Abstract
Introduction:
The spread of drug-resistant pathogens is one of the most serious threats to successful treatment of microbial diseases. Extracts of plants such as flowers, buds, seeds, leaves, twigs, bark, herbs, wood, fruits, and roots have evoked interest as sources of natural products. Irrigation with a broad-spectrum antiseptic substance and inter-appointment intracanal medication has become a standard regimen in root canal therapy.
Aim:
The aim of this study is to compare the antimicrobial efficacy of different natural extracts such as guava leaf extract, Aloe vera extract, papaya leaf extract, and cashew apple extract against Enterococcus faecalis and Candida albicans.
Materials and Methods:
The antimicrobial activity was determined using agar diffusion test. The solutions were divided into four groups: Group I – guava leaf extract, Group II – A. vera extract, and Group III – papaya leaf extract, and Group IV – cashew apple extract. The zones of inhibition of growth were recorded. The strains used for this study were E. faecalis ATCC 29212 and C. albicans ATCC 90028.
Results and Conclusion:
Sodium hypochlorite had demonstrated the best results among the tested solutions. Among the herbal extracts, cashew apple extract and guava leaf extract had shown statistically significant activity against E. faecalis and C. albicans.
Keywords: Antimicrobial efficacy, microbial infection, root canal irrigation
Introduction
Bacteria and their products play an essential role in the pathogenesis of pulpo-periapical diseases. A long-standing endodontic infection allows bacteria to propagate to the entire root canal system, including ramifications, isthmuses, apical deltas, and dentinal tubules.[1] In contemporary endodontics, chemomechanical preparation associated with antiseptic medication has been recommended for infection control. Despite this, residual microorganisms may persist in root canals.[2] Anatomical complexities and microbiological factors often pose serious threats to adequate root canal disinfection. It is a prerequisite to use endodontic irrigants in addition to mechanical preparation to ensure the success of root canal treatment.[3]
An irrigant serves to flush out debris from within the instrumented root canals, dissolve organic tissue remnants, disinfect the root canal space, and provide lubrication during instrumentation, without causing irritation to biological tissues. Sodium hypochlorite (NaOCl) has become the most popular agent for endodontic irrigation even though its optimum working concentration has not been universally agreed.[4] It is a strong proteolytic substance and provides sufficient antimicrobial effect. However, adverse effects of NaOCl have been reported including unpleasant odor and taste, toxicity, possible paresthesia of the mandibular nerve, allergy, and an increase in coronal microleakage of adhesive restorations.[5]
In endodontics, because of the cytotoxic reactions of the most of the commercial intracanal medicaments used and their inability to eliminate bacteria from dentinal tubules, the trend of recent medicine attends to use biologic medication extracted from natural plants.[6] Antibacterial and antioxidant actions of guava leaf extract and Aloe vera extract have been studied and found out that they have antimicrobial actions against oral pathogens.[7,8] Leaf extracts of Carica papaya Linn. exhibited greater activity toward bacteria and fungi.[9] Alcoholic and aqueous extract of false fruit of cashew apple (Anacardium occidentale) displayed significant antimicrobial properties.[10]
Enterococcus faecalis is one of the most prominent bacterial species isolated from root canals of treatment failed teeth. Studies have shown E. faecalis in 30%–89% of teeth with post endodontic treatment failures, mostly as monoculture.[11] Candida albicans have been associated with secondary or persistent endodontic infections.[12]
Aim
The aim of the present study was to explore and compare the antimicrobial activity of newer irrigants such as guava leaf extract, A. vera extract, cashew apple extract, and papaya leaf extract with gold standards such as NaOCl against E. faecalis and C. albicans.
Materials and Methods
This in vitro study was conducted at the Department of Microbiology, Kannur Medical College, Anjarakandy, Kannur. In the present study, A. vera extract (Group I), cashew apple extract (Group II), guava leaf extract (Group III), and papaya leaf extract (Group IV) were selected as the experimental groups and 5.25% NaOCl (Group V) as positive control groups. ATCC 29212 and ATCC 10231 were strains used in this study to check antimicrobial activity of E. faecalis and C. albicans respectively.
