Abstract
Objective
To assess the effect of a multifaceted intervention directed at general practitioners on six year mortality, morbidity, and risk factors of patients with newly diagnosed type 2 diabetes.
Design
Pragmatic, open, controlled trial with randomisation of practices to structured personal care or routine care; analysis after 6 years.
Setting
311 Danish practices with 474 general practitioners (243 in intervention group and 231 in comparison group).
Participants
874 (90.1%) of 970 patients aged ⩾40 years who had diabetes diagnosed in 1989-91 and survived until six year follow up.
Intervention
Regular follow up and individualised goal setting supported by prompting of doctors, clinical guidelines, feedback, and continuing medical education.
Main outcome measures
Predefined clinical non-fatal outcomes, overall mortality, risk factors, and weight.
Results
Predefined non-fatal outcomes and mortality were the same in both groups. The following risk factor levels were lower for intervention patients than for comparison patients (median values): fasting plasma glucose concentration (7.9 v 8.7 mmol/l, P=0.0007), glycated haemoglobin (8.5% v 9.0%, P<0.0001; reference range 5.4-7.4%), systolic blood pressure (145 v 150 mm Hg, P=0.0004), and cholesterol concentration (6.0 v 6.1 mmol/l, P=0.029, adjusted for baseline concentration). Both groups had lost weight since diagnosis (2.6 v 2.0 kg). Metformin was the only drug used more frequently in the intervention group (24% (110/459) v 15% (61/415)).Intervention doctors arranged more follow up consultations, referred fewer patients to diabetes clinics, and set more optimistic goals.
Conclusions
In primary care, individualised goals with educational and surveillance support may for at least six years bring risk factors of patients with type 2 diabetes to a level that has been shown to reduce diabetic complications but without weight gain.
What is already known on this topic
Evidence is increasing that control of hyperglycaemia, hypertension, and dyslipidaemia may postpone the development of diabetic complications in patients with type 2 diabetes
Maintaining good control over a long period can be difficult
What this study adds
Structured individualised personal care with educational and surveillance support for general practitioners reduced levels of risk factors in type 2 diabetic patients after six years
Risk factors were reduced to a level that has been shown to have a beneficial effect on diabetic complications
Participants also showed modest weight loss
Introduction
Efforts to control hyperglycaemia,1 hypertension,2,3 and dyslipidaemia4 may postpone the development of complications in patients with type 2 diabetes.5 However, it is not known whether these results can be implemented over a long period in general practice. General practitioners often do not follow international recommendations,6,7 and the quality of care is not satisfactory even when clinical guidelines are provided.8,9 A combination of interventions, including prompting, may be needed to change general practitioners' behaviour and improve quality of care.10–13
We report the final results of a six year study from general practice examining the effect of structured personal care compared with routine care on overall mortality and on risk factors for and incidence of clinical complications in newly diagnosed diabetic patients aged 40 years or older. Structured care included regular follow up and setting of individualised goals for important risk factors, supported by prompting of doctors, feedback on individual patients, short clinical guidelines, and a brief training programme for general practitioners.
Participants and methods
Recruitment of general practitioners
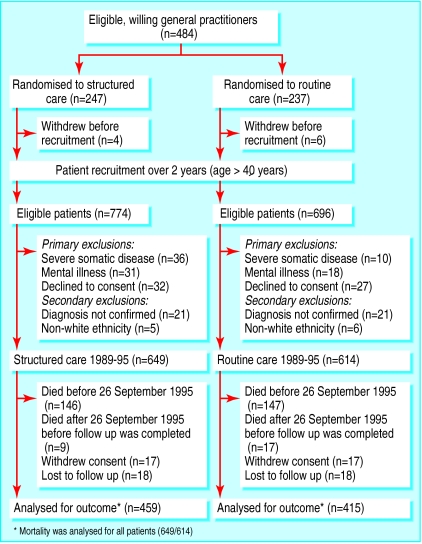
The study was a pragmatic, open, multicentre, cluster randomised controlled trial. In 1988, we sent a random sample of two thirds of Danish general practices, excluding singlehanded practices with a doctor aged ⩾60 years, a written invitation to participate in the study. Of 1902 doctors, 484 (25.4%) volunteered. Their practices were allocated by random numbers to two groups: structured care and routine care. Before randomisation, practices were stratified according to number of partners and spelling of practice address. Immediately after randomisation, 10 doctors dropped out, leaving 474 doctors in 187 single handed practices and 124 group practices (figure). After this no doctors who had study patients withdrew, but 80 and 67 new doctors joined the intervention and comparison group, respectively, when patients moved or new doctors joined or took over a practice. Only one doctor refused to examine a patient who had moved to the practice.
Recruitment of patients
We included all patients aged 40 or older with newly diagnosed diabetes between 1 March 1989 and 28 February 1991 based on hyperglycaemic symptoms or raised blood glucose values measured in general practice, or both, and who were registered with a participating general practitioner. In all, 1470 patients were eligible (figure). The diagnosis was subsequently established by a single fasting whole blood or plasma glucose concentration ⩾7.0/8.0 mmol/l measured at a major laboratory. The doctors were repeatedly instructed not to alter diagnostic practice during the inclusion period and to include all newly diagnosed patients. Patients who were in hospital at the time of diagnosis were also considered for inclusion.
The protocol based exclusion criteria were life threatening somatic disease, severe mental illness, or unwillingness to participate. For this analysis, we also excluded non-white patients and patients whose diagnosis was not established by a blood glucose measurement at a major laboratory within 500 days after diagnosis.
After the recruitment period ended, doctors were asked how many patients they had not included. Intervention and comparison doctors reported 39 and 51 patients, respectively, of whom they considered that 16 and 24 would eventually have been included if they had remembered or managed to do so. Eighteen of the 649 patients in the intervention group started insulin within 180 days after diagnosis. Insulin was discontinued for two of these patients during the observation period. Thus, at least 633 (97.5%) patients were considered to have type 2 diabetes.
Informed consent was obtained from all participants. The protocol was in accordance with the Helsinki declaration and was approved by the ethics committee of Copenhagen and Frederiksberg.
Comparison group: routine care
In the comparison group, doctors were free to choose any treatment and change it over time. From 1988 to 1996, all Danish general practitioners received diabetes guidelines on five occasions.14–16 These differed only slightly from the study guidelines. The study coordinating centre did not contact comparison practices after the end of recruitment (late 1991) until the final follow up examinations started in 1995. During the study period, the study coordinator (NdFO) sent 51 personal letters to doctors in the intervention group and 32 to doctors in the comparison group about study progress and preliminary results. In Denmark, routine care of patients with type 2 diabetes is usually given by general practitioners in ordinary consultations and not in disease management sessions run by nurses.
