Abstract
Background
Red (R) and far-red (FR) light distinctly influence phytochrome-mediated initial tomato growth and development, and more recent evidence indicates that these spectra also modulate responses to a multitude of abiotic and biotic stresses. This research investigated whether different R: FR values affect tomato growth response and salinity tolerance. Tomato seedlings were exposed to different R: FR conditions (7.4, 1.2 and 0.8) under salinity stress (100 mM NaCl), and evaluated for their growth, biochemical changes, active reactive oxygen species (ROS) and ROS scavenging enzymes, pigments, rate of photosynthesis, and chlorophyll fluorescence.
Results
The results showed that under conditions of salinity, tomato seedlings subjected to a lower R: FR value (0.8) significantly increased both their growth, proline content, chlorophyll content and net photosynthesis rate (Pn), while they decreased malondialdehyde (MDA) compared to the higher R: FR value (7.4). Under conditions of salinity, the lower R: FR value caused a decrease in both the superoxide anion (O2•−) and in hydrogen peroxide (H2O2) generation, an increase in the activities of superoxidase dismutase (SOD, EC 1.15.1.1), peroxidase (POD, EC 1.11.1.7) and catalase (CAT, EC 1.11.1.7). Tomato seedlings grown under the lower R: FR value and conditions of salinity showed a higher actual quantum yield of photosynthesis (ΦPSII), electron transport rate (ETR), and photochemical quenching (qP) than those exposed to a higher R: FR, indicating overall healthier growth. However, the salinity tolerance induced at the lower R: FR condition disappeared in the tomato phyB1 mutant.
Conlusion
These results suggest that growing tomato with a lower R: FR value could improve seedlings’ salinity tolerance, and phytochrome B1 play an very important role in this process. Therefore, different qualities of light can be used to efficiently develop abiotic stress tolerance in tomato cultivation.
Keywords: Tomato, R: FR ratio, Phytochrome B1, Antioxidant system, chlorophyll fluorescence
Background
Plant growth and development are controlled by various signaling pathways that enable them to modulate a wide range of molecular and biochemical responses to changes in their environment. Light is one of the most important environmental factors for plant development. To adapt to different light conditions, plants evolved several families of photoreceptors covering both the visible and the UV-A/B region of the spectrum [1–5].
Phytochromes, which absorb red (R) and far-red (FR) light, are the most characterized photoreceptors in plants and are important in mediating many aspects of physiological development. Phytochromes, composed of photochromic proteins, are ~ 130 kDa peptides with a covalently linked linear tetrapyrrole bilin chromophore, that exist as two photo-interconvertible isomeric forms: the red-light-absorbing form (Pr), which is biologically inactive, and the far-red-light-absorbing form (Pfr), which is biologically active [6]. Upon excitation by R or FR light (producing a high or low R: FR ratio, respectively), the phytochrome converts the Pr into the Pfr form, or vice versa [2, 6].The conversion between Pr and PFr synchronizes plant development with the light environment, which causes changes in the expression of genes involved in photomorphogenesis [7–9].
Besides regulating photomorphogenesis, phytochromes also play an essential role in adapting to different sources of abiotic plant stress [2, 10–15]. The tomato phytochromes B1 and B2 mainly act as negative regulators of growth, pigment maintenance and osmoprotectant accumulation during responses to the different abiotic stresses. However, phyA mutant showed similar growth variations under different abiotic stresses when compared to the wild genotype [10]. Indorf et al. [12] found that phyA, phyB and phyAphyB Arabidopsis thaliana mutants showed a reduced expression of salt tolerance genes, and the expression of these genes were also altered by exposure to different light conditions, suggesting that the phytochrome family contributes to salinity stress responses. Compared with the wild type, rice phyB deficiency causes both reduced total leaf area and reduced transpiration per unit leaf area, which reduced water loss and improved drought tolerance of phyB mutants [16]. In fact, phyB seems to be a fundamental component of many plants responses to abiotic stressors.
Soil salinity is a major threat to global food security. During salinity stress, plants have evolved a complex survival response that involves the coordinated action of many physiological and genetic processes, including control of water loss through stomata, ion sequestration, metabolic adjustment, osmotic adjustment, and antioxidative defense [17–20]. The raised level of reactive oxygen species (ROS) under salinity stress such as superoxide (O2•−) and OH• radicals, peroxynitrite, and H2O2, and the failure of ROS-scavenging mechanisms leads to oxidative stress, damage to macromolecules, and eventually cell death [18, 21]. The increasing activity of antioxidant enzymes (SOD, CAT, POD, etc.) correlate with the level of salt tolerance [22, 23]. Moreover, salt tolerance can be improved when some antioxidant enzymes are overexpressed [24, 25]. Salinity tolerant hyperactive phytochrome mutant overexpression lines are associated with decreased H2O2 levels and significantly increased enzymatic activities of the major ROS scavengers, when compared with the wild type [26].
Chlorophyll’s concentration and composition directly influence photosynthetic rate. The effects of environmental stresses on chlorophyll metabolism such as salt stress, light wavelength, and metals, have been studied in plants [27, 28]. There is an observed salinity-induced decrease in chlorophyll, which may be due to a decrease in 5-aminolaevulinic acid accumulation [27]. Knowing the R: FR ratio can provide information about the shade, daylight, and seasonal environment that a plant was grown under, via information about the associated regulation of leaf chlorophyll synthesis, photosynthesis rate, and PSII electron transport [2, 26, 29].
Previous studies have indicated that a well-organized interaction exists between phytochrome and abiotic stresses in plants. However, the exact relationship between the R: FR value and salinity stress on chlorophyll synthesis, photosynthesis rate, and PSII electron transport in the tomato is still elusive. In the present study, the effect of different R: FR values and salinity on tomato seedling growth, biochemicals, ROS and ROS scavenging enzymes, pigment, photosynthesis, and chlorophyll fluorescence is clarified. By clarifying the interaction between R:FR and salinity, we may be able to produce varieties of tomato and other vital food crops that are able to better tolerate and survive increasingly saline soils in diverse environments.
Results
Lower R: FR promoted tomato seedling growth under salinity stress
Data showing the effect of a range of R: FR conditions on the growth parameters of tomato seedlings under salinity stress are in Table 1. In general, salinity caused a significant reduction in plant height, stem diameter, fresh and dry weight of root, stem and leaf, as compared to control plants in the same light conditions. Under normal conditions with no salinity in T3, there was a substantial increase in plant height (42.70%), fresh and dry weight (38.51 and 25.59%, respectively), as compared to T1, whereas in T2, there was a larger increase of 23.87, 47.17 and 39.29% respectively, when compared with T1 (Table 1). Under the salinity condition, in T6, the lower R: FR led to a substantial increase in plant height (49.53%), fresh and dry weight (93.17 and 104.88%, respectively), whereas in T5 there was a lower increase of 22.14, 44.95 and 50.00%, respectively, when compared with T3 (Table 1).
Table 1.
