Abstract
Background:
An amnion membrane is a placenta-derived tissue that consists of numerous growth factors, proteins, and stem cell reserves which help in accelerated wound healing and regeneration. Platelet-rich fibrin (PRF) also releases growth factors after activation from the platelets and gets trapped within fibrin matrix which has been shown to stimulate the mitogenic response in the periosteum for bone repair and regeneration during normal wound healing. This preliminary, controlled, randomized clinical trial with an 18-month follow-up was aimed to evaluate the effectiveness of coronally advanced flap (CAF) with either PRF membrane or bioresorbable amniotic membrane (AM) in treatment of localized gingival recession defects.
Materials and Methods:
Sixteen healthy adult patients presenting with Miller Class I recession defects were treated surgically with CAF along with AM (Group I) or PRF (Group II) for coverage of the recession defects. For all patients, plaque index, gingival index, bleeding on probing, clinical attachment level, depth of recession, width of recession, width of attached gingiva, and gingival thickness were evaluated at 6 months and 18 months postoperatively. Statistical analysis was done using paired t-test, repeated measure analysis of variance test, Bonferroni test for intragroup comparison and unpaired t-test for intergroup comparison.
Results:
The results showed statistically nonsignificant (P < 0.01) difference in all clinical parameters at the 6- and 18-month follow-ups in both groups. Gingival recession in both PRF and amnion group when evaluated individually, significantly reduced from baseline to 6 months (P = 0.000) and from baseline to 18 months (P = 0.000). However, the mean value from 6 months to 18 months was statistically nonsignificant.
Conclusion:
The present study demonstrated that both CAF + PRF and CAF + AM are equally effective in providing clinically significant outcomes with respect to root coverage with AM showing the better percentage of root coverage as compared to PRF.
Keywords: Gingival recession, growth factors, periodontal plastic surgery
Introduction
Gingival recession is defined as “the displacement of marginal tissue apical to the cementoenamel junction (CEJ).”[1] Over the years, various procedures have evolved including pedicle and soft-tissue grafts to obtain root coverage. The most predictable plastic procedure is coronally advanced flap (CAF) with subepithelial connective tissue graft, which remains the “gold standard” of periodontal plastic surgery. It provides excellent predictability and improved long-term root coverage, but it is limited in supply and significantly increases patient morbidity.[2] Owing to this, allografts present an attractive opportunity for coverage of gingival recession.
Use of placental allografts in dentistry is a more recent development. The human placenta comprises two membranes: (1) the inner amniotic membrane (AM) and (2) the outer chorion membrane. With the improvements in the processing, AM has found application in various fields of medicine, constructive surgeries, arthroplasty, etc.[3] Cryopreserved AM is effective in cicatrisation, wound healing epithelization facilitated migration, and reinforced adhesion,[4] thus making it effective in the treatment of periodontal surgery.[5]
Regenerative potential of platelets was introduced in 1974, and Ross et al.[6] were among the pioneers who first described the release of growth factors from platelets which have been shown to stimulate the mitogenic response in the periosteum for bone repair during normal wound healing.[7] Platelet concentrates which include platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) have been found effective in the enhancement of early wound healing[8] and have been speculated as promoters of periodontal tissue regeneration.[9] Among these concentrates, PRF is a second-generation, autologous platelet concentrate that includes a leukocyte aggregate and a high-density fibrin network that provides a slow polymerization system similar to the nature of growth factors including vascular endothelial growth factor, insulin-like growth factor, platelet-derived growth factor (PDGF), transforming growth factor (TGF), epidermal growth factor, and basic fibroblast growth factor. By virtue of this content, PRF accelerates hemostasis and wound healing and has a supportive effect on the immune system, cell migration, and proliferation. PRF preparation is a cost-effective process with short chair-side duration and does not need bovine thrombin and/or anticoagulant addition. Furthermore, the material does not require the biochemical handling of blood and can be formed easily as a regenerative membrane.[10]
The ultimate goal of recession therapy is achieving complete recession coverage in harmony with the adjacent tissues. Given the encouraging effects of PRF and amnion membrane in healing and regeneration, it is hypothesized that PRF and AM might enhance the outcomes obtained with CAF.