Intervention group: structured care
In the intervention group, follow up every three months and annual screening for diabetic complications were supported by sending a questionnaire to the general practitioner one month before the next expected consultation. The general practitioner was also requested to define, together with the patient, the best possible goals for blood glucose concentration, glycated haemoglobin, diastolic blood pressure, and lipids within three predefined categories (table 1). At each quarterly consultation, the general practitioner was asked to compare the achievements with the goal and consider changing either goal or treatment accordingly. In overweight patients, the general practitioner was prompted to get agreement on a small, realistic weight reduction and to follow up on this. However, a specific relative body weight was not strived for.
Table 1.
Treatment goals for intervention group
| Good control | Acceptable control | Poor control | |
|---|---|---|---|
| Fasting blood glucose (mmol/l)* | ⩽7.0 | ⩽8.0 | >8.0 |
| Non-fasting blood glucose (mmol/l)* | ⩽9.0 | ⩽11.0 | >11.0 |
| Glycated haemoglobin (%)† | ⩽7.0 | ⩽8.5 | >8.5 |
| Diastolic blood pressure (mm Hg) | ⩽90 | ⩽100 | >100 |
| Total cholesterol (mmol/l) | ⩽6.0 | ⩽7.0 | >7.0 |
| Fasting triglyceride (mmol/l) | ⩽2.0 | ⩽5.0 | >5.0 |
Capillary whole blood glucose.
Reference range 5.4-7.4%.
Instructions for general practitioners: The aim is normalisation of blood glucose, blood pressure, lipids, and possibly weight. For some patients, it will be impossible or even inappropriate to try to achieve the ideal goal, but prolonged symptoms of hyperglycaemia or hypoglycaemia must not be accepted for any patient. From an overall therapeutic point of view, the general practitioner chooses to aim at the treatment goals in one of the three categories. The choice of category is primarily based on glycated haemoglobin. Good control (normalisation of metabolism) is particularly relevant in young and middle aged patients and in well motivated older patients. Acceptable control applies to some older patients and patients who are difficult to treat or motivate. Poor control (freedom from symptoms) is intended for use when treatment has shown that any other goal is beyond reach.
The doctors received annual descriptive feedback reports on individual patients. They comprised the last six measurements of risk factors, complications, current treatment goal, and pharmacological treatment. No specific advice on treatment was given, but the role of microalbuminuria as a risk marker for cardiovascular disease was underlined.
The general practitioners were introduced to possible solutions to therapeutic problems through clinical guidelines supported by an annual half day seminar. The patients were never approached by the study centre, but four patient leaflets were produced for the doctor to hand out. The doctors were not obliged to follow the guidelines concerning diet and drug treatment (box). Generally, the importance of diet was stressed, and doctors were recommended to postpone, if possible, the start of antidiabetic drugs until at least three months after diagnosis to observe the effect of a possible weight loss.
Summary of treatment guidelines for general practitioners
Diet
Increase complex carbohydrate to at least 50% of the diet, and in particular increase water soluble fibre
Reduce fat content to maximum of 30%
Reduce alcohol intake
Eat 5-6 meals a day
Increase physical exercise
Smoking
Advise patients to cut down or stop
Persistent hyperglycaemia
Metformin for overweight patients
Glipizide or glibenclamide for patients with normal weight
Tolbutamide for patients >70 years
If goal for blood glucose is not met, metformin should be combined with a sulphonylurea before starting insulin
Hypertension
Angiotensin converting enzyme inhibitors or β blockers for most patients
Furosemide (frusemide) for patients with heart failure
Thiazides for patients >70 years
Hyperlipidaemia
Lipid lowering drugs for diet resistant hyperlipidaemia
Assessments
On 26 September 1995, the protocol based final follow up examinations were initiated in both groups and the intervention was terminated. The last examination was made in January 1998. Predefined primary outcomes were overall mortality and incidences of diabetic retinopathy, urinary albumin concentration ⩾15 mg/l, myocardial infarction, and stroke in patients without these outcomes at baseline. Secondary outcomes were new peripheral neuropathy, angina pectoris, intermittent claudication, and amputation. Tertiary outcomes were levels of risk factors included in patients' goals. We did not intend to do subgroup analyses.
The doctors recorded the following information on patients: body height and weight without shoes and outer garments; blood pressure and heart rate by routine methods after a 10 minutes' rest in a sitting position; sense of touch of cotton wool and pin prick on both feet; presence of patellar reflexes; drug treatment; history of myocardial infarction and stroke causing hospital admission; amputation of leg or part before or at the time of diagnosis of diabetes; familiarity with the patient; severe hypoglycaemic events that impaired consciousness and required help from another person during the preceding year; and doctors' background variables.
In questionnaires, patients gave information about whether they lived alone, education, (former) occupation, smoking habits,17 leisure time physical activity, angina pectoris,17 intermittent claudication,17 global self rated health, change of habits, food habits, symptoms of diabetes, and home glucose monitoring.
Eye backgrounds were evaluated by primary care ophthalmologists, who recorded the results of funduscopy in a multiple choice question that listed microaneurysms as the least severe lesions. Hypertension was defined as systolic/diastolic blood pressure ⩾160/90 mm Hg or the use of antihypertensive or diuretic drugs, or any combination of these. Peripheral neuropathy was defined as lack of a sense of touch of cotton wool or pin prick on at least one foot or absent patellar reflex on at least one knee, or any combination of these.
The day of death was taken from the death certificate. We obtained information on hospital admissions for relevant conditions (myocardial infarction, stroke, amputation, etc) since diagnosis from the national hospital discharge registry.
Assays
Fasting blood samples were analysed at Odense University Hospital. Plasma glucose concentration was measured by a glucose dehydrogenase method. Fraction of glycated haemoglobin was determined by ion exchange, high performance liquid chromatography (reference interval: 5.4-7.4%). Serum creatinine concentration was determined by the Jaffe reaction. Serum total cholesterol concentration was measured enzymatically with cholesterol esterase-cholesterol oxidase-peroxidase reagent. Serum triglyceride concentrations were determined enzymatically with a lipase-glycerolkinase-glycerol-3-phosphate oxidase-peroxidase reagent. Urinary albumin concentration was measured in a freshly voided morning urine sample at Århus University Hospital by a polyethyleneglycol radioimmunoassay.18
Statistical analysis and sample size
From the available unpublished clinical experience in 1987, we estimated that we needed between 100 and 1200 patients in each group to detect a 40% difference over 10 years between the groups in the four non-fatal outcomes with 80% power and 95% confidence. On the basis of published estimates of incidence,19 we needed 400 general practitioners to include 1600 patients over two years.