Preparation of Aloe vera extract (Group I)
A. vera leaves were obtained and cut into small pieces with a knife and grinded using an electric grinder into paste form. Aqueous extracts were prepared by dissolving the paste material in sterile distilled water in a ratio of 1:5, i.e., 20 g of plant paste material in 100 ml of water in a sterile 250 ml flask. This was kept in a refrigerator at 4°C for 24 h and was then filtered using filter paper. The extract was then again kept in the refrigerator at 4°C before being reconstituted for further use.
Preparation of cashew apple extract (Group II)
The fruits were cleaned, reduced the size by cutting to small parts, and then dried under shade. It was coarsely powdered with the help of a blender. The coarse powder of fruit was then exhaustively extracted in a Soxhlet apparatus. In this extraction process, 250 g of dried powder was extracted using Soxhlet extraction process with 500 ml of ethyl alcohol and distilled water and chloroform (99:1) as solvent separately. The extracts were concentrated by distilling the solvent and preserved under refrigeration for further studies.[10]
Preparation of papaya leaf extract (Group III)
Plant materials were collected. The disease-free, fresh, young, and green leaves were collected from the papaya plant. The fresh leaves were harvested, properly washed in tap water, and rinsed in sterile distilled water. The leaf was dried in the hot air oven at 40°C for 3 days. The dried leaves were pulverized, using sterile laboratory mortar and pestle, to obtain a powdered form. These were stored in airtight glass containers protected from sunlight until required for analysis.
The crude extract from the leaves of papaya was prepared according to the method proposed by Alabi et al.[6] The aqueous extract was prepared by suspending 100 g of powdered leaves in 200 ml of distilled water. This mixture was diluted with 300 ml of distilled water and then allowed to stand for 24 h. The resulting extract was decanted and filtered through a Whatman filter paper. The filtrate was then concentrated with a rotary evaporator at 45°C.
Preparation of guava leaves extract (Group IV)
Leaves of guava were obtained and dried in fresh open air protecting from direct exposure to sunlight. A 50 g of powdered leaves were taken into a beaker containing 500 ml of sterile distilled water. Hot water extract was prepared by heating this in a water bath till menstruum reduced to about 125 ml which is about one-fourth of the original volume. After the complete evaporation of the water content from extract, the resulting liquid was filtered using filter paper.
Agar-diffusion test
Hundred microliters of test organisms E. faecalis and C. albicans suspensions was obtained from prepared cultures and inoculated in culture plates with previously set layers of Mueller–Hinton Agar and Sabouraud dextrose agar, respectively, for each organism. Sterile spreader was used for inoculation of these organisms across respective media. Disc diffusion assay was conducted. Using pipette, 200 microliters of the extract to be tested was added to the filter paper disk placed in five Petri dishes, respectively, and then dried on the bench at room temperature for 3 h. Gently place the disk on the top of the agar and lightly press it down with tweezers. These plates were incubated for 24 h at 37°C in an incubator. After incubation period, plates were checked for zones of inhibition of bacterial growth and diameters of the zones achieved by each group against E. faecalis and C. albicans were recorded in millimeter (mm) [Figures 1 and 2].
Figure 1.
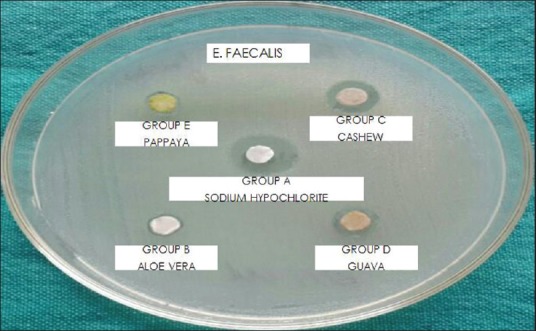
Figure of agar diffusion test against E. faecalis
Figure 2.
