Abstract
We examined the association between mood disorders and risk of herpes zoster in two case-control studies using data from nationwide Danish registries and practices in the UK Clinical Practice Research Datalink. We included incident zoster cases diagnosed in general practice (using systemic antivirals as a proxy in Denmark) or hospital during 1997–2013 in Denmark (n = 190,671) and during 2000–2013 in the United Kingdom (n = 177,361). We risk-set sampled 4 matched population controls per case. Conditional logistic regression analyses adjusting for zoster risk factors showed that the odds ratios for previous mood disorder among cases versus controls were 1.15 (99% confidence interval (CI): 1.12, 1.19; prevalence 7.1% vs. 6.0%) in Denmark and 1.12 (99% CI: 1.11, 1.14; prevalence 31.6% vs. 29.2%) in the United Kingdom. In Denmark, odds ratios were higher for anxiety (1.23; 99% CI: 1.17, 1.30) and severe stress and adjustment disorder (1.24; 99% CI: 1.18, 1.30) than for depression (1.11; 99% CI: 1.07, 1.14). In the United Kingdom, odds ratios for these conditions were similar: 1.12 (99% CI: 1.10, 1.13), 1.12 (99% CI: 1.10, 1.14), and 1.14 (99% CI: 1.10, 1.19) for depression, anxiety, and severe stress and adjustment disorder, respectively. In conclusion, mood disorders were associated with an increased risk of zoster.
Keywords: adjustment disorders, anxiety, depression, herpes zoster, stress disorders
Herpes zoster is a neurocutaneous infection estimated to affect up to 50% of persons who survive until age 85 years (1, 2). In the acute phase, zoster is typically associated with severe pain, which may persist as postherpetic neuralgia, a chronic pain syndrome that can affect quality of life severely (3). Studies of common risk factors for zoster are thus important, because they may help identify high-risk patients who could be targeted with preventive strategies.
Zoster is caused by reactivation of the varicella-zoster virus (VZV) from sensory ganglia when cell-mediated immunity (CMI) wanes below a critical level (1). Mood disorders, such as depression and anxiety, have been associated with impaired CMI (4). In particular, it has been demonstrated that VZV-specific CMI is reduced in major depression (5, 6) and is negatively associated with severity (5). Nevertheless, there are few well-designed epidemiologic studies estimating the risk of zoster in persons with mood disorders.
Registry-based case-control and cohort studies using prospectively collected data have found relative risks of zoster ranging between 1.11 and 1.52 for persons with depression compared with those without depression (7–12); studies using self-reported data on depression found relative risks between 0.93 and 4.15 (13–16). Unfortunately, these studies were hampered by methodological limitations, such as use of broad exposure definitions (e.g., mixing depression with alcohol-related psychotic disorder) (9–11), potential reverse causation (15, 16), selection bias (10, 11, 15, 16), and overadjustment by including possible proxies for exposure (11, 12). Furthermore, no studies have evaluated whether associations depend on time since diagnosis and severity of mood disorders.
We therefore aimed to quantify the risk of zoster among persons with depression, anxiety, and severe stress and adjustment disorder, taking into account time since diagnosis and severity of these disorders.
METHODS
Data sources
We created two case-control data sets using population-based registries in Denmark and the United Kingdom. The study period was January 1, 1997, through December 31, 2013, in Denmark and in January 1, 2000, through December 31, 2013, in the United Kingdom.
In Denmark, we retrieved nationwide data on hospital diagnoses from the National Patient Registry (17) and the Psychiatric Central Register (18). These registries provided data on inpatient psychiatric contacts since 1970, inpatient nonpsychiatric contacts since 1978, and outpatient specialty clinic and emergency department visits since 1995 (17, 18). For each encounter, a primary diagnosis (the main reason for contact) and optional secondary diagnoses are recorded using the International Classification of Diseases, Eighth Revision, until the end of 1993 and the Tenth Revision thereafter. We also obtained hospital data on surgical procedures and specialized treatments (e.g., delivery of chemotherapy). The Danish National Prescription Registry was used to identify prescriptions dispensed at any community pharmacy since 1995 (including date of dispensing, Anatomical Therapeutic Chemical code for the drug, and number and strength of tablets/units) (19). We ascertained diagnoses of diabetes (as a covariate) using the Danish National Diabetes Register. This registry identifies people with diabetes based on hospital-based diabetes diagnoses, reimbursable chiropody and blood-glucose measurements in primary care, and prescriptions for diabetes drugs in order to increase completeness compared to use of hospital diagnoses alone (20). The Danish Population Education Register provided information on the highest level of education attained by study participants as a proxy for socioeconomic status (21). We linked the Danish registries using the unique personal identifier assigned to all residents by the Civil Registration System (22).
In the United Kingdom, we used the Clinical Practice Research Datalink (CPRD) (23), the Hospital Episodes Statistics (HES) database (24), and the Index of Multiple Deprivation (25). The CPRD contains primary health-care data for 11.3 million patients from 674 general practices in the United Kingdom (23). It was established in London in 1987 as a smaller data set, expanding to the General Practice Research Database in 1993 and finally to the CPRD in 2012. Practices collect data on reasons for patient contacts (using Read codes), written prescriptions (using the Multilex Product Dictionary), vaccinations, laboratory and clinical measurements, lifestyle factors, anthropometric data, and referrals to secondary care (23). Internal practice- and patient-level quality checks identify data adequate for research. Approximately 60% of practices allow linkage to other data sets. We obtained inpatient hospital data since 1997 from HES (the date from which HES-linked CPRD is available), in which primary and secondary diagnoses are recorded according to the International Classification of Diseases, Tenth Revision, and procedures according to the Office of Population and Censuses and Surveys Classification of Interventions and Procedures, version 4 (24). The Index of Multiple Deprivation (2010 version) provided data on individual-level and practice-level deprivation. The Index weights 38 indicators within various domains (e.g., income and education) to yield deprivation scores for small geographical areas, which are mapped to practice or home postcode (25).
