Abstract
BACKGROUND
There is increasing evidence showing that chronic non-bacterial prostatic inflammation is involved in the pathogenesis of benign prostatic hyperplasia (BPH) and male lower urinary tract symptoms (LUTS). It has also been reported that estrogen receptor β (ERβ) could have an immunoprotective role in prostatic tissue. Therefore, we investigated the effect of ERβ-activation on not only prostatic inflammation, but also bladder overactive conditions in a rat model with nonbacterial prostatic inflammation.
METHODS
Male Sprague-Dawley rats (8 weeks, n = 15) were divided into three groups: sham-saline group (n = 5), formalin-vehicle group (n = 5), and formalin-treatment group (n = 5). The sham-saline group had sham operation and 50 μl normal saline injected into each ventral lobe of the prostate. The formalin-vehicle group had 50 μl 5% formalin injection into bilateral ventral lobes of the prostate. The formalin-treatment group was treated with 3α-Adiol (a selective ERβ agonist precursor) at a dose of 3 mg/kg daily from 2 days before induction of prostatic inflammation, whereas formalin-vehicle rats received vehicle (olive oil). In each group, conscious cystometry was performed on day 28 after intraprostatic formalin injection or sham treatment. After cystometry, the bladder and prostate were harvested for evaluation of mRNA expression and histological analysis.
RESULTS
In cystometric investigation, the mean number of non-voiding contractions was significantly greater and voiding intervals were significantly shorter in formalin-vehicle rats than those in sham-saline rats (P < 0.05). In RT-qPCR analysis, mRNA expression of NGF, P2X2, and TRPA1 receptors was significantly increased in the bladder mucosa, and mRNA expression of TNF-α, iNOS and COX2 in the ventral lobes of prostate was significantly increased in formalin-vehicle rats compared with sham-saline rats (P < 0.05). In addition, relative mRNA expression ratio of ERβ to ERα (ERβ/ERα) in the ventral lobes of prostate was significantly decreased in formalin-vehicle rats compared with sham-saline rats (P < 0.05). These changes were ameliorated by 3α-Adiol administration in formalin-treatment rats.
CONCLUSIONS
These results indicate that ERβ activation by 3α-Adiol administration, which normalized the ERβ/ERα expression ratio in the prostate, can improve not only prostatic inflammation, but also bladder overactivity. Therefore, ERβ agonists might be useful for treating irritative bladder symptoms in patients with symptomatic BPH associated with prostatic inflammation.
Keywords: prostate, inflammation, estrogen receptor, 3α-Adiol
INTRODUCTION
It have been reported that non-bacterial prostatic inflammation is associated with the development of histological benign prostatic hyperplasia (BPH) [1,2] and male lower urinary tract symptoms (LUTS) [3,4]. Inflammation detected in prostate biopsies performed at baseline assessment in patients enrolled in the Medical Therapies of Prostate Symptoms (MTOPS) study predicted disease progression events such as symptom worsening, acute urinary retention, and need for surgery in placebo-treated patients [5]. Also, in a clinical trial designed to determine the effects of a 5α-reductase inhibitor finasteride on prostate cancer in 8,224 men [6], statistically significant correlations were found between average and maximum chronic inflammation in prostate biopsy specimens with BPH and symptom score (IPSS) variables, and more severe inflammation was associated with higher IPSS scores [7]. Thus, there is increasing evidence pointing toward prostatic inflammation as one of the important etiologic factors of BPH and male LUTS.
Although the etiology of male LUTS associated with BPH is multifactorial, storage LUTS (frequency, urgency, and incontinence) are often associated with urodynamically proven detrusor overactivity (DO), suggesting that the pathophysiological basis of male LUTS associated with BPH could be found not only within the prostate, but also at extra-prostatic sites, most importantly in the bladder [8,9]. Recent studies including ours also showed that rodent models of prostatic inflammation exhibit bladder overactivity as evidenced by frequent urination [10] and that a rat model of formalin-induced prostatic inflammation, which was used in this study, exhibits the upregulation of androgen receptor-responsive genes and TGFβ-related genes in the prostate [11], which is similarly seen in human BPH tissues [12]. Thus, animal models of non-bacterial prostatic inflammation seem to be suitable for the study that examines the role of prostatic inflammation in BPH-associated male LUTS.
It has recently been reported that estrogens acting through two distinct estrogen receptors (ERs), ERα and ERβ [13], modulate tissue inflammation [14]. Furthermore, ERα and ERβ have not only beneficial effects, but also adverse effects on carcinogenesis, aberrant proliferation, and inflammation [13–16]. However, as demonstrated by Prins and co-workers, induction of prostatic inflammation by neonatal administration of estrogen is dependent on ERα, but not ERβ, in mice [15], suggesting the important role of ERα in prostatic inflammation. Alternatively, ERβ has been reported to have an immunoprotective role to limit the tissue damage by modulating immune systems in the rodent prostate [17]. Anti-inflammatory effects of ERβ have also been shown in various animal disease models such as encephalomyelitis, inflammatory bowel disease, cystitis, and skin diseases [18–21]. Thus, we hypothesized that activation of ERβ exerts anti-inflammatory effects in prostatic inflammation and associated bladder overactivity in a rat model. Thus, in this study, we utilized a rat model with non-bacterial prostatic inflammation induced by intraprostatic injection of formalin to investigate whether activation of ERβ can ameliorate bladder overactivity and molecular changes in the bladder by modulating prostatic inflammation.
