Abstract
Casein kinase 1α (CK1α) is a multifunctional protein belonging to the CK1 protein family that is conserved in eukaryotes from yeast to humans. It regulates signaling pathways related to membrane trafficking, cell cycle progression, chromosome segregation, apoptosis, autophagy, cell metabolism, and differentiation in development, circadian rhythm, and the immune response as well as neurodegeneration and cancer. Given its involvement in diverse cellular, physiological, and pathological processes, CK1α is a promising therapeutic target. In this review, we summarize what is known of the biological functions of CK1α, and provide an overview of existing challenges and potential opportunities for advancing theranostics.
Keywords: Casein kinase 1α, Wnt/β-catenin signaling, NF-κB signaling, Hedgehog signaling, Autophagy, Neurodegenerative disease, Cell cycle, Host defense response
Background
Casein kinase 1α (CK1α) (encoded by CSNK1A1 in humans) is a member of the CK1 family of proteins that has broad serine/threonine protein kinase activity [1–4] (Fig. 1a) and is one of the main components of the Wnt/β-catenin signaling pathway. CK1α phosphorylates β-catenin at Ser45 as part of the β-catenin destruction complex for subsequent β-transducin repeat-containing E3 ubiquitin protein ligase (β-TrCP)-mediated ubiquitination and proteasomal degradation [5, 6]. Recent studies have shown that CK1α targets p53 for degradation—which is mediated by murine double minute clone 2 (MDM2) and MDM4 (also known as MDMX) [7–10]—while stabilizing and thereby positively regulating E2F-1, a transcription factor involved in cell cycle progression [7]. Additionally, CK1α was shown to exert dual gating functions by first promoting and then terminating T cell receptor (TCR)-induced nuclear factor κB (NF-κB) activation [11]. Lenalidomide (a thalidomide analog) is a highly effective treatment for myelodysplastic syndrome with deletion of chromosome 5q [MDS del(5q)] that exerts its effects by inducing CK1α ubiquitination and degradation [12, 13]. These findings suggest that CSNK1A1 is a conditionally essential malignancy gene and a potential target for anti-cancer drugs.
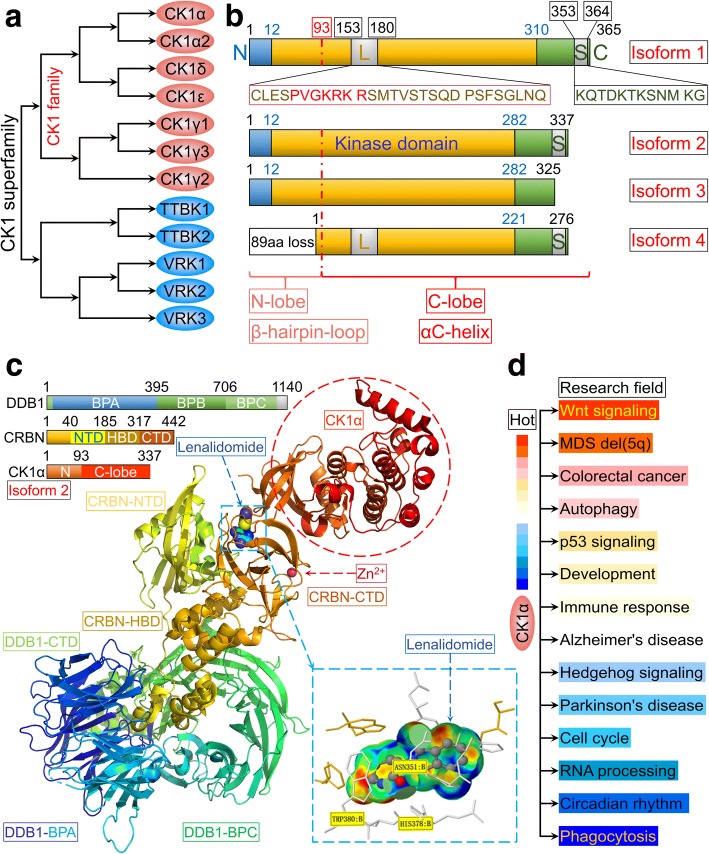
Fig. 1.
Schematic representation of CK1α. a CK1 family and CK1 superfamily. b Four isoforms of CK1α and their functional domains. c Cartoon representation of DNA damage-binding protein (DDB)1ΔBPB-CRBN-lenalidomide-CK1α. Top left, DDB1, CRBN, and CK1α domain color coding and boundaries. Bottom right, enlarged view of the CRBN-lenalidomide-CK1α interface (data were obtained from protein data bank: www.rcsb.org, PDB-ID: 5FQD; and were first published in reference [13]). d Investigations on CK1α in diverse research fields
Overview of CK1α
CSNK1A1 is located on chromosome 5q32 and is expressed as four alternatively spliced transcript variants, yielding four protein isoforms of varying length that mainly differ by the presence or absence of a 28-amino acid “L” insert in the kinase domain and a 12-amino acid “S” insert near the C terminus. The former is unique to vertebrates [14] and contains the sequence of PVGKRKR, which has the characteristics of a nuclear localization signal (NLS) and may target CK1α to the nucleus [15] (Fig. 1b). Isoform 2, which comprises 337 amino acids, is the predominant isoform [11, 13] with a kinase domain located between Ile12 and Ala282 [11]. The 2.45-Å crystal structure revealed that the first 93 amino acids form a β-hairpin loop and (especially residues 35–41) binds cullin 4/really interesting new gene-box 1/DNA damage-binding protein 1/cereblon (CRBN) (also known as CRL4CRBN) E3 ubiquitin ligase for CK1α ubiquitination and degradation [12, 13]. The C-lobe of CK1α is mainly composed of αC helices and contributes to the kinase function (Fig. 1c). CK1α phosphorylates the serine/threonine residue in the canonical motif of pS/T-X(n = 2-4)-pS/T or noncanonical motif of pS/T-X-pS/T (where pS/T is phospho-serine/threonine and X is any amino acid) [16, 17]. The basic residues (K229KQK232) of CK1α are implicated in canonical substrate recognition [17], but the noncanonical substrate with pS/T-X-pS/T motif such as β-catenin is not significantly affected by mutations in the K229KQK232 stretch [17, 18].
CK1α is widely expressed in various organelles including the cell membrane and nucleus [15]. It also localizes to the centrosome, microtubules, the Golgi apparatus, and endoplasmic reticulum in non-neuronal interphase cells [19, 20]; in synaptic vesicles in neurons [20]; spindle microtubules at mitosis [21]; and to nuclear structures (e.g., nuclear speckles) [22]. CK1α is ubiquitously expressed and is constitutively active [23, 24], implying that it has many biological functions besides its role in β-catenin degradation that span diverse research areas (Fig. 1d).
Physiological and pathological expression of CK1α in humans
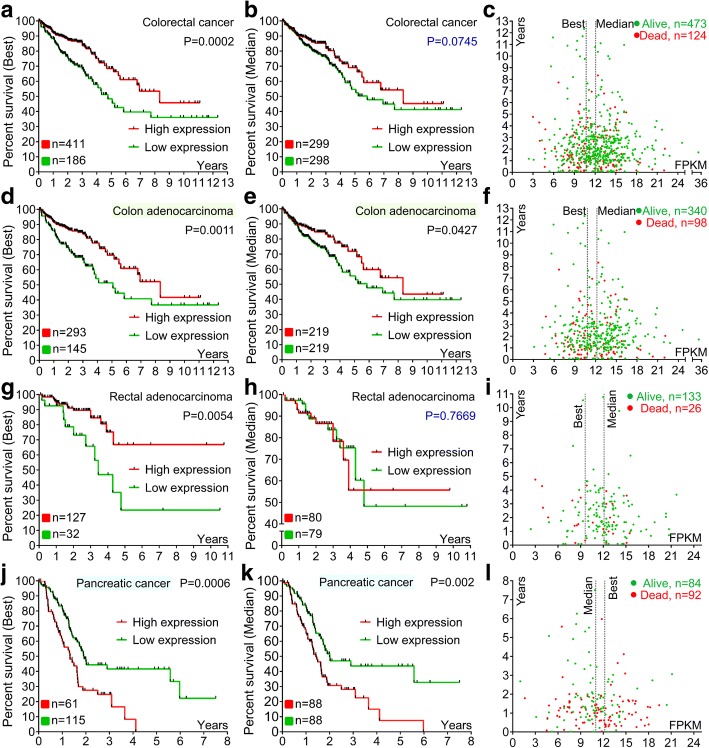
CK1α mRNA is expressed in all tissues in humans under physiological conditions; the levels are high in esophagus and skin, but low in pancreas and liver (Fig. 2a). The protein is highly expressed in adrenal gland, bronchus, testis, placenta, and endometrium but is not detected in smooth muscle, liver, seminal vesicle, or ovary (Fig. 2b). CK1α mRNA is expressed in most cancer tissues (Fig. 2c), and highly expressed in pancreatic cancer but is detected at low levels in colorectal cancer as compared to matched normal tissues with GeneChip arrays (Fig. 2d, e). Interestingly, low CK1α expression was associated with poorer overall survival (OS) in colorectal cancer patients (Fig. 3a–c), especially in colon adenocarcinoma (Fig. 3d–i). On the other hand, high CK1α levels in pancreatic cancer were linked to poorer OS (Fig. 3j–l), providing evidence that CK1α is a conditionally essential malignancy protein. CK1α mRNA was also found to be expressed in various cancer cell lines (Fig. 4a) and was localized to the cytosol (Fig. 4b), suggesting that it mainly functions in the cytoplasm.
Fig. 2.
CK1α expression in normal human tissues and the most common human cancer tissues. a RNA sequencing data for CK1α expressed in normal human tissues are reported as median reads per kilobase per million mapped reads (RPKM). The data were generated by the Genotype-Tissue Expression project (www.gtexportal.org) and were first published in references [238, 239] and deposited in the HPA (www.proteinatlas.org). b Protein expression data from HPA (www.proteinatlas.org), first published in reference [240]. c RNA sequencing data of CK1α levels in 17 cancer types are reported as median number of fragments per kilobase of exon per million reads (FPKM), generated by The Cancer Genome Atlas (TCGA) (https://cancergenome.nih.gov/); data were first published in reference [241], and were deposited in the HPA (www.proteinatlas.org). d, e Microarray data of CK1α expression in normal and cancer tissues in humans were obtained from Oncomine (www.oncomine.org) (reference [242]). Differences in expression levels were evaluated with the Student’s t test using Oncomine software. d Upregulation of CK1α mRNA levels in human cancer tissues relative to matched normal tissues. a, Pancreas, b, pancreatic carcinoma (left, reference [243] and right, reference [244]); c, brain; d, anaplastic astrocytoma; e, oligodendroglioma; f, glioblastoma (reference [245]). e Downregulation of CK1α mRNA levels in human cancer tissues relative to matched normal tissues. g, CD4-positive (n = 5) + CD8-positive (n = 5) + normal T lymphocytes (n = 10); h, angioimmunoblastic T-cell lymphoma; i, anaplastic large cell lymphoma (reference [246]); j, esophagus; k, esophageal squamous cell carcinoma; l, esophageal adenocarcinoma (left, reference [247]; right, reference [248]); m, colon (n = 19) + rectum (n = 3); n, rectal adenocarcinoma; o, colon adenocarcinoma (data obtained from TCGA and deposited in Oncomine); p, bladder mucosa; q, infiltrating bladder urothelial carcinoma (reference [249]); r, buccal mucosa; s, head and neck squamous cell carcinoma (reference [250])
Fig. 3.
Prognostic value of CK1α mRNA level in human colorectal and pancreatic cancers. Data were obtained from The Cancer Genome Atlas and deposited in the HPA (www.proteinatlas.org). P values were estimated with the Kaplan-Meier method. a, b, d, e, g, h, j, k Kaplan-Meier survival analysis of colorectal cancer, colon adenocarcinoma, rectal adenocarcinoma, and pancreatic cancer by best (left) and median (right) separation according to CK1α mRNA expression level. c, f, i, l Interactive survival plot (individual patient data)
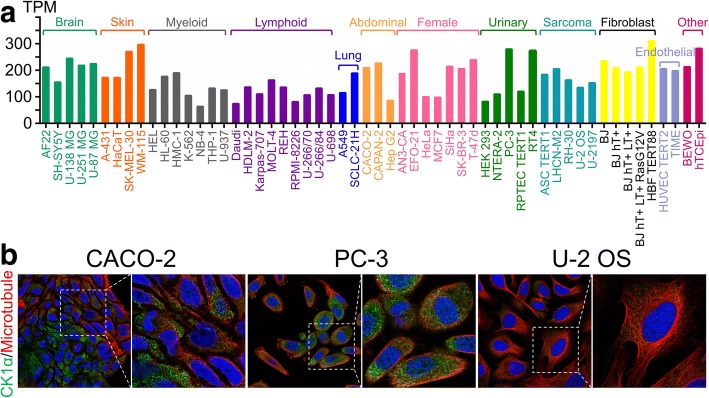
Fig. 4.
CK1α mRNA and protein expression in common cell lines. a RNA sequencing data for CK1α from the HPA (www.proteinatlas.org) are reported as number of transcripts per kilobase million. b Subcellular localization of CK1α in Caco-2, PC-3, and U-2 OS cell lines. Data were obtained from the HPA (www.proteinatlas.org) and were first published in reference [251]
CK1α in Wnt/β-catenin and hedgehog signaling
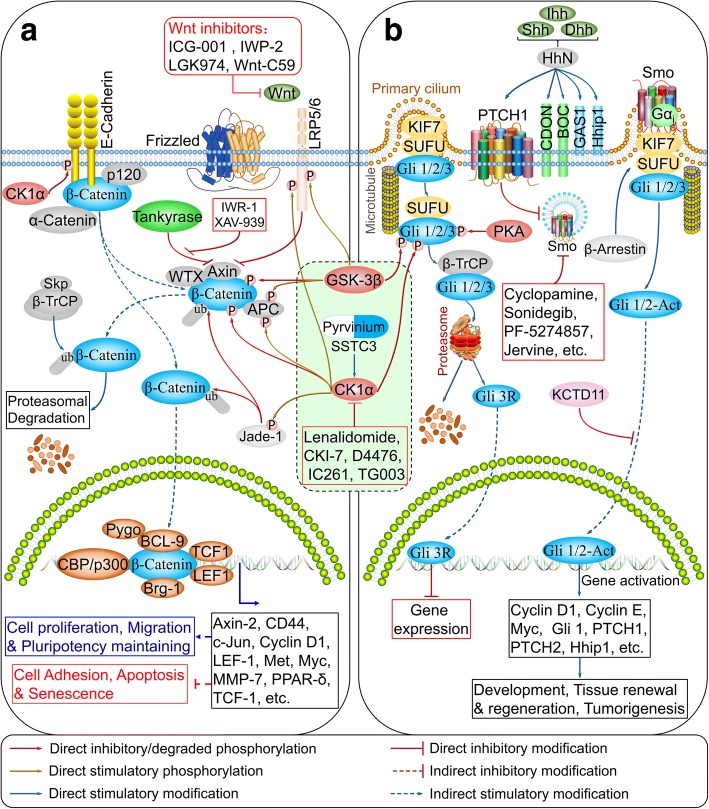
Wnt/β-catenin (also known as canonical Wnt) signaling regulates various physiological processes including embryonic development, adult stem cell maintenance, and genomic stability [25]. Mutations in Wnt pathway components such as adenomatous polyposis coli (APC) result in pathological disturbances, especially in colorectal cancer [26]. β-catenin is a key component of this pathway that binds to the cytoplasmic tail of E-cadherin at the cell membrane to promote cell-cell adhesion [27], and also localizes to the cytoplasm where it forms the destruction complex along with CK1α, glycogen synthase kinase 3β (GSK-3β), APC, Axin, and Wilms tumor gene on X chromosome (WTX, also known as APC membrane recruitment protein 1) to promote the ubiquitination and proteasomal degradation of β-catenin in the absence of extracellular Wnt ligands [28]. β-Catenin is translocated to the nucleus upon activation of Wnt signaling via Rac1 [29], where it forms a complex with T cell factor and co-activators such as cyclic (c)AMP response element-binding protein (CREB)-binding protein and BRM/SWI2-related gene 1 (Brg-1) to activate Wnt target genes [30].
β-Catenin is phosphorylated by CK1α at Ser45, which leads to GSK-3β-dependent phosphorylation at Ser33/37 and Thr41 and subsequent degradation [5]. APC is also phosphorylated at Ser1504/1505/1507 and S1510 (in the R3 region) by CK1α and other CK1 proteins [31], which is essential for β-catenin binding. Thus, CK1α acts as a negative regulator of Wnt signaling [32].
The cytoplasmic domain of E-cadherin is phosphorylated by CK1α at Ser846, which attenuates its interaction with while promoting the release of β-catenin from the cell membrane [33]. Low-density lipoprotein receptor-related protein 6 (LRP6) is a single-pass transmembrane receptor that cooperates with Frizzled proteins for Wnt ligand binding and can be phosphorylated by CK1α and CK1δ at Thr1493, which activates and promotes recruitment of Axin to the membrane in response to the Wnt signal, leading to Wnt pathway activation [34]. The plant homeodomain zinc finger protein Jade-1 functions as an E3 ubiquitin ligase that ubiquitinates both phosphorylated and non-phosphorylated forms of β-catenin [35] and is a substrate of CK1α; it is phosphorylated at Ser18 and Ser20, which reduces its ability to inhibit Wnt/β-catenin signaling [36, 37]. Thus, CK1α can act as a positive regulator of Wnt/β-catenin signaling (Fig. 5a and Table 1).
Fig. 5.
CK1α mediates crosstalk between Wnt/β-catenin and Hedgehog signaling networks. a, b CK1α in Wnt/β-catenin (a) and Hedgehog (b) signaling pathways. (also reviewed in references [41, 252])
Table 1.