Effects of different R: FR values on plant height, stem diameter, fresh and dry weights of root, stem, and leaf in tomato seedlings under salinity stress in wild type (WT) and phytochrome B1 mutant (phyB1 mutant)
| Treatments | T1 | T2 | T3 | T4 | T5 | T6 | ||
|---|---|---|---|---|---|---|---|---|
| WT | Plant height (cm) | 18.43 ± 0.65c | 22.83 ± 0.82b | 26.30 ± 0.75a | 12.92 ± 0.55e | 15.78 ± 0.78d | 19.32 ± 0.65c | |
| Stem Diameter (mm) | 7.37 ± 0.55a | 6.29 ± 0.46b | 5.89 ± 0.50bc | 5.66 ± 0.55c | 5.43 ± 0.53c | 4.61 ± 0.17d | ||
| Fresh mass (g) | Root | 5.66 ± 0.22b | 6.91 ± 0.41a | 5.77 ± 0.40b | 3.00 ± 0.20d | 4.33 ± 0.35c | 5.58 ± 0.30b | |
| Stem | 6.38 ± 0.25c | 9.57 ± 0.34a | 10.94 ± 0.34a | 2.73 ± 0.16e | 4.47 ± 0.28d | 5.89 ± 0.29c | ||
| Leaf | 14.44 ± 0.51c | 22.49 ± 0.55a | 19.96 ± 0.47b | 7.43 ± 0.37e | 10.29 ± 0.47d | 13.96 ± 0.55c | ||
| In total | 26.48 + 0.97c | 38.97 + 0.86a | 36.68 + 1.08b | 13.17 + 0.90e | 19.09 + 1.06d | 25.44 + 1.12c | ||
| Dry mass (g) | Root | 0.28 ± 0.02bc | 0.37 ± 0.02a | 0.30 ± 0.03b | 0.14 ± 0.02e | 0.20 ± 0.02d | 0.26 ± 0.01c | |
| Stem | 0.23 ± 0.02c | 0.35 ± 0.02b | 0.41 ± 0.02a | 0.16 ± 0.03d | 0.24 ± 0.02c | 0.25 ± 0.02c | ||
| Leaf | 1.16 ± 0.05c | 1.61 ± 0.04a | 1.40 ± 0.03b | 0.51 ± 0.04e | 0.78 ± 0.04d | 1.13 ± 0.04c | ||
| In total | 1.68 + 0.07c | 2.34 + 0.06a | 2.11 + 0.04b | 0.82 + 0.08e | 1.23 + 0.08d | 1.68 + 0.02c | ||
| phyB1 mutant | Plant height (cm) | 25.18 ± 0.79a | 24.90 ± 0.74a | 25.21 ± 0.51a | 16.64 ± 0.51b | 16.10 ± 0.55b | 16.61 ± 0.67b | |
| Stem Diameter (mm) | 5.61 ± 0.23a | 5.66 ± 0.23a | 5.50 ± 0.13a | 4.44 ± 0.25b | 4.49 ± 0.30b | 4.30 ± 0.20b | ||
| Fresh mass (g) | Root | 2.52 ± 0.10a | 2.66 ± 0.13a | 2.49 ± 0.15a | 1.68 ± 0.04b | 1.65 ± 0.14b | 1.70 ± 0.06b | |
| Stem | 6.37 ± 0.35a | 6.39 ± 0.24a | 6.33 ± 0.25a | 3.59 ± 0.17b | 3.51 ± 0.21b | 3.42 ± 0.14b | ||
| Leaf | 14.47 ± 0.55a | 14.41 ± 0.45a | 14.12 ± 0.53a | 7.94 ± 0.78b | 8.17 ± 0.17b | 7.96 ± 0.07b | ||
| In total | 23.36 ± 0.47a | 23.47 ± 0.16a | 22.95 ± 0.24a | 13.21 ± 0.58b | 13.33 ± 0.10b | 13.09 ± 0.16b | ||
| Dry mass (g) | Root | 0.138 ± 0.005a | 0.142 ± 0.006a | 0.141 ± 0.015a | 0.087 ± 0.006b | 0.089 ± 0.010b | 0.093 ± 0.015b | |
| Stem | 0.254 ± 0.017a | 0.262 ± 0.026a | 0.265 ± 0.015a | 0.126 ± 0.017b | 0.123 ± 0.005b | 0.128 ± 0.010b | ||
| Leaf | 1.133 ± 0.083a | 1.126 ± 0.101a | 1.066 ± 0.100a | 0.716 ± 0.011b | 0.773 ± 0.015b | 0.763 ± 0.057b | ||
| In total | 1.516 ± 0.083a | 1.533 ± 0.100a | 1.473 ± 0.120a | 0.920 ± 0.017b | 0.980 ± 0.010b | 0.971 ± 0.072b | ||
The values (mean ± SE, n = 6) with different letter within columns are statistically different (P ≤ 0.05) according to Duncan’s multiple range test. T1, 0 mM NaCl + 7.4 R: FR; T2, 0 mM NaCl + 1.2 R: FR; T3, 0 mM NaCl + 0.8 R: FR; T4, 100 mM NaCl + 7.4 R: FR; T5, 100 mM NaCl + 1.2 R: FR; T6, 100 mM NaCl + 0.8 R: FR
Under normal conditions, the growth of tomato seedlings was better with an R: FR of 1.2, compared to a higher ratio of 7.4. However, under the salinity condition, the lower R: FR of 0.8 provided tomato seedlings with greater salinity tolerance.
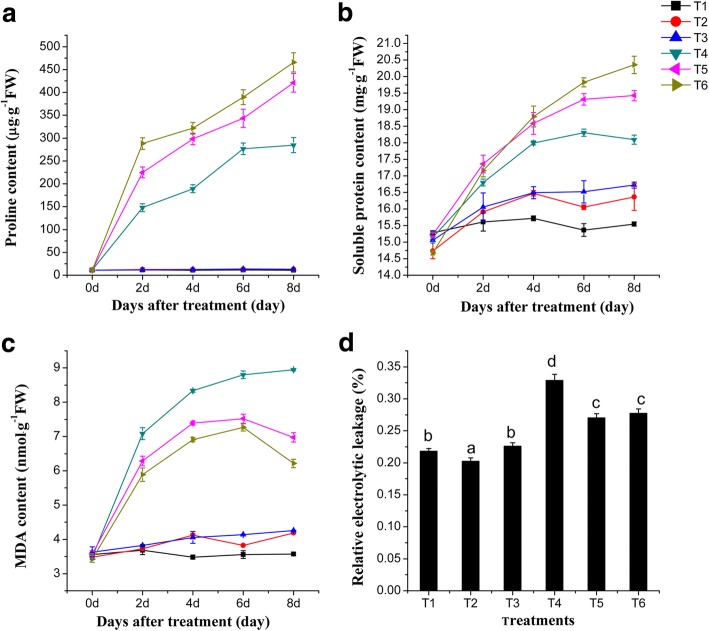
A lower R: FR exhibited higher soluble protein and proline, while MDA amount and electrolytic leakage decreased under the salinity condition
To quantify the effect of salinity stress treatments on the biochemistry of tomato seedlings under different R: FR conditions, the soluble protein, proline, MDA and electrolytic leakage were analyzed. An increased accumulation of proline and soluble protein in plant tissues subjected to stress indicates an effective plant stress response at the metabolic level [26]. It was found that proline and soluble protein accumulated in the greatest quantities under the salinity condition and the lower R: FR of 0.8. After 8 days with the salinity treatment, the amount of proline and soluble protein in the leaf tissues increased by 63.58 and 12.50% under the lower R: FR treatment as compared to the highest ratio tested (Fig. 1a, b). The amount of MDA and the percentage of electrolytic leakage are usually used as tools to assess the severity of oxidative damage, the degree of plant sensitivity, and cell membrane injury [28, 30]. Figure 1 showed that the lower R: FR condition largely decreased MDA and electrolytic leakage percentage under salinity stress. After 8 days of salinity treatment under the lower R: FR, the amount of MDA and electrolytic leakage percentage in the leaf tissue decreased by 30.53 and 15.60%, compared with the highest R: FR condition (Fig. 1c, d). Under the salinity condition, the lower R: FR ratio led to decrease in both MDA amount and electrolytic leakage, but increase in proline amount and accumulation of soluble protein, which made tomato seedlings more tolerant to salinity stress.
Fig. 1.