METHODS
Animals and Surgery
Male Sprague-Dawley rats (8 weeks, n = 15) were divided into three groups; sham-vehicle group (n = 5), formalin-vehicle group (n = 5), formalin-treatment group (n = 5). Rats were housed in plastic cages with soft bedding and free access to food and water under a 12/12 hr reversed light–dark cycle. The sham-vehicle group had sham operations and was injected with 50 μl normal saline into each of bilateral ventral lobes of the prostate. Prostatic inflammation was induced by injection of 5% formalin solution at a volume of 50 μl into each of bilateral ventral lobes of the prostate. Rats with formalin-induced prostatic inflammation in the formalin-treatment group were then treated with 5α-Androstane-3α, 17β-diol (3α-Adiol) (Sigma–Aldrich Co.), an ERβ ligand precursor that is converted to 3ß-Adiol, a high-affinity ligand and agonist of ERβ, by 17β-hydroxysteroid dehydrogenase type [22]. 3α-Adiol was dissolved in olive oil (Wako Pure Chemical Industries, Ltd.) at a dose of 3 mg/kg daily from 2 days before intraprostatic formalin injection for 30 days, whereas rats with prostatic inflammation in the formalin-vehicle group received olive oil only as vehicle treatment. All experimental procedures were in accordance with institutional guidelines and approved by the Oita University Institutional Animal Care and Use Committee.
After cystometry, the bladder and the ventral lobes of the prostate were harvested for evaluation of mRNA expression, Western blot and histological analysis.
Cystometry
Twenty-eight days after intraprostatic injection of formalin or saline, rats were examined by cystometric investigation under a conscious condition. A PE-50 polyethylene catheter (IMAMURA. Co., Ltd.) was inserted into the bladder from the anterior bladder wall under isoflurane anesthesia 2 days before cystometric investigation and, on the day of cystometry, it was used to infuse saline into the bladder and record the intravesical pressure using Chart software with data sampling on a Power-Lab™ (AD Instruments, Castle Hill, New South Wales, Australia). After the rats were placed in a Bollman-type restraining device (NATSUME SEISAKUSHO Co. Ltd.), the intravesical catheter was connected to a pressure transducer and an infusion pump through a three-way stopcock to infuse physiological saline into the bladder at room temperature at a rate of 0.05 ml/min. After rats were acclimated in the restraining device for at least 2 hr, three micturition cycles were recorded to evaluate voiding interval (VI), maximum voiding pressure (MVP), and non-voiding contractions (NVCs). After several micturition cycles, the saline infusion was stopped and post-void residual volume (RV) was measured by withdrawing by gravity through the intravesical catheter followed by manual compression of the bladder through the abdominal wall. NVCs were defined as rises in intravesical pressure that exceeded 10 cm H2O over the baseline without fluid elimination from the urethral orifice.
Immunohistochemistry
Prostate ventral lobes and the bladder were harvested for histological analyses after cystometry. One part of the prostate and the bladder was fixed in buffered 10% formaldehyde solution for 24 hr, embedded in paraffin, cut with a microtome, and stained with hematoxylin-eosin for evaluating tissue inflammation. The remainder of the prostate was used for immunohistochemical staining. Paraffin embedded prostate sections were placed on silicone-coated slides. After deparaffinization in xylene and rehydration using graded alcohol solutions, the sections were fixed with 10 mM sodium citrate pH 6 at 105°C by Autoclave (TOMY SEIKO co. Ltd.) for antigen retrieval. The tissues were rinsed with phosphate buffered saline (PBS), transferred to 0.3% hydrogen peroxide for 10 min to block peroxidase activity, and rinsed with distilled water and PBS. The sections were blocked with 10% normal goat serum (NICHIREI CORPORATION.) for 30 min. These tissues were incubated with an anti-ERβ antibody (1:1,000; Santa Cruz Biotechnology, Inc.) for 1 hr at room temperature. After washing with PBS, the tissue sections were incubated for 30 min with HRP-labeled polymer anti-rabbit antibody (DAKO.) at room temperature. After washing with PBS, the color was developed using the Dako Cytomation Liquid DAB Substrate Chromogen System (DAKO). The tissues were counterstained with hematoxylin.
Real-Time Reverse Transcriptase (RT) PCR
A part of the ventral lobes of prostate and the bladder mucosa was used for Real-Time RT PCR analyses. Total ribonucleic acid (RNA) was isolated from tissue using the Trizol reagent (Invitrogen Life Technologies, Carlsbad, CA) according to the manufacture’s instructions. Total RNA (1 μg) was synthesized into cDNA using the ThermoScript RT-PCR System (Invitrogen Life Technologies) according to the manufacture’s instructions. After the reverse transcription reaction, quantitative real-time PCR was performed with a LightCycler1 Fast-Start DNA Master SYBR-Green I reaction mix (Roche Molecular Biochemicals, Mannheim, Germany) and QuantiTect Primer Assays (Qiagen Inc., Hilden, Germany) on a LightCycler system (Roche Diagnostics Corp., Indianapolis, IN). Each cycle included denatureation at 95°C for 15 sec, annealing at 55°C for 5 sec and polymerization at 72°C for 10 sec. The primers used were ERβ (NM_012754), ERα (NM_012689), TNFα (NM_01 2675), iNOS (XM_003750865), COX2 (NM_017008), P2X2 (NM_207608), TRPA1 (XM_008769306), NGF (NM_001277055), and the reference gene, GAPDH (NM_017008). The primers, ERβ, ERα, TNFα, COX2, NGF, and GAPDH were pre-designed and validated by QIAGEN (QuantiTect Primers, QIA-GEN). The others were designed by Primer 3 software (Table I). Real-time PCR data were analyzed by the comparative CT method.
