosol particles.
Short abstract
Light-initiated reactions of organic α-keto acids in water drive chemistry in mixtures with non-photoactive lipids relevant to environmental interfaces.
Introduction
The Sun is by far the largest source of energy to the planet, and it controls, directly or indirectly, the vast majority of physical, chemical, and biological processes that take place on the Earth.1−3 Atmospheric chemistry is driven by photochemical processing, often from secondary reactions involving photochemically generated radical species.4−6 Indeed, photochemistry in the literature has often been synonymous with photo-oxidation by reactive oxygen species, including the predominant hydroxyl radical (OH).7,8 Recently, however, there has been increased interest in the direct photochemistry of organic species, in both the gas and particle phase in the atmosphere, as well as at the sea surface microlayer.4,9−18 These photoactive organic species have also been shown to be capable of initiating further reactions with species that are not themselves photoactive.10,13,14,19−22 Such indirect photochemical processes include either energy transfer from the initially excited molecule to the excited state of another molecule (photosensitization), or, as is studied here, the reaction of one photochemically excited species with another, non-photoactive species (photoinitiation).
Reaction with OH is often considered to be the controlling factor governing the fate of species in the atmosphere because of its ability to react indiscriminately with most species, even those that are not particularly reactive.7 Photoexcited organic species may act similarly,23 but such processes have been subject to much less investigation in atmospheric chemistry.24,25 With notable exceptions,26,27 the contributions from organic radical reactions have rarely been compared to those of the ubiquitous OH radical reactions.26 It has been shown that, in the aqueous phase, pyruvic acid is capable of initiating polymerization of methyl vinyl ketone (MVK) that is comparable to that from the corresponding reaction initiated by OH.27 Both MVK and pyruvic acid are oxidation products of isoprene.28−31 MVK is a highly reactive species that, upon radical initiation, rapidly forms high molecular weight products.19,27,29,32−44 Pyruvic acid, an α-keto acid whose photochemistry has been studied extensively,9,16,45−56 reacts in the aqueous phase to generate oligomeric species via radical recombination.9,51−56 In this study, we show that α-keto acids can act as radical initiators, not only for highly reactive species like MVK, but also for relatively unreactive lipids, such as fatty acids and fatty alcohols, leading to the generation of covalently bonded molecules from the cross-reaction.
The reactions of these surface-active species are of particular interest because of the ubiquity of fatty acids and fatty alcohols in the sea surface microlayer18,57,58 as well as their ability to partition and persist on atmospheric aerosol particles.58−62 The chemistry of these simple lipids, especially the fatty acids, has been studied to examine their volatile products, but comparatively little attention has been paid to the condensed phase products. The formation of higher molecular weight products through photoinitiated processes, as shown here, has the potential to modify the surfactant layer and contribute to secondary organic aerosol formation. Beyond this, the cross-reactions between a photoinitiator species and non-photoactive species that are discussed in this work show the disproportionately large effect a species may have on the overall reactivity of a mixture, even when the photoactive species is only a minor component.
Results and Discussion
The aqueous phase photochemistry of α-keto acids, including pyruvic acid and OOA, has been studied in detail,9,51−56,63,64 allowing for mechanistic understanding of the available reactive pathways52−54,65 and their dependence on reaction conditions, such as solution pH56 and the presence of oxygen.54 The photochemistry of these species is characterized largely by the formation of radicals that recombine to form oligomeric species, including covalently bonded dimers and trimers.66 Here, we examine the ability of these α-keto acids to initiate reactions with the fatty acids and fatty alcohols, hexanoic acid, nonanoic acid, 1-hexanol, and 1-nonanol, which themselves do not absorb solar actinic radiation.
In addition to the mixed solutions of α-keto acids and fatty acid or alcohol, control solutions consisting of the individual fatty acids and alcohols were also photolyzed. While fatty acids and fatty alcohols are not generally considered photoactive species within the relevant actinic spectrum, it has recently been suggested that some fatty acids, including nonanoic acid, may undergo photochemistry themselves, perhaps due to an enhancement of the triplet state at the air–water interface.11,21,67 Our experimental conditions were chosen to probe bulk phase photochemistry and, therefore, used relatively dilute concentrations of all species. The concentrations used here for the C9 species may prevent observation of minor photochemical pathways. Under our experimental conditions, the distilled fatty acids and fatty alcohols do not appear to undergo photochemistry, with no changes observed in either NMR or ESI– MS between the pre- and post-photolysis solutions (Figures S1–S8, Tables S1–S5). We also note that the higher concentration control of 100 mM nonanoic acid dissolved in methanol shows no photochemical reactivity under our experimental conditions (Figure S9).
Each of the fatty acids and fatty alcohols investigated has weak absorbance in their respective UV–vis absorption spectra at ∼270 nm (Figures S10 and S11), which is slightly decreased in intensity following distillation (Figure S12). For nonanoic acid, this peak has previously been assigned to its triplet state.67,68 It is intriguing that the fatty alcohols also have a peak in this same wavelength region given that their electronic structures are quite different. Interestingly, upon photolysis of 20 mM hexanoic acid, while no new photoproducts are detected by NMR or ESI– MS, the 270 nm peak in the UV–vis absorption spectra is preferentially depleted compared to the acid peak at ∼204 nm (Figure S13). Such behavior can be explained if the observed 270 nm peak was due, at least in part, to an impurity. Regardless, these experimental controls make it clear that under our conditions, any observed photochemistry for the fatty acids and fatty alcohols stems from the α-keto acids acting as radical initiators.