Effects of different R: FR values on proline, soluble protein, and MDA content, and relative electrolyte leakage in tomato seedlings under salinity stress. a proline content. b soluble protein content. c MDA content. Proline, soluble protein and MDA contents were measured 0, 2, 4, 6, and 8 days after exposure to the salinity treatment. d relative electrolyte leakage, measured 8 days after salinity treatment. T1, 0 mM NaCl + 7.4 R: FR; T2, 0 mM NaCl + 1.2 R: FR; T3, 0 mM NaCl + 0.8 R: FR; T4, 100 mM NaCl + 7.4 R: FR; T5, 100 mM NaCl + 1.2 R: FR; T6, 100 mM NaCl + 0.8 R: FR. Vertical bars on the lines represent the SE (n = 5), Bars with different letters are significantly different at the 0.05 level (Duncan’s multiple range test)
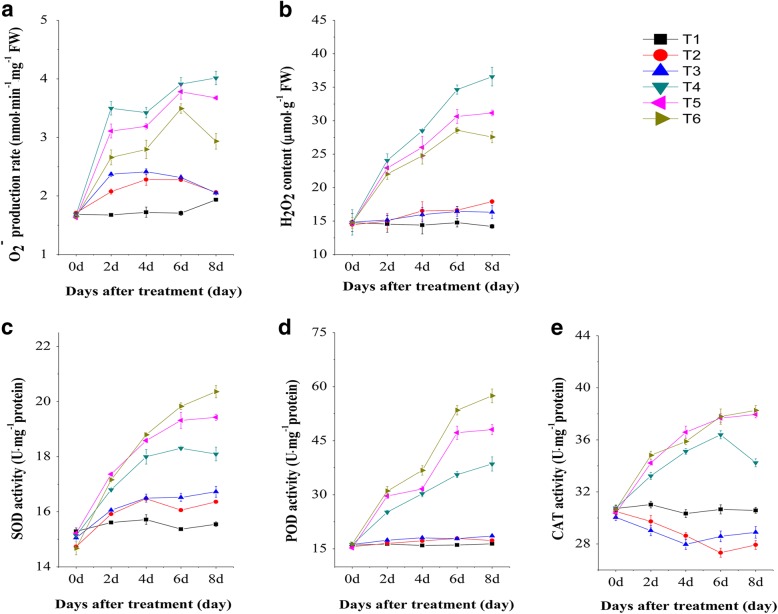
The lower R: FR treatment accumulated less ROS and showed a higher activity of ROS scavenging enzymes under the salinity condition
To observe the effect of salinity stress treatments on plant antioxidant system under the lower R: FR condition, H2O2 and O2•− levels and enzymatic activities of the major ROS scavengers were measured. H2O2 and O2•− are common ROS produced in plants, and are indicators of optimal health, as they are typically produced following exposure to salinity stress [31, 32]. In this experiment, the accumulation of H2O2 and O2•− were significantly enhanced after salinity treatment, and after 8 days, the seedlings grown under the higher R: FR treatment showed almost 1.3–1.5-fold higher levels of H2O2 and O2•− than the corresponding tomato seedlings grown under the lower R: FR treatment (Fig. 2a, b). The activity of SOD, POD and CAT enzymes were notably increased in response to salinity alone, but in combination with the lower R: FR treatment, enzyme activity was comparatively much higher (Fig. 2c, d, e). The activity of major ROS-scavenging enzymes revealed that tomato seedlings grown under the lower R: FR treatment exhibited an improved ROS scavenging system under salinity stress compared to that of tomato seedlings grown under the higher R: FR treatment.
Fig. 2.
Effects of different R: FR values on on ROS (O2•− and H2O2) and ROS scavenging enzymes (SOD, POD and CAT) activities in tomato seedlings under salinity stress. a O2•− content. b H2O2 content. c SOD activity. d POD activity. e CAT activity.These parameters were measured 0, 2, 4, 6, 8 days after exposure to salinity treatment. T1, 0 mM NaCl + 7.4 R: FR; T2, 0 mM NaCl + 1.2 R: FR; T3, 0 mM NaCl + 0.8 R: FR; T4, 100 mM NaCl + 7.4 R: FR; T5, 100 mM NaCl + 1.2 R: FR; T6, 100 mM NaCl + 0.8 R: FR.Vertical bars on the lines represent the SE (n = 5)
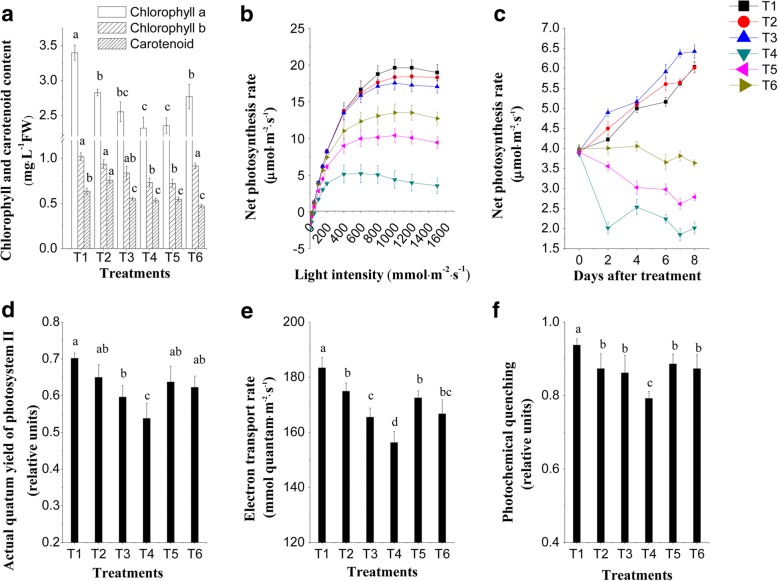
The lower R: FR treatment accumulated more photosynthetic pigments and exhibited increased photosynthetic efficiency
Measurements of chlorophyll, carotenoid, photosynthesis rate, and fluorescence were taken using leaf number two below the youngest fully expanded leaf. Under normal conditions, chlorophyll a had a tendency to decrease with the decrease of R: FR values, no significant differences in chlorophyll b and net photosynthesis rate were observed under different R: FR values, and carotenoid showed a significant difference, not correlated to the R: FR values (Fig. 3a, b, c). However, after the salinity treatment, chlorophyll a, chlorophyll b, and the net photosynthesis rate increased significantly when R: FR value is 0.8, compared with R: FR value is 7.4 (Fig. 3a, b, c). The actual quantum yield of photosynthesis (ΦPSII), electron transport rate (ETR), and photochemical quenching (qP) are inversely proportional to the damage in the PSII reaction centers [31, 32]. These widely used chlorophyll fluorescence parameters indicate how well a plant grows under conditions when higher than normal salinity is present. In the present study, a similar trend was also observed with ΦPSII, ETR, and qP, in which these parameters increased substantially under the lower R: FR treatment (Fig. 3d, e, f), suggesting that a lower R: FR could activate PSII reaction centers and consequently alleviate negative impacts of salinity stress on tomato seedlings.
Fig. 3.
