Abstract
Background:
Innovating newer methods to diagnose a multifactorial disease such as periodontitis is always challenging for a clinician. Gingival crevicular fluid (GCF) which is closely associated with the periodontal tissue environment has been used a viable alternative to saliva for the diagnosis of periodontitis.
Aim:
The aim of the present study was to estimate and compare the interleukin-35 (IL-35) levels in GCF and serum among healthy, gingivitis, and chronic periodontitis (CP) individuals as well as to evaluate the effect of nonsurgical periodontal treatment (NSPT) on IL-35 level among patients with CP.
Settings and Design:
The study was conducted at the Department of Periodontics, Srirama Chandra Bhanja Dental College and Hospital, Cuttack, Odisha, India. It is a comparative study.
Materials and Methods:
A total of 60 participants were divided into healthy (Group I; n = 20), gingivitis (Group II; n = 20), and CP (Group IIIA; n = 20). GCF samples collected from each individual at baseline and 6 weeks after NSPT for Group III individuals (Group IIIB; n = 20) were quantified for IL-35 levels using enzyme-linked immunosorbent assay.
Statistical Analysis:
All analyses were performed using Shapiro–Wilk test, analysis of variance, Tukey's honestly significant difference post hoc test, and multiple regression analysis.
Results:
The mean IL-35 concentration in GCF was significantly high (P < 0.05) for Group IIIA (70.26 ± 4.0 pg/ml), as compared to Group I (54.81 ± 22.3 pg/ml) and Group IIIB (55.72 ± 10.2 pg/ml).
Conclusion:
In the present study, GCF and serum IL-35 concentration among CP individuals was highest among all the groups. Individuals receiving NSPT showed a significant reduction in IL-35 levels as compared to CP individuals.
Keywords: Cytokines, gingival crevicular fluid, nonsurgical periodontal therapy, periodontitis
Introduction
Periodontal disease is the most common form of bone pathology worldwide. Being a chronic inflammatory disorder, it causes destruction of tooth-supporting structures and also acts as a modifying factor of the systemic health of patients.[1] Although the initiation of the disease process is due to periodontopathogens present in the dental plaque,[2] the tissue destruction is mainly caused by the host response to the etiologic microorganism through a variety of inflammatory cytokines.[2,3,4,5]
Cytokines are a broad group of soluble factors that function in an autocrine or paracrine manner.[6] They play pivotal roles in coordinating activities of diverse immune cell types by coupling extracellular stimuli to intracellular signal transduction networks and mediate multiple physiological processes including differentiation, cell growth, and development of target cells.[6] They are produced by host immune-inflammatory cells such as T-helper (Th) cells and macrophages in response to the endotoxins produced by the pathogens. These Th cells are mainly Th1 and Th2 cells.[7] Cytokines produced by Th1 cells (interleukin [IL]-2, interferon gamma, IL-12, tumor necrosis factor-alpha) induce cell-mediated responses while cytokines produced by Th2 cells (IL-4, IL-13) induce antibody-mediated responses.[7,8,9]
IL-35 is a newer generation of signal molecule belonging to IL-12 cytokine family. It is produced by T-regulatory cells (Treg) consisting of α chain (p35) and β chain (Ebi3).[10] It is characterized by sharing of three α (p19, p28, p35) and two β (p40 and Ebi3) subunits.[10] Recent studies have shown that IL-35 is an anti-inflammatory cytokine which suppresses the immune response through the expansion of Treg and suppression of Th17 cell development.[11,12] This is suggestive of a possible role of IL-35 in chronic inflammatory disorder such as periodontitis. However, there is limited information regarding the exact mechanism.
In periodontal diagnostics, recently, there has been a steady growth in trends to develop tools for monitoring periodontitis. So far, diagnosis of periodontal disease relies primarily on clinical and radiographic parameters. These measures are useful in detecting evidence of past disease, or verifying periodontal health, but provide only limited information about patients and sites at risk for future periodontal breakdown. Numerous biomarkers in the saliva, gingival crevicular fluid (GCF) have been proposed and used as diagnostic tests for periodontal disease.
The aim of the present study was to estimate and compare the IL-35 levels in GCF and serum among healthy, gingivitis, and chronic periodontitis (CP) individuals and also to evaluate the effect of nonsurgical periodontal treatment (NSPT) on IL-35 levels among patients with CP to explore the possibility of using IL-35 as a biomarker for periodontal disease activity.