Analysis was by intention to treat. Quoted P values are not adjusted for multiple comparisons. Since there are five primary outcome variables we used the Bonferroni method and accepted P<0.01 as significant. All other outcomes were interpreted at the 5% level, but only to show tendencies. We compared intervention and comparison groups at follow up using a Wald test for binary and continuous variables. We did the analyses with the software PROC GENMOD, SAS version 6.12, using generalised estimating equations (GEE) methods to account for clustering at doctor level. Similarly, we used logistic regression analysis with non-fatal outcomes as responses to adjust for allocation of treatment group, age, sex, occupation, smoking habits, and time from diagnosis to measurement of outcome. We used a generalised linear mixed model (restricted maximum likelihood methods) with the predefined outcomes and explanatory variables as fixed effects and doctor identification as random effect to model the clustering. The time from diabetes diagnosis to death was taken into account by using a Cox regression model with no random effects.
Results
Doctors
When the study started, the general practitioners in the intervention and comparison groups had similar characteristics. There were no differences in sex (P=0.46) or median age (44 v 44 years, P=0.56), years of practice experience (10 v 10, P=0.95), years of hospital experience (6 v 6 years, P=0.40), number of doctors in the practice (2 v 2, P=0.10), number of patients per doctor (1158 v 1151, P=0.51), and weekly hours of work (43 v 43, P=0.69).
Patients
In all, 1316 (85.9%, range 0-12 per doctor) of 1470 eligible newly diagnosed diabetic patients were considered for this analysis. More patients in the intervention group than the comparison were excluded (P=0.002, χ2 test), mainly because of severe somatic disease (figure). The two groups did not differ in total number of patients included (P=0.33, χ2 test), inclusion activity over time (P=0.32, log rank test), and number of patients per doctor (P=0.51, χ2 test). Of 39 baseline variables, only occupation (P=0.01, χ2 test) and smoking habits (P=0.039) differed between the two groups (table 2).
Table 2.
Baseline characteristics of patients. Values are medians (interquartile ranges) unless stated otherwise
| Characteristic | No of respondents (structured/routine care) | Structured care | Routine care |
|---|---|---|---|
| Sociodemographic | |||
| No (%) men | 649/614 | 340 (52.4) | 326 (53.1) |
| Age (years) | 649/614 | 65.5 (55.3-74.0) | 65.3 (56.3-73.5) |
| No (%) live alone | 634/600 | 211 (33.3) | 197 (32.8) |
| No (%) basic school education only | 621/583 | 491 (79.1) | 453 (77.7) |
| (Former) occupation (No (%)) | 631/596 | ||
| Self employed | 153 (24.2) | 95 (15.9) | |
| Salaried employee | 158 (25.0) | 186 (31.2) | |
| Worker | 246 (39.0) | 231 (38.8) | |
| Housewife and other | 74 (11.7) | 84 (14.1) | |
| Doctor's familiarity with patient (No (%)) | 648/614 | ||
| Thorough | 306 (47.2) | 311 (50.7) | |
| Moderate | 251 (38.7) | 229 (37.3) | |
| Poor | 91 (14.0) | 74 (12.0) | |
| Clinical | |||
| Body mass index (kg/m2) | 647/614 | 29.4 (26.2-33.0) | 28.8 (26.0-32.3) |
| Body weight (kg) | 647/614 | 81.5 (71.3-94.3) | 82.1 (72.0-92.2) |
| No (%) with hypertension | 649/614 | 487 (75.0) | 456 (74.3) |
| Systolic blood pressure (mm Hg) | 649/614 | 150 (130-164) | 148 (130-160) |
| Diastolic blood pressure (mm Hg) | 649/614 | 85 (80-90) | 85 (80-90) |
| No (%) with diabetic retinopathy | 577/559 | 29 (5.0) | 25 (4.5) |
| No (%) with peripheral neuropathy | 645/610 | 121 (18.8) | 120 (19.7) |
| Resting heart rate (beats/min) | 647/613 | 76 (68-84) | 76 (68-84) |
| No (%) with known cardiovascular disorders: | |||
| History of myocardial infarction | 649/613 | 43 (6.6) | 47 (7.7) |
| Angina pectoris | 633/596 | 74 (11.7) | 71 (11.9) |
| History of stroke | 649/614 | 23 (3.5) | 26 (4.2) |
| Intermittent claudication | 634/598 | 25 (3.9) | 20 (3.3) |
| Amputation | 649/614 | 2 (0.3) | 4 (0.7) |
| Biochemical | |||
| Diagnostic fasting glucose (mmol/l) | 649/614 | 13.8 (10.7-17.0) | 13.7 (10.7-17.0) |
| Glycated haemoglobin (%)* | 534/506 | 10.2 (8.6-11.6) | 10.2 (8.7-11.9) |
| Total cholesterol (mmol/l) | 628/604 | 6.2 (5.4-7.1) | 6.2 (5.5-7.2) |
| Fasting triglyceride (mmol/l) | 625/604 | 2.03 (1.44-2.91) | 1.98 (1.39-2.95) |
| Urinary albumin (mg/l) | 615/589 | 11.7 (6.0-32.5) | 11.8 (5.7-27.5) |
| Serum creatinine (μmol/l) | 628/605 | 90 (81-101) | 88 (79-100) |
| Behavioural | |||
| No (%) of smokers | 633/598 | ||
| Never | 210 (33.2) | 167 (27.9) | |
| Former | 198 (31.3) | 225 (37.6) | |
| Current | 225 (35.5) | 206 (34.5) | |
| Tobacco consumption (g/day)† | 394/400 | 17.7 (10.0-23.0) | 15.7 (10.0-22.0) |
| Activity (No (%)) | 632/598 | ||
| Low | 182 (28.8) | 159 (26.6) | |
| Moderate | 405 (64.1) | 403 (67.4) | |
| High | 45 (7.1) | 36 (6.0) | |
| Self rated health (No (%)) | 635/600 | ||
| Very good | 71 (11.2) | 75 (12.5) | |
| Good | 219 (34.5) | 195 (32.5) | |
| Average | 286 (45.0) | 267 (44.5) | |
| Poor or very poor | 59 (9.3) | 63 (10.5) | |
Measured within 45 days of diabetes diagnosis. With a time limit of 365 days, glycated haemoglobin is 9.6% (8.1-11.5%) / 9.7%(8.2-11.5%), n=634/604, P=0.40. Reference range 5.4-7.4%.
