Abstract
Objective:
Peripheral artery disease (PAD) is associated with systemic atherosclerosis and indicates an increased risk of mortality in peritoneal dialysis (PD) patients. A high leptin level accelerates atherosclerosis in apoE-deficient mice. The purpose of this study was to examine the association of serum leptin level and PAD in adult PD patients.
Materials and Methods:
The clinical characteristics of sixty PD patients recruited from June 2015 to October 2016 were obtained. Serum leptin concentrations were determined. Ankle–brachial index (ABI) values were measured and those with a left or right ABI <0.9 were defined as the low ABI group.
Results:
Twenty of these 60 PD patients (33.3%) had diabetes mellitus and 32 patients (53.3%) had hypertension. Thirteen PD patients (21.7%) were in the low ABI group. Higher serum leptin (P = 0.002) and C-reactive protein (CRP, P < 0.001) levels were found in the low ABI group compared with those in the normal ABI group. More number of patients with diabetes (P = 0.015) and current smokers (P = 0.037) were noted in the low ABI group than in the normal ABI group. After adjustment for factors that were significantly associated with PAD in multivariate logistic regression analysis, each increase of 1 ng/mL in the serum leptin level (odds ratio [OR], 1.062; 95% confidence interval [CI], 1.014–1.114; P = 0.012) and each increase of 0.1 mg/dL in the serum CRP level (OR, 1.107; 95% CI, 1.011–1.211; P = 0.028) were found to be independent predictors of PAD in PD patients.
Conclusion:
Higher serum leptin and CRP levels correlated positively with the diagnosis of PAD in PD patients.
KEYWORDS: C-reactive protein, Leptin, Peripheral artery disease, Peritoneal dialysis
INTRODUCTION
Peripheral artery disease (PAD) is a disease in which atherosclerotic plaques cause arterial obstruction and reduce blood flow [1]. PAD represents systemic atherosclerosis which can affect the functional activity of the limbs and heightens the risk of cardiovascular disease [2]. Escalated morbidity and 2–3-fold higher overall mortality were found in patients with PAD compared to patients without PAD [3].
PAD is more common in patients with end-stage renal disease (ESRD) than in the general population [4]. In the general population, the prevalence of PAD increases with age, smoking, and diabetes [5,6]. The duration of dialysis is another risk factor in ESRD patients [7]. A recent study revealed that the prevalence of PAD was 27.4% among all patients on maintenance ambulatory peritoneal dialysis (PD) and was higher (45%) in patients older than 70 years [8]. The ankle–brachial pressure index (ABI) is a reliable, initial test to diagnose PAD if a patient has abnormal physical findings, such as claudication, impaired walking function, or ischemic rest pain. An ABI <0.9 is an indicator of PAD when assessing asymptomatic patients [9].
Leptin is a kind of adipokine which is produced and secreted by white adipocytes [10,11]. Several studies reported that leptin is related to the pathogenesis of atherosclerosis and coronary artery disease from its actions to stimulate oxidative stress, vascular inflammation, and vascular smooth muscle hypertrophy [12,13,14]. Hyperleptinemia has been associated with atherosclerosis, endothelial dysfunction, and insulin resistance [15].
The relationship of serum leptin and PAD in PD patients is not clear. Therefore, our study aimed to examine the correlation between serum leptin and ABI in PD patients.
MATERIALS AND METHODS
Patients
Sixty PD patients were recruited from Hualien and Dalin Tzu Chi Hospital from June 2015 to October 2016. All patients had undergone regular PD for more than 3 months. Twenty-six patients were men and 34 were women with ages ranging from 23 to 89 years. Exclusion criteria included acute infection, malignancy, acute myocardial infarction, pulmonary edema, or heart failure at the time of blood sampling or refusal to sign informed consent. The mean duration of PD treatment was 53.92 ± 41.65 months. The study was conducted in accordance with the Declaration of Helsinki and was approved by the Protection of Human Subjects Institutional Review Board of Tzu-Chi University and Hospital (IRB103-136-A). Informed written consent was obtained from all patients prior to their enrolment in this study. Among the patients, 42 received continuous ambulatory PD (Dianeal, Baxter Health Care, Taiwan), with 3–5 dialysate exchanges per day, while the other 18 patients underwent 4–5 dialysate exchanges each night with an automated device (automated PD). The weekly fractional clearance index for urea, total clearance of creatinine, and peritoneal clearance of creatinine were obtained from the medical records.