Thus, the aim of this preliminary randomized clinical trial with an 18-month follow-up was to evaluate the effectiveness of CAF with either PRF membrane or bioresorbable AM in treatment of localized Miller's Class I gingival recession defects.
Materials and Methods
The clinical trial has been registered with CTRI/2017/04/008349 on: 13/04/2017. The protocol was approved by the Institutional Review Committee for human subjects and the study was conducted in accordance with the Helsinki Declaration of 1975, as revised in 2013.
A total number of 16 systemically healthy patients with sufficient vestibular depth and presence of adequate width of attached gingiva between the age group of 20 and 45 years were selected from the Outpatient Department of Periodontology, having Miller's Class I gingival recession, and were included in the study based on the following inclusion and exclusion criteria. Inclusion criteria included systemically healthy patients with the presence of at least one Millers Class I recession, sufficient vestibular depth, and presence of adequate width of keratinized gingiva. Exclusion criteria included the patients who had undertaken any periodontal treatment before 6 months of initial treatment, pregnant and lactating mothers, smokers, and patients with poor oral hygiene.
At day 0, full mouth plaque index (PI), gingival index (GI), and bleeding on probing index were recorded, and ultrasonic scaling was done in all the selected 16 patients. After 4 weeks, patients who maintained their oral hygiene and had fair plaque, gingival, and bleeding on probing index scores were finally included in the study. Among the selected 16 patients (12 males and 4 females) who were included in the study, 6 patients (2 male and 4 female) were excluded from the trial since they did not maintain their oral hygiene [Figure 1]. Finally, 10 male patients with twenty sites (11 maxillary and 9 mandibular) underwent parallel mouth root coverage study. Acrylic stent was fabricated at the selected site for reproducibility of measurements to determine the treatment outcome. Written informed consent was taken from all patients after giving detailed information regarding PRF and amniotic membrane and methodology to be used in the study. The patients were then divided randomly using flip of coin into two groups keeping participants blinded. A parellel mouth design was used for the study. For all patients, PI, GI, bleeding on probing, clinical attachment level, depth of recession, width of recession, width of attached gingiva, and gingival thickness were evaluated at 6 months and 18 months postoperatively. The recruitment and follow-up of all subjects were done from August 2014 to March 2016. The trial was for a period of 18-month recall and was completed within that time.
Figure 1.
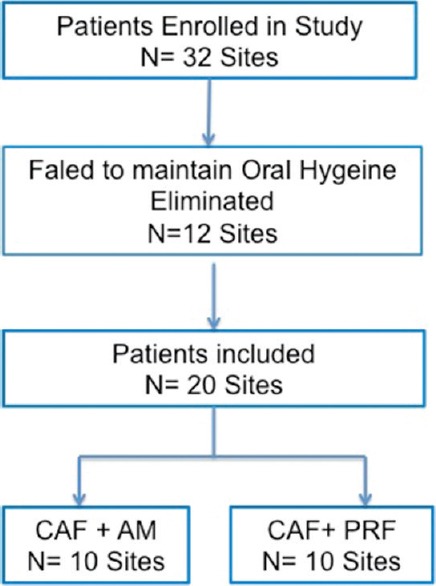
Study flow chart
Surgical procedure
All the participants in the study were blinded to the treatment. The patients were asked to do a presurgical rinse with 10 ml of 0.2% chlorhexidine diluted solution. The selected site was locally infiltrated using (2% lignocaine hydrochloride with adrenaline 1:80,000) before initiating the surgical procedure. All the selected Miller Class I recession defects underwent root coverage procedure by CAF. Primary two horizontal incisions were made in mesial and distal directions from the CEJ up to 1 mm past the proximal line angle of the adjacent teeth leaving the interdental papillae intact. Two vertical releasing incisions were given interdentally on the labial aspect of the involved tooth connecting the horizontal incisions and extending beyond the mucogingival junction. Subcrestal crevicular incision was given using BP blade No. 12/15 at the tooth of interest connecting horizontal and vertical incision. A full-thickness flap was elevated 3–4 mm beyond the marginal bone crest using blunt dissection and then partial thickness flap was extended apically into the vestibule using sharp dissection so that the flap would be easily repositioned as far coronally as needed. The buccal part of the intact papillae was de-epithelialized to act as a connective tissue recipient site for the coronally advanced repositioned flap. Root planing of exposed root surface was done using universal curette 2R/2L and or 4R/4L (Hu-Friedy). The recession defect was either treated with the formed autologous PRF membrane or freeze-dried, irradiated amnion membrane which was procured from tissue bank of Tata Memorial Hospital, Mumbai. The membrane was trimmed and placed to cover the bony recession defect extending from the CEJ to cover the adjacent bone mesially, distally, and apically by 2–3 mm. The buccal flap was coronally repositioned to cover the membrane, and the CAF was retained in position with nonabsorbable 4–0 braided silk [Figure 2].