Former and current smokers together.
The numbers completing the final follow up examination were similar in the two groups (459 v 415, P=0.21, χ2 test). A generalised linear mixed model was constructed with treatment group allocation, age, sex, whether living alone, education, and self rated health as fixed effects and doctor identification as random effect, but none of these variables predicted whether follow up was done or not for surviving patients. At the final follow up examination, the median (interquartile range) duration of diabetes was 5.75 (5.25-6.32) years for the intervention group and 5.86 (5.30-6.47) years for the comparison group (P=0.023).
Process of treatment
In the comparison group, no information was collected about the treatment process from diagnosis until the final examination. In the intervention group, the proportion of patients who had an annual clinical examination fell to 79% (327/412) over four years, and attendance at three monthly consultations was even less, despite prompting. The proportion of patients aiming at “good control” fell from 68% (401/587) to 63% (218/348) over four years. Of 459 intervention patients, 44 had a doctor who did not attend any of the six annual half day seminars, 104 had doctors who attended 1-2 seminars, 155 had doctors who attended 3-4 seminars, and 156 patients had doctors who attended 5-6 seminars.
Table 3 shows the drugs prescribed at the end of the study. Metformin was more widely used in the intervention group, the only group difference observed. Of those given oral antidiabetic drugs, 55% (58/105) of those with body mass ⩾30 in the intervention group and 32% (28/87) in the comparison group had metformin, P=0.001, χ2 test); for patients with a body mass index <30, the proportions were 31% (52/169) and 23% (33/142), respectively (P=0.14). The dose of the drugs was similar in both groups, except for in the eight intervention patients and 12 comparison patients receiving a combination of insulins, where the dose in the intervention group was lower. Insulin and lipid lowering drugs were recommended increasingly explicitly at the seminars, but doctors or patients were reluctant to comply.
Table 3.
Numbers (percentages) of patients receiving drug treatment at end of study
| Age 40-69 years
|
Age ⩾70 years
|
||||||
|---|---|---|---|---|---|---|---|
| Structured care (n=325) | Routine care (n=298) | P value* | Structured care (n=134) | Routine care (n=117) | P value* | ||
| Treatments to lower blood glucose | |||||||
| Diet only | 92 (28) | 92 (31) | 0.53 | 42 (31) | 39 (33) | 0.74 | |
| Oral antidiabetic drugs, total | 196 (60) | 165 (55) | 0.22 | 79 (59) | 64 (55) | 0.52 | |
| Sulphonylureas (SU) only | 101 (31) | 111 (37) | 0.11 | 61 (46) | 54 (46) | 0.92 | |
| Metformin only | 32 (10) | 14 (5) | 0.013 | 7 (5) | 2 (2) | — | |
| Sulphonylurea and metformin | 61 (19) | 39 (13) | 0.073 | 10 (7) | 6 (5) | 0.46 | |
| Other (combinations of) oral antidiabetics | 2 (1) | 1 (0) | — | 1 (1) | 2 (2) | — | |
| Insulin† | 39 (12) | 42 (14) | 0.42 | 14 (10) | 15 (13) | 0.55 | |
| No of different oral antidiabetic drugs | |||||||
| 1 | 132 (41) | 125 (42) | 0.74 | 67 (50) | 56 (48) | 0.74 | |
| 2 | 64 (20) | 40 (13) | 0.05 | 12 (9) | 8 (7) | 0.54 | |
| Drugs to lower blood pressure | |||||||
| Total | 170 (52) | 144 (48) | 0.35 | 86 (64) | 77 (66) | 0.80 | |
| Angiotensin converting enzyme inhibitor‡ | 72 (22) | 73 (25) | 0.50 | 17 (13) | 15 (13) | 0.98 | |
| β blocker‡ | 28 (9) | 22 (7) | 0.59 | 10 (7) | 6 (5) | 0.44 | |
| Calcium channel blocker‡ | 47 (14) | 44 (15) | 0.92 | 17 (13) | 17 (15) | 0.68 | |
| Diuretic‡ | 107 (33) | 90 (30) | 0.46 | 74 (55) | 63 (54) | 0.84 | |
| Other antihypertensive drugs‡ | 6 (2) | 5 (2) | 0.88 | 0 | 5 (4) | — | |
| No of different drugs to lower blood pressure: | |||||||
| 1 | 93 (29) | 71 (24) | 0.18 | 50 (37) | 45 (38) | 0.86 | |
| 2 | 55 (17) | 53 (18) | 0.78 | 31 (23) | 28 (24) | 0.88 | |
| ⩾3 | 22 (7) | 20 (7) | 0.98 | 5 (4) | 4 (3) | — | |
| Lipid lowering drugs | |||||||
| Total | 20 (6) | 14 (5) | 0.45 | 0 | 0 | — | |
Wald test. Not shown when cells have expected counts less than five.
In five cases in combination with an oral drug
Alone or in combination with other antihypertensive drugs.
Intervention doctors used their patients' participation in the study during consultations with patients more than comparison doctors (table 4). Intervention doctors set more optimistic goals for blood glucose concentration (P=0.0003, Wilcoxon test) and were less likely to regard their patients' motivation as very good than comparison doctors, but the doctors in the two groups were equally satisfied with their achievements (table 4).
Table 4.