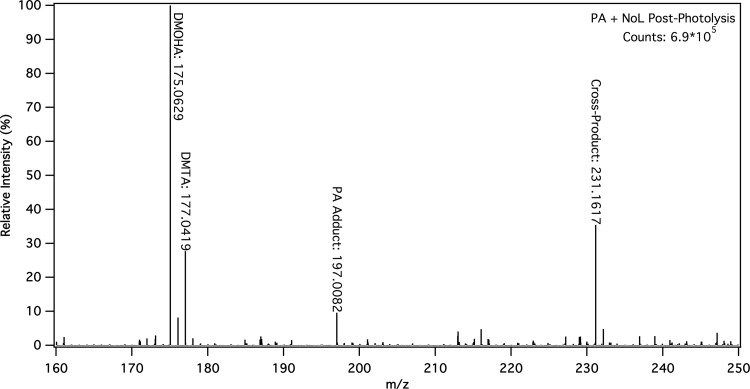
As observed both by NMR and ESI– MS (Figure 1, Figures S14–S23), the mixed solutions of fatty acids/alcohols with an α-keto acid initiator are dominated by photoproducts generated by radical recombination reactions between α-keto acid species (Figures S24 and S25).53,65 Detailed ESI– MS results are given in the Supporting Information (Tables S1–S5). It is not surprising that reactions between photoactive species dominate the observed results for these photolyses. However, new photoproducts are observed for the mixed solutions that are not present in either the α-keto acid or fatty acid/alcohol control photolyses. Namely, as shown in Table 1, for each of the mixed solutions under consideration here, a new analyte is observed with an m/z corresponding to the molecular formula expected for the cross-product between the α-keto acid and the fatty acid/alcohol, evidence that organic radicals generated by photolysis of α-keto acids can react with fatty acids and alcohols. Figure 1 shows representative ESI– MS data for the post-photolysis solution of 0.5 mM pyruvic acid and 0.8 mM nonanol, showing both the expected products for the photolysis of pyruvic acid, as well as the cross-product between pyruvic acid and nonanol (theoretical m/z = 231.1602). Full spectra, including pre-photolysis and methanol controls, are given for all photolyses between α-keto acid and fatty acid/alcohol in Figures S19–S23.
Figure 1.
Representative ESI– MS of a solution of 0.5 mM pyruvic acid and 0.8 mM nonanol after 5 h of photolysis highlighting the formation of the mixed cross-product between the two species compared to major pyruvic acid photolysis products, dimethyl-tartaric acid (DMTA) and 2,4-dihydroxy-2-methyl-5-oxohexanoic acid (DMOHA).52,53
Table 1. ESI– MS Data for the Cross-Product between α-Keto Acid Initiator and Fatty Acid or Fatty Alcohol Species.
| assigned formula [M – H]− | theor. m/z | exp. m/z | mass diff. (ppm) | pre-hνa | post-hνa | |
|---|---|---|---|---|---|---|
| pyruvic acid + hexanoic acid | C9H15O5− | 203.0925 | 203.0926 | 0.5 | Mb | |
| pyruvic acid + hexanol | C9H17O4− | 189.1132 | 189.1143 | 5.6 | M | |
| pyruvic acid + nonanoic acid | C12H21O5− | 245.1394 | 245.1400 | 2.3 | M | |
| pyruvic acid + nonanol | C12H23O4− | 231.1602 | 231.1607 | 2.2 | M | |
| OOA + nonanol | C17H33O4− | 301.2384 | 301.2390 | 1.9 | M | |
| OOA + methanol | C9H17O4− | 189.1132 | 189.1139 | 3.5 | W | S |
Signal intensities are given as weak (W), medium (M), or strong (S) as defined in the Supporting Information. Blank entries mean no signal was observed above the threshold intensity.
There is also weak overlapping photoproduct generated with the same chemical formula in the control photolysis of pyruvic acid.
While not included in these results, we expect the cross-product of OOA and nonanoic acid to also be formed. However, the expected molecular formula from this product matches that of one of the major photoproducts of OOA, making its detection difficult. Similarly, there is a very minor product generated by the photolysis of pyruvic acid with the same chemical formula as the cross-product of pyruvic acid and hexanoic acid. The intensity observed by ESI– MS is, however, increased dramatically in the mixed photolysis case, suggesting the new cross-product is, indeed, formed. It is unlikely that this change is simply due to differences in ionization efficiency, given the similar intensities observed for the other analytes across solution conditions. The cross-products for pyruvic acid with hexanol, nonanoic acid, and nonanol, as well as for OOA with nonanol, are observed following photolysis without any interference from photoproducts generated from the individual α-keto acids.
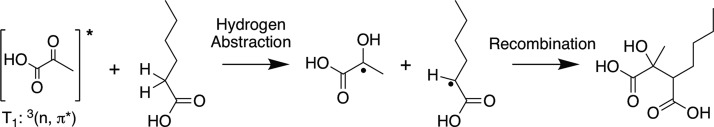
We propose that this cross-product is formed by hydrogen abstraction from the ground state fatty acid/alcohol by the triplet state α-keto acid (T1), leading to radical formation. This radical can then recombine with an α-keto acid radical in solution, forming the cross-product. A proposed mechanism for this process is shown for hexanoic acid and pyruvic acid in Scheme 1. The favored site of hydrogen abstraction on carboxylic acids is a matter of some debate in the literature.69−73 We have drawn hydrogen abstraction as occurring from the α-position of hexanoic acid, as is generally assumed to be favored for longer-tailed carboxylic acids.69,71
Scheme 1. Proposed Hydrogen Abstraction and Recombination Pathway to Form the Cross-Product between Hexanoic Acid and Pyruvic Acid.
There is sufficient energy for the α-keto acid triplet state to induce hydrogen abstraction. Electronic structure calculations suggest that this is, in fact, possible. CBS-QB3 calculations for hydrogen abstraction from the α-position of propionic acid by triplet state pyruvic acid show that because of the relatively strong O–H bond that is formed after abstraction, this process is exothermic by ∼17 kcal/mol. It is worth noting that the calculation of the OH bond strength in this context requires that the heat of formation of the radical be compared with that of a hydrogen atom and pyruvic acid in its T1 state, not the ground state as would be usual for a bond energy calculation. The longer-tailed fatty acids ought to behave similarly to propionic acid, and it is likely that hydrogen abstraction can occur analogously for the fatty alcohols. While hydrogen abstraction may be favored at the α-position, ESI– MS does not give structural information. Therefore, it is possible that the observed cross-products are due to a mix of constitutional isomers resulting from hydrogen abstraction at other sites on the molecule.