Materials and Methods
This was a comparative study conducted over a period of 6 months (October 2015 to March 2016); the study participants were selected from the outpatient Department of Periodontology, Srirama Chandra Bhanja Dental College and Hospital, Cuttack, India. A total of 60 subjects (31 males and 29 females, aged 25–60 years) were divided into three clinical groups, based on the periodontal parameters.
CP patients were diagnosed based on the criteria of American Academy of Periodontology classification of periodontal diseases (1999). The subjects for sampling were selected at random from individuals scheduled for a routine oral examination. Periodontal evaluation included recording of full-mouth gingival index (GI) score (Loe and Silness, 1963), probing pocket depth (PPD), and clinical attachment loss (CAL) using a graduated periodontal probe (University of North Carolina [UNC]-15 periodontal probe, Hu-Friedy, Chicago, IL, USA). All clinical measurements were performed by a single examiner (SCR). For intra-examiner calibration, 20 sites were examined twice, 24 h apart, before the commencement of the study. Calibration was accepted when 95% of differences were within 1.0 mm.
On the basis of GI score, PPD, and CAL measurements, participants were initially categorized into three groups each having 20 individuals (n = 20).
Group I: healthy individuals (GI = 0, PD < 3 mm, and CAL = 0)
Group II: Gingivitis (GI > 1, PD < 3 mm, and CAL = 0 mm)
Group III: CP (GI > 1, PD > 3 mm, CAL > 3 mm).
Subjects in Group III underwent NSPT, i.e., scaling and root planing using periodontal curettes (Gracey curettes; Hu-Friedy, Chicago, IL, USA), completed in two sessions within 24 h in accordance with the Quirynen's one-stage, full-mouth debridement protocol.[13]
Site selection and gingival crevicular fluid collection
In Group I, GCF was collected from multiple sites (3–5 sites per subject) to ensure the collection of an adequate amount of sample. For Group II and Group III, only one site per subject was selected having the greatest GI score or CAL, respectively, determined with the help of UNC-15 probe.
Sterile cotton rolls were used to clean and isolate the selected site. The supragingival plaque was removed gently using a Gracey curette (Universal Gracey curette #4R/4 L, Hu-Friedy, Chicago, IL, USA) so as to avoid contamination of the site. Three microliters of GCF was then collected using color-coded 1–5-μL calibrated volumetric microcapillary pipettes (Sigma-Aldrich Chemical Co. Ltd, St Louis, MO, USA) from the predetermined sites by intracrevicular method. The GCF collected was immediately transferred to a plastic vial and stored at −70°C, until the assay was performed. Microcapillary pipettes contaminated with blood and saliva were discarded.
For the subjects of Group III, sample collection was repeated 6 weeks after performing NSPT. For ease of understanding, the subjects of Group III have been referred to as Group IIIA at baseline and IIIB for recording data 6 weeks after the NSPT. The site selection and sample collection were done by an independent examiner (SMP).
Blood collection
Two milliliters of blood was collected from the antecubital fossa by venipuncture using a 20-gauge needle with 2-ml syringe and immediately transferred to the laboratory. The serum was prepared from the blood sample after 1 h when the blood was allowed to clot by centrifuging at 3000 × g for 5 min. The serum was immediately transferred to a plastic vial and stored at −70°C until the time of assay.[14]
Human interleukin-35 analysis
The GCF and serum samples were then assayed for IL-35 using enzyme-linked immunosorbent assay kit (Wuhan Fine Biological Technology Co., Ltd, China) according to the manufacturer's instructions by an examiner who was blinded to the groups allotted and was not involved in sample collection (KP). The concentrations of IL-35 in the tested samples were estimated using the reference calibrated standard curve, plotted using the optical density values of the standards (provided with the kit).
Statistical analysis
The statistical analysis was performed using statistical software (SPSS version 16). The level of significance was set to 0.05 for all statistical inferences. All continuous data were tested for normality by Shapiro–Wilk test before using inferential statistics. For comparison of two means, Student's t-test was used. For comparison of more than two means, analysis of variance (ANOVA) was used for normal data followed by Tukey's honestly significant difference post hoc test for results which was found to be significant. A multiple regression analysis was carried out to determine the strength of intergroup difference of IL-35 levels.
