Abstract
Objective:
The present study investigated the therapeutic potential and underlying mechanisms of human umbilical cord mesenchymal stem cells (HUCMSCs) on joint cartilage destruction induced by monosodium iodoacetate (MIA) in mice.
Materials and Methods:
HUCMSCs were tested for mesenchymal stem cell (MSC) characteristics including surface markers by flow cytometry and mesoderm differentiation (adipogenesis, osteogenesis, and chondrogenesis). Terminal deoxynucleotidyl transferase dUTP nick end labeling assay and Western blot assay were used to evaluate MIA-induced chondrocyte apoptosis. In the in vivo study, 18 mice were divided into three groups (n = 6 each); normal saline (control), MIA-treated, and MIA-treated/HUCMSC-transplantation. Rota-Rods tests were used to evaluate MIA-induced cartilage destruction behaviors in mice. Histological changes in the mice cartilage were examined by immunohistochemistry.
Results:
HUCMSCs had an immunophenotype similar to bone marrow-derived MSCs and were able to differentiate into adipocytes, osteocytes, and chondrocytes. Conditioned medium of the HUCMSCs exhibited an anti-apoptotic effect and inhibited expression of caspase 3 in MIA-treated chondrocytes. HUCMSC transplantation assisted in recovery from movement impairment (from 30% on day 7 to 115% on day 14) and in regeneration and repair of cartilage damaged by MIA. (International Cartilage Repair Society score: 3.8 in the MIA group vs. 10.2 in the HUCMSC-treated group); HUCMSC transplantation ameliorated cartilage apoptosis through the caspase 3 pathway in MIA-induced cartilage destruction in mice.
Conclusion:
Taken together, these observations suggest that HUCMSC transplantation appears to be effective in protecting cartilage from MIA damage.
KEYWORDS: Apoptosis, Capase3, Human umbilical cord stromal cells, Monosodium iodoacetate, Osteoarthritis
INTRODUCTION
Osteoarthritis (OA) is a common chronic degenerative joint disorder involving mostly the weight-bearing joints such as the knees and hips. More than 10% of American adults have clinical OA, and it has become the fourth most common cause of hospitalization and the most common cause of total knee and hip joint replacement surgeries [1]. Osteochondral transplantation, autologous perichondrial and periosteal grafts, and autologous chondrocyte implantation, however, may not be applicable in OA because the cartilage defects are often too large [2,3,4].
Human umbilical cord mesenchymal stem cells (HUCMSCs) have emerged as a source of mesenchymal stem cells (MSCs), and they have many advantages over bone marrow-derived MSCs (BMSCs). The harvesting procedure is noninvasive, and umbilical cords are discarded tissues and in abundant supply [5,6]. In addition, HUCMSCs strongly express MSC surface markers similar to BMSCs. HUCMSCs are negative for hematopoietic markers such as cluster of differentiation (CD) 34 and CD45 [6,7,8,9]. Furthermore, HUCMSCs are capable of differentiating into mesenchymal lineages, and the number of fibroblast colony-forming units is significantly higher in HUCMSCs than in BMSCs [10]. HUCMSCs are capable of chondrogenic differentiation in vitro [8,11,12,13,14] and in vivo [15] even better than BMSCs [16]. HUCMSCs also have anti-apoptotic potential [17,18,19]. These findings indicate that HUCMSCs may be a potential stem cell source for OA therapy [20,21,22,23,24]. It has not yet been clarified if HUCMSC transplantation assists in recovery from cartilage damage and movement disorders in monosodium iodoacetate (MIA)-induced OA.
HUCMSCs support the expansion of hematopoietic stem cells and are well-tolerated by the immune system [25]. HUCMSCs survived 5 months after injection in an immunogenic rat model [26,27]. We previously showed that HUCMSCs possess human leukocyte antigen-G molecules and may have immunosuppressive characteristics [28].
Intra-articular injection of MIA is widely employed to induce OA-like lesions in the knee [29,30,31,32], which are similar to the pathologic changes of OA in humans [29]. MIA disrupts glycolysis by inhibiting glyceraldehyde-3-phosphate dehydrogenase, subsequently causing chondrocyte death in vitro and in vivo [33]. By modifying MIA concentrations, the MIA-induced OA model holds the great advantage of easy modulation of the progression and severity of articular lesions [29]. In addition, MIA can quickly induce pain-like responses in the rat, and the level of pain can be controlled by different dosages [34,35].
In the present study, the effects of intra-articular injection of HUCMSCs on MIA-induced cartilage destruction in mice were assessed. Histological analyses and behavioral tests were applied to evaluate joint structure and pain behavior, respectively. The underlying mechanism involving apoptosis was also investigated.
MATERIALS AND METHODS
Isolation and expansion of human umbilical cord mesenchymal stem cells
The protocols for the procurement and use of human umbilical cords were approved by the Institutional Review Board of Buddhist Tzu Chi General Hospital (IRB 100-166). The study was conducted in accordance with the Declaration of Helsinki and was approved by the local ethics committee of the institution. Informed written consent was obtained from all patients before their enrollment in this study.
The detailed derivation protocol of HUCMSCs has been reported previously with modifications [6,8,36]. Briefly, one human umbilical cord sample (20 cm in length, 20 g in weight) was collected in sterile boxes containing Hanks' balanced salt solution (Gibco/BRL 14185-052, Grand Island, NY, USA), and separation of Wharton's jelly (WJ) from the vessels and amniotic membrane was conducted within 24 h. The human umbilical cord was washed three times with Ca2+ and Mg2+-free phosphate-buffered saline (PBS, Biowest, Nuaille, France). It was then cut using scissors in a midline direction, and the vessels of the umbilical artery, vein, and outlining membrane were dissociated from WJ. The jelly was then cut into pieces smaller than 0.5 cm3, treated with collagenase type I (Sigma, St Louis, MO, USA), and incubated for 14–18 h at 37°C in a 95% air/5% CO2 humidified atmosphere. The explants were then cultured in low-glucose Dulbecco's Modified Eagle's Medium (DMEM-LG) (Sigma) containing 10% fetal bovine serum (FBS, Biological Industries, Kibbutz Beit Haemek, Israel) and antibiotics at 37°C in a 95% air/5% CO2 humidified atmosphere. They were left undisturbed for 5–7 days to allow for migration of cells from the explants. The resulting HUCMSCs were designated as passage 1. The medium was changed twice a week. Cells were passaged when 90% of confluence was reached.
Flow cytometry
Surface molecules of HUCMSCs cultured on the third or fourth passage were characterized by flow cytometry. The cells were detached using Accutase (Millipore, Billerica, MA, USA) in PBS, washed with PBS containing 2% bovine serum albumin (Sigma) and 0.1% sodium azide (Sigma), and incubated with the respective antibodies conjugated with fluorescein isothiocyanate or phycoerythrin, including CD29, CD34, CD44, CD45, CD90, CD105, HLA-ABC, and HLA-DR (BD PharMingen, Franklin Lakes, NJ, USA). The cells were then analyzed using a flow cytometer (Becton Dickinson, San Jose, CA, USA).