Attitudes and opinions of general practitioners. Values are numbers (percentages) of respondents
| For patient in question, doctor's indication of: | Structured care | Routine care | P value* |
|---|---|---|---|
| Realistic goal for fasting whole blood glucose (mmol/l) | |||
| ⩽7.0 | 158 (35) | 107 (27) | 0.025 |
| >7.0-8.0 | 108 (24) | 88 (22) | 0.56 |
| >8.0-9.0 | 70 (15) | 61 (15) | 0.94 |
| >9.0 | 120 (26) | 146 (36) | 0.010 |
| Satisfaction with own efforts to obtain best possible diabetes control | |||
| Completely satisfied | 132 (29) | 128 (31) | 0.51 |
| Fairly satisfied | 256 (56) | 213 (52) | 0.29 |
| Not satisfied | 70 (15) | 69 (17) | 0.55 |
| Patient's attitude to study participation | |||
| Happy with the attention | 219 (48) | 91 (22) | <0.0001 |
| No special importance | 184 (40) | 287 (69) | <0.0001 |
| Irritated or bothered | 44 (10) | 18 (4) | 0.006 |
| Other | 12 (3) | 18 (4) | 0.16 |
| Use of fact that patient was participating in a study during consultations | |||
| Used vigorously | 60 (13) | 6 (1) | <0.0001 |
| Used moderately | 206 (45) | 34 (8) | <0.0001 |
| Only mentioned when necessary | 189 (42) | 368 (90) | <0.0001 |
| Patient's motivation for best possible control and treatment over past year | |||
| Very good | 86 (19) | 118 (29) | 0.002 |
| Good | 169 (37) | 128 (31) | 0.062 |
| Fair | 124 (27) | 96 (23) | 0.24 |
| Poor | 78 (17) | 71 (17) | 0.96 |
Wald test.
Primary and secondary outcomes
When multiple outcomes were taken into account with Bonferroni's adjustment, we found no differences in the predefined outcomes (table 5). The treatment group allocation was not a significant predictor of death in a Cox regression model adjusted for age, sex, body mass index, glycated haemoglobin, diastolic and systolic blood pressure, cholesterol concentration, triglyceride concentration, smoking habits, and physical activity (hazard ratio 0.91, 95% confidence interval 0.72 to 1.14; P=0.41). In logistic regression analyses taking in account clustering at doctor level and adjusted for age, sex, occupation, smoking habits, and duration of diabetes, there was no difference between groups in diabetic retinopathy (odds ratio 0.90, 95% confidence interval 0.53 to 1.52; P=0.69), microalbuminuria (0.63, 0.41 to 0.98; P=0.042), non-fatal myocardial infarction (0.65, 0.31 to 1.35; P=0.25), non-fatal stroke (0.89, 0.39 to 2.01; P=0.77), peripheral neuropathy (0.86, 0.57 to 1.28; P=0.45), angina pectoris (0.90, 0.49 to 1.66; P=0.74), or intermittent claudication (0.81, 0.35 to 1.88; P=0.63).
Table 5.
Outcomes at end of study.* Values are numbers (%) of group (mortality) or numbers (%) who completed follow up examination and did not have the outcome at baseline (all other outcomes)
| No (%) in structured care group | No (%) in routine care group | P value† | |
|---|---|---|---|
| Primary outcomes: | |||
| Overall mortality | 216/649 (33.3) | 208/614 (33.9) | 0.82 |
| Diabetic retinopathy | 43/359 (12.0) | 45/330 (13.6) | 0.55 |
| Urinary albumin ⩾15 mg/l | 56/249 (22.5) | 72/234 (30.8) | 0.04 |
| Myocardial infarction | 15/437 (3.4) | 18/393 (4.6) | 0.40 |
| Stroke | 18/446 (4.0) | 16/405 (4.0) | 0.95 |
| Secondary outcomes: | |||
| Peripheral neuropathy | 69/375 (18.4) | 69/329 (21.0) | 0.41 |
| Angina pectoris | 22/371 (5.9) | 23/346 (6.7) | 0.68 |
| Intermittent claudication | 13/382 (3.4) | 13/374 (3.5) | 0.96 |
| Amputation | 2/459 (0.44) | 4/414 (0.97) | 0.35 |
Median follow up period for structured care group was 7.41 years for mortality and 5.75 years for other outcomes; median follow up for routine care group was 7.32 years and 5.86 years, respectively.
Wald test. As there are five outcomes we accepted P<0.01 as significant.
Other outcomes
Median fraction of glycated haemoglobin was 8.5% in the intervention group, which is 1.1% higher than the upper limit of the reference range(5.4-7.4%) and 0.5% lower than in the comparison group (table 6). The difference of 0.5% corresponds to about 0.8 mmol/l in fasting plasma glucose concentration (table 6). The group differences for median systolic and diastolic blood pressures were 5 mm Hg and 4 mm Hg. Adjustment for baseline level of the outcome, duration of diabetes, age, sex, occupation, and smoking habits in linear regression analyses confirmed the treatment group difference for the logarithm of glycated haemoglobin (difference (log %) −0.056, 95% confidence interval −0.081 to −0.031; P<0.0001), systolic blood pressure (−5.0 mm Hg, −7.6 to −2.4 mm Hg; P=0.0002), and cholesterol concentration (−0.15 mmol/l, −0.29 to −0.02 mmol/l; P=0.029), but not for weight (−0.83 kg, −1.75 to 0.09 kg; P=0.076), diastolic blood pressure (−0.6 mm Hg, −1.9 to 0.7 mm Hg; P=0.35), logarithm of triglyceride concentration (−0.05 log mmol/l, −0.12 to 0.02 log mmol/l; P=0.19), or logarithm of serum creatinine concentration (−0.004 log μmol/l, −0.033 to 0.025 log μmol/l; P=0.79). Intracluster correlation coefficients varied from −0.021 to 0.054. Compared with weight at diagnosis, the weight at follow up was on average 2.6 kg lower in the intervention group and 2.0 kg lower in the comparison group.
Table 6.