Results
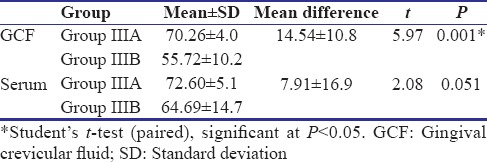
Of 247 patients screened for the study, 84 participants were recruited, and finally, data of 60 participants were subjected to statistical analysis [Figure 1]. Table 1 shows the descriptive statistics (mean ± standard deviation) of the study population. It was found that the mean IL-35 concentration in GCF as well as serum was highest in Group IIIA and least in Group I. Table 2 shows the results of ANOVA test carried out to find the equality of means of IL-35 concentration of GCF and serum between healthy, gingivitis, and CP individuals (Group I, II, and IIIA). It was seen that difference in mean was statistically significant among three groups only for the IL-35 concentration in GCF (P = 0.03). Subsequently, post hoc analysis was performed for pairwise comparisons between groups for IL-35 concentrations in GCF [Table 3]. It was found that IL-35 levels in GCF were significantly higher in Group IIIA as compared to Group I (P = 0.002). Multiple regression analysis was performed to find the strength of association for IL-35 levels in GCF among the four groups [Table 4]. It was found that individuals having periodontal disease are likely to have 17.283 times the level of IL-35 than healthy controls. Table 5 shows the results of Student's t-test (paired) carried out to compare the GCF and serum IL-35 concentration of CP group before and after NSPT (Group IIIA and Group IIIB). Difference in mean between these two group was statistically significant only for IL-35 levels in GCF concentration (P = 0.001).
Figure 1.
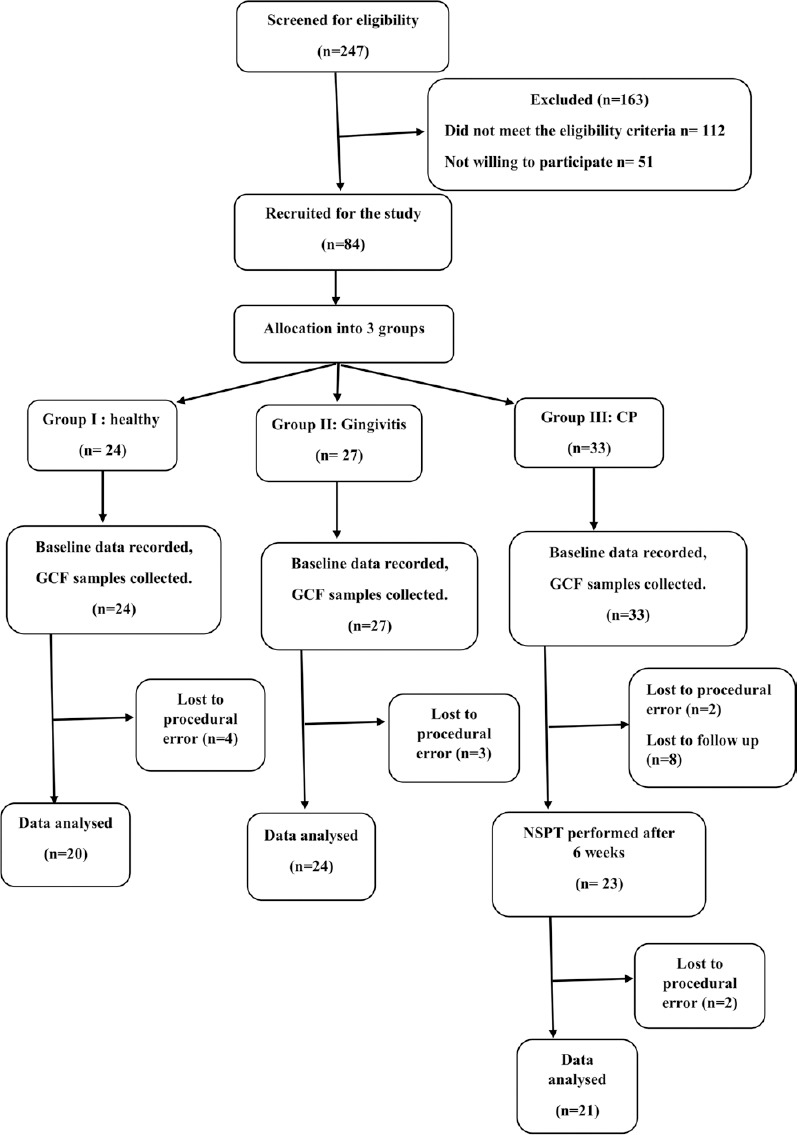
Flow diagram following patient recruitment and follow-up
Table 1.