Study protocols, including variable definitions and code lists, are available from the corresponding author upon request and were made available to the Journal during the review process. The Danish study was approved by the Danish Data Protection Agency (2013-41-1719). Danish legislation does not require approval by an ethical review board or informed consent from patients for registry-based studies. The British study was approved by the CPRD Independent Scientific Advisory Committee (15_248) and the London School of Hygiene and Tropical Medicine Ethics Committee (11219).
Cases
Diagnoses of zoster from primary care are not reported to the Danish registries. We therefore identified zoster cases by prescriptions recorded in the National Prescription Registry for systemic antivirals at doses most compatible with zoster treatment (35 tablets of 800 mg acyclovir or packets with 500-mg tablets of valacyclovir or famciclovir) (26). Because these agents are also used for severe primary and reactivated herpes simplex infections (27), which occur most commonly in young people (28), persons eligible for the study had to be aged ≥40 years at the time of dispensing their first-time prescription for one of the antivirals ever. We retrieved hospital diagnoses of zoster from the Danish National Patient Registry, restricted to patients aged ≥40 years for consistency. We aimed to capture only incident zoster by excluding cases with previous records potentially representing chronic complication from zoster (defined as postherpetic neuralgia or any previous secondary zoster hospital diagnosis). The index date for cases was the date of prescription, hospital admission, or start of outpatient follow-up, whichever came first for persons with multiple records.
In the United Kingdom, cases were those with a record of zoster in the CPRD or a hospital diagnosis in HES, and no previous record of chronic complications from zoster (defined as above). The index date was the first occurring consultation or admission date for zoster. To avoid including prevalent zoster recorded shortly after registration with a new practice, cases had to have ≥12 months of registration with their current practice (29). In both Denmark and the United Kingdom, we also used the complete registration history available before study start to ensure that cases were incident and thus minimize the risk of reverse causation (i.e., that zoster leads to or provokes mood disorder).
Controls
We used risk-set sampling (30) from the Civil Registration System in Denmark and the CPRD in the United Kingdom to match up to 4 controls to each case according to age, sex, and general practice (United Kingdom only). Controls received an index date identical to their case. We applied the same eligibility criteria to controls as used for cases. In the United Kingdom, we excluded matched controls who had no contact with their CPRD practice within the 6 months before and 12 months after the index date, because they were considered inactive (7).
Mood disorder
We identified all records of depression, anxiety, and severe stress and adjustment disorder recorded at any time before the index zoster diagnosis in the Danish hospital registries and in the UK CPRD or HES. To accommodate trends in coding of depression in UK primary care (31), we included Read codes in the CPRD for both diagnoses and symptoms of depression. We categorized mood disorder in subgroups of timing and severity (Table 1) to examine our hypothesis that persons with current (“active”) and severe mood disorders have a higher risk of zoster. Of note, “mild severity” may be least comparable between countries, because persons treated for a mood disorder in general or private practice alone were not included in the Danish data.
Table 1.
Exposure Definitions Used in the Analysis of Mood Disorders and Risk of Herpes Zoster
| Main definition | |
| Never (referent) | No previous health-care record of mood disorder before the index date |
| Ever | Any previous health-care record of mood disorder in the registries at any time before the index datea |
| Timing | |
| Current | “Ever mood disorder” and most recent record within ≤90 days before the index date |
| Recent | “Ever mood disorder” and most recent record within 91–365 days before the index date |
| Former | “Ever mood disorder” and most recent record any time >365 days before the index date. Codes stating “in remission” were classified in this subgroup. |
| Severityb | |
| Severe | “Ever mood disorder” and hospitalization with the condition documented as the primary diagnosis within ≤90 days before the index date |
| Moderate |
|
| Mild | Remaining patients classified with “ever mood disorder” but not severe or moderate disease |
a The index date is the zoster diagnosis or prescription date (in Denmark) for cases and their matched controls.
b In the United Kingdom, the severity classification was examined only among patients with data linked to the Hospital Episodes Statistics database.
Statistical analysis
We used conditional logistic regression to compute unadjusted odds ratios with 99% confidence intervals, associating zoster with history of the 3 mood disorders separately and combined. Given the risk-set matching, odds ratios provide unbiased estimates of incidence rate ratios (30). In multivariable analyses, we additionally adjusted for zoster risk factors (7), listed and defined in Table 2. We stratified results for current mood disorders by age and sex. Finally, to assess the public health relevance, we computed the absolute age-specific rate of zoster for persons with any previous mood disorder diagnosis by multiplying the age-specific effect estimates in the Danish and UK study populations by the age-specific rate of zoster in the CPRD population in 2010 (7).
Table 2.