Induction of adipogenesis
A total of 5 × 104 HUCMSCs were seeded onto a 12-well plate with adipogenic medium (DMEM supplemented with 10% FBS, 5 μg/mL insulin, 0.5 mmol/L isobutylmethylxanthine, 1 μmol/L dexamethasone, and 60 μmol/L indomethacin [all compounds purchased from Sigma]). The HUCMSCs was grown in adipogenic medium for 14 days, and the medium was changed every 3 days. After 14 days of differentiation, the differentiated adipocytes were stained with oil red O (Sigma) and photographed.
Induction of osteogenesis
A total of 1 × 104 HUCMSCs were seeded into each one well of a 12-well plate with osteogenic medium (DMEM supplemented with 10% FBS, 0.1 μmol/L dexamethasone, 10 mmol/L β-glycerol phosphate, and 50 μmol/L ascorbic acid). The medium was changed every 3 days. Following differentiation for 14 days, the osteocytes were stained with Alizarin red (Sigma) and photographed.
Micromass method (pellet) of chondrogenesis
For chondrogenesis assays, micromass cultures were established. HUCMSCs were seeded in a total volume of 30 μL onto the bottom of dry 15-mL test tubes (BD Pharmingen) at a density of 25 × 106 cells/mL. The plate was placed in a humidified CO2 incubator at 37°C for 2 h and additional chondrogenic medium (0.75 mL) was added to each tube. The media were changed every 48 h. Micromass (mm) cartilages were formed and retrieved after 3 weeks of culturing. After being photographed, the micromass cartilages were fixed in 4% paraformaldehyde at 4°C for 24 h. They were then washed in PBS, transferred to 70% ethanol, and processed for histology. The paraffin sections (5 μm) were assessed for cartilage by aggrecan (1:100, GeneTex, Irvine CA, USA) immunohistochemistry (IHC) staining.
Human umbilical cord mesenchymal stem cell-condition medium collection
HUCMSC-conditioned medium (HUCMSC-CM) was generated as follows: 80% confluent, passage 4–6 HUCMSCs in a 15-cm culture dish were washed 3 times with PBS and transferred to a serum-free DMEM-LG (Sigma) culture medium for 48 h. CM from different dishes was harvested and pooled.
Human chondrocyte derivation
The protocols for the procurement and use of human chondrocytes were approved by the Institutional Review Board of Buddhist Tzu Chi General Hospital (IRB 104-158-A).
Chondrocytes were collected from the knee cartilage of two male donors, 67 and 70 years old, who were undergoing total knee replacement for OA. Cartilage fragments were minced into 1 mm3 pieces and digested with type II collagenase (0.1%, Worthington, Lakewood, NJ, USA) solution overnight at 37°C. The digested contents were then filtered through a 100-μm filter and washed with PBS. The isolated chondrocytes were then plated at 5000 cells per cm2 and grown to confluence with DMEM: F12 (Gibco) containing 2 mM L-glutamine and 10% FBS (Gibco), 1X penicillin/streptomycin, 50 μg/mL ascorbic acid, and 0.1 M nonessential amino acids (Gibco, Invitrogen, Grand Island, NY, USA).
Monosodium iodoacetate-induced chondrocyte apoptosis in vitro experiment
The experimental conditions for chondrocyte treatment were divided into four groups, those chondrocytes without CM and MIA, those with CM but no MIA, those with both CM and MIA, and those with no CM but with MIA. In the MIA treatment group, MIA (conc. 0.01 mg/mL, Sigma) was used to treat chondrocytes for 1 h and then washed off with PBS three times and replaced with normal growth medium or with HUCMSC-CM for 24 h. After 24 h, XTT was used for chondrocyte proliferation. The apoptosis of chondrocytes was evaluated by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay. The cell lysates of chondrocytes were processed for Western blot analysis.
XTT assay
Chondrocytes were plated in a 96-well microtiter plate at a density of 2 × 103 cells per well in a final volume of 100 μl of DMEM/F12 (1:1). After treatment with the above four conditions, the cells were incubated with XTT solution (Biological Industries), 150 μL for 3 h at 37°C in accordance with the manufacturer's instructions. The absorbance was read at 450 nm in a microplate reader (Bio-Rad Model 3550, Hercules, CA, USA). Growth curves expressed by the optical density values were constructed.
Transferase dUTP nick end labeling assay
Apoptotic cells of chondrocytes were detected with the Click-iT Plus TUNEL Assay Kit (Life Technologies, Waltham, MA, USA) in accordance with the manufacturer's instructions. The percentage of TUNEL-positive cells was calculated as the number of TUNEL-positive cells divided by the total number of DAPI-positive cells in three non-overlapping areas (2 mm2 per well).
Western blot
The cell lysates of the chondrocytes were then loaded onto a 5%–20% gradient sodium dodecyl sulfate-polyacrylamide gel, subjected to electrophoresis under reducing conditions, and blotted onto a polyvinylidene difluoride membrane (Bio-Rad). The blots were blocked with a solution of 3% nonfat dry milk/2PBS/0.1% Tween-20 at room temperature, rinsed twice with PBS/0.1% Tween-20, and incubated with 1:200 diluted polyclonal anti-caspase 3 or anti-cleaved caspase 3, 8, and 9 antibodies (St. John's Lab, London, UK), followed by 1:5000 diluted anti-rabbit immunoglobulin G horseradish peroxidase (HRP) (Amersham GE, Taipei, Taiwan). Detection of actin by anti-actin antibodies (Santa Cruz, Dallas, TX, USA) was utilized as a loading control. Membranes were rinsed three times in PBS/0.1% Tween-20. Signals were detected with HRP using an electrochemiluminescence kit (Promega, Fitchburg, WI, USA). The intensities of cleaved caspase 3 were quantified using ImageJ processing [37].
Monosodium iodoacetate-induced osteoarthritis in mice
The Institutional Animal Care and Use Committee of Buddhist Tzu Chi General Hospital approved the animal experiments in this study. All experiments with animals were performed using relevant guidelines and regulations. Female mice (6–8 weeks old, weighing 18–22 g) were used to examine the cartilage repair effect of HUCMSCs in vivo. To prevent HUCMSC rejection by mice, nonobese diabetic-severe combined immune deficiency (NOD-SCID [strain name: NOD.CB17-Prkdcscid/JTcu]) mice obtained from Tzu Chi University were chosen for the experiment. The mice were divided into three groups: those injected with normal saline only (control group, n = 6); MIA-injected mice without HUCMSC transplantation (MIA group, n = 6); MIA-injected mice with HUCMSC transplantation (HUCMSC group, n = 6). For MIA-induced arthritis, adult NOD-SCID mice were subjected to a single intra-articular injection of MIA (Sigma) or normal saline (0.9%, control group) through the infrapatellar ligament of both knees of the hind legs. The MIA was dissolved in normal saline and administered in a volume of 10 μL using a 30-gauge needle (BD Pharmingen), at a dose of 0.1 mg per joint in mice. In the control mice, both knees were injected with 10 μL of normal saline. Seven days after MIA injection, the mice that developed significant movement disabilities were prepared for further studies [38].
