Abstract
Background
Compared to other pulmonary function tests, there is a lack of standardization regarding how a maximum voluntary ventilation (MVV) maneuver is performed. Specifically, little is known about the variation in breathing frequency (fR) and its potential impact on the accuracy of test results. This study examines the effect of several preselected values for fR and one self-selected fR (fRself) on MVV.
Methods
Ten participants performed MVV maneuvers at various fR values, ranging from 50 to 130 breaths·min− 1 in 10 breaths·min− 1 intervals and at one fRself. Three identical trials with 2-min rest periods were conducted at each fR, and the sequence in which fR was tested was randomized. Ventilation and related parameters were measured directly by gas exchange analysis via a metabolic measurement system.
Results
A third-order polynomial regression analysis showed that MVV = − 0.0001(fR)3 + 0.0258(fR)2–1.38(fR) + 96.9 at preselected fR and increased up to approximately 100 breaths·min− 1 (r2 = 0.982, P < 0.001). Paired t-tests indicated that average MVV values obtained at all preselected fR values, but not fRself, were significantly lower than the average maximum value across all participants. A linear regression analysis revealed that tidal volume (VT) = − 2.63(MVV) + 300.4 at preselected fR (r2 = 0.846, P < 0.001); however, this inverse relationship between VT and MVV did not remain true for the self-selected fR. The VT obtained at this fR (90.9 ± 19.1% of maximum) was significantly greater than the VT associated with the most similar MVV value (at a preselected fR of 100 breaths·min− 1, 62.0 ± 10.4% of maximum; 95% confidence interval of difference: (17.5, 40.4%), P < 0.001).
Conclusions
This study demonstrates the shortcomings of the current lack of standardization in MVV testing and establishes data-driven recommendations for optimal fR. The true MVV was obtained with a self-selected fR (mean ± SD: 69.9 ± 22.3 breaths·min− 1) or within a preselected fR range of 110–120 breaths·min− 1. Until a comprehensive reference equation is established, it is advised that MVV be measured directly using these guidelines. If an individual is unable to perform or performs the maneuver poorly at a self-selected fR, ventilating within a mandated fR range of 110–120 breaths·min− 1 may also be acceptable.
Keywords: Pulmonary function test, Standardization, Breathing frequency, Exercise testing, Ventilatory reserve, Maximal exercise ventilation, Forced expiratory volume in 1 s
Background
Formerly referred to as maximum breathing capacity, maximum voluntary ventilation (MVV) is a pulmonary function test (PFT) that measures the maximum amount of air a person can inhale and then exhale with voluntary effort. The test is measured in liters per minute (L·min− 1), but data is only collected for 12–15 s and then extrapolated to 1 m in order avoid prolonged hyperventilation by the participant. While the test has been used less over the recent decades due to its fewer applications than the forced expiratory volume in 1 s (FEV1), MVV still possesses clinical utility. Performing the maneuver is contingent on several factors, including respiratory system mechanics (obstructive or restrictive) and ventilatory muscle endurance. Therefore, the test provides a broad assessment of respiratory system function [1]. Abnormal MVV results are valuable in evaluating various neuromuscular disorders [2–4] and predicting the risk for postoperative complications [1, 5]. MVV also remains useful in cardiopulmonary exercise testing as a measure of ventilatory capacity, especially in determining an individual’s ventilatory reserve—the difference between MVV and maximal exercise ventilation ()—which aids in the diagnosis and differentiation of pulmonary and cardiovascular diseases [6–10].
What primarily distinguishes MVV from other PFTs, however, is the lack of standardization regarding how the maneuver is performed. A major component of the test is breathing frequency (fR), but little consensus exists on precisely what fR or range of fR values yield optimal results. The majority of both past and current research that employed MVV does not provide the frequency at which participants ventilated as part of the methodology [11–13]. Moreover, the few studies that do provide this information offer diverging suggestions. One of the earliest investigations on this matter concluded that ventilating at 70 breaths·min− 1 maximized MVV [14]. A later study utilized an fR range of 60–120 breaths·min− 1 [15] while another argued against accepting results obtained with a frequency of less than 65 breaths·min− 1 [16]. A third investigation suggested a narrower window of 70–110 breaths·min− 1 [17]. The American Thoracic Society/European Respiratory Society (ATS/ERS) Task Force offered perhaps the most widely accepted range of 90–110 breaths·min− 1 with an ideal rate of approximately 90 breaths·min− 1, but also declared that no specific fR can be mandated due to a lack of research on the topic [18].