Substrates of human CK1α in major cell signaling pathways
| Gene | Protein | Phosphorylation site | Pathway | Reference |
|---|---|---|---|---|
| APC | APC | S1504, S1505, S1507, S1510 | Wnt/β-Catenin | [31] |
| CTNNB1 | β-Catenin | S45 | [5, 6] | |
| CDH1 | E-cadherin | S846 | [33] | |
| LRP6 | LRP6 | T1493 | [34] | |
| JADE1 | JADE1 | S18, S20 | [36, 37] | |
| FOXO3A | FOXO3A | S318, S321 | Autophagy | [72] |
| DEPTOR | DEPTOR | S286, S287, S291 | [74, 75] | |
| SQSTM1 | SQSTM1/p62 | S349 | [79] | |
| BCL10 | BCL10 | N/A | NF-κB | [11, 260] |
| CARD11 | CARMA1 | S608 | [11, 260] | |
| MALT1 | MALT1 | N/A | [11, 260] | |
| FADD | FADD | S194 | [80, 86] | |
| RELA | NF-κB/p65 | S316 | [261] | |
| RIPK1 | RIP1 | a.a. 293–558 | [83] | |
| TNFRSF1A | TNFR1/p55 | N/A | [83] | |
| TNFRSF1B | TNFR2/p75 | N/A | [84] | |
| YBX1 | YB-1 | S176 | [89] | |
| CDC25A | CDC25A | S79, S82 | Cell cycle | [92, 93] |
| MDM2 | MDM2 | N/A | [7, 95] | |
| MDM4 | MDMX | S289 | [8, 9, 96] | |
| MYC | c-Myc | S252 | [94] | |
| TP53 | P53 | S20 | [97] | |
| YWHAQ | 14-3-3τ | S233 | [101] | |
| YWHAZ | 14-3-3ζ | T233 | [101] | |
| BACE1 | β-Secretase 1 | S498 | Alzheimer’s disease | [113] |
| KCNIP3 | Calsenilin | S63 | [115] | |
| CREB1 | CREB | S108, S111, S114 | Parkinson’s disease | [128] |
| LRRK2 | PARK8 | S910, S935, S955, S973 | [131] | |
| PARK2 | Parkin | S101, S378 | [132] | |
| SNCA | α-Synuclein | S87, S129 | [126] | |
| CDK5 | CDK5 | S159 | [134] |
The development of the Cre-LoxP system has enabled detailed investigations of the opposing functions of CK1α in Wnt signaling. For example, gut-specific knockout of CK1α using the Villin 1 promoter resulted in Wnt hyperactivation due to decreased phosphorylation of β-catenin at Ser45, Ser33/37, and Thr41 and an increment in total β-catenin levels. Accordingly, target genes of Wnt signaling such as cyclin D1, c-myc, and CD44 were induced at both the mRNA and protein levels in CK1α knockout mice [10]. Reporter-based screens of haploid human cells revealed that CK1α and APC were the rate-limiting negative regulators of Wnt signaling [38].
Hedgehog signaling is aberrantly activated in basal cell carcinomas, the most common cancer in humans [39] and in medulloblastoma, the most common pediatric brain malignancy [40]. Gli transcription factors are key mediators of Hedgehog signaling and are phosphorylated by CK1α, GSK-3β, and protein kinase A (PKA), which promote the proteolysis of the active form of Gli1/2 and induction of a repressive form of Gli3 receptor [41]. In Drosophila, CK1α suppresses Hedgehog signaling in the absence of a ligand [42, 43] and is also required for Smoothened (Smo) phosphorylation upon pathway activation [44–48]. However, Smo in mammals lacks CK1α phosphorylation sites [47].
Hedgehog signaling shares many components with the Wnt/β-catenin pathway, including CK1α, GSK-3β, and β-TrCP [49, 50]. Pyrvinium, a CK1α agonist that is known to block Wnt signaling [51], suppresses Hedgehog signaling by attenuating Gli activity [52]. Thus, CK1α functions as a negative regulator of Hedgehog signaling in mammals (Fig. 5b).
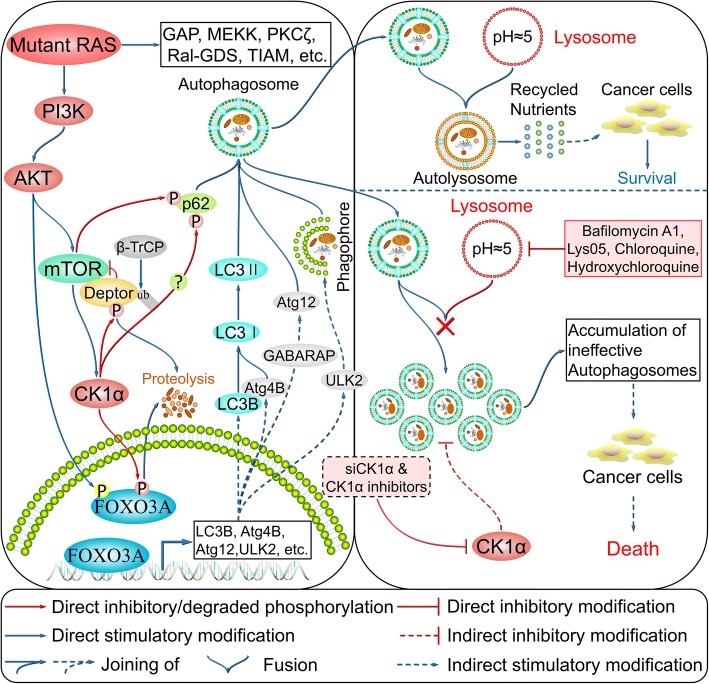
CK1α in the regulation of autophagy
Autophagy plays an important role in the maintenance of organismal homeostasis through regulation of cellular protein and organelle turnover, with their subsequent degradation by lysosomes providing macromolecular precursors and energy to cells [53]. Aberrant autophagy leads to various diseases such as cancer and neurodegeneration [54]. Autophagy is an evolutionarily conserved catabolic process that has five distinct stages: initiation, vesicle nucleation, vesicle elongation, vesicle fusion, and cargo degradation [54]. It is induced by nutrient deficiency, oxidative stress, and infection, among other factors. Vesicle nucleation is induced by an activated Unc-51-like autophagy activating kinase 1 (ULK1) complex, which consists of ULK1/2 (ortholog of yeast autophagy-related 1 [Atg1]), focal adhesion kinase family interacting protein of 200 kDa (ortholog of yeast Atg17) [55], Atg13, and Atg101 [56, 57], which is released from mammalian target of rapamycin (mTOR) inhibition [58]. Beclin-1 is then phosphorylated by ULK1 and serves as a scaffold for the class III phosphatidylinositol-3 kinase (PI3K) complex, promoting the localization of autophagy proteins to the phagophore [59]. During this process, autophagy and Beclin-1 regulator 1 binds to Beclin-1 (ortholog of yeast Atg6) to stabilize the PI3K complex, while Barkor (ortholog of yeast Atg14), ultraviolet radiation resistance-associated gene protein, and p150 (ortholog of yeast vacuolar protein sorting-associated protein 15 [Vps15]) bind to Beclin-1 to promote its interaction with Vps34 and phagophore formation [59–64]. Vesicle elongation is mediated by Atg12–Atg5 [65] and microtubule-associated protein 1A/1B-light chain 3-II (LC3-II) [66] along with LC3-like molecules such as gamma-aminobutyric acid type A receptor-associated proteins (GABARAPs) [67], leading to the formation of an autophagosome. Atg12–Atg5 conjugation is mediated by the E1-like enzyme Atg7 and E2-like enzyme Atg10 [65], while LC3B (ortholog of yeast Atg8) is cleaved at the C terminus by Atg4B protease to generate cytosolic LC3-I, which is conjugated to phosphatidylethanolamine by Atg7–Atg3, yielding lipidated LC3-II [66]. Finally, syntaxin 17 facilitates autophagosome fusion with the lysosome for autophagolysosome formation [68], with the cargo then degraded under low-pH conditions (also reviewed in references [53, 54]).
Among the above-mentioned autophagy-related genes, LC3B, GABARAPs (including GABARAP, GABARAPL1, and GABARAPL2), Atg4B, Atg12, and ULK2 were shown to be directly regulated by the transcription factor Forkhead box protein O3A (FOXO3A) [69–71], which is a CK1α substrate that is phosphorylated at Ser318 and Ser321. Treatment with the CK1 inhibitor D4476 or short interfering RNA siRNA-mediated knockdown of CK1α results in nuclear accumulation of FOXO3A and increased expression of autophagy-related genes. CK1α protein abundance is regulated by PI3K/mTOR signaling induced by activated or oncogenic/mutant RAS [72]. DEP domain-containing mTOR-interacting protein (DEPTOR), an mTOR inhibitor [73], is also phosphorylated by CK1α at Ser286/287/291 after priming phosphorylation by mTOR for subsequent degradation mediated by β-TrCP [74–76]. CK1α inhibition by D4476 or siRNA treatment results in upregulation of DEPTOR followed by suppression of mTOR signaling and induction of autophagy [75, 76]. CK1α is a key modulator of autophagic flux, and CSNK1A1 knockout mediated by transcription activator-like effector nucleases accelerated the turnover of long-lived proteins [77]. A similar observation was made in a previous study demonstrating that CSNK1A1 knockdown strongly induced autophagic flux [78]. Thus, CK1α negatively regulates autophagy.
Sequestosome 1 (SQSTM1) (also known as p62)—an autophagy adaptor/receptor and LC3-binding protein that targets specific substrates to autophagosomes [53]—is also phosphorylated by CK1 isoforms at Ser349 upon accumulation of dysfunctional proteins. Phosphorylated SQSTM1 accelerates the formation of inclusion bodies and autophagic protein clearance [79]. However, the induction of autophagy by CK1-mediated phosphorylation of SQSTM1 requires confirmation by co-immunoprecipitation and loss-of-function studies. The combination of CK1α suppression and treatment with lysosome inhibitors such as chloroquine leads to accumulation of ineffective autophagosomes that deprive cancer cells of nutrients required for growth, resulting in their death [72]. CK1α therefore is a promising target for drugs that can be used in combination with lysosome inhibitors, especially in RAS-driven and mTOR-activated cancers [72, 80] (Fig. 6 and Table 1). Notably, there is a discrepancy in the action modes of CK1α in non-small-cell lung cancer (NSCLC) versus RAS-driven colon cancer. CK1α overexpression potently induces autophagic flux in NSCLC via the PTEN/AKT/FOXO3A/Atg7 axis. It stabilizes phosphatase and tensin homolog deleted on chromosome ten (PTEN) by abrogating PTEN phosphorylation and antagonizing neural precursor cell expressed, developmentally down-regulated 4-1 (NEDD4-1) induced PTEN polyubiquitination, which suppresses NSCLC cell growth [81]. CK1α exhibits dual functions in autophagy regulation based on these evidences.
Fig. 6.
Regulation of autophagy by CK1α. (also reviewed in references [54, 72])
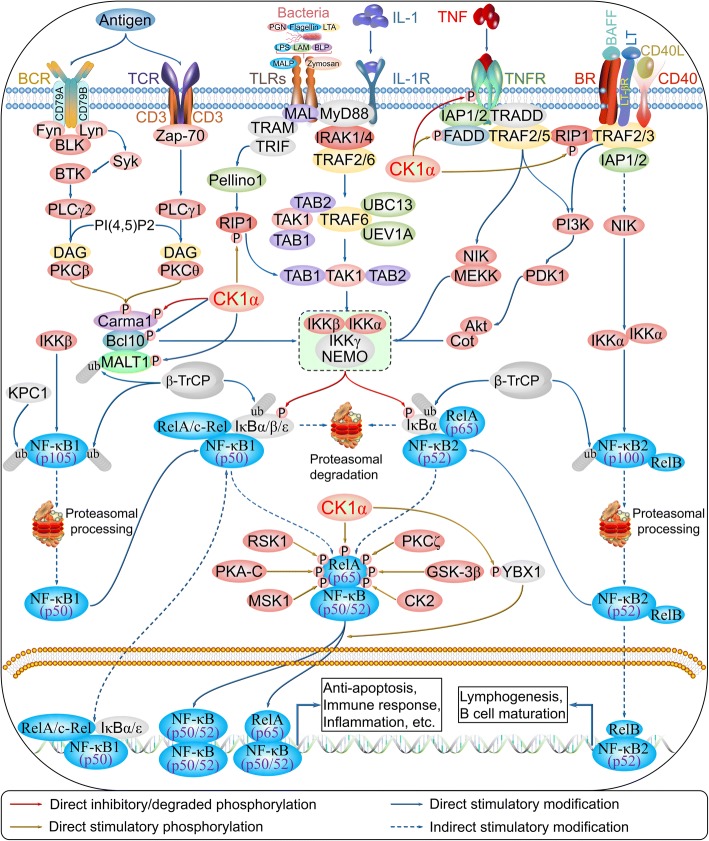
CK1α in NF-κB signaling
NF-κB signaling is a complex signaling pathway involved in innate and adaptive immunity, inflammation, lymphocyte development, and lymphoid organogenesis, and includes the components NF-κB (RelA/p65), NF-κB1 (p105/p50), NF-κB2 (p100/p52), RelB, and c-Rel [82]. NF-κB signaling is activated by various extracellular ligands and their receptors—e.g., tumor necrosis factor receptor (TNFR), interleukin (IL)-1 receptor, Toll-like receptors, B cell receptor (BCR), and TCR. This activates the inhibitor of κB kinase (IKK) complex (IKKα, IKKβ, IKKγ/NF-kappa-B essential modulator), which phosphorylates inhibitor of κBs (IκBs) and targets them for ubiquitination and proteasomal degradation. The free NF-κB/Rel complex is then modified by a series of kinases and translocated to the nucleus, where its activation alone or in combination with other transcription factors induces the expression of target genes.
TNF-α is a pro-inflammatory cytokine that activates two distinct cell surface receptors—namely, TNFR1 (p55) and TNFR2 (p75). CK1α binds to and phosphorylates TNFR1 and TNFR2, which negatively regulate TNF-α-mediated NF-κB activation [83, 84]. Receptor-interacting serine/threonine kinase 1 (RIP1) is a critical factor in programmed necrosis, but also mediates TNF-α activation of NF-κB [85]. However, RIP1 is phosphorylated by CK1α at amino acids 293–558, which potentiates TNF-α-mediated NF-κB activation [83]. These two opposing activities suggest that NF-κB signaling regulates CK1α, which also exhibits dual functions in immunoregulation. Fas-associated death domain (FADD) is an adaptor protein that transmits apoptotic signals through death receptors; it directly binds to RIP1, and mediates both necrosis and NF-κB activation. CK1α phosphorylates FADD at Ser194 [80, 86], which is essential for NF-κB activation [87]. The caspase recruitment domain family member 11 (CARD11)/B-cell chronic lymphocytic leukemia/lymphoma 10 (BCL10)/mucosa-associated lymphoid tissue lymphoma translocation gene 1 (MALT1) (CBM) signalosome complex functions as an adaptor to activate IKKs in antigen-receptor-induced NF-κB activation. Notably, CK1α has been shown to directly bind to the CBM complex leading to NF-κB activation in response to TCR stimulation in normal lymphocytes, which largely depends on the association of phosphorylated BCL10 and ubiquitinated MALT1 with CK1α. Inhibitory phosphorylation of caspase recruitment domain-containing membrane-associated guanylate kinase protein 1 at Ser608 by CK1α impairs its ability to activate NF-κB. Activated B cell-like subtype of diffuse large B-cell lymphoma (ABC DLBCL) cells require CK1α for constitutive NF-κB activity [11, 88]; additionally, the oncoprotein Y box-binding protein 1 is phosphorylated by CK1α at Ser176, resulting in NF-κB activation [89]. These findings provide evidence that CK1α has dual functions in NF-κB signaling (Fig. 7 and Table 1).
Fig. 7.
Regulation of NF-κB signaling by CK1α. (also reviewed in references [253–257])
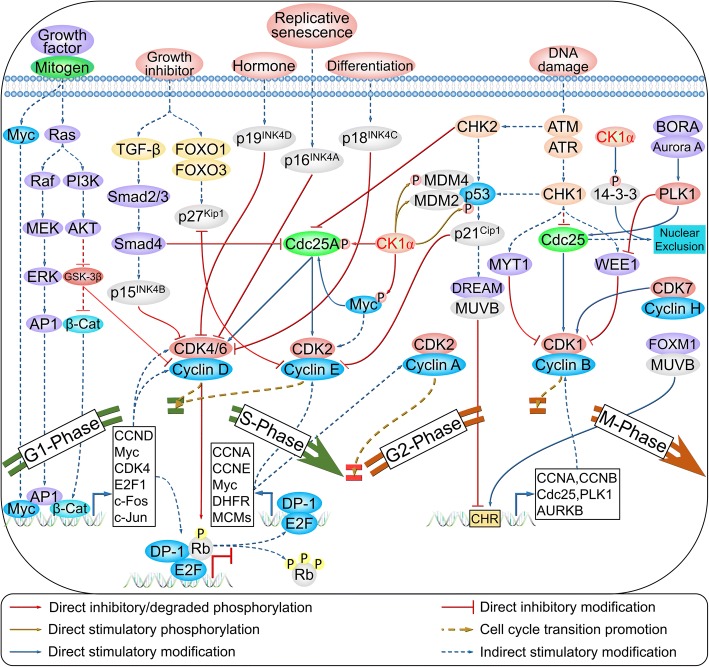
CK1α in cell cycle regulation
The mammalian cell cycle is a highly organized and regulated process initiated by mitogenic, growth, or survival signals [90] that activate downstream signaling pathways including mitogen-activated protein kinase signaling and induce the transcription of early-response genes including Myc, activator protein 1, β-catenin, c-Fos, and c-Jun. These in turn activate the expression of delayed-response genes including E2F1, cyclin D-cyclin-dependent kinase 4/6 (CDK4/6, also known as G1-CDK) complex, and cyclin E-CDK2 (also known as G1/S-CDK) complex. Cell division cycle 25 homolog A (CDC25A) potentiates the activity of G1- and G1/S-CDK to promote G1-S transition; G1/S-CDK then inactivates cyclin-dependent-kinase inhibitors (CKIs) by phosphorylation and removes the inhibition of the cyclin A-CDK2 complex (also known as S-CDK). The pre-replication complex is phosphorylated by S-CDK and dissociates to ensure duplication of genetic material and cell division. During G2 phase, the multi-vulval class B (MUVB) complex associates with forkhead box M1 (FOXM1), which binds to promoters containing a cell cycle genes homology region (CHR). This induces the transcription of genes required for G2-M cell cycle transition such as cyclin B-CDK1 (also known as M-CDK), which is activated by CDC25 family members that dephosphorylate Thr14 and Tyr15 via membrane-associated tyrosine/threonine 1 (MYT1, also known as PKMYT1) and WEE1, respectively. Meanwhile, CDK1 is phosphorylated at Thr161 by the cyclin H-CDK7 complex, leading to M phase entry.
CK1α exhibits cell cycle-dependent subcellular localization, including association with cytosolic vesicles and the nucleus during interphase and with the spindle during mitosis [20, 21, 91]. As stated above, β-catenin is a substrate of CK1α, and early-response genes including Myc and c-Jun are targets of Wnt/β-catenin signaling. CK1α also phosphorylates CDC25A at Ser79 and Ser82, which stimulates the binding of β-TrCP for subsequent ubiquitin-mediated proteolysis [92, 93]. Additionally, c-myc is phosphorylated by CK1α at Ser252 through glioma pathogenesis-related protein 1 (GLIPR1) regulation, which is critical for its degradation [94]. Thus, CK1α functions as a negative regulator in the early stages of the G1-S transition.