Human umbilical cord mesenchymal stem cell transplantation
Seven days after MIA injection, the mice in the HUCMSC group were anesthetized with intraperitoneal injections of ketamine (50 mg/kg) and xylazine (15 mg/kg). A single intra-articular injection of 1 × 105 undifferentiated HUCMSCs in 50 μL normal saline was then given through the infrapatellar ligament of both knees of the hind legs. The mice were allowed to move and eat freely in their cages after HUCMSC transplantation.
Behavior assessments
Testing was performed during the light phase and at 7-day intervals after induction of OA. For at least 30 min before testing, all animals were allowed to habituate to testing conditions. For behavior assessments, the mice were subjected to forced ambulation (Rota-Rod test). Before the study, all the mice received 3 days of training on a Rota-Rod system (3376-4R, TSE Systems, Chesterfield, MO, USA) and those that passed were included in this study. Behavior assessments were performed for all groups on days 1, 7, 14, and 21 after MIA or normal saline injection. For the HUCMSC group, functional assessments were performed before HUCMSC transplantation. The Rota-Rod test was then repeated five times for each transplanted mouse on days 0, 7, 14, 28, and 35. The results from days 7, 14, 28, and 35 were then compared with the mean duration on day 0 in each mouse. The time each mouse remained on the rotating bar was recorded for a maximum period of 1200 s per trial. The speed was set at 20 rpm. Data were presented as the meantime on the rotating bar over five test trials.
Tissue harvesting
After the mice were euthanized on day 35, the joint surfaces were grossly examined. The distal femoral and the proximal tibial plateau were removed. After fixation with 10% buffered formalin (Sigma) for 48 h, the specimens were decalcified with 10% ethylenediaminetetraacetic acid (Gibco) for 2 weeks and cut into four pieces. All pieces were embedded in paraffin. Serial sagittal sections were prepared and stained with hematoxylin and eosin (H and E) (Sigma) and toluidine blue (Sigma). Histological changes were directly observed under microscope.
Histological evaluation
The sections were examined and evaluated in a blinded fashion using the International Cartilage Repair Society (ICRS) scoring system described previously [39]. The surface, matrix, cell distribution, cell population viability, subchondral bone, and cartilage mineralization were evaluated. Scores were given in these six categories with possible total scores of 0–18 with higher scores indicating better function. Two independent researchers evaluated the scores without being aware of any other information. The scores were then completed and averaged.
Immunohistochemical staining
Anti-type II collagen, aggrecan, and caspase 3 monoclonal antibody (1: 100, GeneTex), were used for IHC. A diaminobenzidine tetrahydrochloride substrate was used after incubation with a HRP-linked secondary antibody to detect reactivity. Photographs of the stained sections were recorded by a light microscope (Nikon TE2000-U fitted with a digital camera [Nikon DXM1200F], Nikon, Tokyo, Japan). The intensities of type II collagen, aggrecan, and caspase 3 were quantified using ImageJ processing [40].
Statistical analysis
The results were expressed as mean ± standard error of the mean. Raw data from the Rota-Rod duration and histological scores were analyzed using one-way repeated measures ANOVA and ANOVA with the post hoc test with Fisher's least significant difference, where a P < 0.05 denotes statistical significance.
RESULTS
Human umbilical cord mesenchymal stem cells exhibited mesenchymal stem cell characteristics and differentiation capability
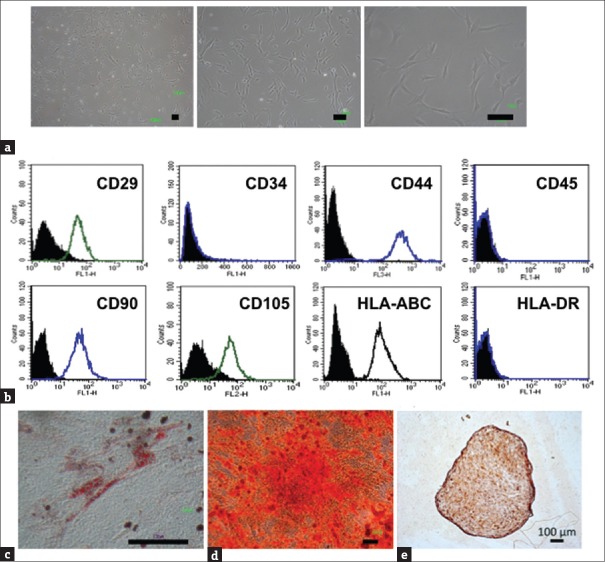
To investigate the MSC characteristics of HUCMSCs, morphology, surface markers, and differentiation capability were evaluated. HUCMSCs isolated from human umbilical cord stroma were characterized by fibroblastic morphology [Figure 1a] and flow cytometry analysis [Figure 1b]. The HUCMSCs were negative for CD34, CD45, and HLA-DR and positive for CD29, CD44, CD90, CD105, and HLA-ABC. Following induction of differentiation, the HUCMSCs readily differentiated into fat, bone, and cartilage. By 14 days postinduction in adipogenic and osteogenic conditions, the differentiated HUCMSCs showed large, oil red O-positive lipid droplets within the cytoplasm [Figure 1c] and became positive for Alizarin red staining with a change of cell morphology to a cuboid shape [Figure 1d]. The HUCMSCs conglobulated into a 3D pallet after chondrogenic induction for 21 days and became positive for aggrecan staining [Figure 1e]. These findings indicated that HUCMSCs could differentiate into adipocytes, osteocytes, and chondrocytes.
Figure 1.