Anthropometric analysis
All anthropometric factors were measured three times, during the morning after overnight fasting, without dialysate in the abdominal cavity. Body weight was measured in light clothing and without shoes to the nearest 0.5 kg; height was measured to the nearest 0.5 cm. Body mass index was calculated as weight (kg) divided by height in meters squared [16,17,18].
Biochemical investigations
Biochemical parameters were determined in the morning before dialysis exchange. Blood samples (~5 mL) were immediately centrifuged at 3000 × g for 10 min. Serum samples were stored at 4°C and used for biochemical analyses within 1 h of collection. Serum levels of blood urea nitrogen, creatinine, fasting glucose, albumin, total cholesterol, triglycerides, total calcium, phosphorus, and C-reactive protein (CRP) were measured using an autoanalyzer (Siemens Advia 1800, Siemens Healthcare GmbH, Henkestr, Germany). Serum leptin (SPI-BIO, Montigny le Bretonneux, France) concentrations were checked using a commercially available enzyme immunoassay [16,19,20,21]. Serum intact parathyroid hormone levels (iPTHs) were measured using enzyme-linked immunosorbent assays (Diagnostic Systems Laboratories, Webster, Texas, USA) [16,19,20,21].
Ankle–brachial index measurements
ABI values were obtained using an oscillometric method. The ABI-form device (VaSera VS-1000; Fukuda Denshi Co., Ltd., Tokyo, Japan) measures blood pressure (BP) in both arms and ankles automatically and simultaneously [22]. During examination, participants were in the supine position and cuffs were placed tightly around the four extremities. Meanwhile, an electrocardiogram was obtained and heart sounds were recorded for at least 10 min. The ABI was derived from the calculation of the ratio of the ankle systolic BP (SBP) divided by the arm SBP. Diagnostic criterion for PAD was an ABI <0.9 and our study adapted left or right ABI values <0.9 to define the low ABI group.
Statistical analysis
The Kolmogorov–Smirnov test was used to examine the normal distribution of data. Normally distributed data were expressed as means ± standard deviation and comparisons between patients were performed using the Student's independent t-test. Nonnormally distributed data were expressed as medians and interquartile ranges and comparisons between patients were performed using the Mann–Whitney U-test. Data expressed as the number and percentage of patients were analyzed by the Chi-square test. The fasting glucose, iPTH, and leptin datasets showed skewed nonnormal distributions and therefore were recalculated by transformation to the logarithm to the base 10; after this transformation, the log-glucose, log-iPTH, and log-leptin became normally distributed. Variables that were significantly associated with PAD were tested for independence using multivariate logistic regression analysis (adapted factors: gender, smoking, age, diabetes, hypertension, CRP, and leptin). The receiver operating curve (ROC) was used to calculate the area under the curve (AUC) to identify the log-leptin levels and serum log-CRP levels which predicted PAD in PD patients. SPSS for Windows (version 19.0; SPSS Inc., Chicago, IL, USA) was used for all statistical analyses and P < 0.05 was considered statistically significant.
RESULTS
Table 1 shows the laboratory and clinical characteristics of the sixty enrolled PD patients. Examination of their medical histories indicated that twenty patients (33.3%) had diabetes mellitus (DM) and 32 (53.3%) had hypertension. Thirteen PD patients (21.7%) were included in the low ABI group. PD patients in the low ABI group had higher serum CRP (P < 0.001) levels and higher serum leptin (P = 0.002) levels than those in the normal ABI group. Compared with the normal ABI group, there were statistically significant differences in diabetes (P = 0.015) and current smoking (P = 0.037) in the low ABI group among PD patients.
Table 1.