MDM2 and MDM4 together inhibit DNA binding and transcriptional activation of p53. Inhibition or knockdown of CK1α was shown to increase p53, MDM2, and p21 levels and lead to dephosphorylation of RB, an inhibitor of the G1-S transition [7]. It was later confirmed that treatment with D4476 triggered an increase in nuclear p53 protein level, although the upregulation of MDM2 was mainly cytoplasmic rather than nuclear [95]. This implies that CK1α interacts with MDM2 to stimulate its binding to p53, leading to ubiquitination and degradation of the latter. Moreover, MDMX is phosphorylated by CK1α at Ser289, which is necessary for the MDMX–p53 interaction and inhibition of the DNA-binding and transcriptional activity of p53 [8, 9, 96]. Thus, CK1α is a positive regulator of the G2-M transition.
p53 is directly phosphorylated by CK1α at Ser20 upon infection with human herpesvirus 6B viral [97]. Additionally, the Ser20 residue of p53 is phosphorylated by checkpoint kinase 1/2 in response to DNA damage, which enhances its tetramerization, stability, and activity [98, 99]. To date, there is no in vivo or in vitro evidence for direct phosphorylation of p53 at Ser15 by CK1α; however, this is thought to occur through regulation of F-box and WD repeat domain-containing 7 (FBXW7), which influences the cell cycle and drug resistance [100]. CK1α also phosphorylates 14-3-3τ and 14-3-3ζ at Ser23 and Thr233, respectively [101], thereby modulating their interaction with and nuclear exclusion of M-CDK (Fig. 8 and Table 1).
Fig. 8.
Cell cycle regulation by CK1α. (also reviewed in reference [90])
Jade-1 phosphorylation by CK1α and polo-like kinase 1 (PLK1) is an important biological event for cell cycle progression that involves phosphorylated FADD, which is most abundant during the G2/M phase. CK1α colocalizes with its substrate FADD, which is phosphorylated at Ser194 in metaphase and early anaphase. Suppression of kinase activity by CKI-7 or siRNA-mediated CK1α knockdown abrogates G2/M arrest induced by taxol [80, 86].
Less is known about the function of CK1α in meiosis. CK1α localizes to the spindle poles, which may not be required for meiotic progression in mammalian oocytes since RNA interference (RNAi) or overexpression of CK1α results in invalid spindle organization and chromosome segregation [102]. CK1α is activated in fertilized mouse oocytes but not in metaphase II-arrested mouse oocytes. Microinjection of a blocking antibody against CK1α during metaphase II arrest and G2 phase had no effect on the completion of the second meiosis or first division; however, injection during the early pronuclear stage prior to S phase blocked kinase entry into pronuclei and interfered with timely cell cycle progression to the first cleavage [91]. However, another study showed that CK1α was upregulated in metaphase and colocalized with condensed chromosomes during oocyte maturation and embryonic development; blocking CK1α resulted in the failure of polar body 1 (PB1) extrusion, chromosome misalignment, and metaphase II plate incrassation, while activating CK1α by pyrvinium pamoate treatment inhibited oocyte meiotic maturation and caused severe abnormalities in congression and chromosome misalignment [103].
Suppression of CK1α in the gut triggers Wnt hyperactivation but does not lead to tumorigenesis, since the DNA damage response and cellular senescence are activated via induction of p53 and its downstream effector p21 [10]. Notably, CSNK1A1 deficiency caused hematopoietic stem cells (HSCs) to exit quiescence and re-enter the cell cycle; meanwhile, CSNK1A1 haploinsufficiency induced HSCs expansion and increased the S/G2/M-phase fractions, whereas homozygous deletion induced significant induction of early and late apoptosis and led to HSCs failure [104]. CK1α loss was associated with cell cycle arrest in human colorectal polyps [105], and inhibition of CK1α kinase activity in multiple myeloma cells by D4476 or siRNA treatment triggered G0/G1 arrest, prolonged G2/M phase, and increased apoptosis [106]. These findings indicate that CK1α has dual functions in cell cycle progression and cell division.
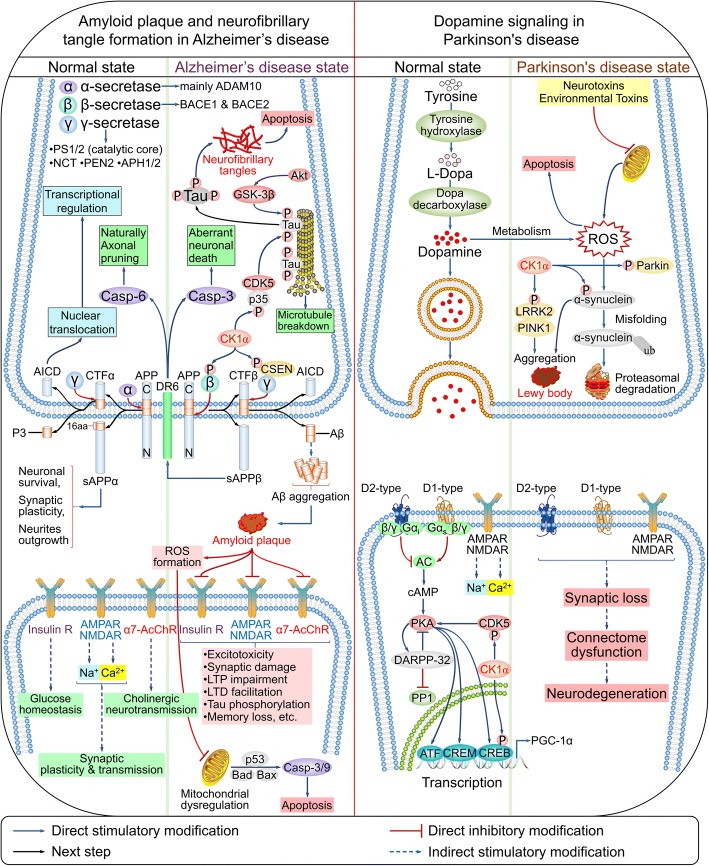
CK1α in neurodegenerative diseases
Alzheimer’s disease (AD) is a progressive neurologic disease and leading cause of dementia that is characterized by the irreversible loss of neurons—particularly in the cortex and hippocampus [107]—leading to memory disorder, personality changes, and cognitive dysfunction [108]. Additional histopathological hallmarks include the presence of extracellular senile plaques containing the amyloid-β (Aβ) peptides and neurofibrillary tangles (NFTs) [107].
Aβ peptides are generated by the sequential cleavage of Aβ precursor protein (APP). In a normal state, the Aβ domain of APP is cleaved by α-secretases (mainly A disintegrase and metalloprotease 10 [ADAM10]), releasing soluble N-terminal (s)APPα and C-terminal fragment α (CTFα). The latter is cleaved by the γ-secretase complex composed of catalytic presenilin 1/2 (PS1/2), nicastrin (NCT), PS enhancer 2 (PEN2), and anterior pharynx defective 1/2 (APH1/2), yielding a soluble extracellular p3 peptide and the APP intracellular domain (AICD). When the amyloidogenic pathway is activated in AD, APP is cleaved by β-secretase 1/2, which releases the ectodomains sAPPβ and CTFβ; subsequent cleavage of CTFα by γ-secretase yields Aβ and AICD [109, 110]. CK1 isoforms are upregulated in the brain of AD patients [111, 112] and directly phosphorylate β-secretase at Ser498, thereby regulating trafficking of β-secretase in the secretory and endocytic pathways [113]. Calsenilin (CSEN) binds PS1/2, the catalytic core of γ-secretase complex, and regulates its APP cleavage activity [114]; it is primarily phosphorylated at Ser63 by CK1, which protects it from cleavage by caspase 3 between Asp61 and Asp64 and generates an ~ 28-kDa C-terminal fragment. Thus, upregulation of CK1 may underlie AD pathology by modulating the phosphorylation state of AD-related proteins [115]. In addition, the sAPPβ ectodomain is phosphorylated by CK1 at Ser206 during secretory cleavage [116], while Aβ in turn stimulates the kinase activity of CK1 [117].
NFTs are another characteristic of AD. In the normal state, tau is dephosphorylated and binds microtubules; hyperphosphorylation by CDK5 and GSK-3β inhibits its microtubule-binding capacity, resulting in the release of tau from axonal microtubules into the cytosol, with a consequent reduction in its solubility and microtubule destabilization [109, 118]. Tau oligomerization leads to the formation of NFTs and neuronal apoptosis [119]. CK1 isoforms also contribute to the hyperphosphorylation of tau, leading to its conversion to an abnormal AD-like state [120]. CK1α was found to be closely associated with paired helical filaments (PHFs) purified from the brain tissue of AD patients. Thus, CK1α is one of the major kinases responsible for the pathological hyperphosphorylation of tau protein [121].
Parkinson’s disease (PD) is the second most common late-onset neurodegenerative disease after AD and is characterized by an accumulation of α-synuclein—also known as Parkinson disease protein 1 (PARK1)—and mitochondrial dysfunction [122] as well as bradykinesia, rigidity, and tremor due to the loss of dopaminergic neurons in the substantia nigra [123]. Other pathological hallmarks include progressive neuronal loss in a subset of brainstem and mesencephalic nuclei and aggregation of α-synuclein in the form of Lewy bodies and neurites [124].
α-Synuclein phosphorylated at Ser87 and especially Ser129 is the predominant form of the protein in Lewy bodies [125]. CK1s (mainly CK1α) and CK2 phosphorylate α-synuclein at both residues [126, 127]. CREB, a transcription factor that induces the expression of peroxisome proliferator-activated receptor gamma coactivator-1α (PGC-1α) and confers protection to dopaminergic neurons, is also phosphorylated by CK1α at Ser108/111/114 [128], which may be critical for CRE-mediated gene expression induced by dopamine and calcium [129].
Mutations in PARK proteins (PARK1–PARK8)—especially α-synuclein, Parkin (also known as PARK2), phosphatase and tensin homolog-induced putative kinase 1 (PARK6), DJ-1 (also known as PARK7), and leucine-rich repeat kinase 2 (LRRK2) (also known as PARK8)—have been detected in both familial and sporadic PD [107, 130]. LRRK2 is phosphorylated by CK1α at Ser910/935/955/973 [131], whereas Parkin is phosphorylated by CK1 at Ser101/378 under okadaic acid treatment [132].
CDK5 is implicated in both AD and PD [133]. CDK5 is phosphorylated by CK1δ at Ser159 [134], whereas p35—the catalytic and regulatory subunit of CDK5—is phosphorylated by CK1α. Additionally, CK1α controls metabotropic glutamate receptor (mGluR)-mediated Ca2+ currents in the CK1α/CDK5/dopamine- and cAMP-regulated neuronal phosphoprotein 32 cascade [135]. A recent genome-wide analysis identified CSNK1A1 as a gene linked to language impairment [136]. Thus, CK1α plays an important role in the pathogenesis of AD and PD (Fig. 9 and Table 1).
Fig. 9.
Signaling pathways regulated by CK1α in neurodegenerative diseases. (also reviewed in references [109, 110, 118, 124, 258, 259])
CK1α in the host defense response
In addition to NF-κB signaling, CK1α is also involved in the host defense response against infectious pathogens. CK1α phosphorylates type I interferon receptor 1 (IFNAR1) at Ser535 and thereby induces its ubiquitination and degradation via recruitment of β-TrCP E3 ubiquitin ligase in response to endoplasmic reticulum stress as well as infection [137, 138] by the protozoan Leishmania major or vesicular stomatitis virus (VSV) in human cells [137] and by infectious bursal disease virus in chicken [139]. Newly research have demonstrated that CK1α mediates degradation of IFNAR1 and type II IFN (IFN-γ) receptor 1 (IFNGR1) caused by hemagglutinin of influenza A virus (IAV) [140]. CK1α also acts as a specific host factor and is required for the spread of Listeria monocytogenes between cells, which occurs via formation of productive membrane protrusions [141]. In Toxoplasma gondii, CK1α is essential for replication in host cells; loss of CK1α enhances the virulence of T. gondii in mice via upregulation of rhoptry proteins (ROPs), activation of signal transducer and activator of transcription 3, and suppression of IL-12 production [142].
CK1α phosphorylates rotavirus non-structural protein 5 at Ser67 [143]; the hyperphosphorylated form of the protein is required for rotavirus RNA replication [144]. Similarly, CK1α phosphorylates non-structural protein 5A (NS5A) of hepatitis C virus (HCV) at Ser232 and NS5 of yellow fever virus (YFV) at Ser56, leading to hyperphosphorylation of NS5A [145, 146] and NS5 [147] for RNA replication. Thus, CK1α is required for pathogen infection, and specifically for viral RNA replication (Table 2).
Table 2.
Substrates of human CK1α in various biological events
| Gene | Protein | Phosphorylation site | Function | Reference |
|---|---|---|---|---|
| IFNAR1 | IFNAR1 | S535 | Leishmania major/VSV/ IAV | [137, 138, 140] |
| IFNGR1 | IFNGR1 | N/A | IAV | [140] |
| NS5A | NS5A | S232 | HCV | [145, 146] |
| NS5 | NS5 | S56 | YFV | [147] |
| NSP5 | NSP5 | S67 | Rotavirus | [143, 144] |
| HNRNPC | hnRNP C1/C2 | S240/253, S247/260, S286/S299 | mRNA metabolism | [183] |
| TUT1 | Star-PAP, RBM21 | S6 | [184] | |
| AGO2 | AGO2 | S824, S828, T830, S831, S834 | MiRNA-mediated silencing of target mRNA | [185] |
| KDM1A | LSD1 | S687 | Glioblastoma | [173] |
| PHLPP1 | PHLPP1 | S1359, T1363, S1379, S1381 | Colorectal cancer | [166] |
| RAPGEF2 | RAPGEF2 | S1244, S1248 | Cancer metastasis | [174] |
| RXRA | RXRα | N/A | Cancer apoptosis | [175] |
| Bid | Bid | N/A | [176] |
CK1α in cancer
CK1α is a component of the Wnt/β-catenin signaling pathway that functions as a tumor suppressor [148]. Low levels of CSNK1A1 may contribute to tumorigenesis and poor prognosis, especially in colorectal cancer according to the data from open-source databases. However, nearest research reported that CSNK1A1 overexpression correlates with poor survival in colorectal cancer [149]. The opposite conclusions both lack the protein data. Notably, the P value of overall survival calculated by Kaplan-Meier method that divided according to relative CSNK1A1 RNA expression in tumor tissue are both very close to 0.05. Thus, the opposite conclusions need a large sample approach based on protein data for final verdict. CK1α interacts with MDMX to inhibit the DNA-binding and transcriptional activity of p53 [8, 9, 96], resulting in p53 ubiquitination and degradation via interaction with MDM2 [7]. CSNK1A1 was unrelated to the survival of sporadic colon cancer patients with functional p53, but those with low CSNK1A1 expression had very poor prognosis compared to patients with high CSNK1A1 levels and non-functional p53 [150]. Loss of CK1α does not lead to colorectal cancer due to induction of p53, unless both p53 and CK1α genes are deleted [10]. CK1α ablation also leads to activation of the IFN signaling pathway, which prevents unlimited proliferation of intestinal epithelial cells even when β-catenin is constitutively active. Concurrent loss of CK1α and IFNAR1 leads to intestinal hyperplasia, inhibition of apoptosis, and rapid and lethal loss of the intestinal barrier function [151]. Thus, CK1α maintains a balance among Wnt/β-catenin, p53, and IFN signaling. It is also implicated in RAS-driven cancers such as colon cancer—which depends on autophagy [72]—and acts as a negative regulator in prostate cancer [94], liposarcoma [152], and ultraviolet radiation-induced skin tumors [153].
CSNK1A1 is located on chromosome 5q32 and is downregulated [154] or mutated [155, 156] in patients with in MDS del(5q). CSNK1A1 mutations have also been detected in adult T cell leukemia/lymphoma (ATL) [157], clear cell renal cell carcinoma [158], colon cancer [159], and esophageal adenocarcinoma [160, 161]. Haploinsufficiency of CSNK1A1 leads to β-catenin activation and expansion of the HSC pool, whereas homozygous deletion leads to inhibition of HSC proliferation [104]. The observation that over 50% of patients treated with lenalidomide experienced remission [162–164] was attributable to the fact that CSNK1A1 haploinsufficiency heightens sensitivity to the effects of lenalidomide-induced CK1α degradation [12], which was shown to be mediated by valosin-containing protein (VCP)/p97 [165].
CK1α phosphorylates pleckstrin homology domain leucine-rich repeat protein phosphatase 1 (PHLPP1) at Ser1359, Thr1363, Ser1379, and Ser1381 leading to its ubiquitination and degradation, which may promote colon cancer progression [166]. It also interacts with hematopoietic pre-B cell leukemia transcription factor-interacting protein (HPIP) to stimulate renal cell carcinoma growth and metastasis via activation of mTOR signaling [167]. CK1α is more highly expressed in and can serve as a diagnostic marker for malignant melanoma [168]; however, CK1α suppression in melanoma cells causes a switch in β-catenin signaling to promote metastasis [169, 170]. It is also highly expressed in multiple myeloma and plasma cell leukemia [171], and has an oncogenic role in these malignancies. Likewise, ABC DLBCL requires CK1α for constitutive NF-κB activity and survival; lenalidomide may have therapeutic effects in ABC DLBCL by inducing the degradation of CK1α [11, 12, 172], as well as in pancreatic cancer in which CK1α is upregulated. The current evidence suggests that CK1α dependency resembles non-oncogenic addiction in which the cancer cell phenotype depends on hyperactivation of specific genes including NF-κB [11].
GSK-3β phosphorylates lysine-specific histone demethylase 1A (KDM1A, also known as LSD1) at Ser683 after priming phosphorylation at Ser687 by CK1α. This leads to KDM1A deubiquitination by ubiquitin-specific protease 22 (USP22) and subsequent stabilization, which is essential for glioblastoma development [173]. IKKβ stimulates the CK1α-mediated degradation of Rap guanine exchange factor 2 (RAPGEF2) via phosphorylation at Ser1244 and Ser1248 in response to hepatocyte growth factor (HGF), and may promote the dissemination and metastasis of human breast cancer cells [174].
CK1α interacts with retinoid X receptor α (RXRα) and enhances cell survival by preventing RXR agonist-induced apoptosis in cancer cells [175]. CK1α exerts an anti-apoptotic function by phosphorylating and preventing the caspase-8 dependent cleavage of BH3-interacting domain death agonist (Bid) in HeLa cells [176] (Table 2).
CK1α has also been implicated in lung [80, 148, 177–179], breast [180], esophageal [181], and urothelial [182] cancers. It was found to promote KRASG12D-induced lung cancer through phosphorylation of FADD at Ser194 [80]; CK1α inhibition prevented acquired drug resistance to erlotinib in epidermal growth factor receptor-mutant NSCLC [179]. On the other hand, the Ki-67-interacting protein Nucleolar protein interacting with the FHA domain of pKi-67 (NIFK) enhanced Ki-67-dependent cell migration and invasion in vitro and metastasis in vivo by reducing CK1α level in lung cancer [148]. Thus, CK1α is a potential therapeutic target due to its role as a conditionally essential malignancy protein.