Distribution of Matching Factors and Herpes Zoster Risk Factors Among Herpes Zoster Cases and Matched Controls in Denmark (1997–2013) and the United Kingdom (2000–2013)
| Variable | Denmark | United Kingdom | ||||||
|---|---|---|---|---|---|---|---|---|
| Cases (n = 190,671) | Controls (n = 762,684) | Cases (n = 177,361) | Controls (n = 674,503) | |||||
| No. | % | No. | % | No. | % | No. | % | |
| Sex | ||||||||
| Male | 65,145 | 34.2 | 260,580 | 34.2 | 72,005 | 40.6 | 263,377 | 39.0 |
| Female | 125,526 | 65.8 | 502,104 | 65.8 | 105,356 | 59.4 | 411,126 | 61.0 |
| Age group at index date, yearsa | ||||||||
| 18–39 | 27,154 | 15.3 | 97,625 | 14.5 | ||||
| 40–49 | 34,838 | 18.3 | 139,352 | 18.3 | 20,844 | 11.8 | 77,009 | 11.4 |
| 50–59 | 41,898 | 22.0 | 167,592 | 22.0 | 33,632 | 19.0 | 127,508 | 18.9 |
| 60–69 | 45,662 | 23.9 | 182,648 | 23.9 | 38,437 | 21.7 | 150,110 | 22.3 |
| 70–79 | 39,264 | 20.6 | 157,056 | 20.6 | 34,767 | 19.6 | 136,694 | 20.3 |
| 80–89 | 23,968 | 12.6 | 95,872 | 12.6 | 19,454 | 11.0 | 755,66 | 11.2 |
| ≥90 | 5,041 | 2.6 | 20,164 | 2.6 | 3,073 | 1.7 | 9,991 | 1.5 |
| Practice-level IMD score (quintiles)b | ||||||||
| 1 (least deprived) | 34,965 | 19.7 | 132,980 | 19.7 | ||||
| 2 | 34,942 | 19.7 | 132,805 | 19.7 | ||||
| 3 | 37,861 | 21.3 | 144,034 | 21.4 | ||||
| 4 | 36,017 | 20.3 | 136,752 | 20.3 | ||||
| 5 (most deprived) | 33,576 | 18.9 | 127,932 | 19.0 | ||||
| Herpes zoster risk factorsc | ||||||||
| Rheumatoid arthritis | 4,091 | 2.1 | 9,157 | 1.2 | 4,031 | 2.3 | 10,187 | 1.5 |
| SLE | 464 | 0.2 | 664 | 0.1 | 525 | 0.3 | 1,093 | 0.2 |
| IBD | 2,710 | 1.4 | 7,324 | 1.0 | 2,499 | 1.4 | 6,879 | 1.0 |
| COPD | 10,966 | 5.8 | 31,252 | 4.1 | 9,479 | 5.3 | 28,342 | 4.2 |
| Asthma | 2,875 | 1.5 | 8,019 | 1.1 | 13,714 | 7.7 | 42,857 | 6.4 |
| CKD | 2,826 | 1.5 | 6,235 | 0.8 | 11,196 | 6.3 | 37,490 | 5.6 |
| Diabetes | 18,827 | 9.9 | 68,492 | 9.0 | 14,880 | 8.4 | 54,085 | 8.0 |
| Inhaled glucocorticoids | 11,255 | 5.9 | 31,515 | 4.1 | 13,987 | 7.9 | 41,828 | 6.2 |
| Solid organ transplantation | 195 | 0.1 | 158 | 0.02 | 29 | 0.2 | 347 | 0.05 |
| HIV infection | 361 | 0.2 | 364 | 0.04 | 196 | 0.1 | 206 | 0.03 |
| Leukemia | 782 | 0.4 | 807 | 0.1 | 392 | 0.2 | 565 | 0.08 |
| Lymphoma | 1,222 | 0.6 | 1,034 | 0.1 | 757 | 0.4 | 799 | 0.1 |
| Myeloma | 486 | 0.3 | 327 | 0.04 | 291 | 0.2 | 254 | 0.04 |
| HSCT | 692 | 0.4 | 486 | 0.1 | 278 | 0.2 | 138 | 0.02 |
| Other cellular immune deficiency | 283 | 0.1 | 382 | 0.1 | 345 | 0.2 | 668 | 0.1 |
| Oral glucocorticoids | 8,881 | 4.7 | 16,247 | 2.1 | 7,795 | 4.4 | 16,676 | 2.5 |
| Other immunosuppressants | 4,309 | 2.3 | 6,482 | 0.8 | 3,313 | 1.9 | 5,830 | 0.9 |
Abbreviations: CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; HIV, human immunodeficiency virus; HSCT, hematopoietic stem cell transplantation; IBD, inflammatory bowel disease; IMD, Index of Multiple Deprivation; SLE, subacute/systemic lupus erythematosus.
a In Denmark, only adults aged 40 years or older at index date were eligible.
b By matching on general practice in the UK data.
c At any time prior to index date, except for leukemia, lymphoma, and myeloma (any diagnosis within prior 2 years); and inhaled glucocorticoids, oral glucocorticoids, and other immunosuppressive treatment (any record within prior 90 days). Distributions of variables used in sensitivity analyses (lifestyle factors and measures of socioeconomic status) are shown in Web Table 11.