In principle, the radicals generated from hydrogen abstraction from the fatty acid/alcohol could recombine with each other to form the corresponding dimer product; however, these products are not observed here by ESI– MS. This is likely because under our experimental conditions it is more probable that these radicals would first encounter a radical generated from an α-keto acid species. Also, any such oligomeric products may not be detected by ESI– MS, especially those derived from the fatty alcohols, if they are not readily ionizable.
In addition to the solutions of α-keto acids and fatty acids dissolved in water, 6 mM OOA was dissolved in methanol and photolyzed at 4 °C. The photoproducts observed with the highest intensity by ESI– MS are the same species generated during the aqueous photolysis of OOA (Figure S26, Table S6). However, there is also an observable analyte that corresponds to the cross-product between OOA and methanol (exp. m/z = 189.1139). It is difficult to speculate on the relative ability of OOA to abstract a hydrogen from methanol compared to the other fatty alcohols under study here because the ratio of methanol is much larger than the 2:1 used in the other cases. However, the ability of OOA to cross-react with methanol is intriguing. Methanol is generally thought of as quite inert, so this observed cross-reaction is suggestive that the organic intermediates generated by the photolysis of α-keto acids are highly capable of hydrogen abstraction for many species beyond those considered here.
The ability of a few photoactive molecules to induce further reactions of non-photoactive species is of importance when considering the chemistry of air–water interfaces, such as those found on the SML or atmospheric aerosols. Lipids, such as those under study here, partition preferentially to air–water interfaces, and there is a considerable enrichment of organic material at surfaces.74 Higher localized concentrations make it more likely for an organic radical to encounter and react with another species before being quenched, which may further enhance the effect of a low concentration of photoinitiators. Beyond favorable reaction conditions, the SML, aerosols, and clouds provide a rich mixture of species for potential reaction,17,58,75,76 expanding beyond simple single-molecule systems. In this way, we are able to bring our fundamental mechanistic understanding of a model chemical system to bear on more complicated reactions in the natural environment.
Conclusions
Here, we have focused on the condensed phase products generated by photoinitiated reactions between α-keto acids and other, non-photoactive organic surface active species, driving the abiotic increase in the molecular complexity of the system through broadband irradiation by a solar simulator. We have shown for five different cases that the cross-product between α-keto acid initiator and non-photoactive species is formed photochemically in aqueous solution. In addition, photolysis of 2-oxooctanoic acid dissolved in methanol is also observed to form the corresponding cross-product. The higher molecular weight products formed from photoinitiated processes are likely to be more surface-active than the simple lipids used as starting materials. This chemistry will further modify aqueous surfaces and generates organic material that could contribute to the formation of secondary organic aerosol. Direct photochemistry of organic molecules, such as α-keto acids, appears to be readily able to initiate reactions with even relatively unreactive species, including methanol. The impact of such photoinitiation by organic species on overall reactivity is not currently considered in atmospheric chemical processing. The results presented here suggest that under some conditions organic photochemistry may be competitive with other reactive processes, including reaction with hydroxyl radical. Further work is needed to better quantify the role that organic radical reactions and photoinitiated processes may play in the overall reactivity of organic species in the atmosphere, especially with comparison to OH.
Methods
Pyruvic acid (98%), hexanoic acid (>99.5%), 1-hexanol (hexanol, 98%), nonanoic acid (96%), and 1-nonanol (nonanol, 98%) were obtained from Sigma-Aldrich and distilled by heating under reduced pressure (<1 Torr) to remove impurities. Pyruvic acid was distilled at <55 °C and used within one month of distillation to ensure oligomers from dark processes were minimized.77 Hexanol was heated to ∼50 °C, hexanoic acid to ∼80 °C, and nonanoic acid and nonanol were heated to ∼180 °C during distillation. In each case noticeable yellow impurities were removed upon distillation, leaving a clear, colorless liquid. Representative NMR spectra before and after distillation are shown in Figures S27–S30. 2-Oxooctanoic acid (OOA, ≥ 99.0%, Sigma-Aldrich) was used without further purification.
Photolysis solutions were made in a ∼1:2 ratio of α-keto acid initiator to fatty acid or alcohol, in the combinations reported in Table 2. The ratio of α-keto acid initiator to nonanol was slightly less than 1:2 because of solubility concerns. Control solutions of 10 mM pyruvic acid, 0.5 mM OOA, 20 mM hexanoic acid, 20 mM hexanol, 1 mM nonanoic acid, and 0.8 mM nonanol were also photolyzed, as were solutions of 6 mM OOA and 100 mM nonanoic acid dissolved in methanol (Fisher Scientific, 99.9%). Solutions were made using 18.2 MΩ (3 ppb TOC) water and then sonicated until fully dissolved. All solutions were used at the natural pH of the solution without further adjustment.