Descriptive data for study population (mean±standard deviation)
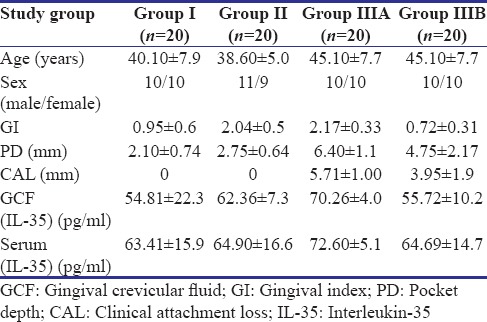
Table 2.
Comparison of mean gingival crevicular fluid interleukin-35 concentration (pg/ml) among study groups
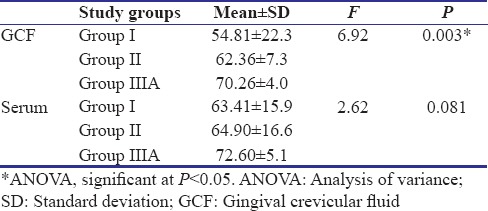
Table 3.
Post hoc analysis for comparison of mean gingival crevicular fluid Interleukin-35 concentration

Table 4.
Regression coefficients for effect of different study groups, age and gender on GCF (IL 35)
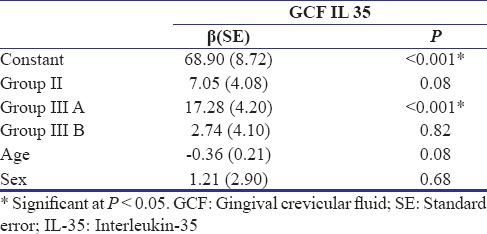
Table 5.
Comparison of level of interleukin-35 in gingival crevicular fluid and plasma (pg/ml)
Discussion
Biomarkers are defined as objective, quantifiable characteristics of biological process. They may not correspond to patient's clinical condition or his/her perception of the state of health.[15] However, they assess the underlying disease process with more accuracy. Hence, it is a commonplace in basic and clinical research to use biomarkers as a diagnostic tool and as well as for assessing prognosis. Such biomarkers, are of more relevance for inflammatory conditions like periodontitis as they assess the current disease activity and not just the cumulative tissue destruction.[16]
IL-35 is one such inflammatory biomarker. It is a suppressive cytokine integral to the negative feedback loops and tolerance-promoting pathways.[17] Its role in inflammatory disorders is being explored widely in recent times.
Role of IL-35 in periodontitis is a relatively unexplored area. One of the pioneering studies was done by Kalburgi et al. in 2013.[18] They compared IL-35 levels in gingival tissues of healthy controls and CP and aggressive periodontitis patients. The result showed that IL-35 mRNA was expressed in gingival tissues of all the three groups; CP exhibited highest IL-35 expression followed by aggressive periodontitis and healthy patients.[18]
In our study, we assessed the IL-35 levels in GCF of healthy controls and gingivitis and CP subjects; the results showed that the mean IL-35 concentration in GCF among CP subjects was highest followed by gingivitis and healthy subjects, while the posttreatment concentration was similar to healthy subjects.
In another study by Mitani et al., IL-35 production in GCF and their gene expression levels in human gingival tissue were investigated.[19] They reported that GCF from CP subjects had significantly higher IL-35 levels as compared to healthy participants, and the gene expression of EBI3, IL-12A mRNA in inflamed gingival tissue was significantly higher in healthy control tissues.[19]
A recent study by Köseoǧlu et al. aimed to compare the expression of IL-35 in plasma, saliva, and GCF among healthy, gingivitis, and CP subjects.[20] The CP subjects exhibited significantly higher volume of IL-35 in GCF as compared to gingivitis and healthy group. However, on comparing the concentration, it was found that levels of IL-35 in GCF were higher in healthy subjects than gingivitis and CP group. This discrepancy was attributed to the fact that GCF was collected with periopaper strips inserted in the gingival sulcus for 30 s. The amount of GCF collected in healthy subjects in the time interval was less than that in gingivitis and CP group. In our study, a standardized volume of GCF was collected from the subjects in all four groups as it can be a better indicator of the concentration of the cytokine in the GCF.[20]
Exact role of IL-35 in etiopathogenesis of periodontal disease is not clear, but it is reported to be secreted from Treg cells as negative feedback regulation.[17] According to Nakajima et al., there is an increase in expression of Treg cell in CP as compared to gingivitis and healthy subjects because these cells are recruited by immune system for containing the disease and arresting tissue destruction.[21] Therefore, reduction in inflammatory load reduces the expression of Treg cells; hence, IL-35 levels in GCF also decrease. This could explain the significant reduction in GCF IL-35 levels in CP patients 6 weeks after NSPT was performed in our study.