Figure 2.
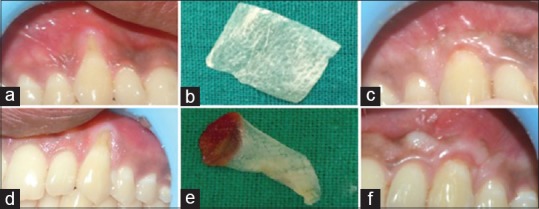
(a) Preoperative recession site for placement of amniotic membrane. (b) Amniotic membrane. (c) Postoperative site after 18 months. (d) Preoperative recession site for placement of PRF membrane. (e) Platelet-rich fibrin membrane. (f) Postoperative site after 18 months
Platelet-rich fibrin preparation
The PRF in the present study was prepared in accordance with the protocol developed by Choukroun et al. in 2001. Intravenous blood (by venipuncture of the antecubital vein) was collected in 10 ml sterile tube without anticoagulant and immediately centrifuged in centrifugation machine at 3000 revolutions/min (approximately: 400 g) for 10 min. After centrifugation, the resultant product formed consisted of three layers. The topmost layer consisted of acellular platelet-poor plasma (PPP), PRF clot was present in the middle, and RBCs were seen to be settled at the bottom of the test tube. PRF clot was easily separated from red corpuscles base (preserving a small red blood cell layer) using a sterile Tweezer and scissor just after removal of PPP. The clot was then transferred onto a sterilized gauge piece which was compressed between two sterilized glass slabs, to transform it to the shape of a membrane.[9]
Postoperative instructions
An extra-oral cold compress and analgesic plus anti-inflammatory drugs were given for pain and edema control. Patients were asked not to use a toothbrush in the surgical region for 4 weeks; instead, mouthwash was prescribed. Instructions were given to the patients to protect the surgical area from excessive trauma or traction. Sutures were removed after 10 days followed by evaluation at 6 months and 18 months. All data collected were statistically evaluated for the comparison of the outcome of the treatment.
Statistical analysis
The collected data at baseline, 6 months, and 18 months postoperative were tabulated and analyzed statistically. The software used for the statistical analysis were SPSS (Statistical; Package for Social Sciences) version 19.0 and GraphPad Quick Calcs Software (Online Software© 2012, Graph Pad Software Inc.) from IBM company, New York, USA. The statistical tests used were paired t-test, repeated measure analysis of variance test, Bonferroni test for intragroup comparison, and unpaired t-test for intergroup comparison between control and experimental groups.