TABLE I.
Primer Sequences
| Gene name | Primer sequence | Accession number |
|---|---|---|
| iNOS | F:AGACGCACAGGCAGAGGT | XM_003750865 |
| R:AGGCACACGCAATGATGG | ||
| TRPA1 | F:ATCAGGAGACCCTGCTTCAC | NM_207608 |
| R:GTTGATGTCTGCTCCCACTG | ||
| P2X2 | F:GCATCATCACCAGGATCGAG | XM_008769306 |
| R:GTCTTGGAGTCCCCATGGTA |
Statistical Analysis
Data are presented as means ±SEM. Statistical analysis was performed using Steel-Dwass test. P-values less than 0.05 were considered statistically significant.
RESULTS
Cystometric Investigation
The mean number of NVCs was significantly greater in formalin-vehicle rats than that in sham-saline rats (2.7±0.66 and 0.4±0.32 per micturition cycle, respectively, P < 0.05), and VI was significantly decreased in formalin-vehicle rats compared to sham-saline rats (1,232±126 sec and 2,389±323 sec, respectively, P < 0.05). However, these changes were improved by 3α-Adiol administration in formalin-treatment rats compared to formalin-vehicle rats (1,232±126 sec and 2,471±235 sec, respectively, P < 0.05). There was no significant difference in MVP and RV among groups (Fig. 1).
Fig. 1.
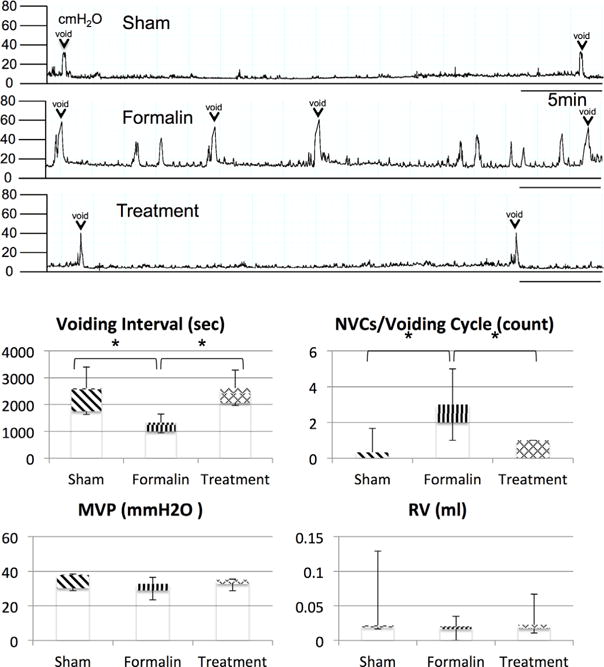
Cystometrograms obtained at 28 days after induction of prostatic inflammation. The mean number of NVCs was significantly greater in formalin-vehicle rats (Formalin) than that in sham-saline rats (Sham) (P < 0.05), and VI was significantly decreased in formalin-vehicle rats compared to sham-saline rats (P < 0.05). The 3α-Adiol administration in formalin-treatment rats (Treatment) recovered these changes. There was no significant difference in MVP and RV among three groups. VI, voiding Interval; NVCs, non voiding contractions; MVP, maximum voiding pressure; RV, residual volume; N = 5 per group. P < 0.05 between groups.
Histopathology
There were regular shaped acini and intact basement membrane in the ventral lobes of prostate tissue from the sham-saline group. (Fig. 2A), whereas prostate tissue from formalin-vehicle rats showed stromal infiltration of mast cells and lymphocytes and irregular shaped acini (Fig. 2B). However, these changes were not observed in the prostate tissue from formalin-treatment rats (Fig. 2C). Bladder tissues from all three groups of rats showed no inflammatory cell accumulation or epithelial formation changes (Fig. 2D–F).
Fig. 2.
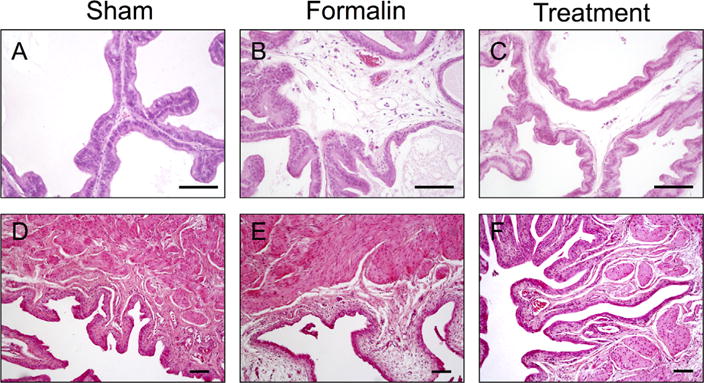
Photomicrographs showing hematoxylin and eosin staining of prostatic ventral lobe tissue sections (A–C) and bladder wall sections (D–F) in each group. There were regular shaped acini and intact basement membrane in the prostate tissue from the control group (Fig. 2A), whereas prostate tissues from formalin-vehicle rats showed stromal infiltration of mast cells and lymphocytes and irregular shaped acini (Fig. 2B). These changes were not observed in the prostate tissue from formalin-treatment rats (Fig. 2C). Bladder tissues from any of three groups did not show inflammatory cell accumulation or epithelial formation changes (D–F). Images were taken at magnification of 100 (D–F), or 200 (A–C). Scale bars: 100 μm. Sham, sham-saline treatment; Formalin, formalin-induced prostatic inflammation with vehicle treatment; Treatment, formalin-induced prostatic inflammation with 3α-Adiol treatment.