Additional analyses
Web Appendix 1 (available at https://academic.oup.com/aje) provides a detailed account of additional analyses. In a subgroup analysis, we examined whether odds ratios of zoster for current mood disorders were more pronounced for persons with their first-ever record of a mood disorder (i.e., “new-onset” conditions) within 90 days before the index date (zoster diagnosis date for cases and their matched controls). We also conducted various sensitivity analyses. We included data on antidepressant prescriptions to: 1) capture mood disorders treated in Danish general or private practice, and 2) examine the following alternative definition of severity: very severe (“severe” group in main analysis), severe (“moderate” group in main analysis), moderate (antidepressant prescription within the 14–90 days prior to index date), or mild (remaining patients). Second, we changed the defining cutoff for current mood disorder to within 7, 14, 30, or 180 days of the index date. Third, we excluded persons with only possible/unspecific codes for mood disorder (e.g., “suspected depression”). Fourth, we excluded persons with more than 1 type of mood disorder. Fifth, we excluded Danish cases identified by antiviral prescriptions without a zoster indication code (codes were considered too incomplete for use in the main analyses). Sixth, we excluded case-control sets included after marketing of the zoster vaccine on August 31, 2013, in the United Kingdom (not available in Denmark during study). Finally, we additionally adjusted for: 1) individual-level socioeconomic status (highest achieved education in Denmark and Index of Multiple Deprivation score in the United Kingdom); and 2) smoking status, alcohol consumption, and body mass index (United Kingdom only).
Against our expectations, we found the lowest odds ratios for those with recent admission (i.e., severe mood disorders). To examine whether this lack of association could be explained by underascertainment of zoster diagnosed during hospital follow-up of patients recently admitted with mood disorder, we performed a post-hoc analysis where we included secondary hospital diagnoses of zoster in the case definitions.
RESULTS
The study included 190,671 cases (people with incident zoster) and 762,684 controls (without incident zoster) in Denmark, and 177,361 cases and 674,503 controls in the United Kingdom (Web Figure 1). Although the prevalence of some covariates differed between Denmark and the United Kingdom, the relative differences between cases and controls were similar (Table 2).
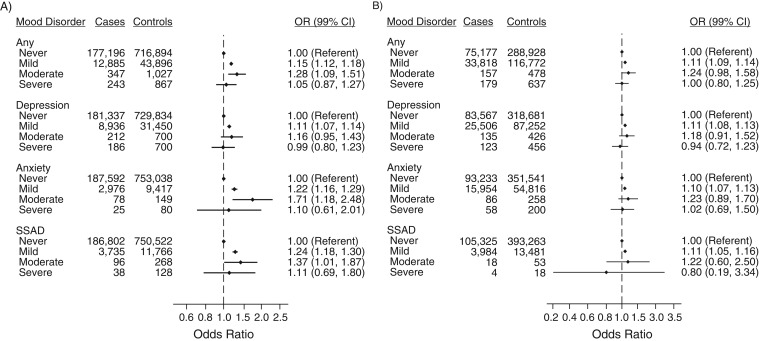
Cases had a higher recorded lifetime prevalence of mood disorder than controls (7.1% vs. 6.0% in Denmark; 31.6% vs. 29.2% in the United Kingdom), with depression being the most frequent mood disorder (Figure 1). Adjustment for risk factors had little effect on odds ratios (Web Table 1 and Web Table 2). The adjusted odds ratios for history of mood disorder among zoster cases compared with controls was 1.15 (99% confidence interval (CI): 1.12, 1.19) in Denmark and 1.12 (99% CI: 1.11, 1.14) in the United Kingdom (Figure 1). In Denmark, we observed slightly higher adjusted odds ratios for anxiety (1.23; 99% CI: 1.17, 1.30) and severe stress and adjustment disorder (1.24; 99% CI: 1.18, 1.30) than for depression (1.11; 99% CI: 1.07, 1.14). In the United Kingdom, estimates were similar for the 3 disorders. No substantial difference was observed when considering current, recent, or former mood disorders separately, although slightly higher adjusted odds ratios were observed for current and recent diagnoses than for former diagnoses in the United Kingdom (Figure 1). Analyses according to severity found that the overall increased risk of zoster was restricted to those classified with mild and moderate mood disorders, but confidence intervals were wide (Figure 2).
Figure 1.
Adjusted odds ratios (ORs) for previous diagnosis of mood disorder among herpes zoster cases compared with matched controls in Denmark, 1997–2013 (A), and the United Kingdom, 2000–2013 (B), according to timing of mood disorder. Odds ratios were adjusted for rheumatoid arthritis, lupus erythematosus, inflammatory bowel disease, chronic obstructive pulmonary disease, asthma, diabetes, chronic kidney disease, human immunodeficiency virus infection, hematopoietic stem cell/bone marrow transplantation, solid organ transplantation, other cellular immune deficiency, leukemia, lymphoma, myeloma, oral glucocorticoids, other immunosuppressant drugs, and inhaled glucocorticoids. CI, confidence interval; SSAD, severe stress and adjustment disorders.
Figure 2.
Adjusted odds ratios (ORs) for previous diagnosis of mood disorder among herpes zoster cases compared with matched controls in Denmark, 1997–2013 (A), and the United Kingdom, 2000–2013 (B), according to severity of mood disorder. Odds ratios were adjusted for rheumatoid arthritis, lupus erythematosus, inflammatory bowel disease, chronic obstructive pulmonary disease, asthma, diabetes, chronic kidney disease, human immunodeficiency virus infection, hematopoietic stem cell/bone marrow transplantation, solid organ transplantation, other cellular immune deficiency, leukemia, lymphoma, myeloma, oral glucocorticoids, other immunosuppressant drugs, and inhaled glucocorticoids. CI, confidence interval; SSAD, severe stress and adjustment disorders.
In analyses according to sex, the adjusted odds ratios for current mood disorder among zoster cases compared with controls were increased only among women (Figure 3). Furthermore, the adjusted odds ratios decreased with increasing age in the United Kingdom (Figure 3), which seemed to be explained by the results for depression (Web Table 3). The differences in absolute rates between persons with any previous mood disorder and the general population was 0.46–0.63 per 1,000 person-years in Denmark and 0.63–0.92 per 1,000 person-years in the United Kingdom, without variation by age (Table 3).