The mean plasma IL-35 concentration was found to be 63.4–72.6 pg/ml, which was similar to earlier reports.[20,22,23] In our study, the mean plasma concentration of IL-35 was highest in CP followed by gingivitis and healthy subjects; however, the difference was statistically not significant (P = 0.08). Further, the mean plasma concentration of IL-35 in CP subjects after NSPT was less compared to CP group, but the difference was not statistically significant (P = 0.051). This is similar to earlier reports by Köseoǧlu et al. They reported that periodontitis and gingivitis do not affect the plasma level of IL-35.[20]
In the present study, we found a predictable association between concentration of IL-35 and presence of periodontal inflammation. Multiple regression analysis showed a significant beta coefficient even after adjusting for age and gender, suggesting that GCF IL-35 can be considered as a predictable biomarker of established periodontal disease. However, the difference between mean GCF and serum IL-35 was not significant for gingivitis subjects, which suggests that IL-35 may not be a predictable marker in initial stages of periodontal disease.
This was the first study of its kind to estimate level of GCF and serum IL-35 in CP subjects after NSPT. However, IL-35 is relatively a newcomer among suppressive cytokines.[17] Estimation of other established cytokines such as transforming growth factor-β along with IL-35 may give a clearer picture of its position in etiopathogenesis of periodontitis. Further multicenter, longitudinal prospective studies should be carried out to affirm these findings and further validate the role of IL-35 in periodontal inflammation.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
We are particularly thankful to Dr. Hemamalini Rath, Associate Professor, Department of Public Health Dentistry, SCB Dental College, Cuttack and Dr. Gaurav Sharma, Senior Resident, Department of Public Health Dentistry, SCB Dental College, Cuttack for providing invaluable scientific advice and insights.
References
- 1.Tonetti MS, Claffey N European Workshop in Periodontology Group C. Advances in the progression of periodontitis and proposal of definitions of a periodontitis case and disease progression for use in risk factor research. Group C consensus report of the 5th European Workshop in Periodontology. J Clin Periodontol. 2005;32(Suppl 6):210–3. doi: 10.1111/j.1600-051X.2005.00822.x. [DOI] [PubMed] [Google Scholar]
- 2.Cekici A, Kantarci A, Hasturk H, Van Dyke TE. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontol 2000. 2014;64:57–80. doi: 10.1111/prd.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seymour GJ. Importance of the host response in the periodontium. J Clin Periodontol. 1991;18:421–6. doi: 10.1111/j.1600-051x.1991.tb02310.x. [DOI] [PubMed] [Google Scholar]
- 4.Ebersole JL, Kirakodu S, Novak MJ, Stromberg AJ, Shen S, Orraca L, et al. Cytokine gene expression profiles during initiation, progression and resolution of periodontitis. J Clin Periodontol. 2014;41:853–61. doi: 10.1111/jcpe.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graves DT. The potential role of chemokines and inflammatory cytokines in periodontal disease progression. Clin Infect Dis. 1999;28:482–90. doi: 10.1086/515178. [DOI] [PubMed] [Google Scholar]
- 6.Taniguchi T. Cytokine signaling through nonreceptor protein tyrosine kinases. Science. 1995;268:251–5. doi: 10.1126/science.7716517. [DOI] [PubMed] [Google Scholar]
- 7.Ohyama H, Kato-Kogoe N, Kuhara A, Nishimura F, Nakasho K, Yamanegi K, et al. The involvement of IL-23 and the Th17 pathway in periodontitis. J Dent Res. 2009;88:633–8. doi: 10.1177/0022034509339889. [DOI] [PubMed] [Google Scholar]
- 8.Carter LL, Dutton RW. Type 1 and type 2: A fundamental dichotomy for all T-cell subsets. Curr Opin Immunol. 1996;8:336–42. doi: 10.1016/s0952-7915(96)80122-1. [DOI] [PubMed] [Google Scholar]