The limited evidence supporting an ideal fR range is problematic. Considering both the diagnostic and prognostic value of MVV, as well as its ability to assess participant compliance during pulmonary function testing [19], inaccurate results due to poor standardization may have substantial clinical implications. Consequently, it is imperative that the variety of current guidelines be evaluated. The aim of this investigation, therefore, was to determine whether there is an optimal fR at which the MVV maneuver ought to be performed, i.e., to consistently provide the highest outcome value. This was accomplished by conducting MVV tests at a wide range of fR utilizing a repeated-measures design. In addition to examining preselected fR, this study also explored the effect of a self-selected fR (fRself). Given that synchronizing breathing frequency to a rhythm-keeping device at a preset rate may feel unnatural or interfere with performance, we hypothesized that participants would maximize MVV by ventilating at a fR uniquely self-selected by each individual rather than breathing within a predetermined range.
Methods
Study design
Ten healthy, non-smoking adults (six men) were recruited from the University of California, Los Angeles (UCLA) community to participate in this study. Nine were undergraduate students aged 18–22 years old and one was a 46 year-old university employee (height 1.72 ± 0.13 m; mass 67.1 ± 16.3 kg). The UCLA Institutional Review Board approved this study, and all participants provided informed consent prior to enrollment.
Each participant performed MVV maneuvers using nine different preselected fRs ranging from 50 to 130 breaths·min− 1 in increments of 10 breaths·min− 1 as measured by a metronome. Additionally, all participants performed a maneuver using fRself. In this condition, subjects performed the test without the aid of a metronome, and were instead instructed to breathe as rapidly and deeply as possible without inducing significant discomfort. The resulting frequency was then recorded. For each frequency, including fRself, participants performed three trials for a total of 30 tests (10 per day with 2-min rest between each) over three consecutive days to allow for adequate recovery. To limit order and practice effects, a random number generator determined the sequence in which fR was tested for each participant.
Data acquisition
All data were obtained using a metabolic measurement system (Oxycon Pro™; CareFusion, Yorba Linda, CA) that underwent volume and gas composition calibrations prior to each testing session. All tests occurred in a laboratory setting where the ambient temperature and humidity were measured and entered into the system before calibration. The volume calibration was done mechanically with known volumes of air, while the composition calibration was performed using ambient air and a gas tank of 16% O2, 4% CO2 to encompass the range found in normal exhaled air. Participants were seated upright in a stationary chair, wore a nose clip, and breathed through a mouthpiece that connected to the metabolic cart. Participants were also instructed to refrain from eating or engaging in vigorous exercise at least before testing.
Prior to performing the first MVV maneuver, slow vital capacity (SVC) was measured using the same system. Participants were instructed to inhale as much air as possible in a single breath and then exhale, completely emptying the lungs. This value was obtained to compare the ratio of the tidal volume (VT) during MVV to SVC. In addition, participants took normal breaths to establish a baseline respiratory exchange ratio, which was used as a metabolic indicator to determine when a participant had achieved sufficient rest between trials. Once this value had been established, MVV testing began. For each trial, participants were instructed to maximize ventilation by inhaling and exhaling as deeply as possible for 12 s. As frequent vigorous respiration can cause dry mouth, water was provided if requested between tests. During trials defined by a preselected fR, participants listened to a corresponding preset tempo from a metronome and timed their breaths accordingly. In between each beat, both an inhalation and an exhalation were performed. During the trials associated with fRself, no metronome was used— participants freely maximized breathing while the metabolic cart recorded fR, the value of which was blinded until after a maneuver was completed. In addition to fR and MVV, which were recorded and analyzed for all trials, breath-by-breath measurements of VT and partial pressure of end-tidal CO2 (PETCO2) were also obtained for all maneuvers except those at the preselected fR of 130 breaths·min− 1 (fR130) (Table 1).