Figure 3.
Adjusted odds ratios (ORs) for current mood disorder among herpes zoster cases compared with matched controls in Denmark, 1997–2013 (A), and the United Kingdom, 2000–2013 (B), in subgroups defined by sex and age. Odds ratios are adjusted for rheumatoid arthritis, lupus erythematosus, inflammatory bowel disease, chronic obstructive pulmonary disease, asthma, diabetes, chronic kidney disease, human immunodeficiency virus infection, hematopoietic stem cell/bone marrow transplantation, solid organ transplantation, other cellular immune deficiency, leukemia, lymphoma, myeloma, oral glucocorticoids, other immunosuppressant drugs, and inhaled glucocorticoids. CI, confidence interval.
Table 3.
Age-Specific Rate of Herpes Zoster in Persons With Any Mood Disorder in Denmark (1997–2013) and the United Kingdom (2000–2013)
| Country | Age Group, years | |||||||
|---|---|---|---|---|---|---|---|---|
| <50a | 50–59 | 60–69 | ≥70 | |||||
| Rate per 1,000 Person-Years | 99% CI | Rate per 1,000 Person-Years | 99% CI | Rate per 1,000 Person-Years | 99% CI | Rate per 1,000 Person-Years | 99% CI | |
| Referentb | 2.08 | 1.74, 2.49 | 4.37 | 3.72, 5.12 | 6.69 | 5.76, 7.76 | 8.84 | 7.49, 10.43 |
| Denmark | 2.73 | 2.15, 3.47 | 5.29 | 3.91, 7.15 | 7.49 | 5.59, 10.02 | 9.47 | 7.02, 12.79 |
| United Kingdom | 2.54 | 2.06, 3.13 | 4.92 | 3.89, 6.22 | 7.32 | 5.85, 9.15 | 9.40 | 7.42, 11.92 |
Abbreviation: CI, confidence interval.
a In Denmark, only adults aged ≥40 years at the index date were eligible. However, in the United Kingdom, persons could be as young as 18 years.
b The United Kingdom general population, 2010.
In the additional analyses, estimates were not more pronounced for those with first-time diagnosis in the current exposure period (Web Table 1). When including antidepressant prescriptions as a proxy for mood disorder in Denmark, lifetime prevalence approached that in the United Kingdom (27.0% of cases and 22.2% of controls), and the adjusted odds ratio for any previous mood disorder increased to 1.26 (99% CI: 1.24, 1.28) (Web Table 4). The increase was observed across subgroups defined by timing and severity but remained lowest for patients with recent hospitalization (1.11, 99% CI: 0.91, 1.34). Results were robust in remaining sensitivity analyses (Web Table 5 to Web Table 12).”
In post-hoc analyses including secondary hospital diagnoses of zoster in the case definitions (Web Table 13 to Web Table 15), we identified 576 and 1,137 additional zoster cases in Denmark and the United Kingdom. In Denmark, the adjusted odds ratio for inpatient admission for mood disorder increased to 1.12 (99% CI: 0.93, 1.35) and to 1.19 (99% CI: 0.98, 1.43) when excluding antidepressant users from the reference group. The estimates for current mood disorder remained low for men but decreased with increasing age, as was observed in the UK main analyses. In the United Kingdom, the adjusted odds ratio for severe mood disorders increased to 1.16 (99% CI: 0.94, 1.43). Furthermore, adjusted odds ratios for current mood disorder became similar for men (1.25; 99% CI: 1.13, 1.37) and women (1.29; 99% CI: 1.22, 1.36).
DISCUSSION
In two large population-based studies from Denmark and the United Kingdom, we found that several mood disorders were associated with increased risk of zoster. The risk was increased even for those with more than 1 year since last health-care contact for mood disorder and among those classified as having mild disease.
Previous registry-based studies from Europe (7, 8), the United States (9), and East Asia (10–12) have reported 11%–52% increases in relative risk of zoster among depressed persons. Although most studies thus report estimates similar to those found in our study, direct comparison with existing evidence is hampered by previous studies using broad definitions of depression (9–11), which included bipolar disorder, personality disorders, and psychotic disorder due to alcohol use. Furthermore, in one cohort study, investigators adjusted for antidepressant use within 6 months before the endpoint (12). Finally, in two cohort studies, comparison cohorts were selected based on factors that might predict zoster (e.g., diagnosis of cancer (10) or absence of psychiatric diagnosis during follow-up (11)).
Four other studies used self-reported data on mood disorders (13–16). The hazard ratios of zoster were not increased among depressed persons in two cohort studies from the United States (0.93 (95% CI: 0.51, 1.71)) (13) and Australia (1.01 (95% CI: 0.95, 1.08)) (14). However, the Australian study adjusted for self-rated health, which may inevitably have caused adjustment for depression itself (1, 13, 21). Two smaller studies found associations between zoster and symptoms of depression (odds ratios = 2.00–4.15) (15, 16) but not anxiety (odds ratio = 1.07) (15). Besides potential selection bias in these studies, symptoms of mood disorder were assessed up to 3 weeks after rash onset. This design may have led to recall bias and reverse causation (15, 16), because zoster neuralgia may affect mental health (3, 32). However, we cannot rule out that their self-reported measures better capture perceived stress than other studies.