Characterization and mesoderm differentiation of human umbilical cord mesenchymal stem cells. (a) Fibroblastic morphology of human umbilical cord mesenchymal stem cells with different degrees of magnification. (b) Representative flow cytometry histograms of human umbilical cord mesenchymal stem cells at passage 3 were negative for CD34, CD45, and HLA-DR but positive for CD29, CD44, CD90, CD105, and HLA-ABC. (c) After culture in adipogenic media (Dulbecco's Modified Eagle's Medium supplemented with 10% fetal bovine serum, 5 ug/mL insulin, 0.5 mmol/L isobutylmethylxanthine, 1 μmol/L dexamethasone, and 60 μmol/L indomethacin) for 14 days, adipogenesis of human umbilical cord mesenchymal stem cells was positive with Oil Red staining. (d) After culture in osteogenic media for 14 days, osteogenesis of human umbilical cord mesenchymal stem cells was positive with Alizarin red staining. (e) After 21 days of chondrogenesis, the formatted 3D pallet showed positive aggrecan staining. Scale bar = 100 μm
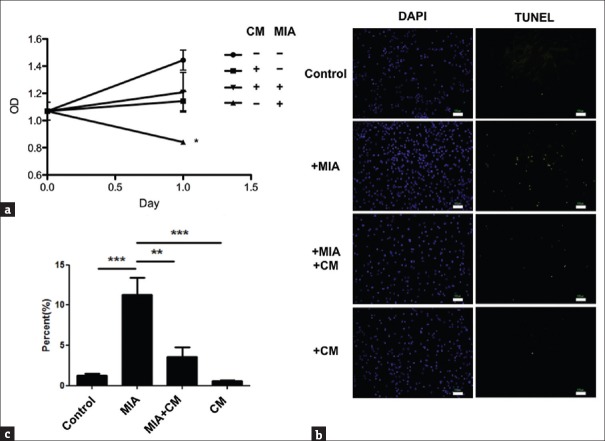
Monosodium iodoacetate-treated chondrocytes recovered from impaired proliferation and increased apoptosis in human umbilical cord mesenchymal stem cells- conditioned medium in vitro
The viability of primary cultured human chondrocytes in vitro was evaluated to determine the effects of MIA. Proliferation of these chondrocytes was impaired after treatment with MIA for 1 h, but this impairment was partially reduced in the HUCMSC-CM (P< 0.05) [Figure 2a]. Findings suggested that HUCMSCs could assist MIA-treated chondrocytes to recover from impaired proliferation. Evaluation was done to determine if MIA-induced impairment of cell proliferation was caused by apoptosis. After MIA treatment for 1 h, the chondrocytes were switched into normal growth media (control) or HUCMSC-CM for 24 h. The percentage of apoptotic cells in each group was calculated [Figure 2b]. The percentage of apoptotic cells reached 12% in the MIA treatment group compared with <5% in the control (P < 0.001) or HUCMSC-CM groups (P < 0.01) [Figure 2c]. Findings suggest that HUCMSCs can assist MIA-treated chondrocytes to recover from the increased apoptosis.
Figure 2.
Effect of human umbilical cord mesenchymal stem cell-conditioned medium on proliferation and anti-apoptosis of monosodium iodoacetate-treated chondrocytes. (a) After treatment with human umbilical cord mesenchymal stem cell-conditioned medium for 24 h, the 0.01 mg/mL monosodium iodoacetate-induced detrimental effect on chondrocyte proliferation was ameliorated. *P < 0.05. OD: optical density. (b) Apoptosis in monosodium iodoacetate-treated chondrocytes with or without adding human umbilical cord mesenchymal stem cell-conditioned medium. Apoptotic cells were examined by transferase dUTP nick end labeling assays and visualized as green spots. (c) The ratio of transferase dUTP nick end labeling-positive cells to DAPI-positive chondrocytes (expressed as percentages) with or without adding human umbilical cord mesenchymal stem cell-conditioned medium. The results were from three independent experiments and expressed as mean ± standard deviation, **P < 0.01, ***P < 0.001. Scale bar = 100 μm
Monosodium iodoacetate-enhanced caspase 3 expression was decreased in human umbilical cord mesenchymal stem cell-conditioned medium
To clarify whether MIA could induce apoptosis via caspase 3 expression, the effects of MIA (0.01 mg/ml) on caspase 3 expression were examined by Western blot [Figure 3a]. A greater increase in caspase 3 [Figure 3b] expression was observed in the MIA-treated group compared with control media or HUCMSCs-CM group (P < 0.05). Findings suggest that MIA-induced apoptosis was mediated through caspase 3 signaling pathways and was decreased by the treatment with HUCMSCs-CM.
Figure 3.
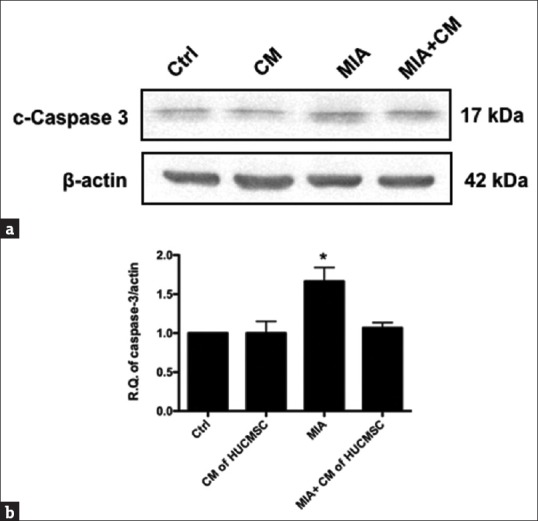
Changes in levels of cleaved caspase 3 in the chondrocytes in the four groups. The chondrocytes were treated with monosodium iodoacetate for 20 min with or without adding human umbilical cord mesenchymal stem cell-conditioned medium for 24 h. After 24 h, the levels of cleaved caspase 3 proteins were examined using Western blotting. (a) Representative Western blots for the expression of caspase 3 proteins in chondrocytes. The expression was increased in the monosodium iodoacetate-treated (b) but effectively decreased in the human umbilical cord mesenchymal stem cell-conditioned medium-treated chondrocytes (P < 0.05). The experiments were repeated in triplicate
Movement impairment in monosodium iodoacetate- induced osteoarthritis mice could be attenuated by human umbilical cord mesenchymal stem cell transplantation
Based on the above results [Figures 2 and 3] showing that protection can be offered by HUCMSC-CM against MIA-induced chondrocyte apoptosis in vitro, the therapeutic effect of HUCMSC transplantation was evaluated in MIA-induced OA mice in vivo. To retain human cells in mice without rejection (although HUCMSCs have immunomodulation ability), NOD-SCID mice (n = 6 in each group) were chosen for the experiment. Movement impairment in the MIA-induced mice was evaluated by the Rota-Rod test.Figure 4 demonstrates that on day 7 after MIA intra-articular injection, there was a significant impairment in running duration in the NOD/SCID mice (MIA-treated group) compared with the control group (P < 0.05). Running durations in the Rota-Rod test were evaluated after MIA-treatment. Compared with day 0, the duration in the MIA group significantly dropped to 36% on day 7, 40% on day 14, 30% on day 21, 40% on day 28, and 20% on day 35. In contrast, the durations in the HUCMSC transplantation group were significantly improved to 115% on day 14, 90% on day 21, 100% on day 28, and 111% on day 35 compared with day 0 (P < 0.001). These data were not significantly different from those of the control group, in which the running durations were 107% on day 14, 111% on day 21, 107% on day 28, and 112% on day 35 [Figure 4]. These results demonstrated improvement in the running duration of the HUCMSC transplantation group. This may further suggest reduction in mechanical pain due to improvement in the knee joint with HUCMSC transplantation.
Figure 4.