Results
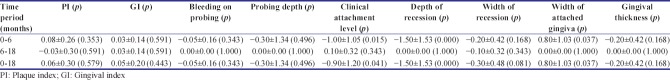
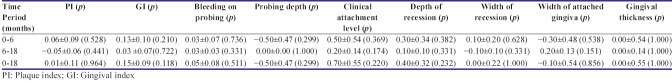
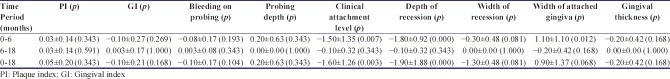
There was no significant difference between the two groups for the plaque, gingival, and bleeding on probing score [Tables 1-3]. No complication was experienced in the surgical sites and the healing was uneventful. The reduction in pocket depth and gain in clinical attachment level was also statistically insignificant from baseline to 6 months, baseline to 18 months, and 6–18 months. Gingival recession in both PRF and amnion group evaluated individually, significantly reduced from baseline to 6 months (P = 0.000) and from baseline to 18 months (P = 0.000). However, the mean value from 6 months to 18 months was statistically nonsignificant. On intergroup comparison of mean value of differences at all time period, the result was statistically nonsignificant. Similar nonsignificant results were obtained for width of recession, width of keratinized gingiva, and gingival thickness.
Table 1.
Intragroup comparison of mean values of all parameters at different time intervals in platelet-rich fibrin membrane group
Table 3.
Intergroup comparison of mean values of all parameter at different time intervals in platelet-rich fibrin and bioresorbable amniotic membrane group
Table 2.
Intragroup comparison of mean values of all parameter at different time intervals in bioresorbable amniotic membrane group
Discussion
The present randomized parallel mouth controlled trial was conducted to compare the relative effectiveness of two treatment modalities in the treatment of facial gingival recessions, namely, CAF along with PRF membrane and a CAF along with a bioresorbable AM. The ultimate goal of periodontal plastic surgical procedures utilized in the treatment of marginal tissue recession is the complete regeneration of all the supportive components of the periodontium, resulting in complete coverage of the denuded root surface in an esthetic as well as functional manner.
In the present study to treat the gingival recession, coronally positioned flap procedure is performed either with PRF membrane or AM. CAF is the first choice surgical technique, when there is the presence of adequate keratinized gingiva apical to the recession defect. Optimum root coverage results, good color blending of the treated area, and recuperation of the original morphology of the soft-tissue margin can be predictably accomplished. Past studies of Pini Prato et al.[11] and Wennström and Zucchelli[12] concluded that the mean root coverage obtained from this technique varies from 60% to 100% and this is one of the most commonly practiced techniques. This procedure, however, does not increase the width of the keratinized gingiva and provides little or no periodontal regeneration in gingival recession defects. To overcome the disadvantage of CAF, concept of guided tissue regeneration (GTR) was introduced for recession treatment along with coronally repositioned flap. Several meta-analytical studies demonstrated that addition of autogenous connective tissue to CAF is the “gold standard” means of root coverage with no antigenic response.[12] However, procurement of CT graft from second donor site increases the patient morbidity and also lengthens the duration of surgery. To overcome this drawback, numerous barrier membranes are commercially available based on GTR concept. Recently, clinical use of other resorbable allograft membranes for GTR have gained popularity with promising results and amends their use with the modern concept of biological GTR. According to Tinti et al.,[13] cryopreserved bioresorbable AM used in the present study was found effective in wound healing and epithelization as it helps in cellular adhesion of gingival cell, growth of fibroblast, and angiogenesis.[14,15,16] With the improvement of bioactive surgical additives to accelerate the therapeutic process is the mainstay in clinical research. In this sense, PRF appears as a natural and satisfactory alternative with encouraging results and low risks. Autologous PRF clot may be used as a membrane in the treatment of gingival recession with various degrees of success rate. PRF is a concentrated aggregate of the growth factors developed in France by Choukroun et al.