Effects of different R: FR values on chlorophyll content, photosynthesis rate, and PSII electron transport in tomato seedlings under salinity stress. a chlorophyll a, chlorophyll b and carotenoid content. b light responsive curve. c net photosynthesis rate. d actual photochemical efficiency of PSII. e electron transport rate. f the photochemical quenching coefficient. Chlorophyll content, the light responsive curve and PSII electron transport parameters were measured eight days after salinity treatment. Net photosynthesis rate was measured 0, 2, 4, 6, and 8 days after exposure to salinity treatment. T1, 0 mM NaCl + 7.4 R: FR; T2, 0 mM NaCl + 1.2 R: FR; T3, 0 mM NaCl + 0.8 R: FR; T4, 100 mM NaCl + 7.4 R: FR; T5, 100 mM NaCl + 1.2 R: FR; T6, 100 mM NaCl + 0.8 R: FR. Vertical bars on the lines represent the SE (n = 5), Bars with different letters are significantly different at the 0.05 level (Duncan’s multiple range test)
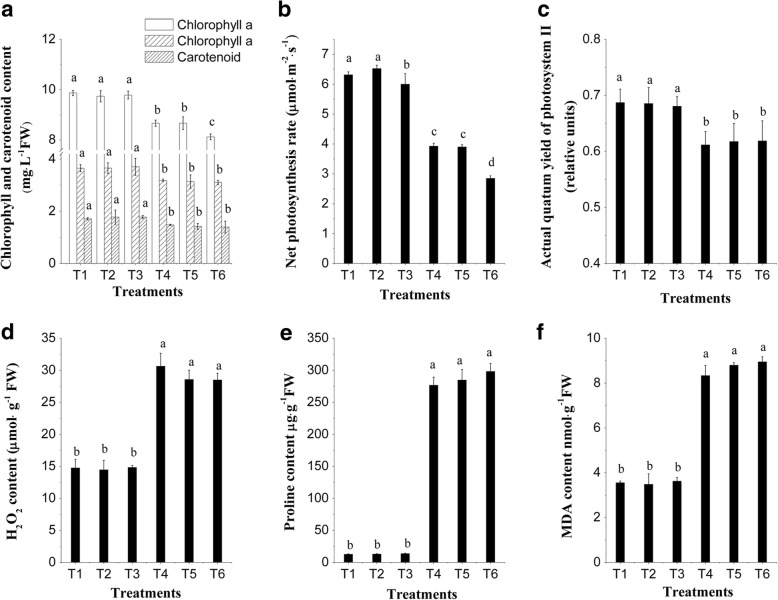
Phytochrome B1 is involved in the regulation of lower R: FR on tomato seedlings salinity tolerance
When phytochrome B1 activity was quantified to test for its influence on tomato salinity tolerance, results showed that in the tomato phyB1 mutant, the salinity treatment decreased plant height, stem diameter, fresh and dry weight of root, stem and leaf, chlorophyll and carotenoid amount, photosynthesis rate, ΦPSII and MDA, and increased proline and H2O2 content, but these parameters did not significantly change when different R: FR treatments were applied under salinity conditions (Table 1, Fig.4). In wild type tomato, different R: FR treatments had a significant influence on these parameters under salinity conditions, however, the influence disappeared in the phyB1 mutant. These results showed that phytochrome B1 mediated tomato seedlings tolerate high salinity conditions under different R: FR treatments.
Fig. 4.
Effects of different R: FR values on on chlorophyll content, photosynthesis rate, actual photochemical efficiency of PSII, and the content of H2O2, proline and MDA in tomato phyB1 mutants under salinity stress. a chlorophyll a, chlorophyll b and carotenoid content. b net photosynthesis rate. c actual photochemical efficiency of PSII. d H2O2 content. e proline content. f MDA content. All these parameters were measured 8 days after salinity treatment. T1, 0 mM NaCl + 7.4 R: FR; T2, 0 mM NaCl + 1.2 R: FR; T3, 0 mM NaCl + 0.8 R: FR; T4, 100 mM NaCl + 7.4 R: FR; T5, 100 mM NaCl + 1.2 R: FR; T6, 100 mM NaCl + 0.8 R: FR. Vertical bars on the lines represent the SE (n = 5), Bars with different letters are significantly different at the 0.05 level (Duncan’s multiple range test)
Discussion
Red (R) and far-red (FR) light are abiotic factors that are particularly important for plant growth and development. The ratio of red to far-red light (R: FR) is often used to signal the proximity of neighboring or canopy vegetation, as chlorophyll selectively absorbs 655–665 nm light while transmitting 725–735 nm light. The ratio can also be used to sense changes in daylength or seasonal variation, as the R: FR is low (about 0.6–0.8) at the beginning and end of the photoperiod, and at its maximum at solar noon (about 1.0 to 1.3) [33, 34]. The R: FR value can trigger many plant morphological responses, including promotion of shoot elongation and reduction of stem diameter in dicotyledonous and ornamental species from a low R: FR, either during the daytime or only at the end of the day [29, 35]. During salinity-induced stress, plants experience reduced growth and development, but can respond in various ways to withstand the stress. In this study, without salinity treatment, plant height was increased, but stem diameter were comparatively reduced under a lower R: FR treatment (Table 1). Similarly, Yan et al. [36] reported that soybean seedling height was significantly increased while the stem diameter was decreased in lower R: FR treatment conditions. However, under a salinity treatment, applying a lower R: FR promoted tomato seedlings growth, increased plant height, fresh and dry weight of root, stem and leaf. Results from this research suggest that a lower R: FR condition could help alleviate the impact of salinity on the development of tomato seedlings, and that more generally, the value of R: FR influences salinity stress tolerance as a consequence of morphological responses. To better quantify and operationalize this finding, further studies on the relationship of salinity tolerance with light quality are necessary.
A given plant’s ability to tolerate salinity is dependent on multiple biochemical pathways that lead to the production of osmotically dynamic metabolites, free radicals, and specific proteins that manage ion and water flux [28, 37]. Likewise, the R: FR ratio also regulates a large range of biochemical processes throughout a plant’s life [29]. Proline plays a crucial role in stabilizing the subcellular structures and scavenging free radicals [26]. MDA is the decomposition product of polyunsaturated fatty acids of biomembranes and is used to assess the severity of oxidative damage [28]. Electrolyte leakage is an important index of the plant cell’s permeability, which plays an important role in the study of plant salt stress [30]. Our results indicated that a lower R: FR of 0.8 under salinity conditions led to a decrease in MDA and electrolytic leakage, and an increase in proline and soluble protein content (Fig. 1). These results suggest that salt-stressed tomato seedlings grown under lower R: FR conditions encountered less cellular damage and lipid peroxidation.
H2O2 and O2•− are reactive oxygen species (ROS), and cause oxidative stress at high concentrations [21]. In this study, accumulation of H2O2 and O2•− from salinity stress was decreased when seedlings were exposed to a lower R: FR condition. Plant cells have developed different mechanisms to alleviate the excess ROS, and keep the balance of the formation and removal of ROS [38]. An increase in antioxidant enzymes (SOD, POD and CAT) protects the plants from oxidative damage, and were shown to be highest in the lower R: FR condition under salinity, suggesting that the lower R: FR condition induced an efficient ROS scavenging mechanism. Similar findings of low accumulation of ROS and higher antioxidant enzyme activity in stress-tolerant plants have been reported previously [26, 28]. Although the mechanism of light quality regulated ROS generation and scavenging remains unclear, there are reports indicating that light quality induces the synthesis of various protective compounds, including antioxidant enzymes and protective proteins [39, 40].
Salinity is known to influence chlorophyll synthesis and photosynthesis in a number of plant species, and chlorophyll content tends to positively correlate with photosynthetic rate [31, 32]. Previous studies have found that salinity stress and a lower R: FR value inhibits the biosynthesis of chlorophyll [29, 37]. However, in our study, the upregulation of Chl a, Chl b, and the net photosynthesis rate were observed in tomato leaves under salinity when exposed to a lower R: FR condition (Fig. 3). To explain these results, further studies on the interaction between salinity and the R: FR value on chlorophyll biosynthesis need to be performed. Maybe salinity and the R: FR value influence chlorophyll biosynthesis in different ways, and a lower R: FR value may help protect chlorophyll biosynthetic enzymes or products that were damaged by salinity. Wang et al. [41] reported that lower R: FR light conditions significantly alleviate PSII and PSI photoinhibition in the shade leaves of tomato plants, and lower R: FR illumination induced nonphotochemical quenching of chlorophyll a fluorescence and increased the activities of Foyer-Halliwell-Asada cycle enzymes and cyclic electron flux (CEF) around PSI. The parameters of the actual quantum yield of photosynthesis (ΦPSII), electron transport rate (ETR), and photochemical quenching (qP) have been used extensively to examine the photosynthetic efficiency of different crop plants subjected to salinity stress [32]. Under salinity, the highest ΦPSII, ETR, and qP values were observed in the lower R: FR condition, suggesting that a lower R: FR condition alleviated the salinity-induced inhibition of PSII electron transport, allowing tomato seedlings to effectively tolerate the stress. These results are in agreement with those of Shu et al. [32], who reported that exogenous materials could relieve salinity-induced inhibition of electron transport at the acceptor side of the PSII reaction center.