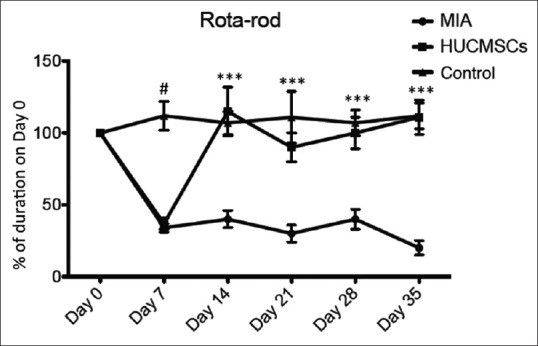
Rota-Rod test performance in mice in the control (injection of normal saline), monosodium iodoacetate (0.1 mg)-injected and human umbilical cord mesenchymal stem cell-transplanted groups (n = 6, each group). The Rota-Rod test was repeated five times each day on days 0, 7, 14, 28, and 35. The results from days 7, 14, 28, and 35 were then compared with the mean duration on day 0 in each mouse. The human umbilical cord mesenchymal stem cell-transplanted mice showed significantly different durations from the monosodium iodoacetate-injected mice without human umbilical cord mesenchymal stem cell transplantation. Motor performance was expressed as a percentage of the duration on day 0. ***P < 0.001, the control and human umbilical cord mesenchymal stem cell groups versus the monosodium iodoacetate group; #P < 0.05, the control group versus the monosodium iodoacetate group
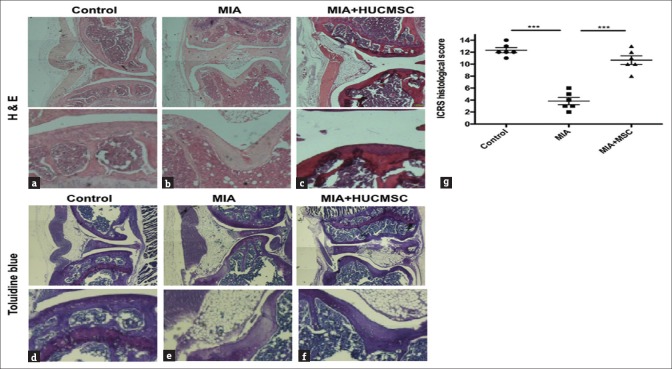
Histological evidence of repair with human umbilical cord mesenchymal stem cell transplantation in monosodium iodoacetate-treated mice
The effects of HUCMSC transplantation on cartilage damage in the MIA-treated OA mice were evaluated by histology and IHC. On day 35 after HUCMSC transplantation, all mice were sacrificed for examination of knee joints. H and E staining showed greater cell loss in both knee joints in the MIA-treated group [Figure 5b] compared with the control [Figure 5a] or HUCMSC transplantation group [Figure 5c]. Toluidine blue staining for cartilage glycosaminoglycans (GAGs) [41] was also markedly more reduced in the MIA-treated group compared with the control and HUCMSC transplantation groups [Figure 5d–f]. To quantify the histological changes in the cartilage, the ICRS grading system was used for comparison [39]. There were higher histological scores in the HUCMSC transplantation group (10.2) than in the MIA-treated group (3.8, P < 0.001) [Figure 5g]. In conclusion, this histological and IHC evidence indicates that HUCMSC transplantation can reduce cell and GAG loss in MIA-treated mouse cartilage.
Figure 5.
Histological changes in the hind knee joints treated with normal saline (control) (a and d), 0.1 mg monosodium iodoacetate (b and e), or 0.1 mg monosodium iodoacetate plus human umbilical cord mesenchymal stem cell transplantation (c and f) at 28 days following normal saline or monosodium iodoacetate injection. The upper panel (a-c) presents H and E staining and the lower panel (d-f) presents toluidine blue staining. Scale bar = 100 μm. There was greater cell loss in the monosodium iodoacetate-injected knees. (d) Histological scores of knee joints of experimental mice. The graph (g) depicts the histological scoring for the six categories in the International Cartilage Repair Society scoring system in the control, monosodium iodoacetate, and human umbilical cord mesenchymal stem cell groups (n = 6 in each group). Comparisons between groups were performed using the one-way ANOVA test. *P < 0.05, **P < 0.01
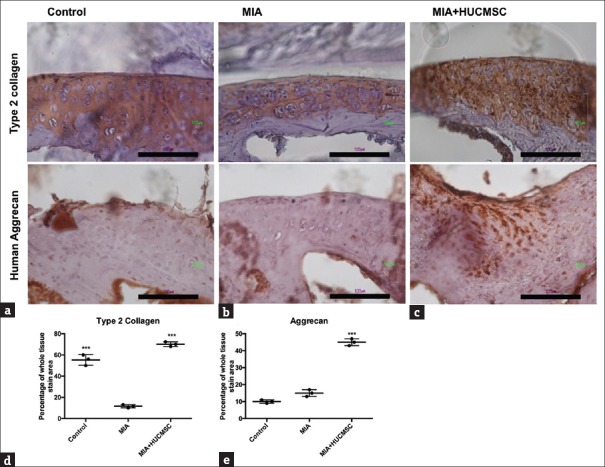
Increased expression of Type II collagen and aggrecan in the cartilage after human umbilical cord mesenchymal stem cell transplantation
To investigate whether HUCMSCs can protect cartilage in vivo, immunostaining of type II collagen and aggrecan [Figure 6] was performed. The cartilage of the MIA group showed weak staining for type II collagen and aggrecan, indicating minimal production of hyaline cartilage. In contrast, the cartilage of the HUCMSC-transplanted group showed a more even distribution and expanding darker staining, indicating the presence of hyaline cartilage in the regenerated tissue. The morphology of cartilage in the HUCMSC-transplanted group was the same as in the control group. Taken together, these results indicated that HUCMSCs could assist in the regeneration of hyaline cartilage and/or repair of hyaline cartilage damage in MIA-treated mice. The amounts of type II collagen [Figure 6d] and aggrecan [Figure 6e] in the HUCMSC-transplanted group were significantly higher than in the MIA-treated group (P < 0.001).
Figure 6.
Immunohistochemistry of the joint cartilage of mice in the (a) control group, (b) monosodium iodoacetate only group, and (c) monosodium iodoacetate + human umbilical cord mesenchymal stem cell group. At 35 days after the experiment, the mice were killed under anesthesia and the knees were removed for staining for collagen type II and aggrecan in the cartilage. The cartilage in the knees of mice with monosodium iodoacetate and transplanted human umbilical cord mesenchymal stem cells and the control group had a strong affinity for type II collagen and aggrecan antibody. The injured tissue in mice after monosodium iodoacetate treatment without human umbilical cord mesenchymal stem cell transplantation was less stained than in the human umbilical cord mesenchymal stem cell transplanted group. The amounts of type II collagen (d) and aggrecan (e) in the human umbilical cord mesenchymal stem cell transplanted group were significantly higher than in the monosodium iodoacetate-treated group (***P < 0.001). Scale bar = 100 μm
Human umbilical cord mesenchymal stem cell transplantation ameliorated cartilage apoptosis in monosodium iodoacetate-treated mice
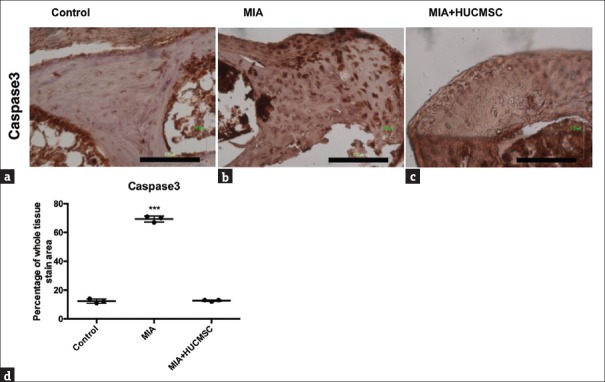
To investigate whether MIA-induced apoptosis could be ameliorated by HUCMSC transplantation in vivo, IHC of caspase 3 in the cartilage was used for the evaluation of apoptosis. We found decreased staining of caspase 3 in HUCMSC-transplanted cartilage compared with MIA injured cartilage as shown in Figure 7 (P < 0.001). The results indicate that HUCMSC transplantation could decrease MIA-induced chondrocyte apoptosis in vivo.
