Abstract
The safety of triple oral therapy with dapagliflozin plus saxagliptin plus metformin versus dual therapy with dapagliflozin or saxagliptin plus metformin was compared in a post‐hoc analysis of 3 randomized trials of sequential or concomitant add‐on of dapagliflozin and saxagliptin to metformin. In the concomitant add‐on trial, patients with type 2 diabetes on stable metformin received dapagliflozin 10 mg/d plus saxagliptin 5 mg/d. In sequential add‐on trials, patients on metformin plus either saxagliptin 5 mg/d or dapagliflozin 10 mg/d received dapagliflozin 10 mg/d or saxagliptin 5 mg/d, respectively, as add‐on therapy. After 24 weeks, incidences of adverse events and serious adverse events were similar between triple and dual therapy and between concomitant and sequential add‐on regimens. Urinary tract infections were more common with sequential than with concomitant add‐on therapy; genital infections were reported only with sequential add‐on of dapagliflozin to saxagliptin plus metformin. Hypoglycaemia incidence was <2.0% across all analysis groups. In conclusion, the safety and tolerability of triple therapy with dapagliflozin, saxagliptin and metformin, as either concomitant or sequential add‐on, were similar to dual therapy with either agent added to metformin.
Keywords: dapagliflozin, DPP‐4 inhibitor, metformin, SGLT‐2 inhibitor, type 2 diabetes
1. INTRODUCTION
Despite the availability of several classes of glucose‐lowering agents, HbA1c goal attainment is suboptimal in type 2 diabetes. Even patients who do achieve their HbA1c targets are unlikely to maintain glycaemic control in the long term without treatment intensification.1
Metformin is the preferred first‐line treatment for patients with type 2 diabetes. Current clinical guidelines recommend stepwise addition of other glucose‐lowering therapies as the disease progresses in order to maintain adequate glycaemic control.2, 3 In patients with high baseline HbA1c, initial dual therapy is recommended, in an attempt to achieve glycaemic control more rapidly than with metformin monotherapy. While several classes of glucose‐lowering agents can be used in combination with metformin, sodium‐glucose cotransporter‐2 (SGLT‐2) inhibitors and dipeptidyl peptidase‐4 (DPP‐4) inhibitors are of particular interest, owing to their favourable efficacy and safety profiles. In phase 3 trials, triple therapy with dapagliflozin (an SGLT‐2 inhibitor), saxagliptin (a DPP‐4 inhibitor) and metformin reduced 24‐week HbA1c to a greater extent than dual therapy with dapagliflozin or saxagliptin in combination with metformin.4, 5, 6 A once‐daily fixed‐dose combination of 5 mg saxagliptin and 10 mg dapagliflozin is approved in Europe and the USA for treatment of type 2 diabetes.
The use of early combination therapy requires careful assessment of potential AEs. Each drug class has a specific AE profile, and combining agents from different drug classes has the potential to change these side effect profiles or to mitigate or cause additive adverse effects. In addition, the timing and sequence of the added agents may have an impact on the side effects. In this post‐hoc pooled analysis, we aimed to evaluate the safety and tolerability of triple therapy with dapagliflozin and saxagliptin added on to metformin. We also assessed the relative safety of concomitant add‐on or sequential add‐on therapeutic regimens.
2. MATERIALS AND METHODS
In this post‐hoc analysis (N = 1169), data from 3 phase 3, randomized, double‐blind trials (Study 1, NCT016060074; Study 2, NCT016463205; Study 3, NCT016190596), the results of which have been reported previously, were pooled. All studies included metformin‐treated patients (aged ≥18 years) with type 2 diabetes and inadequate glycaemic control (HbA1c ≥ 8.0% and ≤12.0%). The studies included a screening period, followed by an open‐label treatment period of varying duration (4‐16 weeks), with subsequent entry into a 24‐week, randomized double‐blind treatment period (Figure 1). The primary efficacy end point of each trial was the change from baseline in HbA1c at 24 weeks. Each study reported the incidences of AEs, serious AEs (SAEs) and hypoglycaemia in patients who received at least 1 dose of the study drug; laboratory parameters and vital signs were also monitored.
Figure 1.