Table 2. Experimental Solution Compositions.
| α-keto acid conc. (mM) | fatty acid/alcohol conc. (mM) | |
|---|---|---|
| pyruvic acid and hexanoic acid (high) | 10 | 20 |
| pyruvic acid and hexanoic acid (low) | 0.5 | 1 |
| pyruvic acid and hexanol | 10 | 20 |
| pyruvic acid and nonanoic acid | 0.5 | 1 |
| pyruvic acid and nonanol | 0.5 | 0.8 |
| OOA and nonanol | 0.5 | 0.9 |
Photolyses were conducted as described in Rapf et al. 2017.53 Briefly, for each experiment, 100 mL of solution were prepared and ∼10 mL were saved as a pre-photolysis control. The remaining solution was photolyzed for 5 h using an unfiltered 450 W Xe arc lamp (Newport). It has previously been shown that photolysis with unfiltered light gives the same photoproducts as are observed when using a lamp equipped with a Pyrex filter to cut out light of wavelengths <300 nm.54,67 Higher concentration solutions were photolyzed in a temperature-stabilized water bath at 4 °C, while the lower concentration solutions were photolyzed at 20 °C due to solubility concerns for nonanoic acid and nonanol. Comparison of hexanoic acid photolyses at both temperatures showed no differences in products with changing water bath temperature. All solutions were purged with N2 beginning 1 h before the start of photolysis and continuing throughout the experiment to eliminate oxygen from the reactor. Oxygen-limited conditions favor the formation of oligomeric products from the aqueous photochemistry of α-keto acids,54 which allows for easier identification and analysis of minor products, such as those under study here. No unexpected or unusually high safety hazards were encountered during the course of this study.
A variety of analytical techniques, including UV–vis and NMR spectroscopy and high-resolution negative mode electrospray ionization mass spectrometry (ESI– MS), were used to characterize solutions. Instrument parameters for these techniques are given in the Supporting Information.
Analysis of mass spectrometry data followed the procedure outlined in Rapf et al., 2017.53 Aqueous solutions were diluted 1:1 with methanol prior to injection in the instrument. Photolysis samples that were conducted in methanol were also diluted with additional methanol (1:1) prior to analysis. In addition to the pre- and post-photolysis samples, methanol blanks of pure methanol were obtained before injection of samples and used to ensure the peaks observed were not due to prior contamination or carry-over of material from previous experiments. It is worth noting that ESI– MS experiments are of limited use for identifying the fatty alcohols, hexanol and nonanol, because they are not expected to deprotonate under the ionization conditions used here. However, no differences were observed in the MS for these control experiments upon irradiation, and this was further confirmed by NMR spectra.
All ions assigned to a particular analyte were within at least 15 ppm of the theoretical mass for each experiment with typical mass differences of <8 ppm. The experimental m/z and mass differences reported in Table 1 and Tables S1–S6 are average values combined across all experiments in which the analyte was detected. The analyses conducted here were not designed to be absolutely quantitative. Therefore, analyte intensities are only used for relative comparison, as they may not correlate directly with species concentration.
In addition to the experimental studies, electronic structure calculations for hydrogen abstraction from the α-position of propionic acid by triplet state pyruvic acid were also conducted. All calculations were performed with the Gaussian 09 suite of programs78 and used the CBS-QB3 method of Petersson and co-workers.79 The details of these calculations are provided in Tables S7–S10.
Acknowledgments
We thank Dr. Allison Reed Harris for her valuable edits to the manuscript. We also thank Dr. Jeremy L. Balsbaugh and the University of Colorado Boulder Central Analytical Laboratory Mass Spectrometry Core Facility (partially funded by NIH S10 RR026641) for mass spectrometry measurements and advice about analysis. Financial support was provided by the National Science Foundation (CHE 1611107), the National Aeronautics and Space Administration under Grant No. NNX15AP20G issued through the Habitable Worlds Program. This material is based upon work supported in part by the U.S. Army Research Laboratory and the U.S. Army Research Office under Grant Number W911NF1710115. R.J.R. also acknowledges support by NASA Headquarters under the NASA Earth and Space Science Fellowship Program – Grant NNX13AP85H, as well as partial support from the Marion L. Sharrah Fellowship and the CIRES Graduate Student Research Award.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acscentsci.8b00124.
Instrument parameters for the analytical techniques (UV–vis and NMR spectroscopy and high resolution ESI– mass spectrometry), UV–vis, NMR, and additional high resolution ESI– mass spectra, as well as detailed tables of the mass spectrometry analysis and electronic structure calculations (PDF)
Author Present Address
§ (R.J.R.) Chemical Sciences Division, Lawrence Berkeley National Lab, Berkeley, CA, 94720, USA.
Author Present Address
⊥ (R.J.P.) Department of Atmospheric Science, Colorado State University, Fort Collins, Colorado, 80523, USA.
Author Present Address
∥ (M.R.D.) Department of Chemistry and Biochemistry, University of California San Diego, La Jolla, California, 92093, USA.
The authors declare no competing financial interest.