Comparison of clinical variables of sixty peritoneal dialysis patients in the normal and low ankle–brachial index groups
| Characteristics | All participants (n=60) | Normal ABI group (n=47) | Low ABI group (n=13) | P |
|---|---|---|---|---|
| Age (years) | 56.88±14.75 | 56.36±15.47 | 58.77±12.15 | 0.607 |
| Peritoneal dialysis duration (months) | 53.92±41.65 | 54.09±35.64 | 53.31±60.49 | 0.953 |
| Height (cm) | 160.27±8.46 | 160.26±8.31 | 160.31±9.32 | 0.984 |
| Body weight (kg) | 63.77±14.07 | 62.56±13.74 | 68.17±14.93 | 0.207 |
| Body mass index (kg/m2) | 24.85±3.91 | 24.79±3.87 | 25.06±4.22 | 0.826 |
| Left ankle–brachial index | 1.09±0.17 | 1.15±0.13 | 0.87±0.14 | <0.001* |
| Right ankle–brachial index | 1.10±0.16 | 1.16±0.11 | 0.88±0.07 | <0.001* |
| SBP (mmHg) | 145.90±23.46 | 147.87±21.62 | 138.77±29.08 | 0.219 |
| Diastolic blood pressure (mmHg) | 85.35±12.65 | 87.00±11.32 | 79.38±16.46 | 0.058 |
| Total cholesterol (mg/dL) | 169.32±35.84 | 171.53±38.16 | 161.31±25.39 | 0.367 |
| Triglycerides (mg/dL) | 190.77±127.94 | 188.55±122.38 | 198.77±151.60 | 0.801 |
| Fasting glucose (mg/dL) | 106.00 (97.00-141.25) | 104.00 (96.00-143.00) | 122.00 (105.00-142.50) | 0.154 |
| Albumin (mg/dL) | 3.71±0.34 | 3.72±0.35 | 3.69±0.33 | 0.788 |
| Blood urea nitrogen (mg/dL) | 58.73±19.87 | 59.94±21.19 | 54.38±13.92 | 0.377 |
| Creatinine (mg/dL) | 11.24±3.02 | 11.54±2.97 | 10.14±3.03 | 0.140 |
| Total calcium (mg/dL) | 9.05±0.71 | 9.13±0.72 | 8.78±0.64 | 0.118 |
| Phosphorus (mg/dL) | 5.18±1.28 | 5.23±1.23 | 5.00±1.51 | 0.567 |
| CRP (mg/dL) | 0.22 (0.07-0.43) | 0.12 (0.06-0.30) | 1.58 (0.47-2.06) | <0.001* |
| Intact parathyroid hormone (pg/mL) | 259.70 (138.60-505.42) | 253.43 (116.93-466.89) | 313.60 (184.40-606.43) | 0.370 |
| Leptin (ng/mL) | 22.55 (11.02-56.43) | 19.76 (7.88-43.72) | 61.12 (35.24-79.73) | 0.002* |
| Weekly Kt/V | 2.15±0.36 | 2.17±0.38 | 2.07±0.29 | 0.416 |
| Peritoneal Kt/V | 1.83±0.43 | 1.85±0.46 | 1.76±0.29 | 0.551 |
| Total clearance of creatinine (L/week) | 57.88±24.79 | 58.31±27.40 | 56.35±11.87 | 0.804 |
| Peritoneal clearance of creatinine (L/week) | 41.61±13.58 | 41.32±14.15 | 42.66±11.71 | 0.757 |
| Female, n (%) | 34 (56.7) | 26 (55.3) | 8 (61.5) | 0.689 |
| Diabetes, n (%) | 20 (33.3) | 12 (25.5) | 8 (61.5) | 0.015* |
| Hypertension, n (%) | 32 (53.3) | 27 (57.4) | 5 (38.5) | 0.225 |
| Smoking, n (%) | 8 (13.3) | 4 (8.5) | 4 (30.8) | 0.037* |
| CAPD model, n (%) | 42 (70.0) | 35 (74.5) | 7 (53.8) | 0.151 |
*Values of P<0.05 were considered statistically significant. Values for continuous variables are shown as mean±SD after analysis by Student’s t-test; variables not normally distributed are shown as median and interquartile range after analysis by the Mann-Whitney U-test; values are presented as n (%) after analysis by the Chi-square test. ABI: Ankle–brachial index, CAPD: Continuous ambulatory peritoneal dialysis, CRP: C-reactive protein, Kt/V: Fractional clearance index for urea, SBP: Systolic blood pressure, SD: Standard deviation
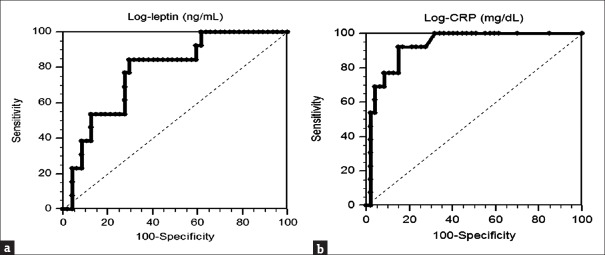
Adjustment of the factors significantly associated with PAD (gender, smoking, age, diabetes, hypertension, CRP, and leptin) in multivariate logistic regression analysis revealed that each increase of 1 ng/mL in the serum leptin level (odds ratio [OR], 1.062; 95% confidence interval [CI], 1.014–1.114; P = 0.012) and each increase of 0.1 mg/dL in the serum level (OR, 1.107; 95% CI, 1.011–1.211; P = 0.028) were independent predictors of PAD in PD patients [Table 2]. ROC curve analysis was applied to estimate the optimal log-leptin levels [Figure 1a] and log-CRP levels [Figure 1b] predicting PAD in PD patients. The AUC for log-leptin was 0.777 (95% CI: 0.651–0.875, P < 0.001) and for log-CRP was 0.930 (95% CI: 0.833–0.980, P < 0.001).