Table 1.
Outcome variables (scaled and absolute values)
| f R | MVV | VT | PETCO2 | ||
|---|---|---|---|---|---|
| (breaths·min− 1) | (%) | (L·min− 1) | (%) | (L) | (mmHg) |
| 50 | 76.2 ± 9.9*† | 125.1 ± 45.3 | 99.1 ± 1.6 | 2.4 ± 0.9 | 15.3 ± 2.0 |
| 60 | 81.7 ± 13.9† | 131.4 ± 52.0 | 88.4 ± 9.0† | 2.2 ± 0.8 | 15.0 ± 3.3 |
| 70 | 84.3 ± 11.9† | 134.1 ± 47.2 | 78.5 ± 5.4† | 1.9 ± 0.7 | 14.5 ± 2.9 |
| 80 | 87.5 ± 8.3† | 139.4 ± 50.2 | 72.1 ± 12.3*† | 1.7 ± 0.6 | 14.2 ± 2.7 |
| 90 | 91.4 ± 5.9† | 144.1 ± 49.6 | 67.7 ± 11.5*† | 1.6 ± 0.5 | 13.3 ± 2.1 |
| 100 | 93.3 ± 3.7† | 147.6 ± 51.5 | 62.0 ± 10.4*† | 1.5 ± 0.5† | 14.2 ± 2.7 |
| 110 | 92.2 ± 9.5† | 144.9 ± 48.4 | 51.9 ± 19.9*† | 1.3 ± 0.6 | 13.6 ± 2.2 |
| 120 | 90.2 ± 10.2† | 144.7 ± 58.3 | 50.5 ± 10.3*† | 1.2 ± 0.5 | 13.0 ± 2.4 |
| 130 | 81.0 ± 11.4† | 129.6 ± 52.1 | n/ab | n/ab | n/ab |
| Self-Selected (69.9 ± 22.3) | 91.1 ± 16.5 | 144.8 ± 59.4 | 90.9 ± 19.1 | 2.1 ± 0.7 | 13.7 ± 2.0 |
| Maximuma | – | 157.7 ± 52.5 | – | 2.4 ± 0.9 | 17.1 ± 2.5 |
Values are presented as mean ± SD both 1) scaled as a percentage of the maximum and 2) absolute. Statistical analysis was performed only on scaled values
All values are reported as the percent of the maximum value obtained using preselected fR values
Abbreviations: fR breathing frequency, MVV maximum voluntary ventilation, VT tidal volume, PETCO2 partial pressure of end-tidal CO2
*P < 0.05 when compared to self-selected fR
†P < 0.05 when compared to maximum
aHighest value obtained across all fR values for every participant
bValues were missing from data collection
Statistical analysis
Data for each subject were scaled relative to the maximum value recorded during preselected fR and reported as the mean ± standard deviation (SD) for all variables. Sample size was estimated from a combination of pilot testing and preliminary power calculations based on an alpha level of 0.05 and a beta level of 0.20 [20]. As suggested by the ATS/ERS Task Force [18], only the test that resulted in the highest MVV out of the three trials per fR was used for data analysis. The overall effect of fR was tested using repeated-measures analysis of variance (ANOVA); comparisons were made between the outcome value at every fR and the maximum value using paired t-tests. The average MVV values were found across subjects for each fR, and the relationship between these values and the corresponding fR and VT were analyzed using polynomial regression. The statistics were calculated in MATLAB (version 8.6.0; MathWorks, Inc., Natick, MA) and significance was determined using an alpha level of 0.05.