Supplementary Material
References
- Miller S. L.; Urey H. C. Organic compound synthesis on the primitive early earth. Science 1959, 130, 245–251. 10.1126/science.130.3370.245. [DOI] [PubMed] [Google Scholar]
- Deamer D.; Weber A. L. Bioenergetics and life’s origins. Cold Spring Harbor Perspect. Biol. 2010, 2, 1–16. 10.1101/cshperspect.a004929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree G. W.; Lewis N. S. Solar energy conversion. Phys. Today 2007, 60, 37–42. 10.1063/1.2718755. [DOI] [Google Scholar]
- George C.; Ammann M.; D’Anna B.; Donaldson D. J.; Nizkorodov S. A. Heterogeneous photochemistry in the atmosphere. Chem. Rev. 2015, 115, 4218–4258. 10.1021/cr500648z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann H.; Hoffmann D.; Schaefer T.; Bräuer P.; Tilgner A. Tropospheric aqueous-phase free-radical chemistry: Radical sources, spectra, reaction kinetics and prediction tools. ChemPhysChem 2010, 11, 3796–3822. 10.1002/cphc.201000533. [DOI] [PubMed] [Google Scholar]
- Vaida V. Spectroscopy of photoreactive systems: Implications for atmospheric chemistry. J. Phys. Chem. A 2009, 113, 5–18. 10.1021/jp806365r. [DOI] [PubMed] [Google Scholar]
- Gligorovski S.; Strekowski R.; Barbati S.; Vione D. Environmental implications of hydroxyl radicals (• OH). Chem. Rev. 2015, 115, 13051–13092. 10.1021/cr500310b. [DOI] [PubMed] [Google Scholar]
- Finlayson-Pitts B. J.; Pitts J. N.. Chemistry of the Upper and Lower Atmosphere; Academic Press: San Diego, 1999. [Google Scholar]
- Reed Harris A. E.; Pajunoja A.; Cazaunau M.; Gratien A.; Pangui E.; Monod A.; Griffith E. C.; Virtanen A.; Doussin J. F.; Vaida V. Multiphase photochemistry of pyruvic acid under atmospheric conditions. J. Phys. Chem. A 2017, 121, 3327–3339. 10.1021/acs.jpca.7b01107. [DOI] [PubMed] [Google Scholar]
- Bernard F.; Ciuraru R.; Boréave A.; George C. Photosensitized formation of secondary organic aerosols above the air/water interface. Environ. Sci. Technol. 2016, 50, 8678–8686. 10.1021/acs.est.6b03520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu R.; Tinel L.; Gonzalez L.; Ciuraru R.; Bernard F.; George C.; Volkamer R. UV photochemistry of carboxylic acids at the air-sea boundary: A relevant source of glyoxal and other oxygenated VOC in the marine atmosphere. Geophys. Res. Lett. 2017, 44, 1079–1087. 10.1002/2016GL071240. [DOI] [Google Scholar]
- Ciuraru R.; Fine L.; Pinxteren M. v.; D’Anna B.; Herrmann H.; George C. Unravelling new processes at interfaces: Photochemical isoprene production at the sea surface. Environ. Sci. Technol. 2015, 49, 13199–13205. 10.1021/acs.est.5b02388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu H.; Ciuraru R.; Dupart Y.; Passananti M.; Tinel L.; Rossignol S.; Perrier S.; Donaldson D. J.; Chen J.; George C. Photosensitized production of atmospherically reactive organic compounds at the air/aqueous interface. J. Am. Chem. Soc. 2015, 137, 8348–8351. 10.1021/jacs.5b04051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciuraru R.; Fine L.; van Pinxteren M.; D’Anna B.; Herrmann H.; George C. Photosensitized production of functionalized and unsaturated organic compounds at the air-sea interface. Sci. Rep. 2015, 5, 12741. 10.1038/srep12741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George C.; D’Anna B.; Herrmann H.; Weller C.; Vaida V.; Donaldson D.; Bartels-Rausch T.; Ammann M., Emerging areas in atmospheric photochemistry. In Atmospheric and Aerosol Chemistry; Springer: Berlin Heidelberg, 2014; pp 1–53. [DOI] [PubMed] [Google Scholar]
- Reed Harris A. E.; Doussin J.-F.; Carpenter B. K.; Vaida V. Gas-phase photolysis of pyruvic acid: The effect of pressure on reaction rates and products. J. Phys. Chem. A 2016, 120, 10123–10133. 10.1021/acs.jpca.6b09058. [DOI] [PubMed] [Google Scholar]
- Giorio C.; Monod A.; Brégonzio-Rozier L.; DeWitt H. L.; Cazaunau M.; Temime-Roussel B.; Gratien A.; Michoud V.; Pangui E.; Ravier S.; et al. Cloud processing of secondary organic aerosol from isoprene and methacrolein photooxidation. J. Phys. Chem. A 2017, 121, 7641–7654. 10.1021/acs.jpca.7b05933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbaghzadeh B.; Upstill-Goddard R. C.; Beale R.; Pereira R.; Nightingale P. D. The atlantic ocean surface microlayer from 50°n to 50°s is ubiquitously enriched in surfactants at wind speeds up to 13 ms–1. Geophys. Res. Lett. 2017, 44, 2852–2858. 10.1002/2017GL072988. [DOI] [Google Scholar]
- Renard P.; Siekmann F.; Gandolfo A.; Socorro J.; Salque G.; Ravier S.; Quivet E.; Clement J. L.; Traikia M.; Delort A. M.; et al. Radical mechanisms of methyl vinyl ketone oligomerization through aqueous phase OH-oxidation: On the paradoxical role of dissolved molecular oxygen. Atmos. Chem. Phys. 2013, 13, 6473–6491. 10.5194/acp-13-6473-2013. [DOI] [Google Scholar]
- Tinel L.; Rossignol S.; Ciuraru R.; Dumas S.; George C. Photosensitized reactions initiated by 6-carboxypterin: Singlet and triplet reactivity. Phys. Chem. Chem. Phys. 2016, 18, 17105–17115. 10.1039/C6CP03119F. [DOI] [PubMed] [Google Scholar]