Table 2.
Multivariate logistic regression analysis of the factors correlated to peripheral artery disease among sixty peritoneal dialysis patients
| Variable | OR | 95% CI | P |
|---|---|---|---|
| Leptin (ng/mL) (each increase of 1 ng/mL) | 1.062 | 1.014-1.112 | 0.012* |
| CRP (mg/dL) (each increase of 0.1 mg/dL) | 1.107 | 1.011-1.211 | 0.028* |
*Values of P<0.05 were considered statistically significant. Analysis of the data was done using multivariate logistic regression analysis (adopted factors: gender, smoking, age, diabetes, hypertension, CRP, and leptin). OR: Odds ratio, CI: Confidence interval, CRP: C-reactive protein
Figure 1.
Receiver operating characteristic curve analysis to predict peripheral artery disease in sixty peritoneal dialysis patients. (a). The area under the receiver operating characteristic curve indicates the diagnostic power of log-leptin to predict peripheral artery disease in peritoneal dialysis patients. The area under the curve for log-leptin was 0.777 (95% confidence interval: 0.651–0.875, P < 0.001). (b). The area under the curve indicates the diagnostic power of log-CRP to predict peripheral artery disease in peritoneal dialysis patients. The area under the curve for log-CRP was 0.930 (95% confidence interval: 0.833–0.980, P < 0.001). CRP: C-reactive protein
DISCUSSION
The results of our study revealed higher serum leptin and CRP levels in the low ABI group than the normal ABI group in these adult PD patients. In addition, significantly higher percentages of patients with DM and smoking were noted in the low ABI group than that of the normal ABI group.
No prior studies have investigated the association between serum leptin and PAD in PD patients. Some studies have revealed a positive correlation between serum leptin and cardiovascular disease [23,24,25]. The serum leptin level was also considered an independent predictor of arterial stiffness in patients with a history of cardiovascular disease [16], possibly due to its atherogenic and thrombotic physiological actions [13,26,27].
Leptin, a 16-kDa peptide product of the ob gene, is produced from white adipocytes [28]. Several studies have explained the possible pathophysiology of the atherogenic and thrombotic properties of leptin. First of all, leptin may stimulate the production of nitric oxide (NO) by upregulating inducible NO synthase, which may induce atherogenesis and injure endothelial function due to reactive oxidative stress [29]. Leptin may also increase oxidative stress by several different mechanisms [15]. Second, leptin potentiates the production of cytokines and growth factors to promote the pro-inflammatory signaling pathway (or pro-inflammatory signals) which may relate to atherosclerosis and endothelial dysfunction [30,31,32]. In addition, leptin may calcify vascular smooth muscle cells by stimulating osteoblastic differentiation and hydroxyapatite production [33]. Moreover, leptin correlates to thrombosis which may be due to platelet hyperactivity and loss of the balance between coagulation and fibrinolysis [15]. Finally, angiotensin II (Ang II) escalates leptin synthesis and both Ang II and leptin potentiate sympathetic activity [34]. Leptin has insulin-resistant properties which lower the antioxidative and lipogenic effects of insulin [35].