Figure 7.
Caspase 3 expression in the joint cartilage of mice in the (a) control group, (b) monosodium iodoacetate only group, and (c) monosodium iodoacetate + human umbilical cord mesenchymal stem cell group. At 35 days after the experiment, the mice were killed under anesthesia and the knees were removed for staining of caspase 3 in the cartilage. After monosodium iodoacetate treatment without human umbilical cord mesenchymal stem cell transplantation, the injured tissue was more strongly stained with caspase 3 than the human umbilical cord mesenchymal stem cell transplanted group and the control group. The amount of caspase 3 (d) in human umbilical cord mesenchymal stem cells-transplanted group was significantly lower than in the monosodium iodoacetate-treated group (***P < 0.001). Scale bar = 100 μm
DISCUSSION
The present experiment demonstrated that HUCMSCs fulfilled the criteria of MSCs and exhibited mesoderm differentiation potential that can differentiate into adipocytes, osteocytes, and chondrocytes; HUCMSC-CM assisted MIA-treated chondrocytes in recovering from impaired proliferation and increased apoptosis and in reducing MIA-enhanced caspase 3 expression. The in vivo experiment substantiated that impaired movements in mice with MIA-induced cartilage destruction could be attenuated by HUCMSC transplantation; the histological and IHC evidence indicated that HUCMSC transplantation reduced cell and GAG loss in MIA-treated mice; HUCMSC transplantation assisted MIA-treated mice in the regeneration of hyaline cartilage and/or repair of cartilage damage and in ameliorating cartilage apoptosis. Thus, HUCMSCs may be a feasible stem cell source for treatment in OA cartilage repair.
HUCMSCs have attracted much attention as a potential cell source for regenerative medicine, including OA [42]. The advantages of HUCMSCs in regenerative medicine include avoidance of ethical issues, painless harvesting process, high cell proliferation, wide differentiation potential, hypo-immunogenicity, and non-tumorigenicity [42,43]. Previous studies found that HUCMSCs are able to differentiate into chondrocytes in 2D and 3D culture systems [24,42,44,45]. Consistent with previous results, the present findings demonstrated that HUCMSCs had MSC characteristics and were capable of differentiation into adipocytes, osteocytes, and chondrocytes [Figure 1]. In view of all these characteristics, HUCMSCs can be regarded as an alternative source of MSCs for OA treatment. HUCMSCs may be a feasible stem cell source for the treatment of OA.
MIA inhibits the activity of glyceraldehyde-3-phosphate dehydrogenase, leading to apoptosis of chondrocytes [46,47,48]. The apoptosis processes through two main pathways, the receptor (extrinsic) pathway and the mitochondrial (intrinsic) pathway involving cytochrome c release [49]. Activation of cell death receptors such as Fas or tumor necrosis factor-α will trigger caspase 8, initiating subsequent caspase 3 activation with an apoptotic reaction. Caspase-dependent apoptosis is involved in MIA-induced OA in the rat model [48,49,50]. In support of these findings, the present in vitro results showed that MIA injection activated caspase 3 and eventually increased apoptosis in primary human chondrocytes [Figure 3], while the in vivo results also showed increased cartilage apoptosis in the MIA-treated group compared with the HUCMSC-treated group [Figure 7].
The present in vitro studies demonstrated that HUCMSC-CM assisted MIA-treated chondrocytes in recovering from impaired proliferation and increased apoptosis [Figure 2] as well as protecting them from caspase 3 overexpression [Figure 3]; the in vivo studies revealed that HUCMSC transplantation assisted MIA-treated mice in regeneration of hyaline cartilage and/or repair of cartilage damage [Figure 6] and in ameliorating cartilage apoptosis [Figure 7]. These findings together with those findings reported in the previous paragraph support the notion that HUCMSCs can be regarded as an alternative source of MSCs for OA cartilage repair treatment.
OA caused by chronic inflammation develops slowly in patients. The limitation of this NOD-SCID model is the rapid destruction of the joint by MIA. In addition, MIA alone can cause cartilage damage without an inflammatory reaction.
Based on the present findings, we conclude that HUCMSCs can fulfill MSC characteristics with mesoderm differentiation capability. HUCMSCs can assist MIA-treated mice in regeneration of hyaline cartilage and/or repair of cartilage damage and in ameliorating cartilage apoptosis. These effects can be associated with motor behavioral improvement. Thus, HUCMSCs may be a feasible source for stem cell treatment for OA cartilage repair.
Financial support and sponsorship
This work was supported by grants from the Intramural Research Project of Buddhist Tzu Chi General Hospital (TCRD 102-27).
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We thank Dr. Jon-Son Kuo for English editing of the manuscript.