CK1α in other biological events
The regulation of mRNA metabolism by CK1α is evidenced by its localization at nuclear speckles and roles in the modification of small nuclear ribonucleoprotein particles (snRNPs) [22] and phosphorylation of heterogeneous nuclear ribonucleoprotein C1/C2 (hnRNP C1/C2)—a nuclear-restricted pre-mRNA-binding protein—at Ser240/253, Ser247/260, and Ser-286/S299, which modulates its mRNA-binding capacity [183]. CK1α phosphorylates speckle-targeted phosphatidylinositol-4, 5-biphosphate K1A-regulated poly(A) polymerase at Ser6 and induces the transcription of hemeoxygenase 1 (HO-1) and NAD(P)H quinone dehydrogenase 1 (NQO1) [184]. It was also shown to phosphorylate argonaute 2 (AGO2) at Ser824-Ser834 (mainly at Ser828), thereby preventing AGO2-associated target mRNA binding and attenuating micro (mi)RNA-mediated gene silencing [185]. Systems biology approaches have also identified CK1α as a regulator of the DNA damage response in embryonic stem cells [186].
CK1α-mediated Wnt/β-catenin signaling is essential for ontogenesis and stem cell fate determination [187]; for instance, its ablation causes the naked cuticle phenotype in Drosophila [188]. Stromal cell derived factor 1α (SDF1α) inhibits CK1α and attenuates CK1α-mediated phosphorylation, destabilization, and degradation of β-catenin, which is important for c-kit+ cardiac stem/progenitor cell (CSPCs) quiescence under normal conditions and for myocardial regeneration following stress or injury [189]. CK1α suppression leads to Wnt activation and transforming growth factor β/mothers against decapentaplegic homolog 2 inhibition, resulting in the conversion of epiblast stem cells into embryonic stem cells (ESCs) [190] and promoting the establishment and maintenance of the pluripotency network [191]. CK1α directly phosphorylates protein arginine methyltransferase 1 (PRMT1) (mainly at Ser284/Thr285/Ser286/289) to suppress grainyhead-like transcription factor 3 (GRHL3)-mediated terminal differentiation and maintain somatic tissue in a state of self-renewal [192]. Additionally, competitive bone marrow repopulation assays have demonstrated that CK1α is essential for long-term HSCs function [193].
Muscarinic acetylcholine receptors (mAChRs) including M1 [194] and M3 [195, 196] are G protein-coupled receptors (GPCRs) [197] that are phosphorylated by CK1α in an agonist-dependent manner. Phosphorylation of adaptor protein 3 (AP3) by CK1α is required for the efficient formation synaptic vesicles from endosomes [198]. CK1α-mediated phosphorylation stimulates the degradation of the clock protein period circadian regulator 1 (PER1), suggesting a function in circadian rhythm [199]. Mice with heterozygous and homozygous CK1α mutations in the adipose lineage developed diabetes as a result of dysregulated glucose metabolism [200]. CK1α also participates in the regulation of human erythrocyte apoptosis by modulating cytosolic Ca2+ activity [201], and promotes homolog pairing and genome organization by inducing the degradation of chromosome-associated protein H2 (Cap-H2) and limiting chromatin-bound Cap-H2 levels in Drosophila [202].
Regulation of CK1α by endogenous factors
CK1α functions as a broad Ser/Thr kinase that regulates multiple biological processes (Tables 1 and 2) and is itself regulated by various factors. For example, the miRNA miR-155 binds to the 3′-untranslated region (3’-UTR) of CK1α mRNA, thereby enhancing Wnt/β-catenin signaling and cyclin D1 expression and promoting liposarcoma cell growth [152]. MiR-155 is also upregulated in systemic and localized scleroderma and may contribute to disease etiology by repressing CK1α and Src homology 2-containing inositol phosphatase 1 (SHIP-1) [203]. Similarly, miR-9-5p binds to the 3’-UTR of both CK1α and GSK-3β, which mediate the migration of mesenchymal stem cells (MSCs) via Wnt/β-catenin signaling [204].
CK1α regulation at the protein level mostly involves transport and subcellular localization, activation/inactivation, and degradation. As stated earlier, CK1α is localized at nuclear speckles and regulates multiple aspects of mRNA metabolism [22, 183]. However, the mechanism underlying CK1α nuclear transport was only recently elucidated: SON DNA-binding protein localizes to nuclear speckles and acts as a scaffold to which CK1α is recruited by family with sequence similarity 83 member H (FAM83H) [205]. Additionally, GLIPR1-mediated redistribution of CK1α from the Golgi apparatus to the cytoplasm as well increased CK1α protein level is essential for β-catenin phosphorylation and destruction [94]. CK1 members were considered as rogue kinases because their enzymatic activity is apparently unregulated. Of note, RNA helicase DDX3 was identified as a binding protein of CK1α which directly stimulates its kinase activity in a Wnt-dependent manner [206]. But no endogenous inhibitor of CK1α has been identified to date, even the degradation of CK1α is mediated by lenalidomide [12, 13, 207].
Small molecules targeting CK1α
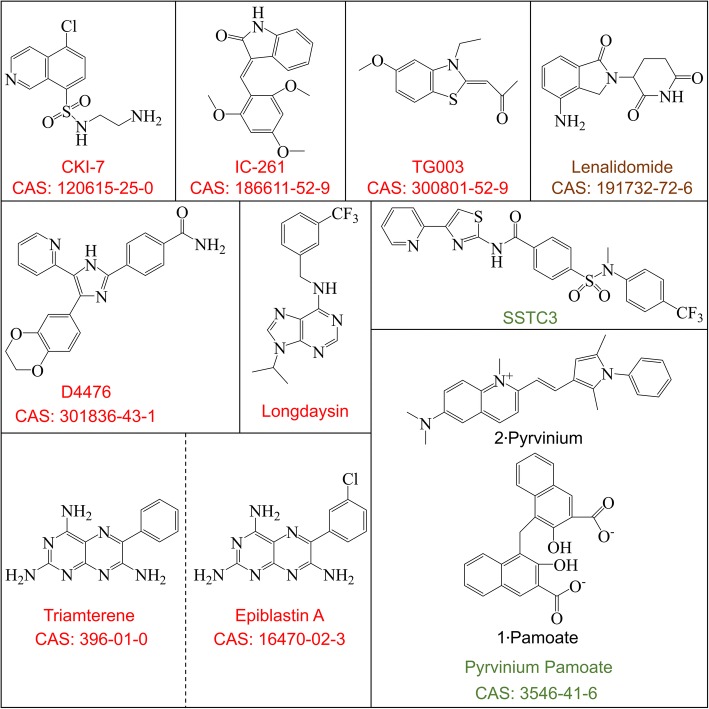
Small molecules are the most useful research tools for investigating protein function, since the clinical application of RNAi and clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein-9 nuclease-mediated gene knockout—while attractive approaches—has numerous challenges or is unfeasible. CKI-7—the first CK1 inhibitor to be developed [208]—is now widely used, with a 50% inhibitory concentration (IC50) of 113–236 μM [80, 209, 210]. IC261 was originally used as a selective inhibitor of CK1ε/δ [211], but has since been shown to block the activity of all CK1 isoforms, with an IC50 of 0.19 μM for CK1α [131, 212]. TG003 was originally identified as a cell division cycle-like kinase inhibitor [213] that suppresses CK1δ/ε activity to a degree equal to or greater than IC261 [214, 215], with an IC50 of 0.33 μM for CK1α [212]. D4476 is the most effective and widely used inhibitor of CK1s, with an IC50 of 200–300 nM [216]. Triamterene—a drug approved by the Food and Drug Administration of the United States (FDA) for the treatment of edematous disorders such as cardiac failure, nephrotic syndrome, and hepatic cirrhosis [217]—was shown to induce epiblast stem cell reprogramming by inhibiting CK1α, with an IC50 of 33.5 μM. However, it also suppressed the kinase activity of CK1δ and CK1ε, with IC50 values of 6.9 and 30.4 μM, respectively [190]. Epiblastin A is a triamterene analog that was developed for more potent inhibition of CK1α; the IC50 values for CK1α, CK1δ, and CK1ε are 3.8, 0.8, and 3.7 μM, respectively [190]. A high-throughput chemical screen identified longdaysin as a small molecule that directly binds CK1α and blocks CK1α-mediated phosphorylation and degradation of PER1, inhibiting CK1α and CK1δ with IC50 values of 5.6 and 8.8 μM, respectively [199].
At present there are no inhibitors that selectively target CK1α or other CK1 isoforms. Nonetheless, the available compounds have been used to study CK1α function. For example, IC261 was used to inhibit CK1α phosphorylation of LRRK2 at Ser935 [131]. In another study, IC261 could not block FADD phosphorylation of FADD at Ser194 by CK1α, although this was achieved by CKI-7 and D4476 [86].
Lenalidomide is a thalidomide analog and FDA approved drug that does not inhibit CK1α but induces CK1α ubiquitination and degradation via CRL4CRBN E3 ubiquitin ligase at concentrations of 0.1–10 μM [12], which has been confirmed by structural analyses [13].
Pyrvinium is an FDA-approved antihelminthic drug that has now been replaced by a more effective, broad-spectrum alternative, although it is still available under the Parke-Davis label in Europe and under the name pamoxan (Sato Pharmaceutical, Tokyo) in Japan [218]. Pyrvinium is a potent inhibitor of Wnt signaling that potentiates the kinase activity of CK1α and stabilizes Axin [51]. Oral administration of pyrvinium was shown to attenuate the expression of Wnt signaling targets and prevent adenoma formation in APCmin mice [219], in addition to stimulating wound repair and myocardial remodeling [220]. Remarkably, subsequent study indicated that pyrvinium did not activates CK1α, but activated GSK3 and down-regulated Akt signaling pathway. However, the study lacks the evidence such as direct interaction between pyrvinium and GSK3 or Akt [221]. SSTC-104 is a functional analog of pyrvinium that activates CK1α, and may be able to counter aberrant Wnt/β-catenin activation by synovial sarcoma (SS) translocation–SSX (also known as SS18-SSX) fusion protein [222]. Later studies reported that poor bioavailability limited the applicability of pyrvinium, and the new CK1α activator SSTC3—which has better pharmacokinetic properties—was developed [223, 224] (Fig. 10). Interestingly, the histone deacetylase 6 inhibitor ACY-1215 was shown to increase Lys49 acetylation and Ser45 phosphorylation by CK1α without affecting Ser33/37 and Thr-41 phosphorylation by GSK-3β [225].
Fig. 10.
Small molecule inhibitors and agonists of CK1α
Conclusions
Human CK1α is an important protein implicated in colorectal cancer [10], MDS del(5q) [12, 13], ABC DLBCL [11], and neurodegenerative diseases [113, 126, 128, 132]. However, there are many open questions regarding the physiological function of CK1α. Firstly, the mechanism of CK1α regulation remains obscure. At the level of transcription, it is unknown whether the regulatory mechanism involves methylation/demethylation of the CSNK1A1 gene promoter. At the post-transcriptional level, a few miRNAs such as miR-155 and -9-5p are known to negatively regulate the CSNK1A1 transcript [152, 203, 204]; however, it is possible that other as-yet unidentified non-coding (nc)RNAs including small nuclear RNAs (snRNAs), small nucleolar RNAs (snoRNAs), long ncRNAs (lncRNAs), and circular RNAs are also involved. CK1α protein expression is controlled at the level of degradation [12, 13] and transport [205]. Although upregulation of PIP2 in the plasma membrane was shown to reduce CK1α activity in erythrocytes and neuronal cells [20, 226–228], there is little known about the endogenous mechanisms of CK1α activation/inactivation. As above mentioned, DDX3 directly stimulates the kinase activity of CK1α in a Wnt-dependent manner [206]. A study of CK1α isoforms in zebrafish (Danio rerio) suggested that the protein kinase activity of CK1α depends on autophosphorylation of C-terminal residues [229]. Clarifying the mechanisms underlying the activation/inactivation of CK1α in different contexts could provide a basis for designing highly targeted and more effective drugs.
CK1α was recently reported that CK1α participates in p53-dependent paracrine factor secretion in skin hyperpigmentation [230]. Future studies will likely provide additional evidence of a role for CK1α in secretion. In addition, downregulation of CK1α in lung cancer, which induced by NIFK is associated with worse prognosis possibly due to activation of Wnt/β-catenin signaling and stimulation of tumorigenesis [148]. On the other hand, the overexpression of CK1α in other malignancies such as pancreatic cancer has also been linked to poor outcome. Whether CK1α induces constitutive activation of NF-κB in pancreatic cancer as in the case of ABC DLBCL, and how it maintains a balance between Wnt/β-catenin, NF-κB, and other signaling pathways remains to be determined.
Splice variants (isoforms) of CK1α have been identified in cell/animal models such as chicken [231], rat [232] and human [233]. All isoforms of CK1α have CK1 catalytic properties, but exhibit different binding activity toward common CK1 substrates [232]. The different isoforms of human CK1α have variable amino acid sequences and distinct functions. CK1α isoform 1 with an NLS in the 28-amino-acid “L” insert (CK1αLS)—but not isoforms 2–4—regulates nuclear signaling in response to H2O2 [14]. CK1αLS also promotes vascular cell proliferation and intimal hyperplasia [234], and mediates the effects of NADPH oxidase on vascular activation [235]. The 12-amino-acid “S” insert near the C terminus may function as a kinase domain for CK1α in zebrafish [229]. A phosphoproteome analysis revealed that isoform 2 of CK1α is phosphorylated at Thr321 [236], which may be linked to endogenous activation/ inactivation of CK1α.
Del(5q) can be detected in not only MDS but also acute lymphoblastic leukemia, especially at 5q32 where the CSNK1A1 gene exists [237]. Thus, CK1α is an attractive molecular target for both diagnosis and monitoring therapy under the treatment of lenalidomide. CK1α is a Ser/Thr kinase with a large number of substrates, some of which have yet to be experimentally verified using approaches such as a pull-down assay, protein interaction domain mapping, and point mutation. A combination of tandem affinity purification and mass spectrometry may facilitate the discovery of new substrates. Additionally, identifying or designing more effective and specific inhibitors, agonists and blocking peptides [95] should enable CK1α targeting in a variety of clinical contexts. Application of small molecule library such as Pfizer compounds and molecular docking algorithm based on the structural information of CK1α may be the most effective approaches so far. Once these inhibitors,agonists and blocking peptides are identified, development of therapy specifically targeting CK1α should open the new avenues for effective management of a broad spectrum of diseases.
Funding
This study was supported by the National Key Basic Research Program (973 Program) (no. 2014CB744505 to X.Y.); Key Program of National Natural Science Foundation of China (no. 81430040 to X.Y.); General Program of National Natural Science Foundation of China (no. 81571738 to J.S.); National Key Research and Development Program of China (no. 2016YFA0100900 to J.S.); and Medical and Health Program of Zhejiang Province (nos. 2016146039 and 2017209189 to M.Z.).
Abbreviations
- 3’-UTR
3′-untranslated region
- ABC DLBCL
Activated B cell-like subtype of diffuse large B-cell lymphoma
- AD
Alzheimer’s disease
- ADAM10
A disintegrase and metalloprotease 10
- AGO2
Argonaute 2
- AICD
APP intracellular domain
- AP3
Adaptor protein 3
- APC
Adenomatous polyposis coli
- APH1/2
Anterior pharynx defective 1/2
- APP
Aβ precursor protein
- Aβ
Amyloid-β
- BCL10
B-cell chronic lymphocytic leukemia/lymphoma 10
- BCR
B cell receptor
- Bid
BH3-interacting domain death agonist
- Brg-1
BRM/SWI2-related gene 1
- Cap-H2
Chromosome-associated protein H2
- CARD11
Caspase recruitment domain family member 11
- CBM
CARD11/BCL10/MALT1
- CBP
CREB binding protein
- CDC25A
Cell division cycle 25 homolog A
- CDK4/6
Cyclin D-cyclin-dependent kinase 4/6
- CHR
Cell cycle genes homology region
- CK1α
Casein kinase 1α
- CKIs
Cyclin-dependent-kinase inhibitors
- CRBN
Cullin 4/really interesting new gene-box 1/DNA damage-binding protein 1/cereblon (also known as CRL4CRBN)
- CREB
Cyclic (c)AMP response element-binding protein
- CRISPR
Clustered regularly interspaced short palindromic repeats
- CSEN
Calsenilin
- CSPCs
Cardiac stem/progenitor cell
- CTFα
C-terminal fragment α
- DARRP-32
Dopamine- and cAMP-regulated neuronal phosphoprotein 32
- DEPTOR
DEP domain-containing mTOR-interacting protein
- ESCs
Embryonic stem cells
- FADD
Fas-associated death domain
- FAM83H
Family with sequence similarity 83 member H
- FBXW7
F-box and WD repeat domain-containing 7
- FOXM1
Forkhead box M1
- FOXO3A
Forkhead box protein O3A
- GABARAPs
Gamma-aminobutyric acid type A receptor-associated proteins
- GLIPR1
Glioma pathogenesis-related protein 1
- GPCRs
G protein-coupled receptors
- GRHL3
Grainyhead-like transcription factor 3
- GSK-3β
Glycogen synthase kinase 3β
- HCV
Hepatitis C virus
- HGF
Hepatocyte growth factor
- hnRNP C1/C2
Heterogeneous nuclear ribonucleoprotein C1/C2
- HO-1
Hemeoxygenase 1
- HPA
The human protein atlas
- HSCs
Hematopoietic stem cells
- IAV
Influenza A virus
- IFNAR1
Interferon receptor 1
- IFNGR1
IFN-γ receptor 1
- IKK
Inhibitor of κB kinase
- IκBs
Inhibitor of κBs (including IκBα, β, and ε)
- KDM1A
Lysine-specific histone demethylase 1A (also known as LSD1)
- LC3-II
Microtubule-associated protein 1A/1B-light chain 3-II
- lncRNA
Long non-coding RNA
- LRP6
Low-density lipoprotein receptor-related protein 6
- LRRK2
Leucine-rich repeat kinase 2
- mAChRs
Muscarinic acetylcholine receptors
- MALT1
Mucosa-associated lymphoid tissue lymphoma translocation gene 1
- MDM2
Murine double minute clone 2
- MDM4
Murine double minute clone 4 (also known as MDMX)
- MDS del(5q)
Myelodysplastic syndrome with deletion of chromosome 5q
- mGluR
Metabotropic glutamate receptor
- MSCs
Mesenchymal stem cells
- mTOR
Mammalian target of rapamycin
- MUVB
Multi-vulval class B
- MYT1
Membrane-associated tyrosine/threonine 1 (also known as PKMYT1)
- NCT
Nicastrin
- NEDD4-1
Neural precursor cell expressed: developmentally down-regulated 4-1
- NFTs
Neurofibrillary tangles
- NF-κB
Nuclear factor κB
- NIFK
FHA domain of pKi-67
- NLS
Nuclear localization signal
- NQO1
NAD(P)H quinone dehydrogenase 1
- NS5A
Non-structural protein 5A
- NSCLC
Non-small-cell lung cancer
- PARK1
Parkinson disease protein 1
- PB1
Polar body 1
- PD
Parkinson’s disease
- PDB
Protein data bank
- PEN2
PS enhancer 2
- PER1
Period circadian regulator 1
- PGC-1α
Proliferator-activated receptor gamma coactivator-1α
- PHFs
Paired helical filaments
- PHLPP1
Pleckstrin homology domain leucine-rich repeat protein phosphatase 1
- PI3K
Class III phosphatidylinositol-3 kinase
- PLK1
polo-like kinase 1
- PRMT1
Protein arginine methyltransferase 1
- PS1/2
Presenilin 1/2
- PTEN
Phosphatase and tensin homolog deleted on chromosome ten
- RAPGEF2
Rap guanine exchange factor 2
- RIP1
Receptor-interacting serine/threonine kinase 1
- RNAi
RNA interference
- ROPs
Rhoptry proteins
- RXRα
Retinoid X receptor α
- SDF1α
Stromal cell derived factor 1α
- SHIP-1
Src homology 2-containing inositol phosphatase 1
- Smo
Smoothened
- snoRNA
Small nucleolar RNA
- snRNA
Small nuclear RNA
- snRNPs
Small nuclear ribonucleoprotein particles
- SQSTM1
Sequestosome 1
- TCGA
The cancer genome atlas
- TCR
T cell receptor
- ULK1/2
Unc-51-like autophagy activating kinase 1
- USP22
Ubiquitin-specific protease 22
- VCP
Valosin-containing protein
- Vps15
Vacuolar protein sorting-associated protein 15
- VSV
Vesicular stomatitis virus
- WTX
Wilms tumor gene on X chromosome (also known as APC membrane recruitment protein 1)
- YFV
Yellow fever virus
- β-TrCP
β-transducin repeat-containing E3 ubiquitin protein ligase
Authors’ contributions
Project planning was done by XY, SJ, and MZ; SJ, and MZ analyzed data and wrote a draft of the paper with the help of JS; XY conceived the ideas, designed the structure and content of review, supervised progress and extensively edited and communicated regarding the manuscript. All authors read and approved the final manuscript.