Older age, hypertension, dyslipidemia, cigarette smoking, and diabetes are established risk factors for PAD [36]. High levels of inflammatory markers such as CRP have been associated with accelerated functional decline in PAD patients [1,36]. Higher CRP levels were associated with PAD in a cross-sectional study among 1611 US adults free of cardiovascular disease, diabetes, and hypertension [37]. In our study, more patients with DM and current smoking and higher serum CRP levels were noted in the low ABI group compared with the normal ABI group among these PD patients. Our results are similar to those in a previous study which showed that PAD is a common disease in PD patients and DM is an independent risk factor for PAD in PD patients [8]. This could be explained by the pathophysiological correlation of arterial stiffness and DM reported in previous studies [16,38]. DM was found to be an independent risk factor for PAD among all ESRD patients in another single-center study in Taiwan; however, DM was not a significant risk factor in the subgroup of PD patients. Possible explanations for this finding are the lower age of the PD patients and lower percentage of PD patients with DM in this study [39].
The limitations of this study include the small number of patients and the cross-sectional design. Therefore, further cohort studies should be conducted to confirm the cause–effect relationship between serum leptin and PAD in PD patients.
CONCLUSION
The present study revealed that serum leptin and CRP levels correlated positively with a diagnosis of PAD in PD patients.
Financial support and sponsorship
This study was financially supported by a grant from the Ministry of Science and Technology, Taiwan (MOST-104-2314-B-303-010).
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Hamburg NM, Creager MA. Pathophysiology of intermittent claudication in peripheral artery disease. Circ J. 2017;81:281–9. doi: 10.1253/circj.CJ-16-1286. [DOI] [PubMed] [Google Scholar]
- 2.Fowkes FG, Murray GD, Butcher I, Heald CL, Lee RJ, et al. Ankle Brachial Index Collaboration. Ankle brachial index combined with Framingham risk score to predict cardiovascular events and mortality: A meta-analysis. JAMA. 2008;300:197–208. doi: 10.1001/jama.300.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Criqui MH, Langer RD, Fronek A, Feigelson HS, Klauber MR, McCann TJ, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326:381–6. doi: 10.1056/NEJM199202063260605. [DOI] [PubMed] [Google Scholar]
- 4.O'Hare AM, Hsu CY, Bacchetti P, Johansen KL. Peripheral vascular disease risk factors among patients undergoing hemodialysis. J Am Soc Nephrol. 2002;13:497–503. doi: 10.1681/ASN.V132497. [DOI] [PubMed] [Google Scholar]
- 5.GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1545–602. doi: 10.1016/S0140-6736(16)31678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1459–544. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Hare AM, Glidden DV, Fox CS, Hsu CY. High prevalence of peripheral arterial disease in persons with renal insufficiency: Results from the National Health and Nutrition Examination Survey 1999-2000. Circulation. 2004;109:320–3. doi: 10.1161/01.CIR.0000114519.75433.DD. [DOI] [PubMed] [Google Scholar]
- 8.Tian SL, Murphy M, Han QF, Lu XH, Wang T. Prevalence and risk factors for peripheral artery disease among patients on maintenance peritoneal dialysis. Blood Purif. 2010;30:50–5. doi: 10.1159/000317121. [DOI] [PubMed] [Google Scholar]
- 9.Paraskevas KI, Kotsikoris I, Koupidis SA, Giannoukas AD, Mikhailidis DP. Ankle – Brachial index: A marker of both peripheral arterial disease and systemic atherosclerosis as well as a predictor of vascular events. Angiology. 2010;61:521–3. doi: 10.1177/0003319710371620. [DOI] [PubMed] [Google Scholar]