Phytochromes are involved in plant tolerate to biotic and abiotic stressors [10, 26, 29, 42]. Tomato contains five phytochrome genes, named PHYA, PHYB1, PHYB2, PHYE and PHYF [43]. In tomato, PHYB1 is mainly involved in the response to R and FR light, and controls seedling hypocotyl elongation, anthocyanin accumulation, cotyledon expansion, flowering, and abiotic tolerance [10, 44]. This study demonstrated that different values of R: FR had a significant influence on the salinity tolerance of wild type tomato seedlings, however, some of the influence disappeared in the tomato phyB1 mutants. In the tomato phyB1 mutant, plant height, stem diameter, fresh and dry weight of root, stem and leaf, chlorophyll b and carotenoid amount, ΦPSII, MDA, proline, and H2O2 content did not significantly change when different R: FR treatments were applied under salinity conditions. However, these parameters significantly changed in wild type tomato seedlings after lower R: FR treatments under salinity conditions. These results suggest that a lower R: FR condition improved tomato salinity stress tolerance, and phytochrome B1 play an very important role in this process. Although the role of phytochromes under abiotic stress conditions is largely unknown, there are many reports indicating that phytochromes might mediate the abiotic stress response in plants to control antioxidant enzymes like peroxidases and non-enzymatic antioxidants such as ascorbate, carotenoids, and flavonoids under stress conditions [10, 42]. Plants exposed to light with different R: FR values have been reported to control phytohormone biosynthesis (gibberellin, auxin, cytokinins and abscisic acid), which are also involved in plant salinity tolerance [2, 45]. Furthermore, phytochrome regulates the expression of some proteins that mediate salt tolerance [12]. These findings suggested a strong correlation between phytochromes and salinity tolerance that might involve a complex signaling network which has yet to be elucidated.
Conclusion
This study identified the effect of R: FR values on tomato salinity tolerance. The results suggested that lower R: FR values could significantly alleviate salt-induced oxidative damage on tomato seedlings, most likely through regulation of antioxidant enzymes and non-enzymatic systems, and phytochrome B1 play an very important role in this process. This was associated with an improvement in the PSII electron transport and promoted tomato seedlings growth under salinity conditions. These findings also indicate possible opportunities to explore the relationship between light quality and salinity stress while also supporting future environmental attempts to improve tolerance to salinity stresses in other crops.
Methods
Plant material and growth conditions
In this study, cv. MoneyMaker (Solanum lycopersicum L.) wild type and MoneyMaker backgrounded phytochrome B1 mutant (phyB1) were used as the experimental organism. The mutant was provided by the Tomato Genetic Resource Center (Department of Vegetable Crops, University of California, Davis), TGR accession number LA4357. Tomato seeds were soaked for 30 min in 50% bleach, then rinsed in running water thoroughly and directly sown on germination paper and incubated at 25 °C. After germination, seedlings were sowed into commercial substrate on polystyrene plugs (plug size: 50 cm × 25 cm × 4.5 cm; 50 cavities very plug, one seed per cavity). Seedlings were grown in a growth chamber under day-neutral conditions (12 h light/12 h dark), maintained constantly at 24–26 °C with 40–45% relative humidity. When the second leaf fully expanded, the same sized seedlings were selected and washed to remove the commercial substrate, and then transplanted on foam plates to be grown hydroponically in the growth chamber (Northwest A&F University, Yangling, Shaanxi, China). The growth chamber was an airtight space, equipped with a lighting system and air conditioner cooling and heating system.
The growth chamber was maintained at 29–31 °C during the day with 70–80% relative humidity, and 24–26 °C during the night with 40–50% relative humidity (12 h light/12 h dark). The growth chamber had six sections, divided by an opaque silvery cloth, with 36 seedlings in each section. The growth chamber used light-emitting diodes with an intensity of 200–230 μmol·m− 2·s− 1. The seedlings were grown with Yamasaki tomato nutrient solution at 0.5 concentration (pH 6.0–6.5, EC: 1.3–1.5 ms·cm− 1), with dissolved oxygen maintained by air pump at 80 ± 0.2 mg·L− 1.
Salinity and R: FR light treatment
After 3 days of pre-culture under normal conditions, treatments with salt (NaCl) and three different R: FR were applied. We use white LED (peaked at 455 and 570 nm) and FR LED (peaked at 730 nm) to justify tomato growth light environment. The experimental plots included six treatments: ① T1, 0 mM NaCl + 7.4 R: FR, ② T2, 0 mM NaCl + 1.2 R: FR, ③ T3, 0 mM NaCl + 0.8 R: FR, ④ T4, 100 mM NaCl + 7.4 R: FR, ⑤ T5, 100 mM NaCl + 1.2 R: FR, ⑥ T6, 100 mM NaCl + 0.8 R: FR. The light intensity and spectrum were measured by a spectroradiometer (PAR-NIR, Apogee Instruments Inc., Logan, UT) and are shown in Fig. 5. For the purposes of this experiment, the R: FR = (photon irradiance between 655 and 665 nm) / (photon irradiance between 725 and 735 nm). The nutrient solutions were renewed every 2 days. After 8d of salinity treatment, the tomato seedlings growth parameters were measured. Tomato wild type samplings were carried out 0, 2, 4, 6 and 8 d after treatments, phyB1 mutant samples were carried out 8 d after treatments, and rapidly frozen these samples in liquid nitrogen and stored at − 80 °C for biochemical analysis, and 3 replicates were used for each analysis.
Fig. 5.
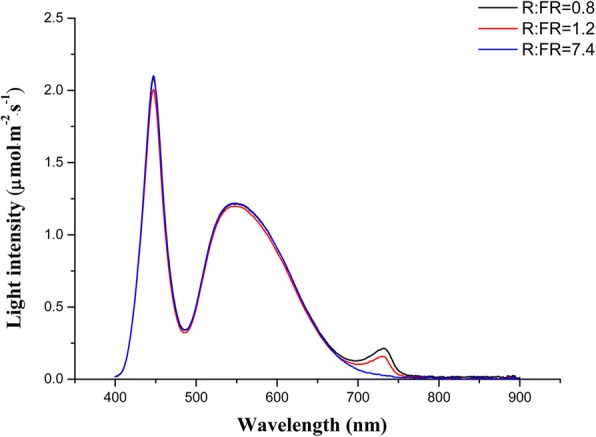
Spectral distribution characteristics of white and FR LED used for different R: FR treatments. The black curve represents R: FR value is 0.8, the red curves represents R: FR value is 1.2; blue curve represents R: FR value is 7.4
Determination of growth parameters
Ten tomato seedlings in each treatment was used to measure the growth parameters. Stem height was measured by a ruler and stem diameter was measured using digital calipers (Digimatic Caliper; Shengli Co., Ltd., Beijing, China). To determine the fresh weight of the root, stem, and leaf, plants were harvested separately, washed with sterile distilled water, and weighed by a balance. The dry weight of plant root, stem, and leaf were determined after drying at 70 °C for 2 days and weighed by a balance.
Determination of proline content
The concentration of proline was prepared and estimated following the method of Gururani et al. [11]. From each treatment, tomato leaf (0.2 g) was homogenized in 2 mL of 3% (w/v) sulfosalicylic acid, then centrifuged (15,000 g, 10 min, 25 °C). Then, 0.5 mL glacial acetic acid and 0.5 mL 2.5% ninhydrin solution were added into 0.5 mL supernatant. The mixture was heated at 100 °C for 30 min in a water bath, cooled in an ice bath, then added to 1.5 mL toluene and allowed to rest for at least 10 min to allow the phases to separate. The absorbance of the toluene fraction was read at 520 nm. The proline content was determined by a calibration curve operated in a similar way with solutions of proline at different concentrations.
Determination of malondialdehyde (MDA) content
Oxidative damage to lipids was estimated by measuring the content of MDA. One gram of plant material from each treatment was homogenized with 5 mL 5% (w/v) trichloroacetic acid (TCA), then centrifuged (15,000 g, 15 min, 4 °C) and added to 5% trichloroacetic acid containing 0.65% (w/v) 2-thiobarbituric acid (TBA) in 2 mL supernatant. The mixture was heated at 100 °C for 15 min in a water bath and then quickly cooled on ice, then the contents were centrifuged (15,000 g, 15 min, 4 °C) and the absorbance was read at 532 nm, as described by Huang et al. [28].