Authors’ information
Corresponding author: Xiaoming Yang, MD, PhD. A principal investigator and an attending physician of radiology of Sir Run Run Shaw Hospital (an affiliated Hospital of Zhejiang University, School of Medicine), also a professor and director of image-guided bio-molecular interventions research in the Department of Radiology at the University of Washington School of Medicine, and holds an appointment as a senior lecturer of radiology at Kuopio University in Finland and as an attending physician of radiology qualified in interventional radiology at UW Medical Center and European community countries.
First author: Shaojie Jiang, MD, PhD. candidate. An MD/PhD candidate student of professor Xiaoming Yang in Zhejiang University.
Co-first author: Miaofeng Zhang, MD. A surgeon of Second Affiliated Hospital (an affiliated Hospital of Zhejiang University, School of Medicine).
Co-author: Jihong Sun, MD, PhD. An attending physician of radiology of Sir Run Run Shaw Hospital (an affiliated Hospital of Zhejiang University, School of Medicine).
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Shaojie Jiang and Miaofeng Zhang contributed equally to this work.
References
- 1.Desjardins PR, Lue PF, Liew CC, Gornall AG. Purification and properties of rat liver nuclear protein kinases. Can J Biochem. 1972;50:1249–1259. doi: 10.1139/o72-170. [DOI] [PubMed] [Google Scholar]
- 2.Matsumura S, Takeda M. Phosphoprotein kinases from rat liver cytosol. Biochim Biophys Acta. 1972;289:237–241. doi: 10.1016/0005-2744(72)90127-1. [DOI] [PubMed] [Google Scholar]
- 3.Hathaway GM, Traugh JA. Cyclic nucleotide-independent protein kinases from rabbit reticulocytes. Purification of casein kinases. J Biol Chem. 1979;254:762–768. [PubMed] [Google Scholar]
- 4.Rowles J, Slaughter C, Moomaw C, Hsu J, Cobb MH. Purification of casein kinase I and isolation of cDNAs encoding multiple casein kinase I-like enzymes. Proc Natl Acad Sci U S A. 1991;88:9548–9552. doi: 10.1073/pnas.88.21.9548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, et al. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–847. doi: 10.1016/S0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- 6.Amit S, Hatzubai A, Birman Y, Andersen JS, Ben-Shushan E, Mann M, et al. Axin-mediated CKI phosphorylation of beta-catenin at Ser 45: a molecular switch for the Wnt pathway. Genes Dev. 2002;16:1066–1076. doi: 10.1101/gad.230302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huart AS, MacLaine NJ, Meek DW, Hupp TR. CK1alpha plays a central role in mediating MDM2 control of p53 and E2F-1 protein stability. J Biol Chem. 2009;284:32384–32394. doi: 10.1074/jbc.M109.052647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu S, Chen L, Becker A, Schonbrunn E, Chen J. Casein kinase 1alpha regulates an MDMX intramolecular interaction to stimulate p53 binding. Mol Cell Biol. 2012;32:4821–4832. doi: 10.1128/MCB.00851-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei X, Wu S, Song T, Chen L, Gao M, Borcherds W, et al. Secondary interaction between MDMX and p53 core domain inhibits p53 DNA binding. Proc Natl Acad Sci U S A. 2016;113:E2558–E2563. doi: 10.1073/pnas.1603838113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elyada E, Pribluda A, Goldstein RE, Morgenstern Y, Brachya G, Cojocaru G, et al. CKIalpha ablation highlights a critical role for p53 in invasiveness control. Nature. 2011;470:409–413. doi: 10.1038/nature09673. [DOI] [PubMed] [Google Scholar]
- 11.Bidere N, Ngo VN, Lee J, Collins C, Zheng L, Wan F, et al. Casein kinase 1alpha governs antigen-receptor-induced NF-kappaB activation and human lymphoma cell survival. Nature. 2009;458:92–96. doi: 10.1038/nature07613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kronke J, Fink EC, Hollenbach PW, MacBeth KJ, Hurst SN, Udeshi ND, et al. Lenalidomide induces ubiquitination and degradation of CK1alpha in del(5q) MDS. Nature. 2015;523:183–188. doi: 10.1038/nature14610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petzold G, Fischer ES, Thoma NH. Structural basis of lenalidomide-induced CK1alpha degradation by the CRL4(CRBN) ubiquitin ligase. Nature. 2016;532:127–130. doi: 10.1038/nature16979. [DOI] [PubMed] [Google Scholar]
- 14.Bedri S, Cizek SM, Rastarhuyeva I, Stone JR. Regulation of protein kinase CK1alphaLS by dephosphorylation in response to hydrogen peroxide. Arch Biochem Biophys. 2007;466:242–249. doi: 10.1016/j.abb.2007.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu Z, Chakraborti T, Morse S, Bennett GS, Shaw G. Four casein kinase I isoforms are differentially partitioned between nucleus and cytoplasm. Exp Cell Res. 2001;269:275–286. doi: 10.1006/excr.2001.5324. [DOI] [PubMed] [Google Scholar]
- 16.Yang W, Garrett L, Feng D, Elliott G, Liu X, Wang N, et al. Wnt-induced Vangl2 phosphorylation is dose-dependently required for planar cell polarity in mammalian development. Cell Res. 2017;27:1466–84. doi: 10.1038/cr.2017.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bustos VH, Marin O, Meggio F, Cesaro L, Allende CC, Allende JE, et al. Generation of protein kinase Ck1alpha mutants which discriminate between canonical and non-canonical substrates. Biochem J. 2005;391:417–424. doi: 10.1042/BJ20050717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bustos VH, Ferrarese A, Venerando A, Marin O, Allende JE, Pinna LA. The first armadillo repeat is involved in the recognition and regulation of beta-catenin phosphorylation by protein kinase CK1. Proc Natl Acad Sci U S A. 2006;103:19725–19730. doi: 10.1073/pnas.0609424104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dubois T, Howell S, Zemlickova E, Aitken A. Identification of casein kinase Ialpha interacting protein partners. FEBS Lett. 2002;517:167–171. doi: 10.1016/S0014-5793(02)02614-5. [DOI] [PubMed] [Google Scholar]
- 20.Gross SD, Hoffman DP, Fisette PL, Baas P, Anderson RA. A phosphatidylinositol 4,5-bisphosphate-sensitive casein kinase I alpha associates with synaptic vesicles and phosphorylates a subset of vesicle proteins. J Cell Biol. 1995;130:711–724. doi: 10.1083/jcb.130.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brockman JL, Gross SD, Sussman MR, Anderson RA. Cell cycle-dependent localization of casein kinase I to mitotic spindles. Proc Natl Acad Sci U S A. 1992;89:9454–9458. doi: 10.1073/pnas.89.20.9454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gross SD, Loijens JC, Anderson RA. The casein kinase Ialpha isoform is both physically positioned and functionally competent to regulate multiple events of mRNA metabolism. J Cell Sci. 1999;112(Pt 16):2647–2656. doi: 10.1242/jcs.112.16.2647. [DOI] [PubMed] [Google Scholar]
- 23.Gross SD, Anderson RA. Casein kinase I: spatial organization and positioning of a multifunctional protein kinase family. Cell Signal. 1998;10:699–711. doi: 10.1016/S0898-6568(98)00042-4. [DOI] [PubMed] [Google Scholar]
- 24.Kang DE, Soriano S, Xia X, Eberhart CG, De Strooper B, Zheng H, et al. Presenilin couples the paired phosphorylation of beta-catenin independent of axin: implications for beta-catenin activation in tumorigenesis. Cell. 2002;110:751–762. doi: 10.1016/S0092-8674(02)00970-4. [DOI] [PubMed] [Google Scholar]
- 25.Sturgeon CM, Ditadi A, Awong G, Kennedy M, Keller G. Wnt signaling controls the specification of definitive and primitive hematopoiesis from human pluripotent stem cells. Nat Biotechnol. 2014;32:554–561. doi: 10.1038/nbt.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phelps RA, Chidester S, Dehghanizadeh S, Phelps J, Sandoval IT, Rai K, et al. A two-step model for colon adenoma initiation and progression caused by APC loss. Cell. 2009;137:623–634. doi: 10.1016/j.cell.2009.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benham-Pyle BW, Pruitt BL, Nelson WJ. Cell adhesion. Mechanical strain induces E-cadherin-dependent Yap1 and beta-catenin activation to drive cell cycle entry. Science. 2015;348:1024–1027. doi: 10.1126/science.aaa4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Major MB, Camp ND, Berndt JD, Yi X, Goldenberg SJ, Hubbert C, et al. Wilms tumor suppressor WTX negatively regulates WNT/beta-catenin signaling. Science. 2007;316:1043–1046. doi: 10.1126/science/1141515. [DOI] [PubMed] [Google Scholar]
- 29.Wu X, Tu X, Joeng KS, Hilton MJ, Williams DA, Long F. Rac1 activation controls nuclear localization of beta-catenin during canonical Wnt signaling. Cell. 2008;133:340–353. doi: 10.1016/j.cell.2008.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nusse R, Clevers H. Wnt/beta-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169:985–999. doi: 10.1016/j.cell.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 31.Ferrarese A, Marin O, Bustos VH, Venerando A, Antonelli M, Allende JE, et al. Chemical dissection of the APC repeat 3 multistep phosphorylation by the concerted action of protein kinases CK1 and GSK3. Biochemistry. 2007;46:11902–11910. doi: 10.1021/bi701674z. [DOI] [PubMed] [Google Scholar]
- 32.Liu J, Xing Y, Hinds TR, Zheng J, Xu W. The third 20 amino acid repeat is the tightest binding site of APC for beta-catenin. J Mol Biol. 2006;360:133–144. doi: 10.1016/j.jmb.2006.04.064. [DOI] [PubMed] [Google Scholar]
- 33.Dupre-Crochet S, Figueroa A, Hogan C, Ferber EC, Bialucha CU, Adams J, et al. Casein kinase 1 is a novel negative regulator of E-cadherin-based cell-cell contacts. Mol Cell Biol. 2007;27:3804–3816. doi: 10.1128/MCB.01590-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeng X, Tamai K, Doble B, Li S, Huang H, Habas R, et al. A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature. 2005;438:873–877. doi: 10.1038/nature04185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chitalia VC, Foy RL, Bachschmid MM, Zeng L, Panchenko MV, Zhou MI, et al. Jade-1 inhibits Wnt signalling by ubiquitylating beta-catenin and mediates Wnt pathway inhibition by pVHL. Nat Cell Biol. 2008;10:1208–1216. doi: 10.1038/ncb1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borgal L, Rinschen MM, Dafinger C, Liebrecht VI, Abken H, Benzing T, et al. Jade-1S phosphorylation induced by CK1alpha contributes to cell cycle progression. Cell Cycle. 2016;15:1034–1045. doi: 10.1080/15384101.2016.1152429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borgal L, Rinschen MM, Dafinger C, Hoff S, Reinert MJ, Lamkemeyer T, et al. Casein kinase 1 alpha phosphorylates the Wnt regulator Jade-1 and modulates its activity. J Biol Chem. 2014;289:26344–26356. doi: 10.1074/jbc.M114.562165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lebensohn AM, Dubey R, Neitzel LR, Tacchelly-Benites O, Yang E, Marceau CD, et al. Comparative genetic screens in human cells reveal new regulatory mechanisms in WNT signaling. elife. 2016;5:e21459. [DOI] [PMC free article] [PubMed]
- 39.Sanchez-Danes A, Hannezo E, Larsimont JC, Liagre M, Youssef KK, Simons BD, et al. Defining the clonal dynamics leading to mouse skin tumour initiation. Nature. 2016;536:298–303. doi: 10.1038/nature19069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Snuderl M, Batista A, Kirkpatrick ND, Ruiz de Almodovar C, Riedemann L, Walsh EC, et al. Targeting placental growth factor/neuropilin 1 pathway inhibits growth and spread of medulloblastoma. Cell. 2013;152:1065–1076. doi: 10.1016/j.cell.2013.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rubin LL, de Sauvage FJ. Targeting the hedgehog pathway in cancer. Nat Rev Drug Discov. 2006;5:1026–1033. doi: 10.1038/nrd2086. [DOI] [PubMed] [Google Scholar]
- 42.Price MA, Kalderon D. Proteolysis of the hedgehog signaling effector Cubitus interruptus requires phosphorylation by glycogen synthase kinase 3 and casein kinase 1. Cell. 2002;108:823–835. doi: 10.1016/S0092-8674(02)00664-5. [DOI] [PubMed] [Google Scholar]
- 43.Lum L, Yao S, Mozer B, Rovescalli A, Von Kessler D, Nirenberg M, et al. Identification of hedgehog pathway components by RNAi in Drosophila cultured cells. Science. 2003;299:2039–2045. doi: 10.1126/science.1081403. [DOI] [PubMed] [Google Scholar]
- 44.Jia J, Tong C, Wang B, Luo L, Jiang J. Hedgehog signalling activity of smoothened requires phosphorylation by protein kinase a and casein kinase I. Nature. 2004;432:1045–1050. doi: 10.1038/nature03179. [DOI] [PubMed] [Google Scholar]
- 45.Zhang C, Williams EH, Guo Y, Lum L, Beachy PA. Extensive phosphorylation of smoothened in hedgehog pathway activation. Proc Natl Acad Sci U S A. 2004;101:17900–17907. doi: 10.1073/pnas.0408093101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Apionishev S, Katanayeva NM, Marks SA, Kalderon D, Tomlinson A. Drosophila smoothened phosphorylation sites essential for hedgehog signal transduction. Nat Cell Biol. 2005;7:86–92. doi: 10.1038/ncb1210. [DOI] [PubMed] [Google Scholar]
- 47.Evangelista M, Lim TY, Lee J, Parker L, Ashique A, Peterson AS, et al. Kinome siRNA screen identifies regulators of ciliogenesis and hedgehog signal transduction. Sci Signal. 2008;1:ra7. doi: 10.1126/scisignal.1162925. [DOI] [PubMed] [Google Scholar]
- 48.Chen Y, Sasai N, Ma G, Yue T, Jia J, Briscoe J, et al. Sonic hedgehog dependent phosphorylation by CK1alpha and GRK2 is required for ciliary accumulation and activation of smoothened. PLoS Biol. 2011;9:e1001083. doi: 10.1371/journal.pbio.1001083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou BP, Hung MC. Wnt, hedgehog and snail: sister pathways that control by GSK-3beta and beta-Trcp in the regulation of metastasis. Cell Cycle. 2005;4:772–776. doi: 10.4161/cc.4.6.1744. [DOI] [PubMed] [Google Scholar]
- 50.Kalderon D. Similarities between the hedgehog and Wnt signaling pathways. Trends Cell Biol. 2002;12:523–531. doi: 10.1016/S0962-8924(02)02388-7. [DOI] [PubMed] [Google Scholar]
- 51.Thorne CA, Hanson AJ, Schneider J, Tahinci E, Orton D, Cselenyi CS, et al. Small-molecule inhibition of Wnt signaling through activation of casein kinase 1alpha. Nat Chem Biol. 2010;6:829–836. doi: 10.1038/nchembio.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li B, Fei DL, Flaveny CA, Dahmane N, Baubet V, Wang Z, et al. Pyrvinium attenuates hedgehog signaling downstream of smoothened. Cancer Res. 2014;74:4811–4821. doi: 10.1158/0008-5472.CAN-14-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Galluzzi L, Bravo-San Pedro JM, Levine B, Green DR, Kroemer G. Pharmacological modulation of autophagy: therapeutic potential and persisting obstacles. Nat Rev Drug Discov. 2017;16:487–511. doi: 10.1038/nrd.2017.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Levy JMM, Towers CG, Thorburn A. Targeting autophagy in cancer. Nat Rev Cancer. 2017;17:528–542. doi: 10.1038/nrc.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hara T, Mizushima N. Role of ULK-FIP200 complex in mammalian autophagy: FIP200, a counterpart of yeast Atg17? Autophagy. 2009;5:85–87. doi: 10.4161/auto.5.1.7180. [DOI] [PubMed] [Google Scholar]
- 56.Mercer CA, Kaliappan A, Dennis PB. A novel, human Atg13 binding protein, Atg101, interacts with ULK1 and is essential for macroautophagy. Autophagy. 2009;5:649–662. doi: 10.4161/auto.5.5.8249. [DOI] [PubMed] [Google Scholar]
- 57.Hosokawa N, Sasaki T, Iemura S, Natsume T, Hara T, Mizushima N. Atg101, a novel mammalian autophagy protein interacting with Atg13. Autophagy. 2009;5:973–979. doi: 10.4161/auto.5.7.9296. [DOI] [PubMed] [Google Scholar]
- 58.Petherick KJ, Conway OJ, Mpamhanga C, Osborne SA, Kamal A, Saxty B, et al. Pharmacological inhibition of ULK1 kinase blocks mammalian target of rapamycin (mTOR)-dependent autophagy. J Biol Chem. 2015;290:28726. doi: 10.1074/jbc.A114.627778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Russell RC, Tian Y, Yuan H, Park HW, Chang YY, Kim J, et al. ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 lipid kinase. Nat Cell Biol. 2013;15:741–750. doi: 10.1038/ncb2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Di Bartolomeo S, Corazzari M, Nazio F, Oliverio S, Lisi G, Antonioli M, et al. The dynamic interaction of AMBRA1 with the dynein motor complex regulates mammalian autophagy. J Cell Biol. 2010;191:155–168. doi: 10.1083/jcb.201002100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhong Y, Wang QJ, Li X, Yan Y, Backer JM, Chait BT, et al. Distinct regulation of autophagic activity by Atg14L and Rubicon associated with Beclin 1-phosphatidylinositol-3-kinase complex. Nat Cell Biol. 2009;11:468–476. doi: 10.1038/ncb1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Matsunaga K, Saitoh T, Tabata K, Omori H, Satoh T, Kurotori N, et al. Two Beclin 1-binding proteins, Atg14L and Rubicon, reciprocally regulate autophagy at different stages. Nat Cell Biol. 2009;11:385–396. doi: 10.1038/ncb1846. [DOI] [PubMed] [Google Scholar]
- 63.Liang C, Feng P, Ku B, Dotan I, Canaani D, Oh BH, et al. Autophagic and tumour suppressor activity of a novel Beclin1-binding protein UVRAG. Nat Cell Biol. 2006;8:688–699. doi: 10.1038/ncb1426. [DOI] [PubMed] [Google Scholar]