[9] in 2001. This platelet concentrate contains PDGF, TGF, and many other unidentified growth factors that modulate factors involved in wound healing. The plaque, gingival, and bleeding on probing score were comparable between the two groups and there was no significant difference between the two groups. This observation of the present study indicates that patients maintained optimum level of hygiene throughout the study. Further, both AM and PRF membrane was well tolerated by the tissue with excellent tissue contour and color blend. When intergroup comparison of mean value of differences was done at baseline for pocket probing depth and CAL, the results were statistically insignificant. The reduction in pocket depth and gain in clinical attachment level was also statistically insignificant from baseline to 6 months, baseline to 18 months, and 6–18 months. These results are in accordance with the case report of Shetty et al.,[17] who reported 100% roots coverage, enhanced gingival biotypes with both the membrane. In the present study, gingival recession in PRF group significantly reduced from baseline to 6 months (P = 0.000) and from baseline to 18 months (P = 0.000). However, the mean value from 6 months to 18 months was statistically nonsignificant (P = 1.000). These results are in agreement with the studies of Padma et al.,[18] Jankovic et al.,[19] and Anilkumar et al.,[20] who stated that the satisfactory improvement in gingival recession may be attributed to the high percentage of undamaged platelets, contained within a fibrin matrix. The maintenance of stable gingival margin between 6 and 18 months in the present study was in accordance with the study by Gupta et al.[21] and Shepherd et al.,[22] who reported no change in mean recession coverage postoperatively between 2 and 4 months follow-up when PRP was used. This may suggest that platelet concentrates promote more rapid attachment to the tooth with the stable result. Furthermore, fibrin matrix in PRF functions such as fibrin glue, which maintains the flap in a constant position, enhances neovascularization, and reduces necrosis. In the present study gingival recession in Amnion group significantly reduced from baseline to 6 months (P = 0.000) and from baseline to 6 months (P = 0.000). However, the mean value from 6 months to 18 months was statistically nonsignificant (P = 0.343). These results are in the accordance to the study of Mehta et al.,[23] Shah et al.,[24] and Gurinsky,[25] who stated that processed dehydrated allograft amnion may provide an effective alternative to autograft tissue in the treatment of shallow-to moderate Miller Class I gingival recession defects. Thus, the authors concluded that the self-adherent nature of the amnion allograft significantly reduced surgical time and made the procedure easier to perform relative to techniques involving the use of autograft or allograft dermis tissue. Nonsignificant results were obtained in our study between 6 and 18 months suggest that bioresorbable AM self-adhesive property provided stable results as was stated by Velez et al.,[4] who analyzed the effects of cryopreserved bioresorbable AM on periodontal soft-tissue healing and observed that it was effective in helping cicatrization and reinforced adhesion. However, on intergroup comparison, the result was statistically nonsignificant. Furthermore, the results were stable even after 18 months postoperatively. This suggests that AM forms a physiologic closure with the host tissue impeding bacterial contamination and multiple studies support amnion's ability to decrease the host immunologic response through localized suppression of polymorph nuclear cell migration. Further, the thinness of amnion membrane resulted in better adaptation of the membrane over the recession site and consequently better coverage of the gingiva in accordance with the study of Agarwal et al.[26]
The intergroup comparison of mean value of differences at all time periods between the group for width of recession, width of keratinized gingiva, and gingival thickness was statistically nonsignificant. These results suggest comparable clinical efficacy of PRF and amnion membrane. These results are in accordance with the report of Shetty et al.,[17] who suggested increase in thickness of the keratinized tissues, reported in both groups, and might contribute to a long-term stable clinical outcome, with reduced probability of the recurrence of recession. Similar results were reported by Shah et al.,[24] who suggested enhancement of gingival biotype 6 months postoperatively after the treatment of gingival recession using amnion membrane. A thick biotype has a tendency toward maintaining a more stable soft tissue in various periodontal surgical procedures. Hence, all the optimum desired results as an allograft for root coverage were achieved by amnion allograft. A recent 6-month study evaluated the use of CAF + PRF against CAF + bioresorbable AM on gingival recession. The site treated with bioresorbable AM showed more stable results than the PRF-treated sites. Within the limitation of the study, use of the AM as an additive material alternate to subepithelial connective tissue in reducing the need for another surgical site and substitute to PRF in reducing the need for preparation of the autologous biomaterial is advocated. However, further testing is needed to confirm their long-term stability.