Clinical, biochemical, behavioural, and process variables at end of study. Values are medians (interquartile ranges) unless stated otherwise
| Clinical | No of patients (structured/routine care) | Structured care | Routine care | P value* |
|---|---|---|---|---|
| Body weight (kg) | 448/404 | 79.9 (69.5-90.4) | 80.5 (70.0-92.0) | 0.72 |
| No (%) with hypertension | 455/409 | 333 (73) | 307 (75) | 0.56 |
| Systolic blood pressure (mm Hg) | 455/409 | 145 (130-160) | 150 (140-165) | 0.0004 |
| Diastolic blood pressure (mm Hg) | 455/409 | 80 (78-90) | 84 (78-90) | 0.40 |
| Resting heart rate (beats/min) | 452/404 | 72 (68-80) | 76 (68-80) | 0.43 |
| Biochemical | ||||
| Fasting plasma glucose (mmol/l)† | 350/296 | 7.9 (6.5-10.6) | 8.7 (7.2-11.6) | 0.0007 |
| Glycated haemoglobin (%)‡ | 450/408 | 8.5 (7.7-9.5) | 9.0 (8.0-10.4) | <0.0001 |
| Total cholesterol (mmol/l) | 449/408 | 6.0 (5.2-6.8) | 6.1 (5.4-6.9) | 0.12 |
| Fasting triglyceride (mmol/l) | 418/350 | 1.78 (1.25-2.52) | 1.89 (1.27-2.75) | 0.32 |
| Serum creatinine (μmol/l) | 449/408 | 89 (81-103) | 91 (80-105) | 0.84 |
| No (%) with glycosuria | 445/400 | 100 (22) | 148 (37) | <0.0001 |
| Behavioural (No (%) of patients) | ||||
| Has altered habits | 417/391 | 344 (82) | 315 (81) | 0.48 |
| Smoking | 419/390 | |||
| Never | 147 (35) | 124 (32) | 0.32 | |
| Former | 138 (33) | 151 (39) | 0.10 | |
| Current | 134 (32) | 115 (29) | 0.45 | |
| Activity | 415/392 | |||
| Low | 122 (29) | 122 (31) | 0.62 | |
| Moderate | 258 (62) | 239 (61) | 0.75 | |
| High | 35 (8) | 31 (8) | 0.78 | |
| Food habits | 416/393 | |||
| Diet with certain amounts of selected foodstuffs | 140 (34) | 121 (31) | 0.59 | |
| Full diet without sugar | 213 (51) | 207 (53) | 0.67 | |
| Diet as non-diabetic subjects | 63 (15) | 65 (17) | 0.36 | |
| Performs home blood or urine glucose monitoring | 416/388 | 117 (28) | 114 (29) | 0.73 |
| Self rated health | 421/394 | |||
| Very good | 68 (16) | 83 (21) | 0.087 | |
| Good | 176 (42) | 150 (38) | 0.29 | |
| Average | 153 (36) | 147 (37) | 0.77 | |
| Poor or very poor | 24 (6) | 14 (4) | 0.15 | |
| Process of care | ||||
| No of consultations/year | 459/414 | 6 (5-10) | 6 (4-9) | 0.002 |
| No of diabetes related consultations/year | 459/414 | 4 (3-6) | 4 (2-6) | <0.0001 |
| No (%) ever treated at diabetes clinic | 459/415 | 79 (17) | 106 (26) | 0.009 |
| No of hospital admissions since diagnosis | 459/415 | 1 (0-3) | 1 (0-3) | 0.79 |
| Total length of stay in hospital (days) | 281/256 | 16 (7-39) | 19 (8-45) | 0.066 |
| No (%) with severe hypoglycaemia since diagnosis | 457/413 | 17 (4) | 15 (4) | 0.94 |
| No (%) with symptoms of diabetes in past two weeks | 419/393 | 194 (46) | 193 (49) | 0.42 |
Wald test.
Including only results from samples analysed one day after sampling, or less.
Reference range 5.4-7.4%.
The patients give similar behavioural reports in both groups, but the intervention seems to have decreased referrals to diabetes clinics and increased the number of consultations (table 6). The main difference was that more intervention patients than control patients had four consultations a year (28% (129/459) v 19% (77/414) of control patients) and fewer had 0-1 consultations a year (10% (44/459) v 23% (95/414)). Hospital admission, severe hypoglycaemia, and experience of symptoms were similar in both groups. Hypoglycaemic episodes were suspected in 23% (12/53) of intervention and 11% (6/57) of comparison patients receiving insulin.
Discussion
This long term randomised trial in primary care shows that a multifaceted intervention directed at general practitioners moderates risk factors of patients with newly diagnosed type 2 diabetes. The interventions included regular follow up and individualised goals for patients supported by prompting of doctors, clinical guidelines, feedback, and continuing medical education. We achieved the same level of risk factors as recent large intervention studies from secondary care without the expected adverse weight gain.1,2,5
The randomisation of practices was successful both on doctor and patient level, and follow up was completed for 90% of surviving patients, which may be because of our simple, precisely defined eligibility criteria and measures.20 The list system with a well defined background population in each practice, the few exclusions, the unchanged inclusion activity over time irrespective of treatment allocation, and doctors' self reports suggest that our patients are likely to be representative of the general population of newly diagnosed diabetic people. This is an advantage over intervention studies in secondary care, which often use selected study populations.1
Predefined outcomes
Modern diabetes care is founded on the results from the UK prospective diabetes study1 2 and Steno type 2 study5 and post hoc analyses of hypertension and lipid trials.3,4 In retrospect, our study was underpowered to detect differences in the primary outcomes in an intention to treat analysis after only six years.1,2 Furthermore, some outcome measures lacked precision because we kept the demands on practitioners and patients to a minimum to prevent attrition.20 Comparison doctors may also have managed their patients more effectively than the average practitioner,21 decreasing the size of any effect.
Risk factors
After almost six years of intervention, the glycaemic control in the intervention group was similar to that achieved in the intervention arms of the Steno type 2 study5 and UK prospective diabetes study at the same point.1 The result is put into perspective by the relatively high median plasma glucose concentration at presentation in our study (13.8 mmol/l) compared with the UK prospective diabetes study (11.3 mmol/l), primarily reflecting the low diagnostic limit in that study.
The glycated haemoglobin fraction in our routine care group, however, was only 0.5% higher than in the structured care group. This reflects the fact that comparison doctors were supposed to “do their best,” and were not under the constraints imposed on doctors treating the comparison group in the UK prospective diabetes study.1 The doctors' reports on their use of study participation and their patients' attitude to it indicate a beneficial effect of study participation in itself. This is highest in the intervention group but also present in the comparison group. The many initiatives taken in Denmark to improve diabetes treatment in primary care may also have contributed.14–16
The favourable weight course, especially in the intervention group, might be ascribed to doctors being taught to await the effect of diet, exercise, and weight loss before starting antidiabetic drugs. This contrast with the strategy in UK prospective diabetes study1 and Steno type 2 study,5 which used stepwise increase of drugs to reach predefined treatment goals. Our individually agreed small, realistic weight losses may have prevented doctors and patients from losing focus on the individual goals for risk factors, in contrast to other approaches to personal care.22
At entry, the average blood pressures in our study (148-150/85 mm Hg) were similar to those in the Steno type 2 study (146-149/85-86 mm Hg)5 but higher than in the UK prospective diabetes study glucose trial(135/82 mm Hg)1 and lower than in its hypertension trial (159-160/94 mm Hg).2 The variation reflects differences in patient age and selection. The average difference between treatment groups in blood pressures was larger than in Steno type 2 study, but smaller than in the UK prospective diabetes study subgroup of hypertensive patients.