Results
All ten participants successfully performed three trials at all ten fR values. As demonstrated in Fig. 1, a third-order polynomial regression analysis showed that MVV = − 0.0001(fR)3 + 0.0258(fR)2–1.38(fR) + 96.9 at preselected fR and increased up to approximately 100 breaths·min− 1 (r2 = 0.982, P < 0.001). A paired t-test revealed that the MVV value obtained at an average fRself of 69.9 ± 22.3 breaths·min− 1 (91.1 ± 16.5% of maximum) was not significantly different from the value measured at the roughly equivalent preselected fR70 (84.3 ± 11.9% of maximum; 95% confidence interval of difference: (− 2.7, 16.3%), P = 0.190). When the MVV values at all fR values were compared to the average maximum value for all subjects, a repeated-measures ANOVA showed a significant effect of fR on MVV (P < 0.001). Further statistical analysis showed that results obtained at every preselected fR were significantly lower than this maximum value, but those measured at fRself were not (Table 1). If multiple comparisons are controlled for using a Bonferroni correction, all preselected fR values other than fR110 and fR120 remain significantly different from the maximum.
Fig. 1.
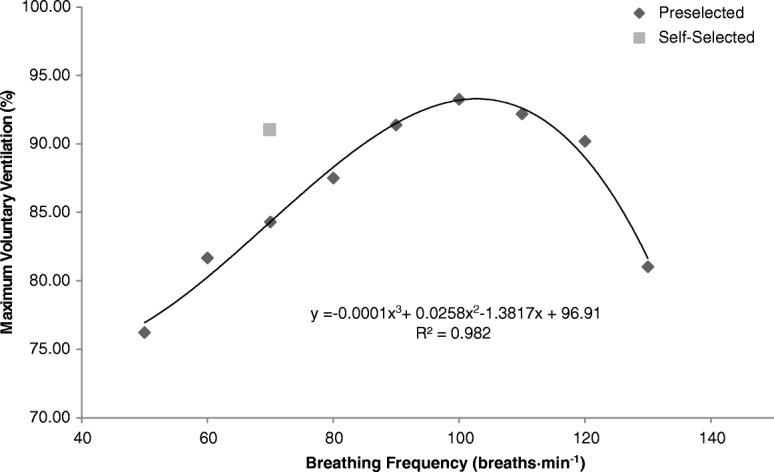
Relationship between the average maximum voluntary ventilation (MVV) across subjects and average breathing frequency (fR). MVV values are presented as a percentage (%) of the maximum. A polynomial regression analysis of the preselected fRs yielded a third-order relationship (R2 = 0.982, P < 0.001)
As demonstrated in Fig. 2, a linear regression analysis revealed that VT = − 2.63(MVV) + 300.4 at preselected fR (r2 = 0.846, P < 0.001). This steadily decreasing trend in VT was observed as the preselected fR and MVV increased, but paired t-tests showed that this inverse relationship did not remain true for fRself. The VT obtained at this fR (90.9 ± 19.1% of maximum) was significantly greater than the VT associated with the most similar MVV value (at fR100, 62.0 ± 10.4% of maximum; 95% confidence interval of difference: (17.5, 40.4%), P < 0.001). By contrast, none of the PETCO2 data measured at the preselected fR differed significantly from that of fRself, and all were significantly less than the average PETCO2 maximum.
Fig. 2.
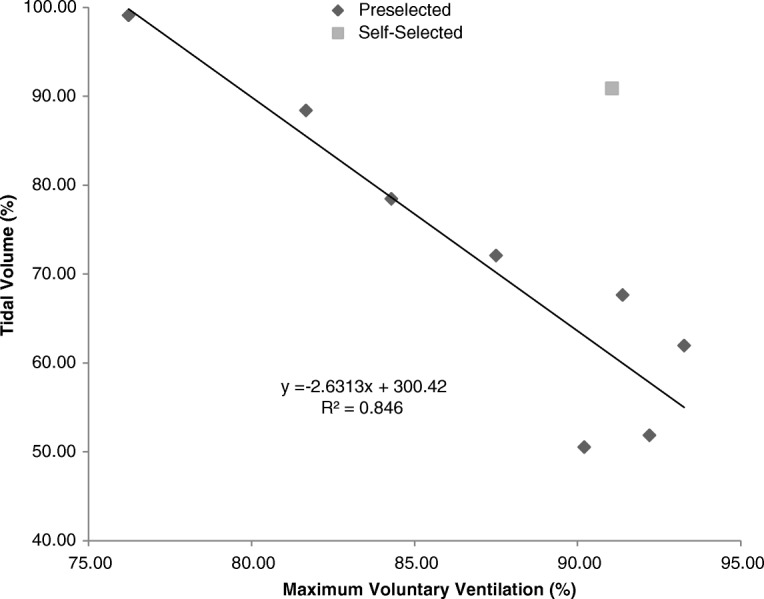
Relationship between the average tidal volume (VT) across subjects and the average maximum voluntary ventilation (MVV). VT and MVV values are presented as a percentage (%) of their respective maxima. A linear regression analysis revealed a significant decreasing trend for the preselected breathing frequencies (R2 = 0.846, P < 0.001)
Discussion
Our study, to the best of our knowledge, is the first that examines the effect of altering fR on MVV. After testing preselected fR values ranging from 50 to 130 breaths·min− 1 as dictated by a metronome and one self-selected fR by each participant, we found that trials conducted at 90–120 breaths·min− 1, and the self-selected rate were equally successful at maximizing MVV. This result differed from our hypothesis where we predicted that only fRself would yield the highest MVV. When compared to the aforementioned guidelines for optimizing MVV of 60–120, 70–110, and 90–110 breaths·min− 1 suggested by Dillard