Conclusion
Soft-tissue maintenance is the primary line of defense in protecting the tissue from bacterial infection. Although the growth factors and the mechanisms involved are still poorly understood, the ease of applying PRF in the dental clinic and its beneficial outcome, including reduction of bleeding and rapid healing, holds promise for further procedures. The biomechanical GTR proposed herein, using the bioresorbable AM, not only maintains the structural and anatomical configuration of the regenerated tissues but also contributes to the enhancement of healing through reduction of postoperative scarring and subsequent loss of function and also provides a rich source of stem cells. The present study demonstrated that both CAF + PRF and CAF + AM are equally effective in providing clinically significant outcomes with respect to root coverage with AM showing better percentage of root coverage as compared to PRF.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.3rd ed. Chicago: American Academy of Periodontology; 1992. The American Academy of Periodontology. Glossary of Periodontal Terms. [Google Scholar]
- 2.Chambrone L, Chambrone D, Pustiglioni FE, Chambrone LA, Lima LA. Can subepithelial connective tissue grafts be considered the gold standard procedure in the treatment of miller class I and II recession-type defects? J Dent. 2008;36:659–71. doi: 10.1016/j.jdent.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 3.Niknejad H, Peirovi H, Jorjani M, Ahmadiani A, Ghanavi J, Seifalian AM, et al. Properties of the amniotic membrane for potential use in tissue engineering. Eur Cell Mater. 2008;15:88–99. doi: 10.22203/ecm.v015a07. [DOI] [PubMed] [Google Scholar]
- 4.Velez I, Parker WB, Siegel MA, Hernandez M. Cryopreserved amniotic membrane for modulation of periodontal soft tissue healing: A pilot study. J Periodontol. 2010;81:1797–804. doi: 10.1902/jop.2010.100060. [DOI] [PubMed] [Google Scholar]
- 5.Brian G. A novel dehydrated amnion allograft for use in the treatment of gingival recession: An observational case series. J Impact Adv Clin Dent. 2009;1:11–6. [Google Scholar]
- 6.Ross R, Glomset J, Kariya B, Harker L. A platelet-dependent serum factor that stimulates the proliferation of arterial smooth muscle cells in vitro . Proc Natl Acad Sci U S A. 1974;71:1207–10. doi: 10.1073/pnas.71.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gassling V, Douglas T, Warnke PH, Açil Y, Wiltfang J, Becker ST, et al. Platelet-rich fibrin membranes as scaffolds for periosteal tissue engineering. Clin Oral Implants Res. 2010;21:543–9. doi: 10.1111/j.1600-0501.2009.01900.x. [DOI] [PubMed] [Google Scholar]
- 8.Toffler M, Toscano M, Holtzclaw D, Corso MD, Ehrenfest DD. Introducing Choukroun's platelet rich fibrin (PRF) to the reconstructive surgery milieu. J Implant Clin Adv Dent. 2009;1:21–32. [Google Scholar]
- 9.Choukroun J, Adda F, Schoeffler C, Vervelle A. An opportunity in perioimplantology: The PRF. Implantodontie. 2001;42:55–62. [Google Scholar]
- 10.Norberg O. Localized gingival recessions: II. Treatment. J West Soc Periodontol Periodontal Abstr. 1977;25:10–21. [PubMed] [Google Scholar]
- 11.Pini Prato G, Rotundo R, Franceschi D, Cairo F, Cortellini P, Nieri M, et al. Fourteen-year outcomes of coronally advanced flap for root coverage: Follow-up from a randomized trial. J Clin Periodontol. 2011;38:715–20. doi: 10.1111/j.1600-051X.2011.01744.x. [DOI] [PubMed] [Google Scholar]
- 12.Wennström JL, Zucchelli G. Increased gingival dimensions. A significant factor for successful outcome of root coverage procedures? A 2-year prospective clinical study. J Clin Periodontol. 1996;23:770–7. doi: 10.1111/j.1600-051x.1996.tb00608.x. [DOI] [PubMed] [Google Scholar]