Determination of electrolyte leakage
The total inorganic ions that leaked out of the leaves were measured by the method described by Abo-Ogiala et al. [17]. Ten leaf disks were cut, weighed, and subsequently placed at room temperature into Falcon tubes containing 10 mL of deionized water. Conductivity of the deionized water (EC0) and soaked plant tissue was measured after 24 h (EC1) using a conductivity meter (DDSJ-308A, Shanghai Jingke Instrument Co., Ltd., China). To determine the maximum electrolyte leakage (EC2), the samples were boiled at 100 °C for 20 min and then cooled at room temperature. The relative electrolyte leakage (%) was calculated as: (EC1-EC0) / (EC2-EC0) × 100%.
Determination of H2O2 and O2•−
To determine O2•−, the assay was done as described by Zhao et al. [46] with some modifications. A section of plant leaf (0.3 g) was homogenized with 3 mL 50 mM phosphate buffer (pH 7.8) and centrifuged (12,000 g, 20 min, 4 °C). One mL of hydroxylamine hydrochloride was added to 1 mL of the supernatant, and the mixture was incubated at 25 °C for 20 min. One mL of 17 mM γ-amino-phenylsulfonic and 1 mL of 7 mM α-amino-phenylsulfonic were added to the mixture for another 20-min incubation at 30 °C. The absorption of the reaction mixture was monitored at 530 nm. O2•− was calculated according to a standard curve based on Sodium nitrite.
The concentration of H2O2 was prepared and estimated following the method of Gururani et al. [26], with slight modifications. Fresh samples of leaf (0.5 g) were homogenized with 5 mL 0.1% (w/v) TCA in an ice bath, and the homogenate was centrifuged at 12,000 g for 15 min. Then, 0.5 mL of 100 mM potassium phosphate buffer (pH 7.0) and 1 mL of 1 M KI were added to 0.5 mL of the supernatant. The absorbance of supernatant was read at 390 nm, and the content of H2O2 was calculated based on a standard curve.
Assay of soluble protein and antioxidant enzymes (SOD, POD, and CAT)
Fresh tomato leaf (0.3 g) from each treatment was crushed into fine powder in a pestle and mortar using liquid nitrogen. The powder was homogenized in 3 mL of 50 mM pre-cooled phosphate buffer (pH 7.8). The homogenate was centrifuged (12,000 g, 20 min, 4 °C), and the supernatant was used for enzyme activity assays. Protein content was evaluated according to the method of Huang et al. [28], using bovine serum albumin as the standard.
Total SOD activity was determined by the inhibition of the photochemical reduction of nitroblue tetrazolium (NBT), as described by Moradi and Ismail [31], with slight modifications. The reaction mixture consisted of 0.1 mL of enzyme extract and 5 mL O2•− generating solution which contained 4 mL 14.5 mM methionine, 0.02 mL 30 μM EDTA-Na2, 0.4 mL 750 μM NBT, and 0.4 mL 20 μM of riboflavin. Extracts were brought to a final volume of 0.18 mL with 50 mM Na-phosphate (pH 7.8). Test tubes were shaken and placed 30 cm under lights for 15-min. The enzyme unit was defined as the inhibition of the photochemical reduction of 50% NBT by the reaction system per minute at 560 nm, using a Multiple-label Multifunctional Microplate reader (spectraMax i3x, Molecular Devices Inc., United States). Enzyme activity was expressed as U·mg− 1 protein.
CAT activity was determined by the reduction of H2O2 at 240 nm spectrophotometrically, as described by Gururani et al. [26], with some modifications. The reaction mixture contained 13.6 mM H2O2 in 15 mM phosphate buffer (pH 7). The reaction was initiated by the addition of the enzyme extract. POD activity was determined with guaiacol as the reducing substrate in a reaction mixture containing 0.2 M Na-phosphate buffer (pH 6), 3 mM guaiacol, and 4.9 mM H2O2. The oxidation of guaiacol was assessed by recording the absorbance increase at 470 nm. One unit of CAT and POD was defined as the amount of enzyme which produced a change of 0.1 in absorbance at 240 nm and 470 nm, respectively, per minute, at 25 °C. The enzyme activities of POD and CAT were expressed as U·mg− 1 protein.
Chlorophyll concentration and photosynthetic rate analysis
Tomato leaf (0.3 g) was soaked in 95% alcohol for extraction. The samples were then stored at 25 °C for 24 h. The absorbance was read at 665, 649 and 470 nm. The concentrations of chlorophyll a, chlorophyll b and cartotenoid were calculated according to Fan et al. [27].
Net photosynthesis rate (Pn) and the light responsiveness curve of tomato leaves were determined using the second fully expanded leaves between 9:00 am and 11:00 am on a portable photosynthesis system (LI6400, LI-COR Inc., USA). In the assimilation chamber, leaf temperature was 25 °C, relative humidity was 85%, ambient CO2 concentration was 400 ± 10 ppm, and photosynthetic photon flux density (PPFD) was 200 μmol·m− 2·s− 1.
Chlorophyll fluorescence parameters
Chlorophyll fluorescence emission from the upper surface of the second fully expanded leaves of intact plants was measured by a modulated fluorimeter (PAM Photosynthesis Yield Analyser, Walz, Effeltrich, Germany), according to Moradi and Ismail [31]. The minimal (Fo) and maximal (Fm) chlorophyll fluorescence emissions were assessed in leaves after 30-min of dark adaptation. To determine the minimal fluorescence level in a leaf during illumination (Fo’), and to allow maximal oxidation of the PSII centers in the presence of far-red light, a black cloth was rapidly placed over the plants to block out light, causing the leaf fluorescence to fall to the Fo’ level, rising again after a few seconds. Then, the leaves were continuously illuminated with a white actinic light, which was equivalent to the actual growth lights used for the tomato plants, to measure both steady-state (Fs) and the maximal chlorophyll fluorescence level in light-adapted leaves (Fm′). From these measurements, the actual photochemical efficiency of PSII [ΦPSII = (Fm′- Fs) / Fm′], the photochemical quenching coefficient [qP = (Fm′- Fs) / (Fm′ − Fo’)], electron transport rate (ETR = ΦPSII ×PPFD × 0.5 × 0.87) were calculated, where PPFD is the photosynthetic photon flux density incident on the leaf, 0.5 is a factor that assumes equal distribution of energy between the two photosystems, and 0.87 is an assumed factor of leaf absorbance.
Statistical analysis
Statistics were calculated using SPSS 20.0 (SPSS, version 20.0, IBM Inc., USA). The data were analyzed by analysis of variance (ANOVA), and the differences between the means were assessed by Duncan’s multiple range test (P < 0.05). Error bars in all figures represent standard deviation from the mean. Graphs were created using OriginPro (version 8.0, Origin Lab, MA, USA).
Acknowledgements
This research was supported financially by the Key Laboratory of Protected Horticultural Engineering in Northwest, Ministry of Agriculture, PR China, We are grateful for tomato mutants obtained from the Genetic Resource Center (Department of Vegetable Crops, University of California, Davis).
Funding
This research was funded by the study on key technologies for healthy production of efficient resources utilization in protected vegetables (2016BZ09), by research on key technologies and supporting equipment of protected vegetable production (CX161002), and by the achievement transformation of multilayer stereo water culture (AA16380048).
Availability of data and materials
Plant materials in this work and datasets analysed during the current study are available from the corresponding author upon request.