- 10.de Faria AP, Demacq C, Figueiredo VN, Moraes CH, Santos RC, Sabbatini AR, et al. Hypoadiponectinemia and aldosterone excess are associated with lack of blood pressure control in subjects with resistant hypertension. Hypertens Res. 2013;36:1067–72. doi: 10.1038/hr.2013.92. [DOI] [PubMed] [Google Scholar]
- 11.Sabbatini AR, Faria AP, Barbaro NR, Gordo WM, Modolo RG, Pinho C, et al. Deregulation of adipokines related to target organ damage on resistant hypertension. J Hum Hypertens. 2014;28:388–92. doi: 10.1038/jhh.2013.118. [DOI] [PubMed] [Google Scholar]
- 12.Seufert J. Leptin effects on pancreatic beta-cell gene expression and function. Diabetes. 2004;53(Suppl 1):S152–8. doi: 10.2337/diabetes.53.2007.s152. [DOI] [PubMed] [Google Scholar]
- 13.Beltowski J. Leptin and atherosclerosis. Atherosclerosis. 2006;189:47–60. doi: 10.1016/j.atherosclerosis.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Bełtowski J. Role of leptin in blood pressure regulation and arterial hypertension. J Hypertens. 2006;24:789–801. doi: 10.1097/01.hjh.0000222743.06584.66. [DOI] [PubMed] [Google Scholar]
- 15.Koh KK, Park SM, Quon MJ. Leptin and cardiovascular disease: Response to therapeutic interventions. Circulation. 2008;117:3238–49. doi: 10.1161/CIRCULATIONAHA.107.741645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsai JP, Wang JH, Chen ML, Yang CF, Chen YC, Hsu BG, et al. Association of serum leptin levels with central arterial stiffness in coronary artery disease patients. BMC Cardiovasc Disord. 2016;16:80. doi: 10.1186/s12872-016-0268-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin YL, Lai YH, Wang CH, Kuo CH, Liou HH, Hsu BG, et al. Triceps skinfold thickness is associated with lumbar bone mineral density in peritoneal dialysis patients. Ther Apher Dial. 2017;21:102–7. doi: 10.1111/1744-9987.12482. [DOI] [PubMed] [Google Scholar]
- 18.Wang CH, Tsai JP, Lai YH, Lin YL, Kuo CH, Hsu BG, et al. Inverse relationship of bone mineral density and serum level of N-terminal pro-B-type natriuretic peptide in peritoneal dialysis patients. Ci Ji Yi Xue Za Zhi. 2016;28:68–72. doi: 10.1016/j.tcmj.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee MC, Chen YC, Ho GJ, Shih MH, Chou KC, Hsu BG, et al. Serum leptin levels positively correlate with peripheral arterial stiffness in kidney transplantation patients. Transplant Proc. 2014;46:353–8. doi: 10.1016/j.transproceed.2013.11.145. [DOI] [PubMed] [Google Scholar]
- 20.Tsai JP, Lee MC, Chen YC, Ho GJ, Shih MH, Hsu BG, et al. Hyperleptinemia is a risk factor for the development of central arterial stiffness in kidney transplant patients. Transplant Proc. 2015;47:1825–30. doi: 10.1016/j.transproceed.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 21.Chen MC, Hsu BG, Lee CJ, Wang JH. Hyperleptinemia positively correlates with cardiometabolic syndrome in hypertensive patients. Int J Clin Exp Pathol. 2016;9:12959–67. [Google Scholar]
- 22.Ho GJ, Chen YC, Yin WY, Chang YJ, Lee MC, Hsu BG, et al. Fasting serum long-acting natriuretic peptide correlates with the ankle brachial index in renal transplant recipients. Exp Clin Transplant. 2013;11:303–9. doi: 10.6002/ect.2012.0224. [DOI] [PubMed] [Google Scholar]
- 23.Wallace AM, McMahon AD, Packard CJ, Kelly A, Shepherd J, Gaw A, et al. Plasma leptin and the risk of cardiovascular disease in the West of Scotland coronary prevention study (WOSCOPS) Circulation. 2001;104:3052–6. doi: 10.1161/hc5001.101061. [DOI] [PubMed] [Google Scholar]