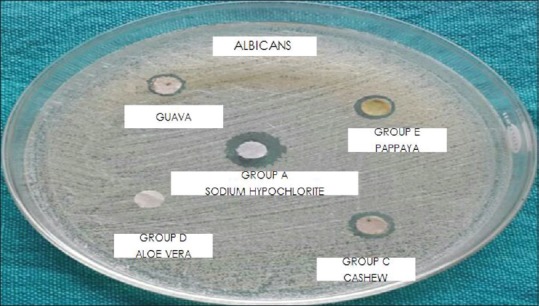
Figure of agar diffusion test against C. albicans
Results
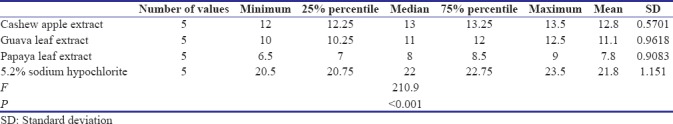
Analysis of variance (one-way) was performed as parametric test to compare different groups for both E. faecalis and C. albicans. Table 1 shows that there exists a significant difference between the diameters of zones of inhibition of bacterial growth obtained for 5.2% NaOCl, A. vera extract, cashew apple extract, papaya leaf extract, and guava leaf extract against E. faecalis (P < 0.05) [Graph 1]. Table 2 shows that there exists a significant difference between the diameters of zones of inhibition of bacterial growth obtained for 5.2% NaOCl, cashew apple extract, papaya leaf extract, and guava leaf extract against C. albicans while A. vera shows no zones of inhibition against C. albicans [Graph 2].
Table 1.
One-way analysis of variance: Enterococcus faecalis
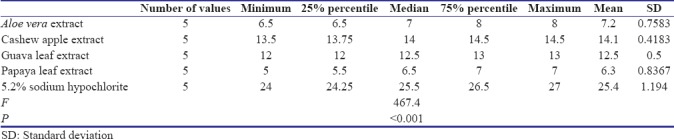
Table 2.
One-way analysis of variance: Candida species
Graph 1.
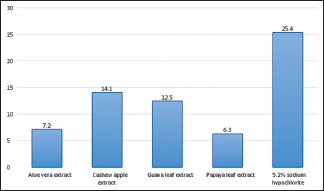
Graph for Enterococcus faecalis
Graph 2.
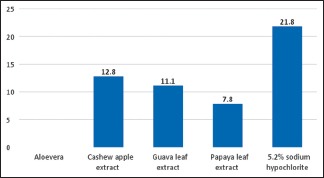
Graph for Candida albicans
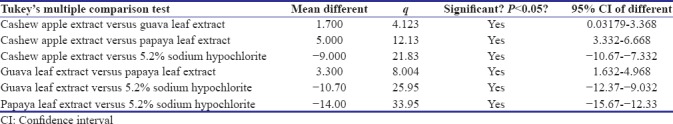
Tables 3 and 4 show post hoc tests-Tukey's honestly significant difference for the intercomparison of the antimicrobial efficacy of different groups against E. faecalis and C. albicans, respectively. Post hoc test revealed that there is statistically significant difference between all groups, except between A. vera extract and papaya leaf extract against E. faecalis.
Table 3.
Post hoc Tukey test for Enterococcus faecalis
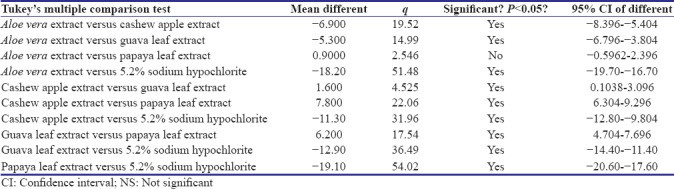
Table 4.
Post hoc Tukey test for Candida albicans
Discussion
The major goal of root canal treatment is to clean canal by considering biological, chemical, and mechanical objectives.[13] After mechanical debridement, biofilm may remain undisturbed in the anatomically challenging areas such as fins, lateral or furcal canals, apical deltas, webs, and isthmus. Effective disinfection in endodontics is only achieved by augmenting mechanical preparation with antimicrobial irrigants.
Root canal infections are multibacterial. The 70% of the isolated bacteria include the anaerobic species. E. feacalis had frequently been isolated from root canals of failed endodontic treatment cases. C. albicans, the common organism associated with therapy-resistant apical periodontitis, is more resistant to disinfecting agents used in endodontics.[14] In the present study, positive control NaOCl, cashew apple extract, and guava leaf extract were shown to inhibit E. faecalis and C. albicans effectively. However, A. vera extract and papaya extract had shown very minimal activity against E. faecalis and C. albicans in the present study.