Despite reduced glycosuria, the symptom burden as well as a simple measure of self rated health was the same in both groups as in the UK prospective diabetes study.23 Our focus on individualised treatment therefore did not affect wellbeing measurably, although wellbeing has been reported to improve in patient centred diabetes care.22 Our failure to show a clinically important improvement in dyslipidaemia may be connected to our adoption of the relatively lax targets for lipid concentrations that were in use in 1988-95.14–16
What caused the reduction in risk factors?
Our flexible approach to the intervention may have maximised not only doctors' ability to participate but also the ultimate generalisability of results. The approach is feasible to implement within the health service24 and the patient sample was non-selective. In complex interventions the effect cannot be ascribed to single elements, although the continuing medical education is probably a core element.12,13 The fact that we used many ways to change doctors' behaviour may be the reason for success.10,11
The intervention apparently did not affect patient behaviour, except that more followed a three monthly follow up scheme, but this could be because of limitations in our measures. Intervention doctors, however, became more focused on lowering risk factors through setting goals, which perhaps prevented doctors from losing professional autonomy20,25 and involved patients in decision making.22,26 The psychological effect of the labelling of care explicitly as good, acceptable, and poor must not be underestimated either.27 Although normoglycaemia was rarely achieved in any of the groups, this was the goal for most intervention patients throughout the study. As a possibly negative side effect of this, intervention doctors tended to regard their patients' motivation as relatively low. Contrary to study recommendations, the referral rate of intervention patients to diabetes clinics was low. This could be because doctors were empowered by structuring care28 or because of patients' improved diabetes status.29
The only major difference in drug treatment between groups was that metformin was used more in the intervention group, especially among obese patients, and this may have contributed to the lower glycated haemoglobin fraction.30 Doctors' reports on their patients' antihypertensive treatment were similar in both groups. Therefore, the effect of the intervention on risk factors may also be partly explained by better compliance with treatment,31 which has been shown to be poor in type 2 diabetic patients.32 The prevalence of severe hypoglycaemia did not differ between groups and was similar to that in other trials.1,5 The tendency among those receiving insulin towards more hypoglycaemic episodes in the intervention group, unrelated to dose, supports the compliance hypothesis mentioned above.
Conclusion
We have shown that even in a group of motivated, volunteering general practitioners that were already supplying acceptable basic patient care, a multifaceted, individualised disease management strategy can provide extra benefit for patients with type 2 diabetes patients for at least six years. The flexible approach to the intervention and the population based patient sample suggest that our model for structured personal care could be applied at population level. Use of the model may reduce risk factors to a level that has been shown to have a beneficial effect on the development of diabetic complications without adverse weight gain.
Figure.
Flow of participants through trial
Acknowledgments
We thank the patients, general practitioners, and ophthalmologists who volunteered to take part in this study. We thank Niels Keiding for statistical advice, Carl Erik Mogensen, Niels Vesti Nielsen, and Dorte Gannik for advice on estimation of renal involvement, diabetic retinopathy, and patient attitudes and behaviour and Klaus Barfoed, Inge Bihlet, Ulla Eithz, Karen Faurfelt, Jørgen Garbøl, Jan Erik Henriksen, Poul Erik Gaarde Madsen, Jens Olesen, and Birthe Palmvig for their contributions to the seminars. We acknowledge the help of Jørgen Bo Nielsen, Lars C Larsen, Charlotte Hindsberger, Lars Jørgen Hansen, Volkert Siersma, and Maeve Drewsen and the expert technical assistance of Merete Møller, Elin Bang, Inge Bihlet, Ulla Johannesen, Klaus Tønning Sørensen, Christina Hundrup, Nann Agerlin Hansen, Birgitte Pedersen, Jesper Løken, Karsten Sørensen, and Lise Bergsøe.
Footnotes
Editorials by Griffin and Wagner
Funding: Danish Medical Research Council, Danish Research Foundation for General Practice, Health Insurance Foundation, Danish Ministry of Health, Novo Nordisk Farmaka Denmark, Pharmacy Foundation, Foundation for General Practice in Copenhagen, Frederiksberg, Tårnby og Dragør, Dr Sofus Carl Emil Friis and his wife Olga Doris Friis Trust, Danish Medical Association Research Fund, Velux Foundation, Rockwool Foundation, Novo Nordisk, Danish Diabetes Association, Oda og Hans Svenningsen Foundation, A P Møller Foundation for Advancement of Medical Science, Novo Nordisk Foundation, Captain Axel Viggo Mørch and his wife's Trust, Danish Eye Health Society, Mogens and Jenny Vissing's Trust, and Bernhard and Marie Klein's Trust.
Competing interests: None declared.