The mechanisms underlying an association between mood disorders and zoster are unknown. Decreased VZV-CMI has been found in patients with major depression (5, 6). Furthermore, the Depression Substudy of the Shingles Prevention Study showed that the boost of VZV-CMI following zoster vaccination was lower in the untreated depressed group compared with persons treated with antidepressants and nondepressed persons (6). Although this immunosuppression in mood disorder may result from persistent activation of the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system during chronic stress (4), death or acute illness of a partner, as a measure of extreme stress, was not associated with zoster in two studies (33, 34). Because grief and mood disorders are distinct psychological states, we hypothesize that these contradictory findings are explained by a greater allostatic load in the latter (4). Indeed, reductions in immune function following negative life events correlate with presence and severity of depressive symptoms (35, 36). However, the increased odds ratios for former mood disorders could also support the explanation that behavioral or shared biological factors (e.g., a genetic susceptibility to both mood disorders and zoster) play a role.
With almost 70,000 exposed cases of zoster, our study is by far the largest on the topic. Furthermore, use of population-based prospective data reduces selection bias, and we adjusted for various zoster risk factors. A concern is that while Denmark has comprehensive hospital registries covering psychiatric and nonpsychiatric wards and specialized outpatient clinics, we had to use prescription data as a proxy for diagnoses from Danish primary care. Conversely, we had comprehensive general practice data from the United Kingdom, but hospital data were limited to admissions and available for only 60% of participants. Because of these methodological differences, we did not pool results in a meta-analysis. Nevertheless, estimates were remarkably similar, and complementary strengths and weaknesses of the data sets allowed us to explore different types of bias (e.g., potential confounding from lifestyle factors, which seemed negligible).
Misclassification of zoster is a possible threat to the validity of both data sets. In a validation study in general practice, we found that 87% of patients with a first-time zoster-specific antiviral prescription had physician-diagnosed zoster as the indication for treatment (37). The validity of zoster in the Danish National Patient Registry, CPRD, and HES is unknown, but previous data generally support a high positive predictive value (of ≥90%) of physician-diagnosed zoster (38–40). It is unlikely that misdiagnosis depends on presence of mood disorder, but completeness may be higher in patients with mood disorder because of increased medical attendance. Such ascertainment bias could explain observed associations and may be more pronounced in Denmark, because the prescribing of antivirals is sensitive to timely health-care seeking. The possibility of bias in the other direction should also be considered—avoidance symptoms or loss of energy could preclude care seeking in some patients. Although that may explain the lack of an association among those with severe mood disorder, our post-hoc analyses including secondary (rather than exclusively primary) zoster hospital diagnoses suggested that we partly introduced this finding by excluding zoster diagnosed in psychiatric hospitals in relation to admission for a mood disorder.
Misclassification of exposure data is another limitation. In the Danish hospital registries, the positive predictive value for single depressive episodes is 75% compared with structured interview (41). For severe stress and adjustment disorder, the positive predictive value varies between 58% for acute stress reaction and 94% for adjustment disorder when applying strict diagnostic criteria to information obtained from medical records (42). Because misclassification occurs mainly between individual mood disorders, the validity of our overall definition is higher. Diagnoses in the United Kingdom stem mainly from general practice. Although validity of mood disorder diagnoses in CPRD is unknown, evidence suggests that nonpsychiatric physicians identify depression with 84% specificity. To increase completeness, we also included depressive symptoms recorded in the CPRD, which may have come at the expense of specificity. On the other hand, completeness is presumably lower in Denmark where we included only hospital-based diagnoses. Regardless, we expect that misclassification of mood disorder in our studies is nondifferential and would therefore bias towards the null. For example, underestimation due to incompleteness (31, 41–43) is evident from the increase in odds ratios when we included antidepressants as a proxy for mood disorder in Denmark. The low odds ratios among men may result from lower completeness than among women, because men seek and receive treatment for mood disorders less frequently (44). The lower odds ratios among the elderly may also be explained by underdiagnosis, because somatic presentation is more common in late-life depression (45). Furthermore, it is possible that persons diagnosed with mood disorder at older ages have already experienced zoster for another reason or simply that stronger risk factors play a relatively greater role than mood disorders. Nonetheless, an absolute increase in rate associated with any mood disorder was observed at all ages.
Analyses according to timing and severity should be interpreted cautiously. Delay from true onset of mood disorder to diagnosis in our data may have occurred if patients seek care late. Furthermore, because severity was not recorded, we categorized patients based on a nonvalidated method using information on treatment patterns (e.g., hospitalizations). Because mild mood disorder treated with psychotherapy in primary care could not be identified in the Danish data, we had expected higher estimates in Denmark, but this may have been mitigated by bias towards the null because of incompleteness. Finally, we lacked data to examine for effect modification by successful psycho- or pharmacotherapy.
In summary, previous studies show that VZV-CMI is reduced in patients with major depression (5, 6). Our study extended these immunological findings and understanding of their potential clinical consequences by showing that several mood disorders were associated with increased risk of zoster. Nevertheless, because the effect of perceived psychological stress on risk of zoster and vaccine efficacy remains controversial, further research on this topic is warranted.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Clinical Epidemiology, Aarhus University Hospital, Aarhus, Denmark (Sigrun A. J. Schmidt, Henrik T. Sørensen); Faculty of Epidemiology and Population Health, London School of Hygiene and Tropical Medicine, London, United Kingdom (Sigrun A. J. Schmidt, Sinéad M. Langan, Sara L. Thomas, Liam Smeeth, Kathryn E. Mansfield, Harriet J. Forbes); Research Unit for General Practice, Department of Public Health, Aarhus University, Aarhus, Denmark (Henrik S. Pedersen, Mogens Vestergaard); Department of Clinical Microbiology, Aalborg University Hospital, Aalborg, Denmark (Henrik C. Schønheyder); Department of Clinical Medicine, Aalborg University, Aalborg, Denmark (Henrik C. Schønheyder); and Section for General Practice, Department of Public Health, Aarhus University, Aarhus, Denmark (Mogens Vestergaard).