NaOCl has been considered as an irrigant of choice for root canal irrigation because of its antimicrobial activity and tissue-dissolving capacity. High pH of NaOCl interferes with the cytoplasmic membrane integrity and causes biosynthetic alterations in cellular metabolism, attributing to its antimicrobial nature. Tissue-dissolving action and dissolution rate of NaOCl are directly proportional to its concentration.[15] However, not only its actions such as antimicrobial activity, tissue-dissolving capacity, and smear layer remove ability but also the caustic potential and toxicity of NaOCl also increase with the increase in concentration.[16]
Phytochemical analysis of the crude extracts of cashew apple revealed the presence of flavonoids, tannins, triterpenoids, and phenolic compounds, and these components are responsible for the antimicrobial activity of the extracts. Phenolic compounds have been shown to be toxic to microorganisms. The site(s) and number of hydroxyl groups on the phenol group are thought to be related to their relative toxicity to microorganisms, with evidence that increased hydroxylation results in increased toxicity.[10]
The flavonoids such as mosin glycosides, quercetin, and quercetin glycosides may contribute to antibacterial action of guava leaf extracts.[7] The resistance to bacterial attacks suggested being as a result of the polygalacturonase inhibitory proteins in the plant cell walls of guava. The aqueous extracts of guava leaf can cause a marked reduction in the adhesion of the early organisms of plaque biofilm formation.[17]
The chemical composition of A. vera includes vitamins, enzymes, minerals, sugars, lignin, saponins, salicylic acids, and amino acids. The latex compound present in A. vera has bacteriostatic property. These constituents may contribute to its antimicrobial activity against various microbes.[14]
C. papaya belonging to family Caricaceae is commonly known as papaya in English. The C. papaya showed the presence of flavonoids (kaempferol and myricetin), alkaloids (carpaine, pseudocarpaine, dehydrocarpaine), phenolic compounds (ferulic acid, caffeic acid, chlorogenic acid), and cyanogenetic compounds (benzylglucosinolate).[18] It is common traditional practice to treat the wound with the leaf extract of papaya to accelerate the healing action. In addition, papaya leaves possess antibacterial activity which might prevent the multiplication of wound infection-causing bacteria.[19]
In the present study, NaOCl had obtained a mean diameter of 20 mm and 24 mm for C. albicans and E. faecalis, respectively. The zones of inhibition of bacterial growth attained by NaOCl were greater than that obtained for other extracts for the tested organisms. This indicates that it has the highest efficacy against the tested organisms than other herbal agents. However, the zones of inhibition of bacterial growth obtained by guava leaf extract and cashew apple extract against C. albicans and E. faecalis are significantly less than NaOCl in our study. This value had some sort of significance as our study emphasized on whether herbal products would provide acceptable antimicrobicity in routine endodontic practice as it is well known that herbal products are more bio-friendly to human tissues.
Hence, from the present study, it can be evaluated that against both C. albicans and E. faecalis, NaOCl was the best antimicrobial irrigant which is followed in descending order by the cashew apple extract, guava leaf extract, A. vera extract, and papaya extract.
Conclusion
Within the limitations of this study, NaOCl had demonstrated the best results amongst the tested solutions. Among the herbal extracts, cashew apple extract and guava leaf extract had shown statistically significant activity against E. faecalis and C. albicans. Further evaluation of the antimicrobial efficacy of cashew apple extract and guava leaf extract is highly recommended before extensive clinical usage.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Segura JJ, Jiménez-Rubio A, Guerrero JM, Calvo JR. Comparative effects of two endodontic irrigants, chlorhexidine digluconate and sodium hypochlorite, on macrophage adhesion to plastic surfaces. J Endod. 1999;25:243–6. doi: 10.1016/S0099-2399(99)80151-4. [DOI] [PubMed] [Google Scholar]