REFERENCES
- 1.Murphy L, Helmick CG. The impact of osteoarthritis in the United States: A population-health perspective. Am J Nurs. 2012;112:S13–9. doi: 10.1097/01.NAJ.0000412646.80054.21. [DOI] [PubMed] [Google Scholar]
- 2.Felson DT, Lawrence RC, Hochberg MC, McAlindon T, Dieppe PA, Minor MA, et al. Osteoarthritis: New insights. Part 2: Treatment approaches. Ann Intern Med. 2000;133:726–37. doi: 10.7326/0003-4819-133-9-200011070-00015. [DOI] [PubMed] [Google Scholar]
- 3.Gilbert JE. Current treatment options for the restoration of articular cartilage. Am J Knee Surg. 1998;11:42–6. [PubMed] [Google Scholar]
- 4.Versier G, Dubrana F French Arthroscopy Society. Treatment of knee cartilage defect in 2010. Orthop Traumatol Surg Res. 2011;97:S140–53. doi: 10.1016/j.otsr.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Can A, Karahuseyinoglu S. Concise review: Human umbilical cord stroma with regard to the source of fetus-derived stem cells. Stem Cells. 2007;25:2886–95. doi: 10.1634/stemcells.2007-0417. [DOI] [PubMed] [Google Scholar]
- 6.Ding DC, Shyu WC, Chiang MF, Lin SZ, Chang YC, Wang HJ, et al. Enhancement of neuroplasticity through upregulation of beta1-integrin in human umbilical cord-derived stromal cell implanted stroke model. Neurobiol Dis. 2007;27:339–53. doi: 10.1016/j.nbd.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 7.Weiss ML, Medicetty S, Bledsoe AR, Rachakatla RS, Choi M, Merchav S, et al. Human umbilical cord matrix stem cells: Preliminary characterization and effect of transplantation in a rodent model of Parkinson's disease. Stem Cells. 2006;24:781–92. doi: 10.1634/stemcells.2005-0330. [DOI] [PubMed] [Google Scholar]
- 8.Wang HS, Hung SC, Peng ST, Huang CC, Wei HM, Guo YJ, et al. Mesenchymal stem cells in the Wharton's jelly of the human umbilical cord. Stem Cells. 2004;22:1330–7. doi: 10.1634/stemcells.2004-0013. [DOI] [PubMed] [Google Scholar]
- 9.Ding DC, Shyu WC, Lin SZ, Liu HW, Chiou SH, Chu TY, et al. Human umbilical cord mesenchymal stem cells support nontumorigenic expansion of human embryonic stem cells. Cell Transplant. 2012;21:1515–27. doi: 10.3727/096368912X647199. [DOI] [PubMed] [Google Scholar]
- 10.Lu LL, Liu YJ, Yang SG, Zhao QJ, Wang X, Gong W, et al. Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis-supportive function and other potentials. Haematologica. 2006;91:1017–26. [PubMed] [Google Scholar]
- 11.Karahuseyinoglu S, Cinar O, Kilic E, Kara F, Akay GG, Demiralp DO, et al. Biology of stem cells in human umbilical cord stroma: In situ and in vitro surveys. Stem Cells. 2007;25:319–31. doi: 10.1634/stemcells.2006-0286. [DOI] [PubMed] [Google Scholar]
- 12.Baksh D, Yao R, Tuan RS. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells. 2007;25:1384–92. doi: 10.1634/stemcells.2006-0709. [DOI] [PubMed] [Google Scholar]
- 13.Bailey MM, Wang L, Bode CJ, Mitchell KE, Detamore MS. A comparison of human umbilical cord matrix stem cells and temporomandibular joint condylar chondrocytes for tissue engineering temporomandibular joint condylar cartilage. Tissue Eng. 2007;13:2003–10. doi: 10.1089/ten.2006.0150. [DOI] [PubMed] [Google Scholar]
- 14.Wang L, Seshareddy K, Weiss ML, Detamore MS. Effect of initial seeding density on human umbilical cord mesenchymal stromal cells for fibrocartilage tissue engineering. Tissue Eng Part A. 2009;15:1009–17. doi: 10.1089/ten.tea.2008.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu S, Jia Y, Yuan M, Guo W, Huang J, Zhao B, et al. Repair of osteochondral defects using human umbilical cord Wharton's jelly-derived mesenchymal stem cells in a rabbit model. Biomed Res Int. 2017;2017:8760383. doi: 10.1155/2017/8760383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berg L, Koch T, Heerkens T, Bessonov K, Thomsen P, Betts D, et al. Chondrogenic potential of mesenchymal stromal cells derived from equine bone marrow and umbilical cord blood. Vet Comp Orthop Traumatol. 2009;22:363–70. doi: 10.3415/VCOT-08-10-0107. [DOI] [PubMed] [Google Scholar]
- 17.Ding DC, Chang YH, Shyu WC, Lin SZ. Human umbilical cord mesenchymal stem cells: A new era for stem cell therapy. Cell Transplant. 2015;24:339–47. doi: 10.3727/096368915X686841. [DOI] [PubMed] [Google Scholar]
- 18.Donders R, Vanheusden M, Bogie JF, Ravanidis S, Thewissen K, Stinissen P, et al. Human Wharton's jelly-derived stem cells display immunomodulatory properties and transiently improve rat experimental autoimmune encephalomyelitis. Cell Transplant. 2015;24:2077–98. doi: 10.3727/096368914X685104. [DOI] [PubMed] [Google Scholar]
- 19.Ren H, Sang Y, Zhang F, Liu Z, Qi N, Chen Y, et al. Comparative analysis of human mesenchymal stem cells from umbilical cord, dental pulp, and menstrual blood as sources for cell therapy. Stem Cells Int. 2016;2016:3516574. doi: 10.1155/2016/3516574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park YB, Ha CW, Kim JA, Han WJ, Rhim JH, Lee HJ, et al. Single-stage cell-based cartilage repair in a rabbit model: Cell tracking and in vivo chondrogenesis of human umbilical cord blood-derived mesenchymal stem cells and hyaluronic acid hydrogel composite. Osteoarthritis Cartilage. 2017;25:570–80. doi: 10.1016/j.joca.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 21.Zhao C, Zhang L, Kong W, Liang J, Xu X, Wu H, et al. Umbilical cord-derived mesenchymal stem cells inhibit cadherin-11 expression by fibroblast-like synoviocytes in rheumatoid arthritis. J Immunol Res. 2015;2015:137695. doi: 10.1155/2015/137695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeong SY, Kim DH, Ha J, Jin HJ, Kwon SJ, Chang JW, et al. Thrombospondin-2 secreted by human umbilical cord blood-derived mesenchymal stem cells promotes chondrogenic differentiation. Stem Cells. 2013;31:2136–48. doi: 10.1002/stem.1471. [DOI] [PubMed] [Google Scholar]
- 23.Wajid N, Mehmood A, Bhatti FU, Khan SN, Riazuddin S. Lovastatin protects chondrocytes derived from Wharton's jelly of human cord against hydrogen-peroxide-induced in vitro injury. Cell Tissue Res. 2013;351:433–43. doi: 10.1007/s00441-012-1540-3. [DOI] [PubMed] [Google Scholar]
- 24.Fong CY, Subramanian A, Gauthaman K, Venugopal J, Biswas A, Ramakrishna S, et al. Human umbilical cord Wharton's jelly stem cells undergo enhanced chondrogenic differentiation when grown on nanofibrous scaffolds and in a sequential two-stage culture medium environment. Stem Cell Rev. 2012;8:195–209. doi: 10.1007/s12015-011-9289-8. [DOI] [PubMed] [Google Scholar]