This work was funded by a Christian and Ottilia Brorsons’ grant for young scientists; Torben and Alice Frimodts’ Fund; a National Institute for Health Research (NIHR) Clinician Scientists Award (grant NIHR/CS/010/014 to S.M.L.); the Lundbeck Foundation (grant R155-2012–11280); the Program for Clinical Research Infrastructure, established by the Lundbeck Foundation and the Novo Nordisk Foundation; and the Wellcome Trust (award to L.S.).
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the UK Department of Health.
L.S. has received grants and personal fees from GSK and other support from AstraZeneca, outside the submitted work. H.T.S. does not report receiving fees, honoraria, grants, or consultancies, but the Department of Clinical Epidemiology is involved in studies with funding from various companies as research grants to (and administered by) Aarhus University; none of these studies are related to the present study. The other authors report no conflicts.
Abbreviations
- CI
confidence interval
- CMI
cell-mediated immunity
- CPRD
Clinical Practice Research Datalink
- HES
Hospital Episodes Statistics
- VZV
varicella-zoster virus
REFERENCES
- 1. Wilson JF. In the clinic. Herpes zoster. Ann Intern Med. 2011;154(5):ITC1-16. [DOI] [PubMed] [Google Scholar]
- 2. Hope-Simpson RE. The nature of herpes zoster: a long-term study and a new hypothesis. Proc R Soc Med. 1965;58:9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Johnson RW, Bouhassira D, Kassianos G, et al. The impact of herpes zoster and post-herpetic neuralgia on quality-of-life. BMC Med. 2010;8:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zorrilla EP, Luborsky L, McKay JR, et al. The relationship of depression and stressors to immunological assays: a meta-analytic review. Brain Behav Immun. 2001;15(3):199–226. [DOI] [PubMed] [Google Scholar]
- 5. Irwin M, Costlow C, Williams H, et al. Cellular immunity to varicella-zoster virus in patients with major depression. J Infect Dis. 1998;178(suppl 1):S104–S108. [DOI] [PubMed] [Google Scholar]
- 6. Irwin MR, Levin MJ, Laudenslager ML, et al. Varicella zoster virus–specific immune responses to a herpes zoster vaccine in elderly recipients with major depression and the impact of antidepressant medications. Clin Infect Dis. 2013;56(8):1085–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Forbes HJ, Bhaskaran K, Thomas SL, et al. Quantification of risk factors for herpes zoster: population based case-control study. BMJ. 2014;348:g2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ogunjimi B, Buntinx F, Bartholomeeusen S, et al. Herpes zoster is associated with herpes simplex and other infections in under 60 year-olds. J Infect. 2015;70(2):171–177. [DOI] [PubMed] [Google Scholar]
- 9. Joesoef RM, Harpaz R, Leung J, et al. Chronic medical conditions as risk factors for herpes zoster. Mayo Clin Proc. 2012;87(10):961–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hata A, Kuniyoshi M, Ohkusa Y. Risk of herpes zoster in patients with underlying diseases: a retrospective hospital-based cohort study. Infection. 2011;39(6):537–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yang YW, Chen YH, Lin HW. Risk of herpes zoster among patients with psychiatric diseases: a population-based study. J Eur Acad Dermatol Venereol. 2011;25(4):447–453. [DOI] [PubMed] [Google Scholar]
- 12. Liao CH, Chang CS, Muo CH, et al. High prevalence of herpes zoster in patients with depression. J Clin Psychiatry. 2015;76(9):e1099–e1104. [DOI] [PubMed] [Google Scholar]
- 13. Schmader K, George LK, Burchett BM, et al. Race and stress in the incidence of herpes zoster in older adults. J Am Geriatr Soc. 1998;46(8):973–977. [DOI] [PubMed] [Google Scholar]
- 14. Liu B, Heywood AE, Reekie J, et al. Risk factors for herpes zoster in a large cohort of unvaccinated older adults: a prospective cohort study. Epidemiol Infect. 2015;143(13):2871–2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lasserre A, Blaizeau F, Gorwood P, et al. Herpes zoster: family history and psychological stress-case-control study. J Clin Virol. 2012;55(2):153–157. [DOI] [PubMed] [Google Scholar]
- 16. Marin M, Harpaz R, Zhang J, et al. Risk factors for herpes zoster among adults. Open Forum Infect Dis. 2016;3(3):ofw119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schmidt M, Schmidt SA, Sandegaard JL, et al. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mors O, Perto GP, Mortensen PB. The Danish Psychiatric Central Research Register. Scand J Public Health. 2011;39(7 suppl):54–57. [DOI] [PubMed] [Google Scholar]
- 19. Pottegård A, Schmidt SAJ, Wallach-Kildemoes H, et al. Data resource profile: the Danish National Prescription Registry. Int J Epidemiol. 2017;46(3):798–798f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Carstensen B, Kristensen JK, Marcussen MM, et al. The National Diabetes Register. Scand J Public Health. 2011;39(7 suppl):58–61. [DOI] [PubMed] [Google Scholar]
- 21. Jensen VM, Rasmussen AW. Danish Education Registers. Scand J Public Health. 2011;39(7 suppl):91–94. [DOI] [PubMed] [Google Scholar]
- 22. Schmidt M, Pedersen L, Sørensen HT. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol. 2014;29(8):541–549. [DOI] [PubMed] [Google Scholar]
- 23. Herrett E, Gallagher AM, Bhaskaran K, et al. Data resource profile: Clinical Practice Research Datalink (CPRD). Int J Epidemiol. 2015;44(3):827–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. National Health Service (NHS) Digital Hospital Episode Statistics. http://www.hscic.gov.uk/hes. Accessed May 11, 2016.