Abbreviations
- CAT
Catalase
- ETR
Electron transport rate
- FR
Far-red
- H2O2
Hydrogen peroxide
- MDA
Malondialdehyde
- O2•−
Superoxide anion
- Pn
Net photosynthesis rate
- POD
Peroxidase
- PSII
Photosystem II
- qP
Photochemical quenching
- R
Red
- ROS
Reactive oxygen species
- SOD
Superoxidase dismutase
- ΦPSII
Actual quantum yield of photosynthesis
Authors’ contributions
ZZ and KC conceived the study. ZZ and KC designed the experiments. KC carried out the chlorophyll concentration, photosynthetic rate and chlorophyll fluorescence parameters studies, and revised the manuscript. JY and DX carried out the biochemicals and antioxidant system studies. KA and EB interpreted the experimental data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Kai Cao and Jie Yu contributed equally to this work.
Contributor Information
Kai Cao, Email: caokai_19871220@163.com.
Jie Yu, Email: ltlsyujie@163.com.
Dawei Xu, Email: 13772050385@163.com.
Kaiqi Ai, Email: akq19950410@163.com.
Encai Bao, Email: baoencai1990@163.com.
Zhirong Zou, Phone: +86-29-87082613, Email: zouzhirong2005@hotmail.com.
References
- 1.Carvalho RF, Takaki M, Azevedo RA. Plant pigments: the many faces of light perception. Acta Physiol Plant. 2011;33(2):241–248. doi: 10.1007/s11738-010-0533-7. [DOI] [Google Scholar]
- 2.Franklin KA, Quail PH. Phytochrome functions in Arabidopsis development. J Exp Bot. 2010;61(1):11–24. doi: 10.1093/jxb/erp304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hayes S, Sharma A, Fraser DP, Trevisan M, Cragg-Barber CK, Tavridou E, Fankhauser C, Jenkins GI, Franklin KA. UV-B perceived by the UVR8 photoreceptor inhibits plant thermomorphogenesis. Curr Biol. 2017;27(1):120–127. doi: 10.1016/j.cub.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu B, Yang Z, Gomez A, Liu B, Lin C, Oka Y. Signaling mechanisms of plant cryptochromes in Arabidopsis thaliana. J Plant Res. 2016;129(2):137–148. doi: 10.1007/s10265-015-0782-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Müller P, Bouly J-P. Searching for the mechanism of signalling by plant photoreceptor cryptochrome. FEBS Lett. 2015;589(2):189–192. doi: 10.1016/j.febslet.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 6.Chen M, Tao Y, Lim J, Shaw A, Chory J. Regulation of phytochrome B nuclear localization through light-dependent unmasking of nuclear-localization signals. Curr Biol. 2005;15(7):637–642. doi: 10.1016/j.cub.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 7.Ádám É, Hussong A, Bindics J, Wüst F, Viczián A, Essing M, Medzihradszky M, Kircher S, Schäfer E, Nagy F. Altered dark-and photoconversion of phytochrome B mediate extreme light sensitivity and loss of photoreversibility of the phyB-401 mutant. PLoS One. 2011;6(11):e27250. doi: 10.1371/journal.pone.0027250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Possart A, Xu T, Paik I, Hanke S, Keim S, Hermann H-M, Wolf L, Hiss M, Becker C, Huq E. Characterization of Phytochrome interacting factors from the Moss Physcomitrella Patens illustrates conservation of Phytochrome signaling modules in land plants. Plant Cell. 2017;29(2):310–330. doi: 10.1105/tpc.16.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheerin DJ, Menon C, zur Oven-krockhaus S, Enderle B, Zhu L, Johnen P, Schleifenbaum F, Stierhof Y-D, Huq E, Hiltbrunner A. Light-activated phytochrome a and B interact with members of the SPA family to promote photomorphogenesis in Arabidopsis by reorganizing the COP1/SPA complex. Plant Cell. 2015;27(1):189–201. doi: 10.1105/tpc.114.134775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gavassi MA, Monteiro CC, Campos ML, Melo HC, Carvalho RF. Phytochromes are key regulators of abiotic stress responses in tomato. Sci Hortic. 2017;222:126–135. doi: 10.1016/j.scienta.2017.04.035. [DOI] [Google Scholar]
- 11.Gururani MA, Upadhyaya CP, Strasser RJ, Yu JW, Park SW. Evaluation of abiotic stress tolerance in transgenic potato plants with reduced expression of PSII manganese stabilizing protein. Plant Sci. 2013;198:7–16. doi: 10.1016/j.plantsci.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 12.Indorf M, Cordero J, Neuhaus G, Rodríguez-Franco M. Salt tolerance (STO), a stress-related protein, has a major role in light signalling. Plant J. 2007;51(4):563–574. doi: 10.1111/j.1365-313X.2007.03162.x. [DOI] [PubMed] [Google Scholar]
- 13.Shibuya T, Itagaki K, Tojo M, Endo R, Kitaya Y. Fluorescent illumination with high red-to-far-red ratio improves resistance of cucumber seedlings to powdery mildew. HortScience. 2011;46(3):429–431. [Google Scholar]
- 14.Wit M, Spoel SH, Sanchez-Perez GF, Gommers CM, Pieterse CM, Voesenek LA, Pierik R. Perception of low red: far-red ratio compromises both salicylic acid-and jasmonic acid-dependent pathogen defences in Arabidopsis. Plant J. 2013;75(1):90–103. doi: 10.1111/tpj.12203. [DOI] [PubMed] [Google Scholar]
- 15.Zhao Y, Zhou J, Xing D. Phytochrome B-mediated activation of lipoxygenase modulates an excess red light-induced defence response in Arabidopsis. J Exp Bot. 2014;65(17):4907–4918. doi: 10.1093/jxb/eru247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu J, Zhang F, Zhou J, Chen F, Wang B, Xie X. Phytochrome B control of total leaf area and stomatal density affects drought tolerance in rice. Plant Mol Biol. 2012;78(3):289–300. doi: 10.1007/s11103-011-9860-3. [DOI] [PubMed] [Google Scholar]
- 17.Abo-Ogiala A, Carsjens C, Diekmann H, Fayyaz P, Herrfurth C, Feussner I, Polle A. Temperature-induced lipocalin (TIL) is translocated under salt stress and protects chloroplasts from ion toxicity. J Plant Physiol. 2014;171(3):250–259. doi: 10.1016/j.jplph.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Adem GD, Roy SJ, Zhou M, Bowman JP, Shabala S. Evaluating contribution of ionic, osmotic and oxidative stress components towards salinity tolerance in barley. BMC Plant Biol. 2014;14(1):113. doi: 10.1186/1471-2229-14-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geilfus CM, Mithöfer A, Ludwig-Müller J, Zörb C, Muehling KH. Chloride-inducible transient apoplastic alkalinizations induce stomata closure by controlling abscisic acid distribution between leaf apoplast and guard cells in salt-stressed Vicia faba. New Phytol. 2015;208(3):803–816. doi: 10.1111/nph.13507. [DOI] [PubMed] [Google Scholar]
- 20.Orsini F, Accorsi M, Gianquinto G, Dinelli G, Antognoni F, Carrasco KBR, Martinez EA, Alnayef M, Marotti I, Bosi S. Beyond the ionic and osmotic response to salinity in Chenopodium quinoa: functional elements of successful halophytism. Funct Plant Biol. 2011;38(10):818–831. doi: 10.1071/FP11088. [DOI] [PubMed] [Google Scholar]
- 21.Miller G, Suzuki N, CIFTCI-YILMAZ S, Mittler R. Reactive oxygen species homeostasis and signallinge during drought and salinity stresses. Plant Cell Environ. 2010;33(4):453–467. doi: 10.1111/j.1365-3040.2009.02041.x. [DOI] [PubMed] [Google Scholar]
- 22.Saxena B, Shukla K, Giri B. Arbuscular Mycorrhizas and Stress Tolerance of Plants. Springer. 2017. Arbuscular mycorrhizal Fungi and tolerance of salt stress in plants; pp. 67–97. [Google Scholar]