- Tinel L.; Rossignol S.; Bianco A.; Passananti M.; Perrier S.; Wang X.; Brigante M.; Donaldson D. J.; George C. Mechanistic insights on the photosensitized chemistry of a fatty acid at the air/water interface. Environ. Sci. Technol. 2016, 50, 11041–11048. 10.1021/acs.est.6b03165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malecha K. T.; Nizkorodov S. A. Feasibility of photosensitized reactions with secondary organic aerosol particles in the presence of volatile organic compounds. J. Phys. Chem. A 2017, 121, 4961–4967. 10.1021/acs.jpca.7b04066. [DOI] [PubMed] [Google Scholar]
- Formosinho S. J. Photochemical hydrogen abstractions as radiationless transitions. Part 1: Ketones, aldehydes and acids. J. Chem. Soc., Faraday Trans. 2 1976, 72, 1313–1331. 10.1039/F29767201313. [DOI] [Google Scholar]
- Chen H.; Ge X.; Ye Z. Aqueous-phase secondary organic aerosol formation via reactions with organic triplet excited states: A short review. Current Pollution Reports 2018, 4, 8–12. 10.1007/s40726-018-0079-7. [DOI] [Google Scholar]
- Silaev M. Kinetics of nonbranched-chain processes of the free-radical addition to molecules of alkenes, formaldehyde, and oxygen with competing reactions of resulting 1:1 adduct radicals with saturated and unsaturated components of the binary reaction system 2017, 4, 569–588. 10.21276/ijirem.2017.4.1.6. [DOI] [Google Scholar]
- Epstein S. A.; Nizkorodov S. A. A comparison of the chemical sinks of atmospheric organics in the gas and aqueous phase. Atmos. Chem. Phys. 2012, 12, 8205–8222. 10.5194/acp-12-8205-2012. [DOI] [Google Scholar]
- Renard P.; Reed Harris A. E.; Rapf R. J.; Ravier S.; Demelas C.; Coulomb B.; Quivet E.; Vaida V.; Monod A. Aqueous phase oligomerization of methyl vinyl ketone by atmospheric radical reactions. J. Phys. Chem. C 2014, 118, 29421–29430. 10.1021/jp5065598. [DOI] [Google Scholar]
- Lee W.; Baasandorj M.; Stevens P. S.; Hites R. A. Monitoring OH-initiated oxidation kinetics of isoprene and its products using online mass spectrometry. Environ. Sci. Technol. 2005, 39, 1030–1036. 10.1021/es049438f. [DOI] [PubMed] [Google Scholar]
- Altieri K. E.; Carlton A. G.; Lim H.-J.; Turpin B. J.; Seitzinger S. P. Evidence for oligomer formation in clouds: Reactions of isoprene oxidation products. Environ. Sci. Technol. 2006, 40, 4956–4960. 10.1021/es052170n. [DOI] [PubMed] [Google Scholar]
- Ervens B.; Carlton A. G.; Turpin B. J.; Altieri K. E.; Kreidenweis S. M.; Feingold G. Secondary organic aerosol yields from cloud-processing of isoprene oxidation products. Geophys. Res. Lett. 2008, 35, L02816. 10.1029/2007GL031828. [DOI] [Google Scholar]
- Carlton A. G.; Turpin B. J.; Lim H.-J.; Altieri K. E.; Seitzinger S. Link between isoprene and secondary organic aerosol (SOA): Pyruvic acid oxidation yields low volatility organic acids in clouds. Geophys. Res. Lett. 2006, 33, L06822. 10.1029/2005GL025374. [DOI] [Google Scholar]
- Carlton A. G.; Wiedinmyer C.; Kroll J. H. A review of secondary organic aerosol (SOA) formation from isoprene. Atmos. Chem. Phys. 2009, 9, 4987–5005. 10.5194/acp-9-4987-2009. [DOI] [Google Scholar]
- Ortiz-Montalvo D. L.; Lim Y. B.; Perri M. J.; Seitzinger S. P.; Turpin B. J. Volatility and yield of glycolaldehyde SOA formed through aqueous photochemistry and droplet evaporation. Aerosol Sci. Technol. 2012, 46, 1002–1014. 10.1080/02786826.2012.686676. [DOI] [Google Scholar]
- Renard P.; Siekmann F.; Salque G.; Demelas C.; Coulomb B.; Vassalo L.; Ravier S.; Temime-Roussel B.; Voisin D.; Monod A. Aqueous-phase oligomerization of methyl vinyl ketone through photooxidation: Part 1: Aging processes of oligomers. Atmos. Chem. Phys. 2015, 15, 21–35. 10.5194/acp-15-21-2015. [DOI] [Google Scholar]
- El Haddad I.; Yao L.; Nieto-Gligorovski L.; Michaud V.; Temime-Roussel B.; Quivet E.; Marchand N.; Sellegri K.; Monod A. In-cloud processes of methacrolein under simulated conditions: Part 2: Formation of secondary organic aerosol. Atmos. Chem. Phys. 2009, 9, 5107–5117. 10.5194/acp-9-5107-2009. [DOI] [Google Scholar]
- Altieri K.; Seitzinger S.; Carlton A.; Turpin B.; Klein G.; Marshall A. Oligomers formed through in-cloud methylglyoxal reactions: Chemical composition, properties, and mechanisms investigated by ultra-high resolution FT-ICR mass spectrometry. Atmos. Environ. 2008, 42, 1476–1490. 10.1016/j.atmosenv.2007.11.015. [DOI] [Google Scholar]
- Perri M. J.; Seitzinger S.; Turpin B. J. Secondary organic aerosol production from aqueous photooxidation of glycolaldehyde: Laboratory experiments. Atmos. Environ. 2009, 43, 1487–1497. 10.1016/j.atmosenv.2008.11.037. [DOI] [Google Scholar]
- Tan Y.; Carlton A. G.; Seitzinger S. P.; Turpin B. J. SOA from methylglyoxal in clouds and wet aerosols: Measurement and prediction of key products. Atmos. Environ. 2010, 44, 5218–5226. 10.1016/j.atmosenv.2010.08.045. [DOI] [Google Scholar]