- 9.Mosmann TR, Coffman RL. TH1 and TH2 cells: Different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–73. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 10.Jones L, Collison L, Vignali D. Interleukin-35 subunit dimerization and stability is unique among IL-12 cytokine family members (134.9) J Immunol. 2010;184(1 Suppl):134–9. [Google Scholar]
- 11.Li X, Mai J, Virtue A, Yin Y, Gong R, Sha X, et al. IL-35 is a novel responsive anti-inflammatory cytokine – A new system of categorizing anti-inflammatory cytokines. PLoS One. 2012;7:e33628. doi: 10.1371/journal.pone.0033628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niedbala W, Wei XQ, Cai B, Hueber AJ, Leung BP, McInnes IB, et al. IL-35 is a novel cytokine with therapeutic effects against collagen-induced arthritis through the expansion of regulatory T cells and suppression of Th17 cells. Eur J Immunol. 2007;37:3021–9. doi: 10.1002/eji.200737810. [DOI] [PubMed] [Google Scholar]
- 13.Quirynen M, Mongardini C, de Soete M, Pauwels M, Coucke W, van Eldere J, et al. The rôle of chlorhexidine in the one-stage full-mouth disinfection treatment of patients with advanced adult periodontitis. Long-term clinical and microbiological observations. J Clin Periodontol. 2000;27:578–89. doi: 10.1034/j.1600-051x.2000.027008578.x. [DOI] [PubMed] [Google Scholar]
- 14.Pradeep AR, Daisy H, Hadge P. Serum levels of monocyte chemoattractant protein-1 in periodontal health and disease. Cytokine. 2009;47:77–81. doi: 10.1016/j.cyto.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 15.Strimbu K, Tavel JA. What are biomarkers? Curr Opin HIV AIDS. 2010;5:463–6. doi: 10.1097/COH.0b013e32833ed177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taba M, Jr, Kinney J, Kim AS, Giannobile WV. Diagnostic biomarkers for oral and periodontal diseases. Dent Clin North Am. 2005;49:551–71. doi: 10.1016/j.cden.2005.03.009. vi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sawant DV, Hamilton K, Vignali DA. Interleukin-35: Expanding its job profile. J Interferon Cytokine Res. 2015;35:499–512. doi: 10.1089/jir.2015.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalburgi NB, Muley A, Shivaprasad BM, Koregol AC. Expression profile of IL-35 mRNA in gingiva of chronic periodontitis and aggressive periodontitis patients: A semiquantitative RT-PCR study. Dis Markers. 2013;35:819–23. doi: 10.1155/2013/489648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitani A, Niedbala W, Fujimura T, Mogi M, Miyamae S, Higuchi N, et al. Increased expression of interleukin (IL)-35 and IL-17, but not IL-27, in gingival tissues with chronic periodontitis. J Periodontol. 2015;86:301–9. doi: 10.1902/jop.2014.140293. [DOI] [PubMed] [Google Scholar]
- 20.Köseoǧlu S, Saǧlam M, Pekbaǧrıyanık T, Savran L, Sütçü R. Level of interleukin-35 in gingival crevicular fluid, saliva, and plasma in periodontal disease and health. J Periodontol. 2015;86:964–71. doi: 10.1902/jop.2015.140666. [DOI] [PubMed] [Google Scholar]
- 21.Nakajima T, Ueki-Maruyama K, Oda T, Ohsawa Y, Ito H, Seymour GJ, et al. Regulatory T-cells infiltrate periodontal disease tissues. J Dent Res. 2005;84:639–43. doi: 10.1177/154405910508400711. [DOI] [PubMed] [Google Scholar]
- 22.Gu X, Tian T, Zhang B, Liu Y, Yuan C, Shao L, et al. Elevated plasma interleukin-35 levels predict poor prognosis in patients with non-small cell lung cancer. Tumour Biol. 2015;36:2651–6. doi: 10.1007/s13277-014-2887-8. [DOI] [PubMed] [Google Scholar]
- 23.Wu H, Li P, Shao N, Ma J, Ji M, Sun X, et al. Aberrant expression of Treg-associated cytokine IL-35 along with IL-10 and TGF-β in acute myeloid leukemia. Oncol Lett. 2012;3:1119–23. doi: 10.3892/ol.2012.614. [DOI] [PMC free article] [PubMed] [Google Scholar]