Expression of NGF, P2X2, and TRPA1 mRNA in the Bladder Mucosa
NGF, P2X2, and TRPA1 receptor mRNA expression was significantly increased by three to ninefold in the bladder mucosa of formalin-vehicle rats compared to sham-saline rats (Fig. 3). The treatment with 3α-Adiol significantly suppressed the upregulation of these genes in the bladder mucosa of formalin-treatment rats compared to formalin-vehicle rats.
Fig. 3.
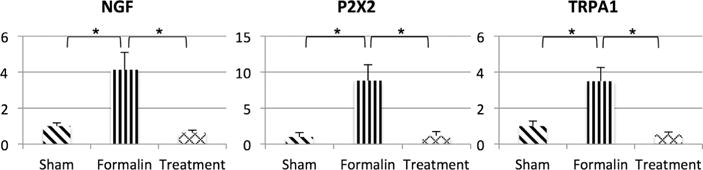
Relative expression of NGF, P2X2, and TRPA1 genes in the bladder. Statistically significant upregulation of these genes was observed in formalin-vehicle rats compared to sham-saline rats. In formalin-treatment rats, mRNA expression of these genes was significantly decreased compared to formalin-vehicle rats. Data are expressed as the relative expression ratio of each target gene to that of GAPDH. Sham; sham-saline treatment, Formalin; formalin-induced prostatic inflammation with vehicle treatment, Treatment; formalin-induced prostatic inflammation with 3α-Adiol treatment. N = 5 per group. P < 0.05 between groups.
Expression of TNFα, iNOS, COX2, and ERs mRNA in the Ventral Lobes of Prostate
In the ventral lobes of prostate, mRNA TNFα, iNOS, COX2, and ERα mRNA expression was significantly increased by two to fivefolds in formalin-vehicle rats compared to sham-saline rats (Fig. 4). However, these genes were downregulated after 3α-Adiol administration in formalin-treatment rats. On the other hand, the expression of ERβ in formalin-vehicle rats was decreased to approximately 30% of the ERβ expression of sham-saline rats (P < 0.05), whereas the treatment with 3α-Adiol restored the expression of ERβ in formalin-treatment rats.
Fig. 4.
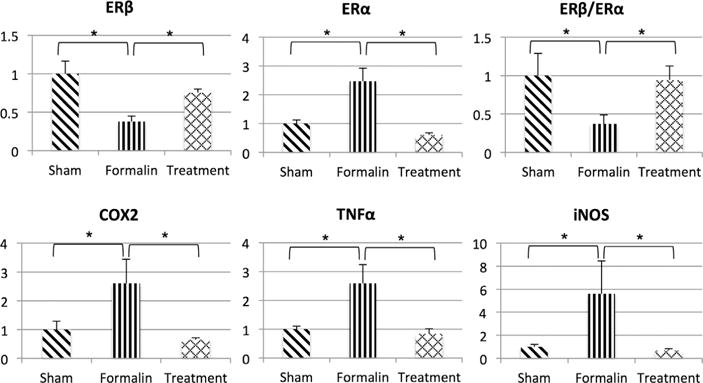
Relative expression of inflammation-related genes and estrogen receptor genes in the ventral lobes of prostate. Statistically significant upregulation of inflammation related genes and ERα gene was observed in the prostate tissue from formalin-vehicle rats compared to sham-saline rats. On the other hand, ERβ mRNA expression in formalin-vehicle rats was significantly decreased compared to sham-saline rats. In contrast, inflammation related genes and ERα gene were significantly decreased in formalin-treatment rats compared to formalin-vehicle rats. However, activation of ERβ by 3α-Adiol reversed the decreased relative expression ratio of ERβ to ERα (ERβ/ERα ratio) in formalin-treatment rats compared to formalin-vehicle rats. Data are expressed as the relative expression ratio of each target gene to that of GAPDH except ERβ/ERα ratio. Sham, sham-saline treatment; Formalin, formalin-induced prostatic inflammation with vehicle treatment; Treatment, formalin-induced prostatic inflammation with 3α-Adiol treatment. N = 5 per group. P < 0.05 between groups.
Immunohistochemistry
In sham-saline rats, ERβ was expressed in epithelial cell nuclei and the cytoplasm of the ventral lobes of prostate tissue (Fig. 5A). However, in formalin-vehicle rats, ERβ expression was lost in epithelial cell nuclei of irregular shaped acini with inflammatory changes in the ventral lobes of prostate (Fig. 5B). After 3α-Adiol administration in formalin-treatment rats, ERβ expression was recovered to the level of sham-saline rats (Fig. 5C).