- 13.Tinti C, Vincenzi G, Cortellini P, Pini Prato G, Clauser C. Guided tissue regeneration in the treatment of human facial recession. A 12-case report. J Periodontol. 1992;63:554–60. doi: 10.1902/jop.1992.63.6.554. [DOI] [PubMed] [Google Scholar]
- 14.Bourne G. The foetal membranes. A review of the anatomy of normal amnion and chorion and some aspects of their function. Postgrad Med J. 1962;38:193–201. doi: 10.1136/pgmj.38.438.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kothari CR, Goudar G, Hallur N, Sikkerimath B, Gudi S, Kothari MC, et al. Use of amnion as a graft material in vestibuloplasty: A clinical study. Br J Oral Maxillofac Surg. 2012;50:545–9. doi: 10.1016/j.bjoms.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 16.Chakraborthy S, Sambashivaiah S, Kulal R, Bilchodmath S. Amnion and chorion allografts in combination with coronally advanced flap in the treatment of gingival recession: A Clinical study. J Clin Diagn Res. 2015;9:ZC98–101. doi: 10.7860/JCDR/2015/12971.6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shetty SS, Chatterjee A, Bose S. Bilateral multiple recession coverage with platelet-rich fibrin in comparison with amniotic membrane. J Indian Soc Periodontol. 2014;18:102–6. doi: 10.4103/0972-124X.128261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Padma R, Shilpa A, Kumar PA, Nagasri M, Kumar C, Sreedhar A, et al. A split mouth randomized controlled study to evaluate the adjunctive effect of platelet-rich fibrin to coronally advanced flap in miller's class-I and II recession defects. J Indian Soc Periodontol. 2013;17:631–6. doi: 10.4103/0972-124X.119281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jankovic S, Aleksic Z, Milinkovic I, Dimitrijevic B. The coronally advanced flap in combination with platelet-rich fibrin (PRF) and enamel matrix derivative in the treatment of gingival recession: A comparative study. Eur J Esthet Dent. 2010;5:260–73. [PubMed] [Google Scholar]
- 20.Anilkumar K, Geetha A, Umasudhakar, Ramakrishnan T, Vijayalakshmi R, Pameela E, et al. Platelet-rich-fibrin: A novel root coverage approach. J Indian Soc Periodontol. 2009;13:50–4. doi: 10.4103/0972-124X.51897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta S, Banthia R, Singh P, Banthia P, Raje S, Aggarwal N, et al. Clinical evaluation and comparison of the efficacy of coronally advanced flap alone and in combination with platelet rich fibrin membrane in the treatment of miller class I and II gingival recessions. Contemp Clin Dent. 2015;6:153–60. doi: 10.4103/0976-237X.156034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shepherd N, Greenwell H, Hill M, Vidal R, Scheetz JP. Root coverage using acellular dermal matrix and comparing a coronally positioned tunnel with and without platelet-rich plasma: A pilot study in humans. J Periodontol. 2009;80:397–404. doi: 10.1902/jop.2009.080438. [DOI] [PubMed] [Google Scholar]
- 23.Mehta TN, Mittal M, Mehta M, Hora BS. A novel dehydrated amnion allograft for use in the treatment of gingival recession: A case report. J Res Adv Dent. 2014;3:176–81. [Google Scholar]
- 24.Shah R, Sowmya NK, Mehta DS. Amnion membrane for coverage of gingival recession: A novel application. Contemp Clin Dent. 2014;5:293–5. doi: 10.4103/0976-237X.137900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gurinsky B. A novel dehydrated amnion allograft for use in the treatment of gingival recession: An observational case series. J Implant Adv Clin Dent. 2009;1:1–5. [Google Scholar]
- 26.Agarwal A, Gupta ND, Jain A. Platelet rich fibrin combined with decalcified freeze-dried bone allograft for the treatment of human intrabony periodontal defects: A randomized split mouth clinical trail. Acta Odontol Scand. 2016;74:36–43. doi: 10.3109/00016357.2015.1035672. [DOI] [PubMed] [Google Scholar]