et al. (1993) [15], Morris (1976) [17], and the ATS/ERS Task Force [18] respectively, our findings disagreed with the first and second recommendations but closely resembled the third. However, our data do not support the ATS/ERS Task Force’s recommended goal of 90 breaths·min− 1. Considering that participants maximized MVV by breathing at fR90–120 and fRself, we recommend choosing either of these approaches rather than aiming for a single value of fR. Furthermore, because maximal values were obtained at fRself, our data suggest that no rhythm-keeping instrument or preselected fR range may be necessary at all.
Interestingly, while the trials defined by a fR70 yielded a significantly lower MVV than the mean maximum for all subjects, they were not statistically different than the MVV value obtained at fRself (69.9 ± 22.3 breaths·min− 1, nearly equivalent to 70 breaths·min− 1). This is likely explained by the variance, which suggests that while breathing at a self-selected rate may increase the likelihood of achieving one’s true MVV, this chosen rate may not be equal among all individuals. Another noteworthy observation stemmed from the differences in VT measured at the preselected and self-selected fR. When plotted against MVV, VT steadily decreased at the preselected fR values. This was unsurprising considering that MVV values were greater at higher fR and as fR increases, the tidal volume may decrease in order to sustain the faster rate. However, it is also possible that during exercise, increased intra-thoracic pressure and work of breathing can permit concomitant increases in fR and VT. Interestingly, the data showed that the VT associated with the MVV value obtained at fRself deviated from the trend observed at the preselected fR values—it was significantly greater than its most similar preselected counterpart (obtained at fR100). This suggests that when breathing at a self-selected rate, individuals are more likely to utilize an optimal combination of slower deep breaths and faster shallower breaths to maximize MVV. Additionally, matching the rate of one’s breathing to a metronome or other rhythm-keeping instrument can feel distracting and unnatural to the participant, especially compared to ventilating at a natural rate during an assessment of , which may decrease the likelihood of an accurate measurement [1, 21].
Unlike MVV and VT, there were no significant relationships observed between fR and PETCO2. This is somewhat unexpected as PETCO2 is known to decrease as the ventilation increases. The lack of this trend in the data may be partially explained by the missing values associated with fR130. It also is plausible that the inverse relationship between fR and PETCO2 is not evident until the rate of ventilation is much greater than what was tested in this study. Moreover, it is important to note that the missing values for VT at fR130 may not have fit the strong, negative linear correlation demonstrated in Fig. 2. Due to the sharp decrease in MVV from fR120 to fR130, it is unlikely that the corresponding reduction in VT would have been of the same magnitude; however, it is also improbable that this single data pair would have rendered the aforementioned relationship between VT and MVV non-significant.