- 25.Troyer DL, Weiss ML. Wharton's jelly-derived cells are a primitive stromal cell population. Stem Cells. 2008;26:591–9. doi: 10.1634/stemcells.2007-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fu YS, Cheng YC, Lin MY, Cheng H, Chu PM, Chou SC, et al. Conversion of human umbilical cord mesenchymal stem cells in Wharton's jelly to dopaminergic neurons in vitro: Potential therapeutic application for Parkinsonism. Stem Cells. 2006;24:115–24. doi: 10.1634/stemcells.2005-0053. [DOI] [PubMed] [Google Scholar]
- 27.Jomura S, Uy M, Mitchell K, Dallasen R, Bode CJ, Xu Y, et al. Potential treatment of cerebral global ischemia with oct-4+ umbilical cord matrix cells. Stem Cells. 2007;25:98–106. doi: 10.1634/stemcells.2006-0055. [DOI] [PubMed] [Google Scholar]
- 28.Ding DC, Chou HL, Chang YH, Hung WT, Liu HW, Chu TY, et al. Characterization of HLA-G and related immunosuppressive effects in human umbilical cord stroma-derived stem cells. Cell Transplant. 2016;25:217–28. doi: 10.3727/096368915X688182. [DOI] [PubMed] [Google Scholar]
- 29.Guingamp C, Gegout-Pottie P, Philippe L, Terlain B, Netter P, Gillet P, et al. Mono-iodoacetate-induced experimental osteoarthritis: A dose-response study of loss of mobility, morphology, and biochemistry. Arthritis Rheum. 1997;40:1670–9. doi: 10.1002/art.1780400917. [DOI] [PubMed] [Google Scholar]
- 30.Janusz MJ, Hookfin EB, Heitmeyer SA, Woessner JF, Freemont AJ, Hoyland JA, et al. Moderation of iodoacetate-induced experimental osteoarthritis in rats by matrix metalloproteinase inhibitors. Osteoarthritis Cartilage. 2001;9:751–60. doi: 10.1053/joca.2001.0472. [DOI] [PubMed] [Google Scholar]
- 31.Kobayashi K, Imaizumi R, Sumichika H, Tanaka H, Goda M, Fukunari A, et al. Sodium iodoacetate-induced experimental osteoarthritis and associated pain model in rats. J Vet Med Sci. 2003;65:1195–9. doi: 10.1292/jvms.65.1195. [DOI] [PubMed] [Google Scholar]
- 32.Zhu Y, Wu X, Liang Y, Gu H, Song K, Zou X, et al. Repair of cartilage defects in osteoarthritis rats with induced pluripotent stem cell derived chondrocytes. BMC Biotechnol. 2016;16:78. doi: 10.1186/s12896-016-0306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grossin L, Etienne S, Gaborit N, Pinzano A, Cournil-Henrionnet C, Gerard C, et al. Induction of heat shock protein 70 (Hsp70) by proteasome inhibitor MG 132 protects articular chondrocytes from cellular death in vitro and in vivo . Biorheology. 2004;41:521–34. [PubMed] [Google Scholar]
- 34.Kalff KM, El Mouedden M, van Egmond J, Veening J, Joosten L, Scheffer GJ, et al. Pre-treatment with capsaicin in a rat osteoarthritis model reduces the symptoms of pain and bone damage induced by monosodium iodoacetate. Eur J Pharmacol. 2010;641:108–13. doi: 10.1016/j.ejphar.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 35.Pitcher T, Sousa-Valente J, Malcangio M. The monoiodoacetate model of osteoarthritis pain in the mouse. J Vis Exp. 2016 doi: 10.3791/53746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mitchell KE, Weiss ML, Mitchell BM, Martin P, Davis D, Morales L, et al. Matrix cells from Wharton's jelly form neurons and glia. Stem Cells. 2003;21:50–60. doi: 10.1634/stemcells.21-1-50. [DOI] [PubMed] [Google Scholar]
- 37.Taylor SC, Berkelman T, Yadav G, Hammond M. A defined methodology for reliable quantification of western blot data. Mol Biotechnol. 2013;55:217–26. doi: 10.1007/s12033-013-9672-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bove SE, Calcaterra SL, Brooker RM, Huber CM, Guzman RE, Juneau PL, et al. Weight bearing as a measure of disease progression and efficacy of anti-inflammatory compounds in a model of monosodium iodoacetate-induced osteoarthritis. Osteoarthritis Cartilage. 2003;11:821–30. doi: 10.1016/s1063-4584(03)00163-8. [DOI] [PubMed] [Google Scholar]
- 39.Mainil-Varlet P, Aigner T, Brittberg M, Bullough P, Hollander A, Hunziker E, et al. Histological assessment of cartilage repair: A report by the Histology Endpoint Committee of the International Cartilage Repair Society (ICRS) J Bone Joint Surg Am. 2003;85-A(Suppl 2):45–57. [PubMed] [Google Scholar]
- 40.Kaczmarek E, Górna A, Majewski P. Techniques of image analysis for quantitative immunohistochemistry. Rocz Akad Med Bialymst. 2004;49(Suppl 1):155–8. [PubMed] [Google Scholar]
- 41.Geyer G, Linss W. Toluidine blue staining of cartilage proteoglycan subunits. Acta Histochem. 1978;61:127–34. doi: 10.1016/S0065-1281(78)80056-7. [DOI] [PubMed] [Google Scholar]
- 42.Chang YH, Liu HW, Wu KC, Ding DC. Mesenchymal stem cells and their clinical applications in osteoarthritis. Cell Transplant. 2016;25:937–50. doi: 10.3727/096368915X690288. [DOI] [PubMed] [Google Scholar]
- 43.Ding DC, Shyu WC, Lin SZ. Mesenchymal stem cells. Cell Transplant. 2011;20:5–14. doi: 10.3727/096368910X. [DOI] [PubMed] [Google Scholar]
- 44.Gauthaman K, Fong CY, Venugopal JR, Biswas A, Ramakrishna S, Bongso A, et al. Propagation and differentiation of human Wharton's jelly stem cells on three-dimensional nanofibrous scaffolds. Methods Mol Biol. 2013;1058:1–23. doi: 10.1007/7651_2012_1. [DOI] [PubMed] [Google Scholar]
- 45.Lin YX, Ding ZY, Zhou XB, Li ST, Xie de M, Li ZZ, et al. In vitro and in vivo evaluation of the developed PLGA/HAp/Zein scaffolds for bone-cartilage interface regeneration. Biomed Environ Sci. 2015;28:1–2. doi: 10.3967/bes2015.001. [DOI] [PubMed] [Google Scholar]
- 46.Bar-Yehuda S, Rath-Wolfson L, Del Valle L, Ochaion A, Cohen S, Patoka R, et al. Induction of an antiinflammatory effect and prevention of cartilage damage in rat knee osteoarthritis by CF101 treatment. Arthritis Rheum. 2009;60:3061–71. doi: 10.1002/art.24817. [DOI] [PubMed] [Google Scholar]
- 47.Kalbhen DA. Chemical model of osteoarthritis – A pharmacological evaluation. J Rheumatol. 1987;14:130–1. [PubMed] [Google Scholar]
- 48.Grossin L, Cournil-Henrionnet C, Pinzano A, Gaborit N, Dumas D, Etienne S, et al. Gene transfer with HSP 70 in rat chondrocytes confers cytoprotection in vitro and during experimental osteoarthritis. FASEB J. 2006;20:65–75. doi: 10.1096/fj.04-2889com. [DOI] [PubMed] [Google Scholar]
- 49.Jiang L, Li L, Geng C, Gong D, Jiang L, Ishikawa N, et al. Monosodium iodoacetate induces apoptosis via the mitochondrial pathway involving ROS production and caspase activation in rat chondrocytes in vitro . J Orthop Res. 2013;31:364–9. doi: 10.1002/jor.22250. [DOI] [PubMed] [Google Scholar]
- 50.Wang XD, Kou XX, He DQ, Zeng MM, Meng Z, Bi RY, et al. Progression of cartilage degradation, bone resorption and pain in rat temporomandibular joint osteoarthritis induced by injection of iodoacetate. PLoS One. 2012;7:e45036. doi: 10.1371/journal.pone.0045036. [DOI] [PMC free article] [PubMed] [Google Scholar]