- 64.Kim J, Kim YC, Fang C, Russell RC, Kim JH, Fan W, et al. Differential regulation of distinct Vps34 complexes by AMPK in nutrient stress and autophagy. Cell. 2013;152:290–303. doi: 10.1016/j.cell.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Suzuki K, Kirisako T, Kamada Y, Mizushima N, Noda T, Ohsumi Y. The pre-autophagosomal structure organized by concerted functions of APG genes is essential for autophagosome formation. EMBO J. 2001;20:5971–5981. doi: 10.1093/emboj/20.21.5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tanida I, Tanida-Miyake E, Ueno T, Kominami E. The human homolog of Saccharomyces cerevisiae Apg7p is a protein-activating enzyme for multiple substrates including human Apg12p, GATE-16, GABARAP, and MAP-LC3. J Biol Chem. 2001;276:1701–1706. doi: 10.1074/jbc.C000752200. [DOI] [PubMed] [Google Scholar]
- 68.Itakura E, Kishi-Itakura C, Mizushima N. The hairpin-type tail-anchored SNARE syntaxin 17 targets to autophagosomes for fusion with endosomes/lysosomes. Cell. 2012;151:1256–1269. doi: 10.1016/j.cell.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 69.Zhao J, Brault JJ, Schild A, Cao P, Sandri M, Schiaffino S, et al. FoxO3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metab. 2007;6:472–483. doi: 10.1016/j.cmet.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 70.Webb AE, Brunet A. FOXO transcription factors: key regulators of cellular quality control. Trends Biochem Sci. 2014;39:159–169. doi: 10.1016/j.tibs.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mammucari C, Milan G, Romanello V, Masiero E, Rudolf R, Del Piccolo P, et al. FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab. 2007;6:458–471. doi: 10.1016/j.cmet.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 72.Cheong JK, Zhang F, Chua PJ, Bay BH, Thorburn A, Virshup DM. Casein kinase 1alpha-dependent feedback loop controls autophagy in RAS-driven cancers. J Clin Invest. 2015;125:1401–1418. doi: 10.1172/JCI78018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Peterson TR, Laplante M, Thoreen CC, Sancak Y, Kang SA, Kuehl WM, et al. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell. 2009;137:873–886. doi: 10.1016/j.cell.2009.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Duan S, Skaar JR, Kuchay S, Toschi A, Kanarek N, Ben-Neriah Y, et al. mTOR generates an auto-amplification loop by triggering the betaTrCP- and CK1alpha-dependent degradation of DEPTOR. Mol Cell. 2011;44:317–324. doi: 10.1016/j.molcel.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhao Y, Xiong X, Sun Y. DEPTOR, an mTOR inhibitor, is a physiological substrate of SCF(betaTrCP) E3 ubiquitin ligase and regulates survival and autophagy. Mol Cell. 2011;44:304–316. doi: 10.1016/j.molcel.2011.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gao D, Inuzuka H, Tan MK, Fukushima H, Locasale JW, Liu P, et al. mTOR drives its own activation via SCF(betaTrCP)-dependent degradation of the mTOR inhibitor DEPTOR. Mol Cell. 2011;44:290–303. doi: 10.1016/j.molcel.2011.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hale CM, Cheng Q, Ortuno D, Huang M, Nojima D, Kassner PD, et al. Identification of modulators of autophagic flux in an image-based high content siRNA screen. Autophagy. 2016;12:713–726. doi: 10.1080/15548627.2016.1147669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Szyniarowski P, Corcelle-Termeau E, Farkas T, Hoyer-Hansen M, Nylandsted J, Kallunki T, et al. A comprehensive siRNA screen for kinases that suppress macroautophagy in optimal growth conditions. Autophagy. 2011;7:892–903. doi: 10.4161/auto.7.8.15770. [DOI] [PubMed] [Google Scholar]
- 79.Watanabe Y, Tsujimura A, Taguchi K, Tanaka M. HSF1 stress response pathway regulates autophagy receptor SQSTM1/p62-associated proteostasis. Autophagy. 2017;13:133–148. doi: 10.1080/15548627.2016.1248018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bowman BM, Sebolt KA, Hoff BA, Boes JL, Daniels DL, Heist KA, et al. Phosphorylation of FADD by the kinase CK1alpha promotes KRASG12D-induced lung cancer. Sci Signal. 2015;8:ra9. doi: 10.1126/scisignal.2005607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cai J, Li R, Xu X, Zhang L, Lian R, Fang L, et al. CK1alpha suppresses lung tumour growth by stabilizing PTEN and inducing autophagy. Nat Cell Biol. 2018;20:465–478. doi: 10.1038/s41556-018-0065-8. [DOI] [PubMed] [Google Scholar]
- 82.Sun SC. The non-canonical NF-kappaB pathway in immunity and inflammation. Nat Rev Immunol. 2017;17:545–558. doi: 10.1038/nri.2017.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang Y, Sun X, Wu J, Xu BE, Gu C, Wang H, et al. Casein kinase 1alpha interacts with RIP1 and regulates NF-kappaB activation. Biochemistry. 2008;47:441–448. doi: 10.1021/bi7010515. [DOI] [PubMed] [Google Scholar]
- 84.Beyaert R, Vanhaesebroeck B, Declercq W, Van Lint J, Vandenabele P, Agostinis P, et al. Casein kinase-1 phosphorylates the p75 tumor necrosis factor receptor and negatively regulates tumor necrosis factor signaling for apoptosis. J Biol Chem. 1995;270:23293–23299. doi: 10.1074/jbc.270.40.23293. [DOI] [PubMed] [Google Scholar]
- 85.Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, et al. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Alappat EC, Feig C, Boyerinas B, Volkland J, Samuels M, Murmann AE, et al. Phosphorylation of FADD at serine 194 by CKIalpha regulates its nonapoptotic activities. Mol Cell. 2005;19:321–332. doi: 10.1016/j.molcel.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 87.Marikar FM, Jin G, Sheng W, Ma D, Hua Z. Metallothionein 2A an interactive protein linking phosphorylated FADD to NF-kappaB pathway leads to colorectal cancer formation. Chin Clin Oncol. 2016;5:76. doi: 10.21037/cco.2016.11.03. [DOI] [PubMed] [Google Scholar]
- 88.Carvalho G, Le Guelte A, Demian C, Vazquez A, Gavard J, Bidere N. Interplay between BCL10, MALT1 and IkappaBalpha during T-cell-receptor-mediated NFkappaB activation. J Cell Sci. 2010;123:2375–2380. doi: 10.1242/jcs.069476. [DOI] [PubMed] [Google Scholar]
- 89.Martin M, Hua L, Wang B, Wei H, Prabhu L, Hartley AV, et al. Novel serine 176 phosphorylation of YBX1 activates NF-kappaB in Colon Cancer. J Biol Chem. 2017;292:3433–3444. doi: 10.1074/jbc.M116.740258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Otto T, Sicinski P. Cell cycle proteins as promising targets in cancer therapy. Nat Rev Cancer. 2017;17:93–115. doi: 10.1038/nrc.2016.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gross SD, Simerly C, Schatten G, Anderson RA. A casein kinase I isoform is required for proper cell cycle progression in the fertilized mouse oocyte. J Cell Sci. 1997;110(Pt 24):3083–3090. doi: 10.1242/jcs.110.24.3083. [DOI] [PubMed] [Google Scholar]
- 92.Honaker Y, Piwnica-Worms H. Casein kinase 1 functions as both penultimate and ultimate kinase in regulating Cdc25A destruction. Oncogene. 2010;29:3324–3334. doi: 10.1038/onc.2010.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Piao S, Lee SJ, Xu Y, Gwak J, Oh S, Park BJ, et al. CK1epsilon targets Cdc25A for ubiquitin-mediated proteolysis under normal conditions and in response to checkpoint activation. Cell Cycle. 2011;10:531–537. doi: 10.4161/cc.10.3.14757. [DOI] [PubMed] [Google Scholar]
- 94.Li L, Ren C, Yang G, Fattah EA, Goltsov AA, Kim SM, et al. GLIPR1 suppresses prostate cancer development through targeted oncoprotein destruction. Cancer Res. 2011;71:7694–7704. doi: 10.1158/0008-5472.CAN-11-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Huart AS, MacLaine NJ, Narayan V, Hupp TR. Exploiting the MDM2-CK1alpha protein-protein interface to develop novel biologics that induce UBL-kinase-modification and inhibit cell growth. PLoS One. 2012;7:e43391. doi: 10.1371/journal.pone.0043391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen L, Li C, Pan Y, Chen J. Regulation of p53-MDMX interaction by casein kinase 1 alpha. Mol Cell Biol. 2005;25:6509–6520. doi: 10.1128/MCB.25.15.6509-6520.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.MacLaine NJ, Oster B, Bundgaard B, Fraser JA, Buckner C, Lazo PA, et al. A central role for CK1 in catalyzing phosphorylation of the p53 transactivation domain at serine 20 after HHV-6B viral infection. J Biol Chem. 2008;283:28563–28573. doi: 10.1074/jbc.M804433200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hirao A, Kong YY, Matsuoka S, Wakeham A, Ruland J, Yoshida H, et al. DNA damage-induced activation of p53 by the checkpoint kinase Chk2. Science. 2000;287:1824–1827. doi: 10.1126/science.287.5459.1824. [DOI] [PubMed] [Google Scholar]
- 99.Shieh SY, Ahn J, Tamai K, Taya Y, Prives C. The human homologs of checkpoint kinases Chk1 and Cds1 (Chk2) phosphorylate p53 at multiple DNA damage-inducible sites. Genes Dev. 2000;14:289–300. [PMC free article] [PubMed] [Google Scholar]
- 100.Li N, Lorenzi F, Kalakouti E, Normatova M, Babaei-Jadidi R, Tomlinson I, et al. FBXW7-mutated colorectal cancer cells exhibit aberrant expression of phosphorylated-p53 at Serine-15. Oncotarget. 2015;6:9240–9256. doi: 10.18632/oncotarget.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dubois T, Rommel C, Howell S, Steinhussen U, Soneji Y, Morrice N, et al. 14-3-3 is phosphorylated by casein kinase I on residue 233. Phosphorylation at this site in vivo regulates Raf/14-3-3 interaction. J Biol Chem. 1997;272:28882–28888. doi: 10.1074/jbc.272.46.28882. [DOI] [PubMed] [Google Scholar]
- 102.Qi ST, Wang ZB, Huang L, Liang LF, Xian YX, Ouyang YC, et al. Casein kinase 1 (alpha, delta and epsilon) localize at the spindle poles, but may not be essential for mammalian oocyte meiotic progression. Cell Cycle. 2015;14:1675–1685. doi: 10.1080/15384101.2015.1030548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang L, Lu A, Zhou HX, Sun R, Zhao J, Zhou CJ, et al. Casein kinase 1 alpha regulates chromosome congression and separation during mouse oocyte meiotic maturation and early embryo development. PLoS One. 2013;8:e63173. doi: 10.1371/journal.pone.0063173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schneider RK, Adema V, Heckl D, Jaras M, Mallo M, Lord AM, et al. Role of casein kinase 1A1 in the biology and targeted therapy of del(5q) MDS. Cancer Cell. 2014;26:509–520. doi: 10.1016/j.ccr.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pribluda A, Elyada E, Wiener Z, Hamza H, Goldstein RE, Biton M, et al. A senescence-inflammatory switch from cancer-inhibitory to cancer-promoting mechanism. Cancer Cell. 2013;24:242–256. doi: 10.1016/j.ccr.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 106.Hu Y, Song W, Cirstea D, Lu D, Munshi NC, Anderson KC. CSNK1alpha1 mediates malignant plasma cell survival. Leukemia. 2015;29:474–482. doi: 10.1038/leu.2014.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nussbaum RL, Ellis CE. Alzheimer's disease and Parkinson's disease. N Engl J Med. 2003;348:1356–1364. doi: 10.1056/NEJM2003ra020003. [DOI] [PubMed] [Google Scholar]
- 108.Winblad B, Amouyel P, Andrieu S, Ballard C, Brayne C, Brodaty H, et al. Defeating Alzheimer's disease and other dementias: a priority for European science and society. Lancet Neurol. 2016;15:455–532. doi: 10.1016/S1474-4422(16)00062-4. [DOI] [PubMed] [Google Scholar]
- 109.Canter RG, Penney J, Tsai LH. The road to restoring neural circuits for the treatment of Alzheimer's disease. Nature. 2016;539:187–196. doi: 10.1038/nature20412. [DOI] [PubMed] [Google Scholar]
- 110.Muller UC, Deller T, Korte M. Not just amyloid: physiological functions of the amyloid precursor protein family. Nat Rev Neurosci. 2017;18:281–298. doi: 10.1038/nrn.2017.29. [DOI] [PubMed] [Google Scholar]
- 111.Yasojima K, Kuret J, DeMaggio AJ, McGeer E, McGeer PL. Casein kinase 1 delta mRNA is upregulated in Alzheimer disease brain. Brain Res. 2000;865:116–120. doi: 10.1016/S0006-8993(00)02200-9. [DOI] [PubMed] [Google Scholar]
- 112.Flajolet M, He G, Heiman M, Lin A, Nairn AC, Greengard P. Regulation of Alzheimer's disease amyloid-beta formation by casein kinase I. Proc Natl Acad Sci U S A. 2007;104:4159–4164. doi: 10.1073/pnas.0611236104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Walter J, Fluhrer R, Hartung B, Willem M, Kaether C, Capell A, et al. Phosphorylation regulates intracellular trafficking of beta-secretase. J Biol Chem. 2001;276:14634–14641. doi: 10.1074/jbc.M011116200. [DOI] [PubMed] [Google Scholar]
- 114.Buxbaum JD. A role for calsenilin and related proteins in multiple aspects of neuronal function. Biochem Biophys Res Commun. 2004;322:1140–1144. doi: 10.1016/j.bbrc.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 115.Choi EK, Miller JS, Zaidi NF, Salih E, Buxbaum JD, Wasco W. Phosphorylation of calsenilin at Ser63 regulates its cleavage by caspase-3. Mol Cell Neurosci. 2003;23:495–506. doi: 10.1016/S1044-7431(03)00072-1. [DOI] [PubMed] [Google Scholar]
- 116.Walter J, Capell A, Hung AY, Langen H, Schnolzer M, Thinakaran G, et al. Ectodomain phosphorylation of beta-amyloid precursor protein at two distinct cellular locations. J Biol Chem. 1997;272:1896–1903. doi: 10.1074/jbc.272.3.1896. [DOI] [PubMed] [Google Scholar]
- 117.Chauhan A, Chauhan VP, Murakami N, Brockerhoff H, Wisniewski HM. Amyloid beta-protein stimulates casein kinase I and casein kinase II activities. Brain Res. 1993;629:47–52. doi: 10.1016/0006-8993(93)90479-7. [DOI] [PubMed] [Google Scholar]
- 118.Polanco JC, Li C, Bodea LG, Martinez-Marmol R, Meunier FA, Gotz J. Amyloid-beta and tau complexity - towards improved biomarkers and targeted therapies. Nat Rev Neurol. 2018;14:22–39. doi: 10.1038/nrneurol.2017.162. [DOI] [PubMed] [Google Scholar]
- 119.de Calignon A, Fox LM, Pitstick R, Carlson GA, Bacskai BJ, Spires-Jones TL, et al. Caspase activation precedes and leads to tangles. Nature. 2010;464:1201–1204. doi: 10.1038/nature08890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Singh TJ, Grundke-Iqbal I, Iqbal K. Phosphorylation of tau protein by casein kinase-1 converts it to an abnormal Alzheimer-like state. J Neurochem. 1995;64:1420–1423. doi: 10.1046/j.1471-4159.1995.64031420.x. [DOI] [PubMed] [Google Scholar]
- 121.Kuret J, Johnson GS, Cha D, Christenson ER, DeMaggio AJ, Hoekstra MF. Casein kinase 1 is tightly associated with paired-helical filaments isolated from Alzheimer's disease brain. J Neurochem. 1997;69:2506–2515. doi: 10.1046/j.1471-4159.1997.69062506.x. [DOI] [PubMed] [Google Scholar]
- 122.Wang C, Telpoukhovskaia MA, Bahr BA, Chen X, Gan L. Endo-lysosomal dysfunction: a converging mechanism in neurodegenerative diseases. Curr Opin Neurobiol. 2017;48:52–58. doi: 10.1016/j.conb.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 123.Herrera A, Munoz P, Steinbusch HWM, Segura-Aguilar J. Are dopamine oxidation metabolites involved in the loss of dopaminergic neurons in the nigrostriatal system in Parkinson's disease? ACS Chem Neurosci. 2017;8:702–711. doi: 10.1021/acschemneuro.7b00034. [DOI] [PubMed] [Google Scholar]
- 124.Dehay B, Bourdenx M, Gorry P, Przedborski S, Vila M, Hunot S, et al. Targeting alpha-synuclein for treatment of Parkinson's disease: mechanistic and therapeutic considerations. Lancet Neurol. 2015;14:855–866. doi: 10.1016/S1474-4422(15)00006-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Anderson JP, Walker DE, Goldstein JM, de Laat R, Banducci K, Caccavello RJ, et al. Phosphorylation of Ser-129 is the dominant pathological modification of alpha-synuclein in familial and sporadic Lewy body disease. J Biol Chem. 2006;281:29739–29752. doi: 10.1074/jbc.M600933200. [DOI] [PubMed] [Google Scholar]
- 126.Mbefo MK, Fares MB, Paleologou K, Oueslati A, Yin G, Tenreiro S, et al. Parkinson disease mutant E46K enhances alpha-synuclein phosphorylation in mammalian cell lines, in yeast, and in vivo. J Biol Chem. 2015;290:9412–9427. doi: 10.1074/jbc.M114.610774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Okochi M, Walter J, Koyama A, Nakajo S, Baba M, Iwatsubo T, et al. Constitutive phosphorylation of the Parkinson's disease associated alpha-synuclein. J Biol Chem. 2000;275:390–397. doi: 10.1074/jbc.275.1.390. [DOI] [PubMed] [Google Scholar]
- 128.Shanware NP, Williams LM, Bowler MJ, Tibbetts RS. Non-specific in vivo inhibition of CK1 by the pyridinyl imidazole p38 inhibitors SB 203580 and SB 202190. BMB Rep. 2009;42:142–147. doi: 10.5483/BMBRep.2009.42.3.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Liu FC, Graybiel AM. Spatiotemporal dynamics of CREB phosphorylation: transient versus sustained phosphorylation in the developing striatum. Neuron. 1996;17:1133–1144. doi: 10.1016/S0896-6273(00)80245-7. [DOI] [PubMed] [Google Scholar]
- 130.Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, et al. Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science. 2004;304:1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- 131.Chia R, Haddock S, Beilina A, Rudenko IN, Mamais A, Kaganovich A, et al. Phosphorylation of LRRK2 by casein kinase 1alpha regulates trans-Golgi clustering via differential interaction with ARHGEF7. Nat Commun. 2014;5:5827. doi: 10.1038/ncomms6827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yamamoto A, Friedlein A, Imai Y, Takahashi R, Kahle PJ, Haass C. Parkin phosphorylation and modulation of its E3 ubiquitin ligase activity. J Biol Chem. 2005;280:3390–3399. doi: 10.1074/jbc.M407724200. [DOI] [PubMed] [Google Scholar]