- Tan Y.; Perri M. J.; Seitzinger S. P.; Turpin B. J. Effects of precursor concentration and acidic sulfate in aqueous glyoxal– OH radical oxidation and implications for secondary organic aerosol. Environ. Sci. Technol. 2009, 43, 8105–8112. 10.1021/es901742f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim Y. B.; Tan Y.; Turpin B. J. Chemical insights, explicit chemistry, and yields of secondary organic aerosol from OH radical oxidation of methylglyoxal and glyoxal in the aqueous phase. Atmos. Chem. Phys. 2013, 13, 8651–8667. 10.5194/acp-13-8651-2013. [DOI] [Google Scholar]
- Donaldson D.; Valsaraj K. T. Adsorption and reaction of trace gas-phase organic compounds on atmospheric water film surfaces: A critical review. Environ. Sci. Technol. 2010, 44, 865–873. 10.1021/es902720s. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Chen Z.; Zhao Y. Laboratory simulation for the aqueous OH-oxidation of methyl vinyl ketone and methacrolein: Significance to the in-cloud SOA production. Atmos. Chem. Phys. 2010, 10, 9551–9561. 10.5194/acp-10-9551-2010. [DOI] [Google Scholar]
- Liu Y.; Siekmann F.; Renard P.; El Zein A.; Salque G.; El Haddad I.; Temime-Roussel B.; Voisin D.; Thissen R.; Monod A. Oligomer and SOA formation through aqueous phase photooxidation of methacrolein and methyl vinyl ketone. Atmos. Environ. 2012, 49, 123–129. 10.1016/j.atmosenv.2011.12.012. [DOI] [Google Scholar]
- Chan K. M.; Huang D. D.; Li Y. J.; Chan M. N.; Seinfeld J. H.; Chan C. K. Oligomeric products and formation mechanisms from acid-catalyzed reactions of methyl vinyl ketone on acidic sulfate particles. J. Atmos. Chem. 2013, 70, 1–18. 10.1007/s10874-013-9248-7. [DOI] [Google Scholar]
- Chang X.-P.; Fang Q.; Cui G. Mechanistic photodecarboxylation of pyruvic acid: Excited-state proton transfer and three-state intersection. J. Chem. Phys. 2014, 141, 154311. 10.1063/1.4898085. [DOI] [PubMed] [Google Scholar]
- Guzman M. I.; Hoffmann M. R.; Colussi A. J. Photolysis of pyruvic acid in ice: Possible relevance to CO and CO2 ice core record anomalies. J. Geophys. Res. 2007, 112, D10123. 10.1029/2006JD007886. [DOI] [Google Scholar]
- Davidson R. S.; Goodwin D.; De Violet P. F. The mechanism of the photo-induced decarboxylation of pyruvic acid in solution. Chem. Phys. Lett. 1981, 78, 471–474. 10.1016/0009-2614(81)85239-6. [DOI] [Google Scholar]
- Yamamoto S.; Back R. A. The photolysis and thermal decomposition of pyruvic acid in the gas phase. Can. J. Chem. 1985, 63, 549–554. 10.1139/v85-089. [DOI] [Google Scholar]
- Vesley G. F.; Leermakers P. A. Photochemistry of alpha-keto acids and alpha-keto esters 3. Photolysis of pyruvic acid in vapor phase. J. Phys. Chem. 1964, 68, 2364–2366. 10.1021/j100790a507. [DOI] [Google Scholar]
- Leermakers P. A.; Vesley G. F. Photolysis of pyruvic acid in solution. J. Org. Chem. 1963, 28, 1160–1161. [Google Scholar]
- Guzman M. I.; Colussi A. J.; Hoffmann M. R. Photoinduced oligomerization of aqueous pyruvic acid. J. Phys. Chem. A 2006, 110, 3619–3626. [DOI] [PubMed] [Google Scholar]
- Griffith E. C.; Carpenter B. K.; Shoemaker R. K.; Vaida V. Photochemistry of aqueous pyruvic acid. Proc. Natl. Acad. Sci. U. S. A. 2013, 110, 11714–11719. 10.1073/pnas.1303206110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapf R. J.; Perkins R. J.; Carpenter B. K.; Vaida V. Mechanistic description of photochemical oligomer formation from aqueous pyruvic acid. J. Phys. Chem. A 2017, 121, 4272–4282. 10.1021/acs.jpca.7b03310. [DOI] [PubMed] [Google Scholar]
- Reed Harris A. E.; Ervens B.; Shoemaker R. K.; Kroll J. A.; Rapf R. J.; Griffith E. C.; Monod A.; Vaida V. Photochemical kinetics of pyruvic acid in aqueous solution. J. Phys. Chem. A 2014, 118, 8505–8516. 10.1021/jp502186q. [DOI] [PubMed] [Google Scholar]
- Eugene A. J.; Guzman M. I. Reactivity of ketyl and acetyl radicals from direct solar actinic photolysis of aqueous pyruvic acid. J. Phys. Chem. A 2017, 121, 2924–2935. 10.1021/acs.jpca.6b11916. [DOI] [PubMed] [Google Scholar]
- Rapf R. J.; Dooley M. R.; Kappes K.; Perkins R. J.; Vaida V. pH dependence of the aqueous photochemistry of α-keto acids. J. Phys. Chem. A 2017, 121, 8368–8379. 10.1021/acs.jpca.7b08192. [DOI] [PubMed] [Google Scholar]
- Cochran R. E.; Laskina O.; Jayarathne T.; Laskin A.; Laskin J.; Lin P.; Sultana C.; Lee C.; Moore K. A.; Cappa C. D.; Bertram T. H.; Prather K. A.; Grassian V. H.; Stone E. A. Analysis of organic anionic surfactants in fine and coarse fractions of freshly emitted sea spray aerosol. Environ. Sci. Technol. 2016, 50, 2477–2486. 10.1021/acs.est.5b04053. [DOI] [PubMed] [Google Scholar]
- Cochran R. E.; Laskina O.; Trueblood J. V.; Estillore A. D.; Morris H. S.; Jayarathne T.; Sultana C. M.; Lee C.; Lin P.; Laskin J.; et al. Molecular diversity of sea spray aerosol particles: Impact of ocean biology on particle composition and hygroscopicity. Chem. 2017, 2, 655–667. 10.1016/j.chempr.2017.03.007. [DOI] [Google Scholar]