Fig. 5.
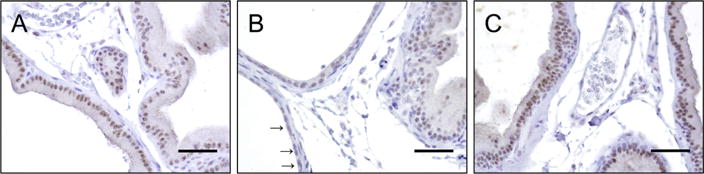
Photomicrographs showing immunohistochemical staining in prostatic ventral lobe tissue sections. A: sham-saline rat, B: formalin-vehicle rat, C: formalin-treatment rat that received 3α-Adiol administration. Positive staining for ERβ was observed in the epithelial nuclei and, at a lesser degree, in the cytoplasm of the prostate tissue from sham-saline rats and formalin-treatment rats (Fig. 5A and C). In contrast, in formalin-vehicle rats, staining for ERβ was weakened in the epithelial nuclei of irregular shaped acini which are indicated by black arrows () in the prostate tissue (Fig. 5B). Images were taken at magnification of 400. Scale bars: 100 μm.
DISCUSSION
In the present study, we investigated the effect of ERβ activation on not only prostatic inflammation, but also bladder overactivity in a rat model with non-bacterial prostatic inflammation.
Patients with BPH often exhibit irritating bladder symptoms such as urinary frequency or urgency. In this study, rats with prostatic inflammation showed bladder overactivity as evidenced by increased NVCs and decreased VI in association with increased expression of NGF, P2X2 and TRPA1 (Figs.1 and 3). NGF is reportedly one of robust biomarkers for overactive bladder (OAB) because of its high concentration in the patients’ urine [23]. The expression of P2X2 and TRPA1 receptors was also examined in this study because these receptors that are predominantly expressed in C-fiber afferent pathways are shown to contribute to afferent sensitization, which has been implicated as a pathophysiological mechanism in OAB and/or bladder inflammation [24,25]. Therefore, in this study, the upregulation of these genes in the bladder might indicate bladder afferent sensitization, resulting enhanced bladder activity after prostatic inflammation.
In a rat model of formalin induced prostatic inflammation, Vera et al. showed that injected formalin was restricted to the prostate using formalin mixed with dye [26]. Moreover, in a rat model of cystitis with transurethral formalin instillation, bladder epithelial damage and tissue edema were observed throughout the bladder wall [27]. In the present study, we did not observe histological changes such as edema or leucocyte infiltration in the bladder wall after intraprostatic formalin injection. These results suggest that there is a mechanism which causes bladder overactivity without direct formalin infiltration into the bladder wall. It has recently been reported that neural cross-talk via the convergence of pelvic afferents is a potential source of the overlap of chronic pelvic pain syndrome including interstitial cystitis and irritable bowel syndrome [28]. Other studies also reported that dichotomized DRG neurons supplying both bladder and prostate may play a role in prostate-to-bladder neural cross-talk after prostatic inflammation [29–31]. Thus, it is possible that prostate-to-bladder cross-organ sensitization through dichotomized afferents might be involved at least in part in the induction of the bladder overactive condition following non-bacterial prostatic inflammation in this study.
In formalin-vehicle rats, bladder overactivity, upregulation of ERα, downregulation of ERβ, and inflammation-related genes such as TNF-α, iNOS, and COX2, were shown in the prostate. However, treatment with an ERβ agonist reduced bladder overactivity and mRNA expression of ERα, TNF-α, iNOS, and COX2 and also restored mRNA expression of ERβ. TNF-α is a pro-inflammatory cytokine, which is rapidly generated by macrophages in response to tissue injury [32]. Upregulation of the expression of COX-2 and iNOS induces production of PGE2 and NO [33]. These products are reported to play important roles in progression of tissue inflammation [34]. We also recently reported that a rat model of formalin-induced prostatic inflammation showed the upregulation of androgen receptor-responsive genes and TGFβ-related genes in the prostate [11], which is similarly found in human BPH tissues [12]. Thus, the animal model used in this study is possibly useful for an investigation of an inflammatory aspect of BPH and male LUTS pathophysiology.
Although estrogen is known to be involved in regulation of prostate growth, it also has adverse effects on prostatic epithelium such as aberrant proliferation, inflammation, and carcinogenesis [13,14]. There are previous reports indicating that these effects of estrogen on the prostate are related to activation of ERα. Prins et al. showed that the prostate of ERα knockout (KO) mice did not respond to neonatal estrogenization, whereas the prostate of ERβKO mice still showed epithelial dysplasia and inflammatory cell infiltrates similar to that observed in wild type animals [13,15], indicating that ERα receptors are involved in induction of prostatic inflammation. Therefore, the alteration of ERα and ERβ expression was investigated using a rat model of nonbacterial prostatic inflammation in this study. We observed upregulation of ERα mRNA expression by formalin injection into the prostate (Fig. 4), whereas the expression of ERβ was downregulated by formalin-induced prostatitis (Figs. 4 and 5B). Thus, it is possible that formalin-induced prostatic inflammation increases the relative expression level of ERα to ERβ, which may contribute to chronic prostatic inflammation, although further studies are needed to clarify this point. A previous study using human BPH tissues also showed increased expression levels of ERα in addition to androgen receptors in prostatic epithelial cells compared to normal prostatic tissue [35], suggesting that activation of both ERα and androgen receptors is an important mechanism in the proliferation associated with BPH.