- 2.Soares JA, Roque de Carvalho MA, Cunha Santos SM, Mendonça RM, Ribeiro-Sobrinho AP, Brito-Júnior M, et al. Effectiveness of chemomechanical preparation with alternating use of sodium hypochlorite and EDTA in eliminating intracanal Enterococcus faecalis biofilm. J Endod. 2010;36:894–8. doi: 10.1016/j.joen.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Peters OA. Current challenges and concepts in the preparation of root canal systems: A review. J Endod. 2004;30:559–67. doi: 10.1097/01.don.0000129039.59003.9d. [DOI] [PubMed] [Google Scholar]
- 4.Gomes BP, Ferraz CC, Vianna ME, Berber VB, Teixeira FB, Souza-Filho FJ, et al. In vitro antimicrobial activity of several concentrations of sodium hypochlorite and chlorhexidine gluconate in the elimination of Enterococcus faecalis. Int Endod J. 2001;34:424–8. doi: 10.1046/j.1365-2591.2001.00410.x. [DOI] [PubMed] [Google Scholar]
- 5.Shakouie S, Eskandarinezhad M, Gasemi N, Milani AS, Samiei M, Golizadeh S, et al. An in vitro comparison of the antibacterial efficacy of triphala with different concentrations of sodium hypochlorite. Iran Endod J. 2014;9:287–9. [PMC free article] [PubMed] [Google Scholar]
- 6.Kamat S, Rajeev K, Saraf P. Role of herbs in endodontics: An update. Endodontology. 2011;23:98–101. [Google Scholar]
- 7.Biswas B, Rogers K, McLaughlin F, Daniels D, Yadav A. Antimicrobial activities of leaf extracts of guava (Psidium guajava L.) on two gram-negative and gram-positive bacteria. Int J Microbiol. 2013;2013:746165. doi: 10.1155/2013/746165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Athiban PP, Borthakur BJ, Ganesan S, Swathika B. Evaluation of antimicrobial efficacy of Aloe vera and its effectiveness in decontaminating gutta percha cones. J Conserv Dent. 2012;15:246–8. doi: 10.4103/0972-0707.97949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vijayakumar1 M, Bharathidasan R, Prince L. Antimicrobial activity of Carica papaya L. Int J Arts Sci Res. 2015;2:37–43. [Google Scholar]
- 10.Aiswarya G, Reza KH, Radhika G, Farook SM. Study for antibacterial activity of cashew apple (Anacardium occidentale) extracts. Pharm Lett. 2011;3:193–200. [Google Scholar]
- 11.George S, Basrani B, Kishen A. Possibilities of gutta-percha-centered infection in endodontically treated teeth: An in vitro study. J Endod. 2010;36:1241–4. doi: 10.1016/j.joen.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 12.Siqueira JF, Jr, Rôças IN, Lopes HP, Magalhães FA, de Uzeda M. Elimination of Candida albicans infection of the radicular dentin by intracanal medications. J Endod. 2003;29:501–4. doi: 10.1097/00004770-200308000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Aniketh TN, Idris M, Geeta IB, Nandakishore KJ, Sahu GK. Root canal irrigants and irrigation techniques: A review. J Evol Med Dent Sci. 2015;4:4694–700. [Google Scholar]
- 14.Jose J, Krishnamma S, Peedikayil F, Amaan S, Tomy N, Mariodan JP. Comparative evaluation of antimicrobial activity of QMiX, 2.5% sodium hypochlorite, 2% chlorhexidine, guava leaf extract and Aloe vera extract against Enterococcus faecalis and Candida albicans – An in-vitro study. J Clin Diagnostic Res. 2016;10:ZC20–3. doi: 10.7860/JCDR/2016/17705.7747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baumgartner JC, Cuenin PR. Efficacy of several concentrations of sodium hypochlorite for root canal irrigation. J Endod. 1992;18:605–12. doi: 10.1016/S0099-2399(06)81331-2. [DOI] [PubMed] [Google Scholar]
- 16.Shetty KP, Satish SV, Kilaru K, Ponangi KC, Venumuddala VR, Ratnakar P. Comparative evaluation of the cytotoxicity of 5.25% sodium hypochlorite, 2% chlorhexidine and mixture of a tetracycline isomer, an acid and a detergent on human red blood corpuscles: An in-vitro study. Saudi Endod J. 2014;4:1–6. [Google Scholar]
- 17.Ravi K, Divyashree P. Psidium guajava: A review on its potential as an adjunct in treating periodontal disease. Pharmacogn Rev. 2014;8:96–100. doi: 10.4103/0973-7847.134233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anjum V, Ansari SH, Naquvi KJ, Arora P, Ahamed A. Development of quality standards of Carica papaya Linn. leaves. Pharm Lett. 2013;5:370–6. [Google Scholar]
- 19.Aruljothi S, Uma C, Sivagurunathan P, Bhuwaneswari M. Investigation on antibacterial activity of Carica Papaya leaf extracts against wound infection-causing bacteria. Int J Res Stud Biosci. 2014;2:8–12. [Google Scholar]