- 133.Cheung ZH, Fu AK, Ip NY. Synaptic roles of Cdk5: implications in higher cognitive functions and neurodegenerative diseases. Neuron. 2006;50:13–18. doi: 10.1016/j.neuron.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 134.Sharma P, Sharma M, Amin ND, Albers RW, Pant HC. Regulation of cyclin-dependent kinase 5 catalytic activity by phosphorylation. Proc Natl Acad Sci U S A. 1999;96:11156–11160. doi: 10.1073/pnas.96.20.11156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Liu F, Ma XH, Ule J, Bibb JA, Nishi A, DeMaggio AJ, et al. Regulation of cyclin-dependent kinase 5 and casein kinase 1 by metabotropic glutamate receptors. Proc Natl Acad Sci U S A. 2001;98:11062–11068. doi: 10.1073/pnas.191353898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Simpson NH, Ceroni F, Reader RH, Covill LE, Knight JC, Consortium SLI, et al. Genome-wide analysis identifies a role for common copy number variants in specific language impairment. Eur J Hum Genet. 2015;23:1370–1377. doi: 10.1038/ejhg.2014.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Liu J, Carvalho LP, Bhattacharya S, Carbone CJ, Kumar KG, Leu NA, et al. Mammalian casein kinase 1alpha and its leishmanial ortholog regulate stability of IFNAR1 and type I interferon signaling. Mol Cell Biol. 2009;29:6401–6412. doi: 10.1128/MCB.00478-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bhattacharya S, HuangFu WC, Liu J, Veeranki S, Baker DP, Koumenis C, et al. Inducible priming phosphorylation promotes ligand-independent degradation of the IFNAR1 chain of type I interferon receptor. J Biol Chem. 2010;285:2318–2325. doi: 10.1074/jbc.M109.071498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhang L, Li H, Chen Y, Gao X, Lu Z, Gao L, et al. The down-regulation of casein kinase 1 alpha as a host defense response against infectious bursal disease virus infection. Virology. 2017;512:211–221. doi: 10.1016/j.virol.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 140.Xia C, Wolf JJ, Vijayan M, Studstill CJ, Ma W, Hahm B. Casein kinase 1alpha mediates degradation of receptors for type I and type II interferons caused by hemagglutinin of influenza a virus. J Virol. 2018;92:e00006–18. doi: 10.1128/JVI.00006-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chong R, Squires R, Swiss R, Agaisse H. RNAi screen reveals host cell kinases specifically involved in listeria monocytogenes spread from cell to cell. PLoS One. 2011;6:e23399. doi: 10.1371/journal.pone.0023399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang Z, Wang S, Wang W, Gu Y, Liu H, Wei F, et al. Targeted disruption of CK1alpha in toxoplasma gondii increases acute virulence in mice. Eur J Protistol. 2016;56:90–101. doi: 10.1016/j.ejop.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 143.Eichwald C, Jacob G, Muszynski B, Allende JE, Burrone OR. Uncoupling substrate and activation functions of rotavirus NSP5: phosphorylation of Ser-67 by casein kinase 1 is essential for hyperphosphorylation. Proc Natl Acad Sci U S A. 2004;101:16304–16309. doi: 10.1073/pnas.0406691101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Campagna M, Budini M, Arnoldi F, Desselberger U, Allende JE, Burrone OR. Impaired hyperphosphorylation of rotavirus NSP5 in cells depleted of casein kinase 1alpha is associated with the formation of viroplasms with altered morphology and a moderate decrease in virus replication. J Gen Virol. 2007;88:2800–2810. doi: 10.1099/vir.0.82922-0. [DOI] [PubMed] [Google Scholar]
- 145.Quintavalle M, Sambucini S, Di Pietro C, De Francesco R, Neddermann P. The alpha isoform of protein kinase CKI is responsible for hepatitis C virus NS5A hyperphosphorylation. J Virol. 2006;80:11305–11312. doi: 10.1128/JVI.01465-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sudha G, Yamunadevi S, Tyagi N, Das S, Srinivasan N. Structural and molecular basis of interaction of HCV non-structural protein 5A with human casein kinase 1alpha and PKR. BMC Struct Biol. 2012;12:28. doi: 10.1186/1472-6807-12-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bhattacharya D, Ansari IH, Striker R. The flaviviral methyltransferase is a substrate of casein kinase 1. Virus Res. 2009;141:101–104. doi: 10.1016/j.virusres.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lin TC, Su CY, Wu PY, Lai TC, Pan WA, Jan YH, et al. The nucleolar protein NIFK promotes cancer progression via CK1alpha/beta-catenin in metastasis and Ki-67-dependent cell proliferation. Elife. 2016;5:e11288. doi: 10.7554/eLife.11288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Richter J, Kretz AL, Lemke J, Fauler M, Werner JU, Paschke S, et al. CK1alpha overexpression correlates with poor survival in colorectal cancer. BMC Cancer. 2018;18:140. doi: 10.1186/s12885-018-4019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sarasqueta AF, Forte G, Corver WE, de Miranda NF, Ruano D, van Eijk R, et al. Integral analysis of p53 and its value as prognostic factor in sporadic colon cancer. BMC Cancer. 2013;13:277. doi: 10.1186/1471-2407-13-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Katlinskaya YV, Katlinski KV, Lasri A, Li N, Beiting DP, Durham AC, et al. Type I interferons control proliferation and function of the intestinal epithelium. Mol Cell Biol. 2016;36:1124–1135. doi: 10.1128/MCB.00988-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhang P, Bill K, Liu J, Young E, Peng T, Bolshakov S, et al. MiR-155 is a liposarcoma oncogene that targets casein kinase-1alpha and enhances beta-catenin signaling. Cancer Res. 2012;72:1751–1762. doi: 10.1158/0008-5472.CAN-11-3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Prasad R, Katiyar SK. Ultraviolet radiation-induced inflammation activates beta-catenin signaling in mouse skin and skin tumors. Int J Oncol. 2014;44:1199–1206. doi: 10.3892/ijo.2014.2275. [DOI] [PubMed] [Google Scholar]
- 154.Boultwood J, Pellagatti A, Cattan H, Lawrie CH, Giagounidis A, Malcovati L, et al. Gene expression profiling of CD34+ cells in patients with the 5q- syndrome. Br J Haematol. 2007;139:578–589. doi: 10.1111/j.1365-2141.2007.06833.x. [DOI] [PubMed] [Google Scholar]
- 155.Bello E, Pellagatti A, Shaw J, Mecucci C, Kusec R, Killick S, et al. CSNK1A1 mutations and gene expression analysis in myelodysplastic syndromes with del(5q) Br J Haematol. 2015;171:210–4. doi: 10.1111/bjh.13563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Smith AE, Kulasekararaj AG, Jiang J, Mian S, Mohamedali A, Gaken J, et al. CSNK1A1 mutations and isolated del(5q) abnormality in myelodysplastic syndrome: a retrospective mutational analysis. Lancet Haematol. 2015;2:e212–e221. doi: 10.1016/S2352-3026(15)00050-2. [DOI] [PubMed] [Google Scholar]
- 157.Kataoka K, Nagata Y, Kitanaka A, Shiraishi Y, Shimamura T, Yasunaga J, et al. Integrated molecular analysis of adult T cell leukemia/lymphoma. Nat Genet. 2015;47:1304–1315. doi: 10.1038/ng.3415. [DOI] [PubMed] [Google Scholar]
- 158.Sato Y, Yoshizato T, Shiraishi Y, Maekawa S, Okuno Y, Kamura T, et al. Integrated molecular analysis of clear-cell renal cell carcinoma. Nat Genet. 2013;45:860–867. doi: 10.1038/ng.2699. [DOI] [PubMed] [Google Scholar]
- 159.Okerberg ES, Hainley A, Brown H, Aban A, Alemayehu S, Shih A, et al. Identification of a tumor specific, active-site mutation in casein kinase 1alpha by chemical proteomics. PLoS One. 2016;11:e0152934. doi: 10.1371/journal.pone.0152934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Dulak AM, Stojanov P, Peng S, Lawrence MS, Fox C, Stewart C, et al. Exome and whole-genome sequencing of esophageal adenocarcinoma identifies recurrent driver events and mutational complexity. Nat Genet. 2013;45:478–486. doi: 10.1038/ng.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Manni S, Carrino M, Piazza F. Role of protein kinases CK1alpha and CK2 in multiple myeloma: regulation of pivotal survival and stress-managing pathways. J Hematol Oncol. 2017;10:157. doi: 10.1186/s13045-017-0529-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.List A, Kurtin S, Roe DJ, Buresh A, Mahadevan D, Fuchs D, et al. Efficacy of lenalidomide in myelodysplastic syndromes. N Engl J Med. 2005;352:549–557. doi: 10.1056/NEJMoa041668. [DOI] [PubMed] [Google Scholar]
- 163.List A, Dewald G, Bennett J, Giagounidis A, Raza A, Feldman E, et al. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med. 2006;355:1456–1465. doi: 10.1056/NEJMoa061292. [DOI] [PubMed] [Google Scholar]
- 164.Fenaux P, Giagounidis A, Selleslag D, Beyne-Rauzy O, Mufti G, Mittelman M, et al. A randomized phase 3 study of lenalidomide versus placebo in RBC transfusion-dependent patients with low-/Intermediate-1-risk myelodysplastic syndromes with del5q. Blood. 2011;118:3765–3776. doi: 10.1182/blood-2011-01-330126. [DOI] [PubMed] [Google Scholar]
- 165.Nguyen TV, Li J, Lu CJ, Mamrosh JL, Lu G, Cathers BE, et al. p97/VCP promotes degradation of CRBN substrate glutamine synthetase and neosubstrates. Proc Natl Acad Sci U S A. 2017;114:3565–3571. doi: 10.1073/pnas.1700949114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Li X, Liu J, Gao T. Beta-TrCP-mediated ubiquitination and degradation of PHLPP1 are negatively regulated by Akt. Mol Cell Biol. 2009;29:6192–6205. doi: 10.1128/MCB.00681-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mai H, Xu X, Mei G, Hong T, Huang J, Wang T, et al. The interplay between HPIP and casein kinase 1alpha promotes renal cell carcinoma growth and metastasis via activation of mTOR pathway. Oncogene. 2016;5:e260. doi: 10.1038/oncsis.2016.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sun D, Zhou M, Kowolik CM, Trisal V, Huang Q, Kernstine KH, et al. Differential expression patterns of capping protein, protein phosphatase 1, and casein kinase 1 may serve as diagnostic markers for malignant melanoma. Melanoma Res. 2011;21:335–343. doi: 10.1097/CMR.0b013e328346b715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sinnberg T, Menzel M, Kaesler S, Biedermann T, Sauer B, Nahnsen S, et al. Suppression of casein kinase 1alpha in melanoma cells induces a switch in beta-catenin signaling to promote metastasis. Cancer Res. 2010;70:6999–7009. doi: 10.1158/0008-5472.CAN-10-0645. [DOI] [PubMed] [Google Scholar]
- 170.Sinnberg T, Wang J, Sauer B, Schittek B. Casein kinase 1alpha has a non-redundant and dominant role within the CK1 family in melanoma progression. BMC Cancer. 2016;16:594. doi: 10.1186/s12885-016-2643-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Manni S, Carrino M, Manzoni M, Gianesin K, Nunes SC, Costacurta M, et al. Inactivation of CK1alpha in multiple myeloma empowers drug cytotoxicity by affecting AKT and beta-catenin survival signaling pathways. Oncotarget. 2017;8:14604–14619. doi: 10.18632/oncotarget.14654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Yang Y, Shaffer AL, 3rd, Emre NC, Ceribelli M, Zhang M, Wright G, et al. Exploiting synthetic lethality for the therapy of ABC diffuse large B cell lymphoma. Cancer Cell. 2012;21:723–737. doi: 10.1016/j.ccr.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zhou A, Lin K, Zhang S, Chen Y, Zhang N, Xue J, et al. Nuclear GSK3beta promotes tumorigenesis by phosphorylating KDM1A and inducing its deubiquitylation by USP22. Nat Cell Biol. 2016;18:954–966. doi: 10.1038/ncb3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Magliozzi R, Low TY, Weijts BG, Cheng T, Spanjaard E, Mohammed S, et al. Control of epithelial cell migration and invasion by the IKKbeta- and CK1alpha-mediated degradation of RAPGEF2. Dev Cell. 2013;27:574–585. doi: 10.1016/j.devcel.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 175.Zhao Y, Qin S, Atangan LI, Molina Y, Okawa Y, Arpawong HT, et al. Casein kinase 1alpha interacts with retinoid X receptor and interferes with agonist-induced apoptosis. J Biol Chem. 2004;279:30844–30849. doi: 10.1074/jbc.M404651200. [DOI] [PubMed] [Google Scholar]
- 176.Desagher S, Osen-Sand A, Montessuit S, Magnenat E, Vilbois F, Hochmann A, et al. Phosphorylation of bid by casein kinases I and II regulates its cleavage by caspase 8. Mol Cell. 2001;8:601–611. doi: 10.1016/S1097-2765(01)00335-5. [DOI] [PubMed] [Google Scholar]
- 177.Grills C, Jithesh PV, Blayney J, Zhang SD, Fennell DA. Gene expression meta-analysis identifies VDAC1 as a predictor of poor outcome in early stage non-small cell lung cancer. PLoS One. 2011;6:e14635. doi: 10.1371/journal.pone.0014635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Srivastava M, Khurana P, Sugadev R. Lung cancer signature biomarkers: tissue specific semantic similarity based clustering of digital differential display (DDD) data. BMC Res Notes. 2012;5:617. doi: 10.1186/1756-0500-5-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lantermann AB, Chen D, McCutcheon K, Hoffman G, Frias E, Ruddy D, et al. Inhibition of casein kinase 1 alpha prevents acquired drug resistance to Erlotinib in EGFR-mutant non-small cell lung Cancer. Cancer Res. 2015;75:4937–4948. doi: 10.1158/0008-5472.CAN-15-1113. [DOI] [PubMed] [Google Scholar]
- 180.Loh YN, Hedditch EL, Baker LA, Jary E, Ward RL, Ford CE. The Wnt signalling pathway is upregulated in an in vitro model of acquired tamoxifen resistant breast cancer. BMC Cancer. 2013;13:174. doi: 10.1186/1471-2407-13-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kuo IY, Wu CC, Chang JM, Huang YL, Lin CH, Yan JJ, et al. Low SOX17 expression is a prognostic factor and drives transcriptional dysregulation and esophageal cancer progression. Int J Cancer. 2014;135:563–573. doi: 10.1002/ijc.28695. [DOI] [PubMed] [Google Scholar]
- 182.Litlekalsoy J, Rostad K, Kalland KH, Hostmark JG, Laerum OD. Expression of circadian clock genes and proteins in urothelial cancer is related to cancer-associated genes. BMC Cancer. 2016;16:549. doi: 10.1186/s12885-016-2580-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kattapuram T, Yang S, Maki JL, Stone JR. Protein kinase CK1alpha regulates mRNA binding by heterogeneous nuclear ribonucleoprotein C in response to physiologic levels of hydrogen peroxide. J Biol Chem. 2005;280:15340–15347. doi: 10.1074/jbc.M500214200. [DOI] [PubMed] [Google Scholar]
- 184.Mohan N, Sudheesh AP, Francis N, Anderson R, Laishram RS. Phosphorylation regulates the star-PAP-PIPKIalpha interaction and directs specificity toward mRNA targets. Nucleic Acids Res. 2015;43:7005–7020. doi: 10.1093/nar/gkv676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Golden RJ, Chen B, Li T, Braun J, Manjunath H, Chen X, et al. An Argonaute phosphorylation cycle promotes microRNA-mediated silencing. Nature. 2017;542:197–202. doi: 10.1038/nature21025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Carreras Puigvert J, von Stechow L, Siddappa R, Pines A, Bahjat M, Haazen LC, et al. Systems biology approach identifies the kinase Csnk1a1 as a regulator of the DNA damage response in embryonic stem cells. Sci Signal. 2013;6:ra5. doi: 10.1126/scisignal.2003208. [DOI] [PubMed] [Google Scholar]
- 187.Van Camp JK, Beckers S, Zegers D, Van Hul W. Wnt signaling and the control of human stem cell fate. Stem Cell Rev. 2014;10:207–229. doi: 10.1007/s12015-013-9486-8. [DOI] [PubMed] [Google Scholar]
- 188.Harwood AJ. Signal transduction in development: holding the key. Dev Cell. 2002;2:384–385. doi: 10.1016/S1534-5807(02)00156-9. [DOI] [PubMed] [Google Scholar]
- 189.Dimova N, Wysoczynski M, Rokosh G. Stromal cell derived factor-1alpha promotes C-kit+ cardiac stem/progenitor cell quiescence through casein kinase 1alpha and GSK3beta. Stem Cells. 2014;32:487–499. doi: 10.1002/stem.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ursu A, Illich DJ, Takemoto Y, Porfetye AT, Zhang M, Brockmeyer A, et al. Epiblastin a induces reprogramming of epiblast stem cells into embryonic stem cells by inhibition of casein kinase 1. Cell Chem Biol. 2016;23:494–507. doi: 10.1016/j.chembiol.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 191.Illich DJ, Zhang M, Ursu A, Osorno R, Kim KP, Yoon J, et al. Distinct signaling requirements for the establishment of ESC pluripotency in late-stage EpiSCs. Cell Rep. 2016;15:787–800. doi: 10.1016/j.celrep.2016.03.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Bao X, Siprashvili Z, Zarnegar BJ, Shenoy RM, Rios EJ, Nady N, et al. CSNK1a1 regulates PRMT1 to maintain the progenitor state in self-renewing somatic tissue. Dev Cell. 2017;43:227–239. doi: 10.1016/j.devcel.2017.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Gui J, Zhao B, Lyu K, Tong W, Fuchs SY. Downregulation of the IFNAR1 chain of type 1 interferon receptor contributes to the maintenance of the haematopoietic stem cells. Cancer Biol Ther. 2017;18:534–543. doi: 10.1080/15384047.2017.1345395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Waugh MG, Challiss RA, Berstein G, Nahorski SR, Tobin AB. Agonist-induced desensitization and phosphorylation of m1-muscarinic receptors. Biochem J. 1999;338(Pt 1):175–183. doi: 10.1042/bj3380175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Tobin AB, Keys B, Nahorski SR. Identification of a novel receptor kinase that phosphorylates a phospholipase C-linked muscarinic receptor. J Biol Chem. 1996;271:3907–3916. doi: 10.1074/jbc.271.7.3907. [DOI] [PubMed] [Google Scholar]