- Tervahattu H.; Hartonen K.; Kerminen V. M.; Kupiainen K.; Aarnio P.; Koskentalo T.; Tuck A. F.; Vaida V. New evidence of an organic layer on marine aerosols. J. Geophys. Res. 2002, 107, 4053–4060. 10.1029/2000JD000282. [DOI] [Google Scholar]
- Tervahattu H.; Juhanoja J.; Vaida V.; Tuck A. F.; Niemi J. V.; Kupiainen K.; Kulmala M.; Vehkamaki H. Fatty acids on continental sulfate aerosol particles. J. Geophys. Res. 2005, 110, D06207 10.1029/2004JD005400. [DOI] [Google Scholar]
- Gilman J. B.; Eliason T. L.; Fast A.; Vaida V. Selectivity and stability of organic films at the air-aqueous interface. J. Colloid Interface Sci. 2004, 280, 234–243. 10.1016/j.jcis.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Gilman J. B.; Tervahattu H.; Vaida V. Interfacial properties of mixed films of long-chain organics at the air-water interface. Atmos. Environ. 2006, 40, 6606–6614. 10.1016/j.atmosenv.2006.05.052. [DOI] [Google Scholar]
- Closs G. L.; Miller R. J. Photo-reduction and photodecarboxylation of pyruvic acid - applications of CIDNP to mechanistic photochemistry. J. Am. Chem. Soc. 1978, 100, 3483–3494. 10.1021/ja00479a033. [DOI] [Google Scholar]
- Griffith E. C.; Rapf R. J.; Shoemaker R. K.; Carpenter B. K.; Vaida V. Photoinitiated synthesis of self-assembled vesicles. J. Am. Chem. Soc. 2014, 136, 3784–3787. 10.1021/ja5006256. [DOI] [PubMed] [Google Scholar]
- Rapf R. J.; Perkins R. J.; Yang H.; Miyake G. M.; Carpenter B. K.; Vaida V. Photochemical synthesis of oligomeric amphiphiles from alkyl oxoacids in aqueous environments. J. Am. Chem. Soc. 2017, 139, 6946–6959. 10.1021/jacs.7b01707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- If not specified, the terms “dimer” and “trimer” are used here to refer to covalently bonded oligomeric species, rather than noncovalently associated species.
- Rossignol S.; Tinel L.; Bianco A.; Passananti M.; Brigante M.; Donaldson D. J.; George C. Atmospheric photochemistry at a fatty acid–coated air-water interface. Science 2016, 353, 699–702. 10.1126/science.aaf3617. [DOI] [PubMed] [Google Scholar]
- Xiao P.; Wang Q.; Fang W.-H.; Cui G. Quantum chemical investigation on photochemical reactions of nonanoic acids at air–water interface. J. Phys. Chem. A 2017, 121, 4253–4262. 10.1021/acs.jpca.7b03123. [DOI] [PubMed] [Google Scholar]
- Brocks J. J.; Beckhaus H.-D.; Beckwith A. L.; Rüchardt C. Estimation of bond dissociation energies and radical stabilization energies by ESR spectroscopy. J. Org. Chem. 1998, 63, 1935–1943. 10.1021/jo971940d. [DOI] [Google Scholar]
- Ogata Y.; Tomizawa K.; Takagi K. Photo-oxidation of formic, acetic, and propionic acids with aqueous hydrogen peroxide. Can. J. Chem. 1981, 59, 14–18. 10.1139/v81-003. [DOI] [Google Scholar]
- Sun W.; Yang L.; Yu L.; Saeys M. Ab initio reaction path analysis for the initial hydrogen abstraction from organic acids by hydroxyl radicals. J. Phys. Chem. A 2009, 113, 7852–7860. 10.1021/jp8090792. [DOI] [PubMed] [Google Scholar]
- Alvarez-Idaboy J. R.; Mora-Diez N.; Boyd R. J.; Vivier-Bunge A. On the importance of prereactive complexes in molecule-radical reactions: Hydrogen abstraction from aldehydes by OH. J. Am. Chem. Soc. 2001, 123, 2018–2024. 10.1021/ja003372g. [DOI] [PubMed] [Google Scholar]
- Butkovskaya N. I.; Kukui A.; Pouvesle N.; Le Bras G. Rate constant and mechanism of the reaction of OH radicals with acetic acid in the temperature range of 229–300 K. J. Phys. Chem. A 2004, 108, 7021–7026. 10.1021/jp048444v. [DOI] [Google Scholar]
- Donaldson D. J.; Vaida V. The influence of organic films at the air-aqueous boundary on atmospheric processes. Chem. Rev. 2006, 106, 1445–1461. 10.1021/cr040367c. [DOI] [PubMed] [Google Scholar]
- Quinn P. K.; Collins D. B.; Grassian V. H.; Prather K. A.; Bates T. S. Chemistry and related properties of freshly emitted sea spray aerosol. Chem. Rev. 2015, 115, 4383–4399. 10.1021/cr500713g. [DOI] [PubMed] [Google Scholar]
- Cochran R. E.; Ryder O. S.; Grassian V. H.; Prather K. A. Sea spray aerosol: The chemical link between the oceans, atmosphere, and climate. Acc. Chem. Res. 2017, 50, 599–604. 10.1021/acs.accounts.6b00603. [DOI] [PubMed] [Google Scholar]
- Perkins R. J.; Shoemaker R. K.; Carpenter B. K.; Vaida V. Chemical equilibria and kinetics in aqueous solutions of zymonic acid. J. Phys. Chem. A 2016, 120, 10096–10107. 10.1021/acs.jpca.6b10526. [DOI] [PubMed] [Google Scholar]
- Frisch M. J.; Trucks G. W.; Schlegel H. B.; Scuseria G. E.; Robb M. A.; Cheeseman J. R.; Scalmani G.; Barone V.; Petersson G. A.; Nakatsuji H., et al. Gaussian 09, revision d.01; Gaussian, Inc.: Wallingford, CT, 2016. [Google Scholar]
- Montgomery J. A.; Frisch M. J.; Ochterski J. W.; Petersson G. A. A complete basis set model chemistry. VI. Use of density functional geometries and frequencies. J. Chem. Phys. 1999, 110, 2822–2827. 10.1063/1.477924. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.