Regarded as one of the most long-standing PFTs, equations developed to predict MVV from more widely applicable parameters, such as FEV, have existed since the mid-twentieth century. The most common and simplistic of these include MVV = FEV1 × 35 [22]; FEV1 × 37.5 [23]; and FEV1 × 40 [24]. And while some still prefer to utilize these equations over a direct assessment, substantial evidence has highlighted the limitations of such practice. These along with many similar reference equations fail to account for a number of physical characteristics that have been shown to influence MVV. The most predominant of these include height, sex, and age [25, 26]. Studies have shown that individuals who smoke [27], suffer from cystic fibrosis [28], and women who are pregnant [29] also exhibit MVV values that deviate from height-, sex- and age-matched controls. Furthermore, a growing body of literature suggests that current reference equations for MVV and other PFTs fail to account for ethnic and socioeconomic disparities in addition to ignoring the trend of increasing racial diversity [30, 31]. Investigations have derived specific prediction equations based on ethnicity, such as in Brazilian [11], Chinese [13], Filipino [32], and African-American adolescent [12] populations, but all possess significant mathematical differences from one another. The recurring shortcoming of these equations is their lack of cohesion—no comprehensive equation exists that successfully incorporates all of the aforementioned variables. As a result, a number of studies have argued that MVV is best measured directly [19, 28, 33].
It is important to note that the results from this investigation possess similar limitations to those outlined above, including a homogenous participant cohort and the inability to account for differences in physical characteristics due to a small sample size. Further research ought to examine whether a self-selected fR is as accurate and more efficient than a preselected range of fR in a larger, more heterogeneous population. Future investigations should also explore other methods to standardize the assessment of MVV, for instance, an optimal test duration and the feasibility of a definitive reference equation. Until then, we advise that participants perform the maneuver preferably at a self-selected fR or within a preselected range of 90–120 breaths·min− 1.
Conclusion
Although not as prominent as in decades past, MVV remains a clinically relevant PFT whose outcomes are valuable in cardiopulmonary exercise testing and aid in the diagnosis of various neuromuscular, cardiovascular, and pulmonary diseases. This study demonstrates the shortcomings of the current lack of standardization in MVV testing and establishes data-driven recommendations for optimal fR. While classic literature has previously investigated this topic, the antiquity of these works warrants modern research. Furthermore, what these studies suggest as an optimal measurement technique has been overlooked or forgotten in the current guidelines. Our paper therefore contributes a new and much-needed focus on an evidence-based approach to selecting the optimal fR for MVV measurement. We recommend that participants perform an MVV maneuver at a self-selected fR as it maximizes the likelihood of an accurate measurement by optimizing a combination of slower deep breaths and faster shallower breaths, eliminates the necessity to synchronize breaths to a rhythm-keeping device, and more closely resembles the procedure to obtain . If an individual is unable to perform or performs the maneuver poorly at a self-selected fR, ventilating within a mandated fR range of 110–120 breaths·min− 1 may also be acceptable.
Acknowledgements
We would like to express our gratitude to Andrew Chang and Jonathan Lee for their assistance with data collection.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- ATS/ERS
American Thoracic Society/European Respiratory Society
- FEV1
Forced expiratory volume in 1 s
- FR
Breathing frequency
- fRself
Self-selected breathing frequency
- fRX
Preselected breathing frequency of X breaths·min−1
- MVV
Maximum voluntary ventilation
- PETCO2
Partial pressure of end-tidal CO2
- PFT
Pulmonary function test
- SD
Standard deviation
- SVC
Slow vital capacity
- UCLA
University of California, Los Angeles
Maximal exercise ventilation
- VT
Tidal volume
Authors’ contributions
All authors made substantial contributions to this study and manuscript. The study was conceived and designed by BAD and CBC. EVN and WS collected and analyzed the data. EVN, BAD, WS, and CBC composed significant portions of the manuscript and made crucial edits. All authors also read and approved the final version of the manuscript.
Ethics approval and consent to participate
This study was approved by the UCLA Institutional Review Board. All participants provided informed consent prior to enrollment.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Eric V. Neufeld, Email: eneufeld8@ucla.edu
Brett A. Dolezal, Phone: +1.310.741.8954, Email: BDolezal@mednet.ucla.edu
William Speier, Email: speier@ucla.edu.
Christopher B. Cooper, Email: CCooper@mednet.ucla.edu
References
- 1.Mottram C. Ruppel’s manual of pulmonary function testing. 11th ed. St. Louis: Elsevier Health Sciences; 2017. p. 66-67.