- 23.Vijayalakshmi T, Vijayakumar A, Kiranmai K, Nareshkumar A, Sudhakar C. Salt stress induced modulations in growth, compatible solutes and antioxidant enzymes response in two cultivars of safflower (Carthamus tinctorius L. cultivar TSF1 and cultivar SM) differing in salt tolerance. Am J Plant Sci. 2016;7(13):1802. doi: 10.4236/ajps.2016.713168. [DOI] [Google Scholar]
- 24.Kim Y, Kim I, Shin S, Park T, Park H, Kim Y, Lee G, Kang H, Lee S, Yoon H. Overexpression of dehydroascorbate reductase confers enhanced tolerance to salt stress in rice plants (Oryza sativa L. japonica) J Agron Crop Sci. 2014;200(6):444–456. doi: 10.1111/jac.12078. [DOI] [Google Scholar]
- 25.Prashanth S, Sadhasivam V, Parida A. Over expression of cytosolic copper/zinc superoxide dismutase from a mangrove plant Avicennia marina in indica rice var Pusa Basmati-1 confers abiotic stress tolerance. Transgenic Res. 2008;17(2):281–291. doi: 10.1007/s11248-007-9099-6. [DOI] [PubMed] [Google Scholar]
- 26.Gururani MA, Ganesan M, Song I-J, Han Y, Kim J-I, Lee H-Y, Song P-S. Transgenic turfgrasses expressing hyperactive Ser599Ala Phytochrome a mutant exhibit abiotic stress tolerance. J Plant Growth Regul. 2016;35(1):11–21. doi: 10.1007/s00344-015-9502-0. [DOI] [Google Scholar]
- 27.Fan X, Zang J, Xu Z, Guo S, Jiao X, Liu X, Gao Y. Effects of different light quality on growth, chlorophyll concentration and chlorophyll biosynthesis precursors of non-heading Chinese cabbage (Brassica campestris L.) Acta Physiol Plant. 2013;35(9):2721–2726. doi: 10.1007/s11738-013-1304-z. [DOI] [Google Scholar]
- 28.Huang C-J, Wei G, Jie Y-C, Xu J-J, Zhao S-Y, Wang L-C, Anjum S. Responses of gas exchange, chlorophyll synthesis and ROS-scavenging systems to salinity stress in two ramie Boehmeria nivea. Photosynthetica. 2015;53(3):455–463. doi: 10.1007/s11099-015-0127-0. [DOI] [Google Scholar]
- 29.Demotes-Mainard S, Péron T, Corot A, Bertheloot J, Le Gourrierec J, Pelleschi-Travier S, Crespel L, Morel P, Huché-Thélier L, Boumaza R. Plant responses to red and far-red lights, applications in horticulture. Environmental and Experimental Botany. 2016;121:4–21. doi: 10.1016/j.envexpbot.2015.05.010. [DOI] [Google Scholar]
- 30.Demidchik V, Straltsova D, Medvedev SS, Pozhvanov GA, Sokolik A, Yurin V. Stress-induced electrolyte leakage: the role of K+−permeable channels and involvement in programmed cell death and metabolic adjustment. J Exp Bot. 2014;65(5):1259–1270. doi: 10.1093/jxb/eru004. [DOI] [PubMed] [Google Scholar]
- 31.Moradi F, Ismail AM. Responses of photosynthesis, chlorophyll fluorescence and ROS-scavenging systems to salt stress during seedling and reproductive stages in rice. Ann Bot. 2007;99(6):1161–1173. doi: 10.1093/aob/mcm052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shu S, Yuan L-Y, Guo S-R, Sun J, Yuan Y-H. Effects of exogenous spermine on chlorophyll fluorescence, antioxidant system and ultrastructure of chloroplasts in Cucumis sativus L. under salt stress. Plant Physiol Biochem. 2013;63:209–216. doi: 10.1016/j.plaphy.2012.11.028. [DOI] [PubMed] [Google Scholar]
- 33.Hertel C, Leuchner M, Menzel A. Vertical variability of spectral ratios in a mature mixed forest stand. Agric For Meteorol. 2011;151(8):1096–1105. doi: 10.1016/j.agrformet.2011.03.013. [DOI] [Google Scholar]
- 34.Holmes M, Smith H. The function of phytochrome in the natural environment—I. Characterization of daylight for studies in photomorphogenesis and photoperiodism. Photochem Photobiol. 1977;25(6):533–538. doi: 10.1111/j.1751-1097.1977.tb09124.x. [DOI] [Google Scholar]
- 35.Nagasuga K, Kubota F. Effects of shading on hydraulic resistance and morphological traits of internode and node of napiergrass (Pennisetum purpureum Schumach.) Plant production science. 2008;11(3):352–354. doi: 10.1626/pps.11.352. [DOI] [Google Scholar]
- 36.Yan Y, Gong W, Yang W, Wan Y, Chen X, Chen Z, Wang L. Seed treatment with uniconazole powder improves soybean seedling growth under shading by corn in relay strip intercropping system. Plant production science. 2010;13(4):367–374. doi: 10.1626/pps.13.367. [DOI] [Google Scholar]
- 37.Parvaiz A, Satyawati S. Salt stress and phyto-biochemical responses of plants-a review. Plant Soil and Environment. 2008;54(3):89. doi: 10.17221/2774-PSE. [DOI] [Google Scholar]
- 38.Abogadallah GM. Insights into the significance of antioxidative defense under salt stress. Plant Signal Behav. 2010;5(4):369–374. doi: 10.4161/psb.5.4.10873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dong C, Fu Y, Liu G, Liu H. Growth, photosynthetic characteristics, antioxidant capacity and biomass yield and quality of wheat (Triticum aestivum L.) exposed to LED light sources with different spectra combinations. J Agron Crop Sci. 2014;200(3):219–230. doi: 10.1111/jac.12059. [DOI] [Google Scholar]
- 40.Manivannan A, Soundararajan P, Halimah N, Ko CH, Jeong BR. Blue LED light enhances growth, phytochemical contents, and antioxidant enzyme activities of Rehmannia glutinosa cultured in vitro. Hortic Environ Biotechnol. 2015;56(1):105–113. doi: 10.1007/s13580-015-0114-1. [DOI] [Google Scholar]
- 41.Wang F, Wu N, Zhang L, Ahammed GJ, Chen X, Xiang X, Zhou J, Xia X, Shi K, Yu J, et al. Light signaling-dependent regulation of Photoinhibition and Photoprotection in tomato. Plant Physiol. 2018;176(2):1311–1326. doi: 10.1104/pp.17.01143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alves FRR, de Melo HC, Crispim-Filho AJ, Costa AC, Nascimento KJT, Carvalho RF. Physiological and biochemical responses of photomorphogenic tomato mutants (cv. Micro-tom) under water withholding. Acta Physiol Plant. 2016;38(6):155. doi: 10.1007/s11738-016-2169-8. [DOI] [Google Scholar]
- 43.Hauser BA, Pratt LH, Cordonnier-Pratt M-M. Absolute quantification of five phytochrome transcripts in seedlings and mature plants of tomato (Solanum lycopersicum L.) Planta. 1997;201(3):379–387. doi: 10.1007/s004250050080. [DOI] [PubMed] [Google Scholar]
- 44.Weller JL, Schreuder ME, Smith H, Koornneef M, Kendrick RE. Physiological interactions of phytochromes a, B1 and B2 in the control of development in tomato. Plant J. 2000;24(3):345–356. doi: 10.1046/j.1365-313x.2000.00879.x. [DOI] [PubMed] [Google Scholar]
- 45.Fahad S, Hussain S, Matloob A, Khan FA, Khaliq A, Saud S, Hassan S, Shan D, Khan F, Ullah N. Phytohormones and plant responses to salinity stress: a review. Plant Growth Regul. 2015;75(2):391–404. doi: 10.1007/s10725-014-0013-y. [DOI] [Google Scholar]
- 46.Zhao H, Ye L, Wang Y, Zhou X, Yang J, Wang J, Cao K, Zou Z. Melatonin increases the chilling tolerance of chloroplast in cucumber seedlings by regulating photosynthetic electron flux and the ascorbate-glutathione cycle. Front Plant Sci. 2016;7 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Plant materials in this work and datasets analysed during the current study are available from the corresponding author upon request.