- 25. Department for Communities and Local Government The English Indices of Deprivation 2010. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/6871/1871208.pdf. Accessed May 11, 2016.
- 26. Sand C, Hædersdal M, Helweg-Larsen J, et al., eds. ATC group J05—antiviral agents. The Danish Institute for Rational Pharmacotherapy; 2007. http://www.irf.dk/dk/rekommandationsliste/baggrundsnotater/infektionssygdomme/atc-gruppe_j05_-__antivirale_midler.htm. Accessed May 11, 2016. [Google Scholar]
- 27. Workowski KA, Bolan GA, Centers for Disease Control and Prevention . Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1–137. [PMC free article] [PubMed] [Google Scholar]
- 28. Solomon L, Cannon MJ, Reyes M, et al. Epidemiology of recurrent genital herpes simplex virus types 1 and 2. Sex Transm Infect. 2003;79(6):456–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lewis JD, Bilker WB, Weinstein RB, et al. The relationship between time since registration and measured incidence rates in the General Practice Research Database. Pharmacoepidemiol Drug Saf. 2005;14(7):443–451. [DOI] [PubMed] [Google Scholar]
- 30. Pearce N. What does the odds ratio estimate in a case-control study? Int J Epidemiol. 1993;22(6):1189–1192. [DOI] [PubMed] [Google Scholar]
- 31. Rait G, Walters K, Griffin M, et al. Recent trends in the incidence of recorded depression in primary care. Br J Psychiatry. 2009;195(6):520–524. [DOI] [PubMed] [Google Scholar]
- 32. Chen MH, Wei HT, Su TP, et al. Risk of depressive disorder among patients with herpes zoster: a nationwide population-based prospective study. Psychosom Med. 2014;76(4):285–291. [DOI] [PubMed] [Google Scholar]
- 33. Harpaz R, Leung JW, Brown CJ, et al. Psychological stress as a trigger for herpes zoster: might the conventional wisdom be wrong? Clin Infect Dis. 2015;60(5):781–785. [DOI] [PubMed] [Google Scholar]
- 34. Schmidt SA, Vestergaard M, Pedersen HS, et al. Partner bereavement and risk of herpes zoster: results from two population-based case-control studies in Denmark and the United Kingdom. Clin Infect Dis. 2016;64(5):572–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zisook S, Shuchter SR, Irwin M, et al. Bereavement, depression, and immune function. Psychiatry Res. 1994;52(1):1–10. [DOI] [PubMed] [Google Scholar]
- 36. Irwin M, Daniels M, Bloom ET, et al. Life events, depressive symptoms, and immune function. Am J Psychiatry. 1987;144(4):437–441. [DOI] [PubMed] [Google Scholar]
- 37. Schmidt SAJ, Vestergaard M, Baggesen LM, et al. Prevaccination epidemiology of herpes zoster in Denmark: quantification of occurrence and risk factors. Vaccine. 2017;35(42):5589–5596. [DOI] [PubMed] [Google Scholar]
- 38. Rübben A, Baron JM, Grussendorf-Conen EI. Routine detection of herpes simplex virus and varicella zoster virus by polymerase chain reaction reveals that initial herpes zoster is frequently misdiagnosed as herpes simplex. Br J Dermatol. 1997;137(2):259–261. [DOI] [PubMed] [Google Scholar]
- 39. Scott FT, Johnson RW, Leedham-Green M, et al. The burden of herpes zoster: a prospective population based study. Vaccine. 2006;24(9):1308–1314. [DOI] [PubMed] [Google Scholar]
- 40. Weinmann S, Chun C, Schmid DS, et al. Incidence and clinical characteristics of herpes zoster among children in the varicella vaccine era, 2005–2009. J Infect Dis. 2013;208(11):1859–1868. [DOI] [PubMed] [Google Scholar]
- 41. Bock C, Bukh JD, Vinberg M, et al. Validity of the diagnosis of a single depressive episode in a case register. Clin Pract Epidemiol Ment Health. 2009;5:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Svensson E, Lash TL, Resick PA, et al. Validity of reaction to severe stress and adjustment disorder diagnoses in the Danish Psychiatric Central Research Registry. Clin Epidemiol. 2015;7:235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gradus JL, Bozi I, Antonsen S, et al. Severe stress and adjustment disorder diagnoses in the population of Denmark. J Trauma Stress. 2014;27(3):370–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Department of Mental Health and Substance Dependence Gender disparities in mental health. World Health Organization; 2002. http://www.who.int/mental_health/media/en/242.pdf?ua=1. Accessed May 11, 2016. [Google Scholar]
- 45. Hegeman JM, Kok RM, van der Mast RC, et al. Phenomenology of depression in older compared with younger adults: meta-analysis. Br J Psychiatry. 2012;200(4):275–281. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.