In addition, there is increasing evidence that supports ERβ as a mediator of the beneficial anti-inflammatory and anti-proliferative effects of estrogen in the prostate [13,17]. Various animal disease models have shown anti-inflammatory effects of ERβ in the brain, intestinal tract, bladder, and skin tissues [19–21,36]. In an animal model of inflammatory bowel disease induced by infection with Helicobacter hepaticus, ERβ agonists improved the inflammatory condition with attenuation of the expression of inflammatory cytokines [20]. Chaudhary et al. showed that the ERβ agonist, ERB41, administration could improve skin inflammation and inhibit photocarcinogenesis induced by ultra violet B (UVB). This study also showed that ERβ stimulation could restore or enhance the expression of ERβ, which was diminished in the skin SCC induced by UVB [21]. An in vivo study using the mouse brain microvascular endothelial cell line, estrogen, which has affinity to both ERα and ERβ, recovered the expression of ERβ that was reduced by lipopolysaccharide-induced inflammation [37]. Taken together, these data suggest that ERβ stimulation could restore the expression of ERβ decreased by inflammation, which might be a mechanism underlying anti-inflammatory effects of ERβ stimulation in various tissues or cell lines. In the present study, we showed the recovered expression of ERβ after 3α-Adiol administration and downregulation of inflammation-associated genes as well as ERα. Furthermore, the 3α-Adiol administration also increased the relative ERβ expression to ERα, which leads to anti-inflammation effects on nonbacterial prostatic inflammation.
There are some limitations of this study. Firstly, 3α-Adiol is known to be not only a precursor of an ERβ ligand, 3β-Adiol, but also an androgenic steroid [22]. Because increased ERα expression after prostatic inflammation was normalized by the 3α-Adiol treatment, it is possible that androgenic stimulation by 3α-Adiol may contribute to the reduction of ERα although it contradicts the findings that inflammation-mediated androgen receptor stimulation shown in our previous study [11] is associated with ERα upregulation, not downregulation, in the rat prostate, as demonstrated in this study. Thus, future studies using specific ER agonists or androgen receptor antagonists are needed to clarify the receptor specific mechanisms or their interactions in the modulation of prostatic inflammation. Secondly, we did not perform the quantitative analysis of inflammatory phenotypes in the prostate including incidence of abnormal acini after prostatic inflammation or 3α-Adiol treatment because this study focused on the bladder phenotypes such as bladder overactivity and molecular changes in the bladder following prostatic inflammation. Thus, we will plan to perform a further study to examine the ER-mediated modulatory mechanisms of prostatic inflammation in future.
CONCLUSION
We observed the increase of inflammation-related gene expression and the decrease of relative ERβ expression to ERα, which lead to progression of non-bacterial prostatic inflammation and bladder overactivity in an animal model. Furthermore, we showed that the 3α-Adiol treatment could increase relative ERβ expression to ERα, and suppressed bladder overactivity and prostatic inflammation. Thus, ERβ stimulation could be a therapeutic strategy for the treatment of prostatic inflammation and irritative bladder symptoms in patients with BPH in which prostatic inflammation is involved in the emergence of LUTS.
Acknowledgments
This work was supported by GSK Japan Research Grant 2015 (SM, KM, and HM), the National Institutes of Health (U54 DK112079) (ZW, NY, and DD) and University of Pittsburgh Physicians (UPP) Foundation Grant (NY).
Grant sponsor: GlaxoSmithKline Foundation; Grant sponsor: National Institutes of Health; Grant number: U54 DK112079; Grant sponsor: University of Pittsburgh Physicians (UPP) Foundation.
Footnotes
Conflicts of interest: Zhou Wang, Teresa Liu, Yasuhito Funahashi, Fuminori Sato, Donald B. DeFranco, or Naoki Yoshimura have no conflict of interest.