- 2.Braun NM, Arora NS, Rochester DF. Respiratory muscle and pulmonary function in polymyositis and other proximal myopathies. Thorax. 1983;38:616–623. doi: 10.1136/thx.38.8.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Serisier DE, Mastaglia FL, Gibson GJ. Respiratory muscle function and ventilatory control. I in patients with motor neurone disease. II in patients with myotonic dystrophy. Q J Med. 1982;51:205–226. [PubMed] [Google Scholar]
- 4.Rochester DF, Esau SA. Assessment of ventilatory function in patients with neuromuscular disease. Clin Chest Med. 1994;15:751–763. [PubMed] [Google Scholar]
- 5.Bevacqua BK. Pre-operative pulmonary evaluation in the patient with suspected respiratory disease. Indian J Anaesth. 2015;59:542–549. doi: 10.4103/0019-5049.165854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh VN. The role of gas analysis with exercise testing. Prim Care. 2001;28:159–179. doi: 10.1016/S0095-4543(05)70012-9. [DOI] [PubMed] [Google Scholar]
- 7.Ferrazza AM, Martolini D, Valli G, Palange P. Cardiopulmonary exercise testing in the functional and prognostic evaluation of patients with pulmonary diseases. Respiration. 2009;77:3–17. doi: 10.1159/000186694. [DOI] [PubMed] [Google Scholar]
- 8.Toma N, Bicescu G, Enache R, Dragoi R, Cinteza M. Cardiopulmonary exercise testing in differential diagnosis of dyspnea. Maedica (Buchar) 2010;5:214–218. [PMC free article] [PubMed] [Google Scholar]
- 9.Arena R, Sietsema KE. Cardiopulmonary exercise testing in the clinical evaluation of patients with heart and lung disease. Circulation. 2011;123:668–680. doi: 10.1161/CIRCULATIONAHA.109.914788. [DOI] [PubMed] [Google Scholar]
- 10.Myers J. Applications of cardiopulmonary exercise testing in the management of cardiovascular and pulmonary disease. Int J Sports Med. 2005;26(Suppl 1):S49–S55. doi: 10.1055/s-2004-830515. [DOI] [PubMed] [Google Scholar]
- 11.Neder JA, Andreoni S, Lerario MC, Nery LE. Reference values for lung function tests. II. Maximal respiratory pressures and voluntary ventilation. Braz J Med Biol Res. 1999;32:719–727. doi: 10.1590/S0100-879X1999000600007. [DOI] [PubMed] [Google Scholar]
- 12.Fulton JE, Pivarnik JM, Taylor WC, Snider SA, Tate AL, Frankowski RF. Prediction of maximum voluntary ventilation (MVV) in African-American adolescent girls. Pediatr Pulmonol. 1995;20:225–233. doi: 10.1002/ppul.1950200405. [DOI] [PubMed] [Google Scholar]
- 13.Kor AC, Ong KC, Earnest A, Wang YT. Prediction of the maximal voluntary ventilation in healthy adult Chinese subjects. Respirology. 2004;9:76–80. doi: 10.1111/j.1440-1843.2003.00532.x. [DOI] [PubMed] [Google Scholar]
- 14.Bernstein L, D'Silva JL, Mendel D. The effect of the rate of breathing on the maximum breathing capacity determined with a new spirometer. Thorax. 1952;7:255–262. doi: 10.1136/thx.7.3.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dillard TA, Hnatiuk OW, McCumber TR. Maximum voluntary ventilation. Spirometric determinants in chronic obstructive pulmonary disease patients and normal subjects. Am Rev Respir Dis. 1993;147:870–875. doi: 10.1164/ajrccm/147.4.870. [DOI] [PubMed] [Google Scholar]
- 16.Miller WF, Johnson RL, Jr, Wu N. Relationships between maximal breathing capacity and timed expiratory capacities. J Appl Physiol. 1959;14:510–516. doi: 10.1152/jappl.1959.14.4.510. [DOI] [PubMed] [Google Scholar]
- 17.Morris JF. Spirometry in the evaluation of pulmonary function. West J Med. 1976;125:110–118. [PMC free article] [PubMed] [Google Scholar]