References
- 1.UK Prospective Diabetes Study Group. Intensive blood glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 2.UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703–713. [PMC free article] [PubMed] [Google Scholar]
- 3.Curb JD, Pressel SL, Cutler JA, Savage PJ, Applegate WB, Black H, et al. Effect of diuretic-based antihypertensive treatment on cardiovascular disease risk in older diabetic patients with isolated systolic hypertension. Systolic Hypertension in the Elderly Program Cooperative Research Group. JAMA. 1996;276:1886–1892. [PubMed] [Google Scholar]
- 4.Pyorala K, Pedersen TR, Kjekshus J, Faergeman O, Olsson AG, Thorgeirsson G. Cholesterol lowering with simvastatin improves prognosis of diabetic patients with coronary heart disease. A subgroup analysis of the Scandinavian simvastatin survival study (4S) Diabetes Care. 1997;20:614–620. doi: 10.2337/diacare.20.4.614. [DOI] [PubMed] [Google Scholar]
- 5.Gæde P, Vedel P, Parving H-H, Pedersen O. Intensified multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: the Steno type 2 randomised study. Lancet. 1999;353:617–622. doi: 10.1016/S0140-6736(98)07368-1. [DOI] [PubMed] [Google Scholar]
- 6.Dunn NR, Bough P. Standards of care of diabetic patients in a typical English community. Br J Gen Pract. 1996;46:401–405. [PMC free article] [PubMed] [Google Scholar]
- 7.Weiner JP, Parente ST, Garnick DW, Fowles J, Lawthers AG, Palmer RH. Variation in office-based quality. A claims-based profile of care provided to Medicare patients with diabetes. JAMA. 1995;273:1503–1508. doi: 10.1001/jama.273.19.1503. [DOI] [PubMed] [Google Scholar]
- 8.Feder G, Griffiths C, Highton C, Eldridge S, Spence M, Southgate L. Do clinical guidelines introduced with practice based education improve care of asthmatic and diabetic patients? A randomised controlled trial in general practices in east London. BMJ. 1995;311:1473–1478. doi: 10.1136/bmj.311.7018.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hetlevik I, Holmen J, Midthjell K. Treatment of diabetes mellitus—physicians' adherence to clinical guidelines in Norway. Scand J Prim Health Care. 1997;15:193–197. doi: 10.3109/02813439709035027. [DOI] [PubMed] [Google Scholar]
- 10.Griffin S. Diabetes care in general practice: meta-analysis of randomised control trials. BMJ. 1998;317:390–396. doi: 10.1136/bmj.317.7155.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wensing M, van der Weijden T, Grol R. Implementing guidelines and innovations in general practice: which interventions are effective? Br J Gen Pract. 1998;48:991–997. [PMC free article] [PubMed] [Google Scholar]
- 12.Davis DA, Thomson MA, Oxman AD, Haynes RB. Changing physician performance. A systematic review of the effect of continuing medical education strategies. JAMA. 1995;274:700–705. doi: 10.1001/jama.274.9.700. [DOI] [PubMed] [Google Scholar]
- 13.Cantillon P, Jones R. Does continuing medical education in general practice make a difference? BMJ. 1999;318:1276–1279. doi: 10.1136/bmj.318.7193.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beck-Nielsen H, Damsgaard EM, Faber O, Jørgensen FS, Sørensen NS. Non-insulin dependent diabetes mellitus. Diagnosis and treatment. Copenhagen: Danish Society of Internal Medicine; 1988. pp. 1–40. [Google Scholar]
- 15.Lauritzen T, Christiansen JS, Faber O. Non-insulin dependent diabetes—NIDDM. Clinical guidelines for practitioners. 1st, 2nd, and 3rd eds. Copenhagen: Danish College of General Practitioners; 1991. , 1994, 1996. [Google Scholar]
- 16.Beck-Nielsen H, Borch-Johnsen K, Damsgaard EM, Deckert T, Fog J, Frøland A, et al. Diabetes care in Denmark. The Danish National Board of Health. Tidsskrift for Diabetesbehandling. 1994;4:1–36. [Google Scholar]
- 17.Rose GA, Blackburn H, Gillum RF, Prineas RJ. Cardiovascular survey methods. WHO: Geneva; 1982. [Google Scholar]
- 18.Mogensen CE. Microalbuminuria and kidney function. Notes on methods, interpretation, and classification. In: Clarke WL, Larner J, Pohl S, editors. Methods in diabetes research. Vol II: clinical methods. New York: Wiley; 1986. [Google Scholar]
- 19.Melton LJ, Palumbo PJ, Chu CP. Incidence of diabetes mellitus by clinical type. Diabetes Care. 1983;6:75–86. doi: 10.2337/diacare.6.1.75. [DOI] [PubMed] [Google Scholar]
- 20.Ross S, Grant A, Counsell C, Gillespie W, Russell I, Prescott R. Barriers to participation in randomised controlled trials: a systematic review. J Clin Epidemiol. 1999;52:1143–1156. doi: 10.1016/s0895-4356(99)00141-9. [DOI] [PubMed] [Google Scholar]
- 21.Pringle M, Stewart-Evans C, Coupland C, Williams I, Allison S, Sterland J. Influences on control in diabetes mellitus: patient, doctor, practice, or delivery of care? BMJ. 1993;306:630–634. doi: 10.1136/bmj.306.6878.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kinmonth AL, Woodcock A, Griffin S, Spiegal N, Campbell MJ Diabetes Care from Diagnosis Research Team. Randomised controlled trial of patient centred care of diabetes in general practice: impact on current wellbeing and future disease risk. BMJ. 1998;317:1202–1208. doi: 10.1136/bmj.317.7167.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.UK Prospective Diabetes Study Group. Quality of life in type 2 diabetic patients is affected by complications but not by intensive policies to improve blood glucose or blood pressure control (UKPDS 37) Diabetes Care. 1999;22:1125–1136. doi: 10.2337/diacare.22.7.1125. [DOI] [PubMed] [Google Scholar]
- 24.Medical Research Council. A framework for development and evaluation of RCTs for complex interventions to improve health. London: MRC; 2000. [Google Scholar]
- 25.Grol R, Dalhuijsen J, Thomas S, Veld C, Rutten G, Mokkink H. Attributes of clinical guidelines that influence use of guidelines in general practice: observational study. BMJ. 1998;317:858–861. doi: 10.1136/bmj.317.7162.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greenfield S, Kaplan SH, Ware JEJ, Yano EM, Frank HJ. Patients' participation in medical care: effects on blood sugar control and quality of life in diabetes. J Gen Intern Med. 1988;3:448–457. doi: 10.1007/BF02595921. [DOI] [PubMed] [Google Scholar]
- 27.Brandt CJ, Ellegaard H, Joensen M, Kallan FV, Sorknaes AD, Tougaard L. Effect of diagnosis of “smoker's lung”. RYLUNG Group. Lancet. 1997;349:253. doi: 10.1016/s0140-6736(05)64863-5. [DOI] [PubMed] [Google Scholar]
- 28.Farmer A, Coulter A. Organization of care for diabetic patients in general practice: influence on hospital admissions. Br J Gen Pract. 1990;40:56–58. [PMC free article] [PubMed] [Google Scholar]
- 29.Majeed A, Bardsley M, Morgan D, O'Sullivan C, Bindman AB. Cross sectional study of primary care groups in London: association of measures of socioeconomic and health status with hospital admission rates. BMJ. 2000;321:1057–1060. doi: 10.1136/bmj.321.7268.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCormack J, Greenhalgh T. Seeing what you want to see in randomised controlled trials: versions and perversions of UKPDS data. BMJ. 2000;320:1720–1723. doi: 10.1136/bmj.320.7251.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagasawa M, Smith MC, Barnes JH, Fincham JE. Meta-analysis of correlates of diabetes patients' compliance with prescribed medications. Diabetes Educ. 1990;16:192–200. doi: 10.1177/014572179001600309. [DOI] [PubMed] [Google Scholar]
- 32.Sclar DA, Robison LM, Skaer TL, Dickson WM, Kozma CM, Reeder CE. Sulfonylurea pharmacotherapy regimen adherence in a Medicaid population: influence of age, gender, and race. Diabetes Educ. 1999;25:531–538. doi: 10.1177/014572179902500406. [DOI] [PubMed] [Google Scholar]