References
- 1.Kramer G, Mitteregger D, Marberger M. Is benign prostatic hyperplasia (BPH) an immune inflammatory disease? Eur Urol. 2007;51(5):1202–1216. doi: 10.1016/j.eururo.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 2.Sciarra A, Di Silverio F, Salciccia S, Autran Gomez AM, Gentilucci A, Gentile V. Inflammation and chronic prostatic diseases: Evidence for a link? Eur Urol. 2007;52(4):964–972. doi: 10.1016/j.eururo.2007.06.038. [DOI] [PubMed] [Google Scholar]
- 3.Mishra VC, Allen DJ, Nicolaou C, Sharif H, Hudd C, Karim OM, Motiwala HG, Laniado ME. Does intraprostatic inflammation have a role in the pathogenesis and progression of benign prostatic hyperplasia? BJU Int. 2007;100(2):327–331. doi: 10.1111/j.1464-410X.2007.06910.x. [DOI] [PubMed] [Google Scholar]
- 4.Taoka R, Tsukuda F, Ishikawa M, Haba R, Kakehi Y. Association of prostatic inflammation with down-regulation of macrophage inhibitory cytokine-1 gene in symptomatic benign prostatic hyperplasia. J Urol. 2004;171(6 Pt 1):2330–2335. doi: 10.1097/01.ju.0000127760.87421.e9. [DOI] [PubMed] [Google Scholar]
- 5.Roehrborn CG. Definition of at-risk patients: Baseline variables. BJU Int. 2006;97(Suppl 2):7–11. doi: 10.1111/j.1464-410X.2006.06098.x. discussion 21–12. [DOI] [PubMed] [Google Scholar]
- 6.Andriole G, Bostwick D, Brawley O, Gomella L, Marberger M, Tindall D, Breed S, Somerville M, Rittmaster R. Chemoprevention of prostate cancer in men at high risk: Rationale and design of the reduction by dutasteride of prostate cancer events (REDUCE) trial. J Urol. 2004;172(4 Pt 1):1314–1317. doi: 10.1097/01.ju.0000139320.78673.2a. [DOI] [PubMed] [Google Scholar]
- 7.Nickel JC, Roehrborn CG, O’Leary MP, Bostwick DG, Somerville MC, Rittmaster RS. The relationship between prostate inflammation and lower urinary tract symptoms: Examination of baseline data from the REDUCE trial. Eur Urol. 2008;54(6):1379–1384. doi: 10.1016/j.eururo.2007.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andersson KE. LUTS treatment: Future treatment options. Neurourol Urodynamics. 2007;26(6 Suppl):934–947. doi: 10.1002/nau.20500. [DOI] [PubMed] [Google Scholar]
- 9.Yoshimura N, Kaiho Y, Miyazato M, Yunoki T, Tai C, Chancellor MB, Tyagi P. Therapeutic receptor targets for lower urinary tract dysfunction. Naunyn-Schmiedeberg’s Arch Pharmacol. 2008;377(4–6):437–448. doi: 10.1007/s00210-007-0209-z. [DOI] [PubMed] [Google Scholar]
- 10.Lee S, Yang G, Bushman W. Prostatic inflammation induces urinary frequency in adult mice. PLoS ONE. 2015;10(2):e0116827. doi: 10.1371/journal.pone.0116827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Funahashi Y, O’Malley KJ, Kawamorita N, Tyagi P, DeFranco DB, Takahashi R, Gotoh M, Wang Z, Yoshimura N. Upregulation of androgen-responsive genes and transforming growth factor-beta1 cascade genes in a rat model of non-bacterial prostatic inflammation. Prostate. 2014;74(4):337–345. doi: 10.1002/pros.22668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Malley KJ, Dhir R, Nelson JB, Bost J, Lin Y, Wang Z. The expression of androgen-responsive genes is up-regulated in the epithelia of benign prostatic hyperplasia. Prostate. 2009;69(16):1716–1723. doi: 10.1002/pros.21034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Risbridger GP, Wang H, Frydenberg M, Cunha G. The meta-plastic effects of estrogen on mouse prostate epithelium: Proliferation of cells with basal cell phenotype. Endocrinology. 2001;142(6):2443–2450. doi: 10.1210/endo.142.6.8171. [DOI] [PubMed] [Google Scholar]
- 14.Ellem SJ, Risbridger GP. The dual, opposing roles of estrogen in the prostate. Ann N Y Acad Sci. 2009;1155:174–186. doi: 10.1111/j.1749-6632.2009.04360.x. [DOI] [PubMed] [Google Scholar]
- 15.Prins GS, Birch L, Couse JF, Choi I, Katzenellenbogen B, Korach KS. Estrogen imprinting of the developing prostate gland is mediated through stromal estrogen receptor alpha: Studies with alphaERKO and betaERKO mice. Cancer Res. 2001;61(16):6089–6097. [PubMed] [Google Scholar]
- 16.Ricke WA, Ishii K, Ricke EA, Simko J, Wang Y, Hayward SW, Cunha GR. Steroid hormones stimulate human prostate cancer progression and metastasis. Int J Cancer. 2006;118(9):2123–2131. doi: 10.1002/ijc.21614. [DOI] [PubMed] [Google Scholar]
- 17.Prins GS, Korach KS. The role of estrogens and estrogen receptors in normal prostate growth and disease. Steroids. 2008;73(3):233–244. doi: 10.1016/j.steroids.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tiwari-Woodruff S, Voskuhl RR. Neuroprotective and anti-inflammatory effects of estrogen receptor ligand treatment in mice. J Neurol Sci. 2009;286(1–2):81–85. doi: 10.1016/j.jns.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Acar D, Cayan S, Aktas S, Tek M, Akbay E. The effect of tamoxifen on bladder functions and histology, and the role of estrogen receptor beta in a rat chemical cystitis model. Neurourol Urodynamics. 2007;26(2):309–316. doi: 10.1002/nau.20247. [DOI] [PubMed] [Google Scholar]