- 18.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 19.Campbell SCA. Comparison of the maximum voluntary ventilation with the forced expiratory volume in one second: an assessment of subject cooperation. J Occup Med. 1982;24:531–533. [PubMed] [Google Scholar]
- 20.Dupont WD, Plummer WD., Jr Power and sample size calculations. A review and computer program. Control Clin Trials. 1990;11:116–128. doi: 10.1016/0197-2456(90)90005-M. [DOI] [PubMed] [Google Scholar]
- 21.Johnson BD, Weisman IM, Zeballos RJ, Beck KC. Emerging concepts in the evaluation of ventilatory limitation during exercise: the exercise tidal flow-volume loop. Chest. 1999;116:488–503. doi: 10.1378/chest.116.2.488. [DOI] [PubMed] [Google Scholar]
- 22.Clark TJ, Freedman S, Campbell EJ, Winn RR. The ventilatory capacity of patients with chronic airways obstruction. Clin Sci. 1969;36:307–316. [PubMed] [Google Scholar]
- 23.Burrows B, Strauss RH, Niden AH. Chronic obstructive lung disease 1, 2: III. Interrelationships of pulmonary function data. Am Rev Respir Dis. 1965;91:861–868. doi: 10.1164/arrd.1965.91.6.861. [DOI] [PubMed] [Google Scholar]
- 24.Jones NL, Jones G, Edwards RH. Exercise tolerance in chronic airway obstruction. Am Rev Respir Dis. 1971;103:477–491. doi: 10.1164/arrd.1971.103.4.477. [DOI] [PubMed] [Google Scholar]
- 25.Cherniack RM, Raber MB. Normal standards for ventilatory function using an automated wedge spirometer. Am Rev Respir Dis. 1972;106:38–46. doi: 10.1164/arrd.1972.106.1.38. [DOI] [PubMed] [Google Scholar]
- 26.Bass H. The flow volume loop: normal standards and abnormalities in chronic obstructive pulmonary disease. Chest. 1973;63:171–176. doi: 10.1378/chest.63.2.171. [DOI] [PubMed] [Google Scholar]
- 27.Hasan S, Rakkah NIAV, Attaur-Rasool S. Effect of smoking on respiratory pressures and lung volumes in young adults. Biomedica. 2013;29:96–100. [Google Scholar]
- 28.Stein R, Selvadurai H, Coates A, Wilkes DL, Schneiderman-Walker J, Corey M. Determination of maximal voluntary ventilation in children with cystic fibrosis. Pediatr Pulmonol. 2003;35:467–471. doi: 10.1002/ppul.10298. [DOI] [PubMed] [Google Scholar]
- 29.Tell A, Bagali S, Aithala M, Khodnapur J, Dhanakshirur GB. Alterations in minute ventilation, maximum voluntary ventilation and dyspneic index in different trimesters of pregnancy. Indian J Physiol Pharmacol. 2014;58:96–99. [PubMed] [Google Scholar]
- 30.Ortega VE, Kumar R. The effect of ancestry and genetic variation on lung function predictions: What is “normal” lung function in diverse human populations? Curr Allergy Asthma Rep. 2015;15:16. doi: 10.1007/s11882-015-0516-2. [DOI] [PubMed] [Google Scholar]
- 31.Duong M, Islam S, Rangarajan S, Teo K, O’Byrne PM, Schunemann HJ, Igumbor E, Chifamba J, Liu L, Li W, et al. Global differences in lung function by region (PURE): an international, community-based prospective study. Lancet Respir Med. 2013;1:599–609. doi: 10.1016/S2213-2600(13)70164-4. [DOI] [PubMed] [Google Scholar]
- 32.Roa Jr CC, Zaldivar CA, Salonga R, Bobadilla J, Lansang MA, Reodica R, Balgos A, Blanco J, TJ Q. Normal standards for ventilatory function test in adult Filipinos. Philipp J Intern Med. 2013;51:1–6. [Google Scholar]
- 33.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, van der Grinten CP, Gustafsson P, Hankinson J, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