- 196.Tobin AB, Totty NF, Sterlin AE, Nahorski SR. Stimulus-dependent phosphorylation of G-protein-coupled receptors by casein kinase 1alpha. J Biol Chem. 1997;272:20844–20849. doi: 10.1074/jbc.272.33.20844. [DOI] [PubMed] [Google Scholar]
- 197.Hauser AS, Attwood MM, Rask-Andersen M, Schioth HB, Gloriam DE. Trends in GPCR drug discovery: new agents, targets and indications. Nat Rev Drug Discov. 2017;16:829–842. doi: 10.1038/nrd.2017.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Turner KM, Burgoyne RD, Morgan A. Protein phosphorylation and the regulation of synaptic membrane traffic. Trends Neurosci. 1999;22:459–464. doi: 10.1016/S0166-2236(99)01436-8. [DOI] [PubMed] [Google Scholar]
- 199.Hirota T, Lee JW, Lewis WG, Zhang EE, Breton G, Liu X, et al. High-throughput chemical screen identifies a novel potent modulator of cellular circadian rhythms and reveals CKIalpha as a clock regulatory kinase. PLoS Biol. 2010;8:e1000559. doi: 10.1371/journal.pbio.1000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ugrankar R, Berglund E, Akdemir F, Tran C, Kim MS, Noh J, et al. Drosophila glucome screening identifies Ck1alpha as a regulator of mammalian glucose metabolism. Nat Commun. 2015;6:7102. doi: 10.1038/ncomms8102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zelenak C, Eberhard M, Jilani K, Qadri SM, Macek B, Lang F. Protein kinase CK1alpha regulates erythrocyte survival. Cell Physiol Biochem. 2012;29:171–180. doi: 10.1159/000337598. [DOI] [PubMed] [Google Scholar]
- 202.Nguyen HQ, Nye J, Buster DW, Klebba JE, Rogers GC, Bosco G. Drosophila casein kinase I alpha regulates homolog pairing and genome organization by modulating condensin II subunit cap-H2 levels. PLoS Genet. 2015;11:e1005014. doi: 10.1371/journal.pgen.1005014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Yan Q, Chen J, Li W, Bao C, Fu Q. Targeting miR-155 to treat experimental scleroderma. Sci Rep. 2016;6:20314. doi: 10.1038/srep20314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Li X, He L, Yue Q, Lu J, Kang N, Xu X, et al. MiR-9-5p promotes MSC migration by activating beta-catenin signaling pathway. Am J Physiol Cell Physiol. 2017;313:C80–C93. doi: 10.1152/ajpcell.00232.2016. [DOI] [PubMed] [Google Scholar]
- 205.Kuga T, Kume H, Adachi J, Kawasaki N, Shimizu M, Hoshino I, et al. Casein kinase 1 is recruited to nuclear speckles by FAM83H and SON. Sci Rep. 2016;6:34472. doi: 10.1038/srep34472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Cruciat CM, Dolde C, de Groot RE, Ohkawara B, Reinhard C, Korswagen HC, et al. RNA helicase DDX3 is a regulatory subunit of casein kinase 1 in Wnt-beta-catenin signaling. Science. 2013;339:1436–1441. doi: 10.1126/science.1231499. [DOI] [PubMed] [Google Scholar]
- 207.Lindner S, Kronke J. The molecular mechanism of thalidomide analogs in hematologic malignancies. J Mol Med (Berl) 2016;94:1327–1334. doi: 10.1007/s00109-016-1450-z. [DOI] [PubMed] [Google Scholar]
- 208.Chijiwa T, Hagiwara M, Hidaka H. A newly synthesized selective casein kinase I inhibitor, N-(2-aminoethyl)-5-chloroisoquinoline-8-sulfonamide, and affinity purification of casein kinase I from bovine testis. J Biol Chem. 1989;264:4924–4927. [PubMed] [Google Scholar]
- 209.Nyati S, Ranga R, Ross BD, Rehemtulla A, Bhojani MS. Molecular imaging of glycogen synthase kinase-3beta and casein kinase-1alpha kinases. Anal Biochem. 2010;405:246–254. doi: 10.1016/j.ab.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Burzio V, Antonelli M, Allende CC, Allende JE. Biochemical and cellular characteristics of the four splice variants of protein kinase CK1alpha from zebrafish (Danio rerio) J Cell Biochem. 2002;86:805–814. doi: 10.1002/jcb.10263. [DOI] [PubMed] [Google Scholar]
- 211.Behrend L, Milne DM, Stoter M, Deppert W, Campbell LE, Meek DW, et al. IC261, a specific inhibitor of the protein kinases casein kinase 1-delta and -epsilon, triggers the mitotic checkpoint and induces p53-dependent postmitotic effects. Oncogene. 2000;19:5303–5313. doi: 10.1038/sj.onc.1203939. [DOI] [PubMed] [Google Scholar]
- 212.Kurihara T, Sakurai E, Toyomoto M, Kii I, Kawamoto D, Asada T, et al. Alleviation of behavioral hypersensitivity in mouse models of inflammatory pain with two structurally different casein kinase 1 (CK1) inhibitors. Mol Pain. 2014;10:17. doi: 10.1186/1744-8069-10-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Muraki M, Ohkawara B, Hosoya T, Onogi H, Koizumi J, Koizumi T, et al. Manipulation of alternative splicing by a newly developed inhibitor of Clks. J Biol Chem. 2004;279:24246–24254. doi: 10.1074/jbc.M314298200. [DOI] [PubMed] [Google Scholar]
- 214.Isojima Y, Nakajima M, Ukai H, Fujishima H, Yamada RG, Masumoto KH, et al. CKIepsilon/delta-dependent phosphorylation is a temperature-insensitive, period-determining process in the mammalian circadian clock. Proc Natl Acad Sci U S A. 2009;106:15744–15749. doi: 10.1073/pnas.0908733106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Anastassiadis T, Deacon SW, Devarajan K, Ma H, Peterson JR. Comprehensive assay of kinase catalytic activity reveals features of kinase inhibitor selectivity. Nat Biotechnol. 2011;29:1039–1045. doi: 10.1038/nbt.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Rena G, Bain J, Elliott M, Cohen P. D4476, a cell-permeant inhibitor of CK1, suppresses the site-specific phosphorylation and nuclear exclusion of FOXO1a. EMBO Rep. 2004;5:60–65. doi: 10.1038/sj.embor.7400048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Sarafidis PA, Georgianos PI, Lasaridis AN. Diuretics in clinical practice. Part I: mechanisms of action, pharmacological effects and clinical indications of diuretic compounds. Expert Opin Drug Saf. 2010;9:243–257. doi: 10.1517/14740330903499240. [DOI] [PubMed] [Google Scholar]
- 218.Ishii I, Harada Y, Kasahara T. Reprofiling a classical anthelmintic, pyrvinium pamoate, as an anti-cancer drug targeting mitochondrial respiration. Front Oncol. 2012;2:137. doi: 10.3389/fonc.2012.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Li B, Flaveny CA, Giambelli C, Fei DL, Han L, Hang BI, et al. Repurposing the FDA-approved pinworm drug pyrvinium as a novel chemotherapeutic agent for intestinal polyposis. PLoS One. 2014;9:e101969. doi: 10.1371/journal.pone.0101969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Saraswati S, Alfaro MP, Thorne CA, Atkinson J, Lee E, Young PP. Pyrvinium, a potent small molecule Wnt inhibitor, promotes wound repair and post-MI cardiac remodeling. PLoS One. 2010;5:e15521. doi: 10.1371/journal.pone.0015521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Venerando A, Girardi C, Ruzzene M, Pinna LA. Pyrvinium pamoate does not activate protein kinase CK1, but promotes Akt/PKB down-regulation and GSK3 activation. Biochem J. 2013;452:131–137. doi: 10.1042/BJ20121140. [DOI] [PubMed] [Google Scholar]
- 222.Barham W, Frump AL, Sherrill TP, Garcia CB, Saito-Diaz K, Van Saun MN, et al. Targeting the Wnt pathway in synovial sarcoma models. Cancer Discov. 2013;3:1286–1301. doi: 10.1158/2159-8290.CD-13-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Li B, Orton D, Neitzel LR, Astudillo L, Shen C, Long J, et al. Differential abundance of CK1alpha provides selectivity for pharmacological CK1alpha activators to target WNT-dependent tumors. Sci Signal. 2017;10:eaak9916. [DOI] [PMC free article] [PubMed]
- 224.Li B, Lee E, Robbins DJ. Casein kinase1alpha activators, a precision weapon for CRC. Oncotarget. 2017;8:96462–96463. doi: 10.18632/oncotarget.21892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Iaconelli J, Huang JH, Berkovitch SS, Chattopadhyay S, Mazitschek R, Schreiber SL, et al. HDAC6 inhibitors modulate Lys49 acetylation and membrane localization of beta-catenin in human iPSC-derived neuronal cells. ACS Chem Biol. 2015;10:883–890. doi: 10.1021/cb500838r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Brockman JL, Anderson RA. Casein kinase I is regulated by phosphatidylinositol 4,5-bisphosphate in native membranes. J Biol Chem. 1991;266:2508–2512. [PubMed] [Google Scholar]
- 227.Bazenet CE, Brockman JL, Lewis D, Chan C, Anderson RA. Erythroid membrane-bound protein kinase binds to a membrane component and is regulated by phosphatidylinositol 4,5-bisphosphate. J Biol Chem. 1990;265:7369–7376. [PubMed] [Google Scholar]
- 228.Knippschild U, Gocht A, Wolff S, Huber N, Lohler J, Stoter M. The casein kinase 1 family: participation in multiple cellular processes in eukaryotes. Cell Signal. 2005;17:675–689. doi: 10.1016/j.cellsig.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 229.Budini M, Jacob G, Jedlicki A, Perez C, Allende CC, Allende JE. Autophosphorylation of carboxy-terminal residues inhibits the activity of protein kinase CK1alpha. J Cell Biochem. 2009;106:399–408. doi: 10.1002/jcb.22019. [DOI] [PubMed] [Google Scholar]
- 230.Chang CH, Kuo CJ, Ito T, Su YY, Jiang ST, Chiu MH, et al. CK1alpha ablation in keratinocytes induces p53-dependent, sunburn-protective skin hyperpigmentation. Proc Natl Acad Sci U S A. 2017;114:E8035–E8E44. doi: 10.1073/pnas.1702763114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Green CL, Bennett GS. Identification of four alternatively spliced isoforms of chicken casein kinase I alpha that are all expressed in diverse cell types. Gene. 1998;216:189–195. doi: 10.1016/S0378-1119(98)00291-1. [DOI] [PubMed] [Google Scholar]
- 232.Yong TJ, Gan YY, Toh BH, Sentry JW. Human CKIalpha(L) and CKIalpha(S) are encoded by both 2.4- and 4. 2-kb transcripts, the longer containing multiple RNA-destablising elements. Biochim Biophys Acta. 2000;1492:425–433. doi: 10.1016/S0167-4781(00)00146-9. [DOI] [PubMed] [Google Scholar]
- 233.Zhang J, Gross SD, Schroeder MD, Anderson RA. Casein kinase I alpha and alpha L: alternative splicing-generated kinases exhibit different catalytic properties. Biochemistry. 1996;35:16319–16327. doi: 10.1021/bi9614444. [DOI] [PubMed] [Google Scholar]
- 234.Panchenko MP, Siddiquee Z, Dombkowski DM, Alekseyev YO, Lenburg ME, Walker JD, et al. Protein kinase CK1alphaLS promotes vascular cell proliferation and intimal hyperplasia. Am J Pathol. 2010;177:1562–1572. doi: 10.2353/ajpath.2010.100327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Wang H, Albadawi H, Siddiquee Z, Stone JM, Panchenko MP, Watkins MT, et al. Altered vascular activation due to deficiency of the NADPH oxidase component p22phox. Cardiovasc Pathol. 2014;23:35–42. doi: 10.1016/j.carpath.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 236.Nousiainen M, Sillje HH, Sauer G, Nigg EA, Korner R. Phosphoproteome analysis of the human mitotic spindle. Proc Natl Acad Sci U S A. 2006;103:5391–5396. doi: 10.1073/pnas.0507066103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Ohyashiki JH, Ohyashiki K, Sandberg AA, Minowada J, Kinniburgh AJ. Human-fms gene is retained in acute lymphoblastic leukemia cells with del(5)(q32) Cancer Genet Cytogenet. 1987;25:341–350. doi: 10.1016/0165-4608(87)90195-6. [DOI] [PubMed] [Google Scholar]
- 238.Consortium GT The genotype-tissue expression (GTEx) project. Nat Genet. 2013;45:580–585. doi: 10.1038/ng.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Consortium GT, Laboratory DA, Coordinating Center -Analysis Working G, Statistical Methods groups-Analysis Working G, Enhancing Gg, Fund NIHC et al. Genetic effects on gene expression across human tissues. Nature. 2017;550:204–213. doi: 10.1038/nature24277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, Mardinoglu A, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347:1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 241.Cancer Genome Atlas Research N. Weinstein JN, Collisson EA, Mills GB, Shaw KR, Ozenberger BA, et al. The Cancer genome atlas Pan-Cancer analysis project. Nat Genet. 2013;45:1113–1120. doi: 10.1038/ng.2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Rhodes DR, Yu J, Shanker K, Deshpande N, Varambally R, Ghosh D, et al. ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia. 2004;6:1–6. doi: 10.1016/S1476-5586(04)80047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Segara D, Biankin AV, Kench JG, Langusch CC, Dawson AC, Skalicky DA, et al. Expression of HOXB2, a retinoic acid signaling target in pancreatic cancer and pancreatic intraepithelial neoplasia. Clin Cancer Res. 2005;11:3587–3596. doi: 10.1158/1078-0432.CCR-04-1813. [DOI] [PubMed] [Google Scholar]
- 244.Pei H, Li L, Fridley BL, Jenkins GD, Kalari KR, Lingle W, et al. FKBP51 affects cancer cell response to chemotherapy by negatively regulating Akt. Cancer Cell. 2009;16:259–266. doi: 10.1016/j.ccr.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Sun L, Hui AM, Su Q, Vortmeyer A, Kotliarov Y, Pastorino S, et al. Neuronal and glioma-derived stem cell factor induces angiogenesis within the brain. Cancer Cell. 2006;9:287–300. doi: 10.1016/j.ccr.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 246.Piccaluga PP, Agostinelli C, Califano A, Rossi M, Basso K, Zupo S, et al. Gene expression analysis of peripheral T cell lymphoma, unspecified, reveals distinct profiles and new potential therapeutic targets. J Clin Invest. 2007;117:823–834. doi: 10.1172/JCI26833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Hu N, Clifford RJ, Yang HH, Wang C, Goldstein AM, Ding T, et al. Genome wide analysis of DNA copy number neutral loss of heterozygosity (CNNLOH) and its relation to gene expression in esophageal squamous cell carcinoma. BMC Genomics. 2010;11:576. doi: 10.1186/1471-2164-11-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Kim SM, Park YY, Park ES, Cho JY, Izzo JG, Zhang D, et al. Prognostic biomarkers for esophageal adenocarcinoma identified by analysis of tumor transcriptome. PLoS One. 2010;5:e15074. doi: 10.1371/journal.pone.0015074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Lee JS, Leem SH, Lee SY, Kim SC, Park ES, Kim SB, et al. Expression signature of E2F1 and its associated genes predict superficial to invasive progression of bladder tumors. J Clin Oncol. 2010;28:2660–2667. doi: 10.1200/JCO.2009.25.0977. [DOI] [PubMed] [Google Scholar]
- 250.Ginos MA, Page GP, Michalowicz BS, Patel KJ, Volker SE, Pambuccian SE, et al. Identification of a gene expression signature associated with recurrent disease in squamous cell carcinoma of the head and neck. Cancer Res. 2004;64:55–63. doi: 10.1158/0008-5472.CAN-03-2144. [DOI] [PubMed] [Google Scholar]
- 251.Thul PJ, Akesson L, Wiking M, Mahdessian D, Geladaki A, Ait Blal H, et al. A subcellular map of the human proteome. Science. 2017;356:eaal3321. [DOI] [PubMed]
- 252.Kahn M. Can we safely target the WNT pathway? Nat Rev Drug Discov. 2014;13:513–532. doi: 10.1038/nrd4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Young RM, Staudt LM. Targeting pathological B cell receptor signalling in lymphoid malignancies. Nat Rev Drug Discov. 2013;12:229–243. doi: 10.1038/nrd3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Li MO, Rudensky AY. T cell receptor signalling in the control of regulatory T cell differentiation and function. Nat Rev Immunol. 2016;16:220–233. doi: 10.1038/nri.2016.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Moynagh PN. The roles of Pellino E3 ubiquitin ligases in immunity. Nat Rev Immunol. 2014;14:122–131. doi: 10.1038/nri3599. [DOI] [PubMed] [Google Scholar]
- 256.Chen J, Chen ZJ. Regulation of NF-kappaB by ubiquitination. Curr Opin Immunol. 2013;25:4–12. doi: 10.1016/j.coi.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 258.Schapira AH, Olanow CW, Greenamyre JT, Bezard E. Slowing of neurodegeneration in Parkinson's disease and Huntington's disease: future therapeutic perspectives. Lancet. 2014;384:545–555. doi: 10.1016/S0140-6736(14)61010-2. [DOI] [PubMed] [Google Scholar]
- 259.Vaughan RA, Foster JD. Mechanisms of dopamine transporter regulation in normal and disease states. Trends Pharmacol Sci. 2013;34:489–496. doi: 10.1016/j.tips.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Bidere N. Role of CK1alpha in adaptive immunity and lymphomagenesis. Med Sci (Paris) 2009;25:454–456. doi: 10.1051/medsci/2009255454. [DOI] [PubMed] [Google Scholar]
- 261.Wang B, Wei H, Prabhu L, Zhao W, Martin M, Hartley AV, et al. Role of novel serine 316 phosphorylation of the p65 subunit of NF-kappaB in differential gene regulation. J Biol Chem. 2015;290:20336–20347. doi: 10.1074/jbc.M115.639849. [DOI] [PMC free article] [PubMed] [Google Scholar]