- 20.Cook LC, Hillhouse AE, Myles MH, Lubahn DB, Bryda EC, Davis JW, Franklin CL. The role of estrogen signaling in a mouse model of inflammatory bowel disease: A Helicobacter hepaticus model. PLoS ONE. 2014;9(4):e94209. doi: 10.1371/journal.pone.0094209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaudhary SC, Singh T, Talwelkar SS, Srivastava RK, Arumugam A, Weng Z, Elmets CA, Afaq F, Kopelovich L, Athar M. Erb-041, an estrogen receptor-beta agonist, inhibits skin photocarcinogenesis in SKH-1 hairless mice by down-regulating the WNT signaling pathway. Cancer Prev Res (Phila) 2014;7(2):186–198. doi: 10.1158/1940-6207.CAPR-13-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muthusamy S, Andersson S, Kim HJ, Butler R, Waage L, Bergerheim U, Gustafsson JA. Estrogen receptor beta and 17beta-hydroxysteroid dehydrogenase type 6, a growth regulatory pathway that is lost in prostate cancer. Proc Natl Acad Sci USA. 2011;108(50):20090–20094. doi: 10.1073/pnas.1117772108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ciftci S, Ozkurkcugil C, Yilmaz H, Ustuner M, Yavuz U, Yuksekkaya M, Cekmen MB. Urinary nerve growth factor and a variable solifenacin dosage in patients with an overactive bladder. Int Urogynecol J. 2016;27(2):275–280. doi: 10.1007/s00192-015-2825-3. [DOI] [PubMed] [Google Scholar]
- 24.Meng M, Zheng J, Yan J, Li Q, Fang Q, Li W. P2X2 and P2X5 receptors mediate bladder hyperesthesia in ICC in female overactive bladder. Cell Biochem Biophys. 2015;72(2):375–383. doi: 10.1007/s12013-014-0471-x. [DOI] [PubMed] [Google Scholar]
- 25.DeBerry JJ, Schwartz ES, Davis BM. TRPA1 mediates bladder hyperalgesia in a mouse model of cystitis. Pain. 2014;155(7):1280–1287. doi: 10.1016/j.pain.2014.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vera PL, Meyer-Siegler KL. Inflammation of the rat prostate evokes release of macrophage migration inhibitory factor in the bladder: Evidence for a viscerovisceral reflex. J Urol. 2004;172(6 Pt 1):2440–2445. doi: 10.1097/01.ju.0000138055.01611.13. [DOI] [PubMed] [Google Scholar]
- 27.Dupont MC, Spitsbergen JM, Kim KB, Tuttle JB, Steers WD. Histological and neurotrophic changes triggered by varying models of bladder inflammation. J Urol. 2001;166(3):1111–1118. [PubMed] [Google Scholar]
- 28.Pezzone MA, Liang R, Fraser MO. A model of neural cross-talk and irritation in the pelvis: Implications for the overlap of chronic pelvic pain disorders. Gastroenterology. 2005;128(7):1953–1964. doi: 10.1053/j.gastro.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 29.Chen Y, Wu X, Liu J, Tang W, Zhao T, Zhang J. Distribution of convergent afferents innervating bladder and prostate at dorsal root ganglia in rats. Urology. 2010;76(3):764, e761–e766. doi: 10.1016/j.urology.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 30.Lee S, Yang G, Xiang W, Bushman W. Retrograde double-labeling demonstrates convergent afferent innervation of the prostate and bladder. Prostate. 2016;76(8):767–775. doi: 10.1002/pros.23170. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz ES, La JH, Young EE, Feng B, Joyce S, Gebhart GF. Chronic prostatitis induces bladder hypersensitivity and sensi-tizes bladder afferents in the mouse. J Urol. 2016;196(3):892–901. doi: 10.1016/j.juro.2016.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beutler B, Cerami A. The biology of cachectin/TNF-a primary mediator of the host response. Annu Rev Immunol. 1989;7:625–655. doi: 10.1146/annurev.iy.07.040189.003205. [DOI] [PubMed] [Google Scholar]
- 33.Au RY, Al-Talib TK, Au AY, Phan PV, Frondoza CG. Avocado soybean unsaponifiables (ASU) suppress TNF-alpha, IL-1beta, COX-2, iNOS gene expression, and prostaglandin E2 and nitric oxide production in articular chondro-cytes and monocyte/macrophages. Osteoarthritis Cartilage. 2007;15(11):1249–1255. doi: 10.1016/j.joca.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 34.Brenner SS, Klotz U, Alscher DM, Mais A, Lauer G, Schweer H, Seyberth HW, Fritz P, Bierbach U. Osteoarthritis of the knee-clinical assessments and inflammatory markers. Osteoarthritis Cartilage. 2004;12(6):469–475. doi: 10.1016/j.joca.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 35.Nicholson TM, Sehgal PD, Drew SA, Huang W, Ricke WA. Sex steroid receptor expression and localization in benign prostatic hyperplasia varies with tissue compartment. Differentiation. 2013;85(4–5):140–149. doi: 10.1016/j.diff.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Marinis E, Acaz-Fonseca E, Arevalo MA, Ascenzi P, Fiocchetti M, Marino M, Garcia-Segura LM. 17beta-Oestradiol anti-inflammatory effects in primary astrocytes require oestrogen receptor beta-mediated neuroglobin up-regulation. J Neuroendocrinol. 2013;25(3):260–270. doi: 10.1111/jne.12007. [DOI] [PubMed] [Google Scholar]
- 37.Holm A, Andersson KE, Nordstrom I, Hellstrand P, Nilsson BO. Down-regulation of endothelial cell estrogen receptor expression by the inflammation promoter LPS. Mol Cell Endocrinol. 2010;319(1–2):8–13. doi: 10.1016/j.mce.2010.01.002. [DOI] [PubMed] [Google Scholar]