- 24.Söderberg S, Ahrén B, Jansson JH, Johnson O, Hallmans G, Asplund K, et al. Leptin is associated with increased risk of myocardial infarction. J Intern Med. 1999;246:409–18. doi: 10.1046/j.1365-2796.1999.00571.x. [DOI] [PubMed] [Google Scholar]
- 25.Söderberg S, Stegmayr B, Ahlbeck-Glader C, Slunga-Birgander L, Ahrén B, Olsson T, et al. High leptin levels are associated with stroke. Cerebrovasc Dis. 2003;15:63–9. doi: 10.1159/000067128. [DOI] [PubMed] [Google Scholar]
- 26.Correia ML, Haynes WG. Leptin, obesity and cardiovascular disease. Curr Opin Nephrol Hypertens. 2004;13:215–23. doi: 10.1097/00041552-200403000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Werner N, Nickenig G. From fat fighter to risk factor: The zigzag trek of leptin. Arterioscler Thromb Vasc Biol. 2004;24:7–9. doi: 10.1161/01.ATV.0000110908.43721.ad. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM, et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 29.Naseem KM. The role of nitric oxide in cardiovascular diseases. Mol Aspects Med. 2005;26:33–65. doi: 10.1016/j.mam.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 30.Loffreda S, Yang SQ, Lin HZ, Karp CL, Brengman ML, Wang DJ, et al. Leptin regulates proinflammatory immune responses. FASEB J. 1998;12:57–65. [PubMed] [Google Scholar]
- 31.Yamagishi SI, Edelstein D, Du XL, Kaneda Y, Guzmán M, Brownlee M, et al. Leptin induces mitochondrial superoxide production and monocyte chemoattractant protein-1 expression in aortic endothelial cells by increasing fatty acid oxidation via protein kinase A. J Biol Chem. 2001;276:25096–100. doi: 10.1074/jbc.M007383200. [DOI] [PubMed] [Google Scholar]
- 32.Sánchez-Margalet V, Martín-Romero C, Santos-Alvarez J, Goberna R, Najib S, Gonzalez-Yanes C, et al. Role of leptin as an immunomodulator of blood mononuclear cells: Mechanisms of action. Clin Exp Immunol. 2003;133:11–9. doi: 10.1046/j.1365-2249.2003.02190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parhami F, Tintut Y, Ballard A, Fogelman AM, Demer LL. Leptin enhances the calcification of vascular cells: Artery wall as a target of leptin. Circ Res. 2001;88:954–60. doi: 10.1161/hh0901.090975. [DOI] [PubMed] [Google Scholar]
- 34.Correia ML, Morgan DA, Sivitz WI, Mark AL, Haynes WG. Leptin acts in the central nervous system to produce dose-dependent changes in arterial pressure. Hypertension. 2001;37:936–42. doi: 10.1161/01.hyp.37.3.936. [DOI] [PubMed] [Google Scholar]
- 35.Muoio DM, Dohm GL, Tapscott EB, Coleman RA. Leptin opposes insulin's effects on fatty acid partitioning in muscles isolated from obese ob/ob mice. Am J Physiol. 1999;276:E913–21. doi: 10.1152/ajpendo.1999.276.5.E913. [DOI] [PubMed] [Google Scholar]
- 36.Krishna SM, Moxon JV, Golledge J. A review of the pathophysiology and potential biomarkers for peripheral artery disease. Int J Mol Sci. 2015;16:11294–322. doi: 10.3390/ijms160511294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shankar A, Li J, Nieto FJ, Klein BE, Klein R. Association between C-reactive protein level and peripheral arterial disease among US adults without cardiovascular disease, diabetes, or hypertension. Am Heart J. 2007;154:495–501. doi: 10.1016/j.ahj.2007.04.060. [DOI] [PubMed] [Google Scholar]
- 38.Schram MT, Henry RM, van Dijk RA, Kostense PJ, Dekker JM, Nijpels G, et al. Increased central artery stiffness in impaired glucose metabolism and type 2 diabetes: The Hoorn Study. Hypertension. 2004;43:176–81. doi: 10.1161/01.HYP.0000111829.46090.92. [DOI] [PubMed] [Google Scholar]
- 39.Lee CC, Wu CJ, Chou LH, Shen SM, Chiang SF, Jen PC, et al. Peripheral artery disease in peritoneal dialysis and hemodialysis patients: Single-center retrospective study in Taiwan. BMC Nephrol. 2012;13:100. doi: 10.1186/1471-2369-13-100. [DOI] [PMC free article] [PubMed] [Google Scholar]