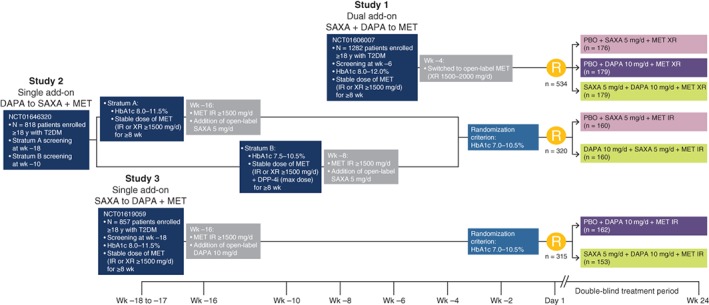
Study design. Abbreviations: d, day; DAPA, dapagliflozin; DPP‐4i, dipeptidyl peptidase‐4 inhibitor; IR, immediate release; MET, metformin; PBO, placebo; SAXA, saxagliptin; T2DM, type 2 diabetes; wk, week; XR, extended release; y, year
Analyses of AEs, SAEs and hypoglycaemic events in the 3 component studies were performed using pooled data sets; details of the data pooling methodology are available in online Appendix S1. Data were summarized by descriptive statistics and no formal statistical comparisons were made between treatment groups.
3. RESULTS
3.1. Patients and changes in HbA1c (all studies)
Baseline characteristics were mostly similar between the 3 studies (Table S1). Patients in the concomitant add‐on trial (Study 1) had higher mean HbA1c and fasting plasma glucose (FPG) values at baseline than did patients in Studies 1 and 2. Abnormal findings in medical histories were reported for 84% to 96% of patients across the 3 studies; a higher proportion of patients in Study 2 had diabetic complications (nephropathy, neuropathy and retinopathy) compared with patients in Studies 1 and 3. Mean and median estimated glomerular filtration rates (eGFRs) were approximately the same in all 3 studies. In the pooled data set, baseline demographic and clinical characteristics were balanced across treatment groups (Table S2). Mean HbA1c progressively decreased from screening to week 24 in all 3 studies (Table S3).
3.2. Safety of triple therapy vs dual therapy
The incidence of AEs and drug‐related AEs was similar across the 3 groups analysed (46.0%‐55.7% and 6.2%‐6.8%, respectively), as was the frequency of study drug discontinuation due to AEs (0.6%‐2.0%) (Table 1). While numerically more AEs led to discontinuation with triple therapy vs dual therapy, no AE preferred term led to discontinuation in more than 2 patients in any group. The incidence of SAEs was low and similar with both triple‐ and dual‐therapy regimens (<3.0%). No SAE was reported in more than 1 patient within each treatment group.
Table 1.
Adverse events for dapagliflozin plus saxagliptin plus metformin triple therapy and saxagliptin plus metformin or dapagliflozin plus metformin dual therapy
| Triple vs dual therapy | Concomitant vs sequential add‐on therapy | ||||
|---|---|---|---|---|---|
| Patients, n (%) | All studies Triple therapy with DAPA + SAXA + MET (N = 492) | Studies 1 and 3 Dual therapy with DAPA + MET (N = 341) | Studies 1 and 2 Dual therapy with SAXA + MET (N = 336) | Study 1 Concomitant add‐on DAPA + SAXA to MET (N = 179) | Studies 2 and 3 Sequential add‐on DAPA to SAXA + MET or SAXA to DAPA + MET (N = 313) |
| Any AE | 250 (50.8) | 157 (46.0) | 187 (55.7) | 87 (48.6) | 163 (52.1) |
| AE related to study medication | 32 (6.5) | 21 (6.2) | 23 (6.8) | 12 (6.7) | 20 (6.4) |
| AE leading to discontinuation | 10 (2.0) | 4 (1.2) | 2 (0.6) | 1 (0.6) | 9 (2.9) |
| Any SAE | 12 (2.4) | 7 (2.1) | 9 (2.7) | 2 (1.1) | 10 (3.2) |
| SAE related to study medication | 1 (0.2) | 0 | 1 (0.3) | 0 | 1 (0.3) |
| SAE leading to discontinuation | 3 (0.6) | 0 | 2 (0.6) | 0 | 3 (1.0) |
| Most common AEsa | |||||
| Nasopharyngitis | 18 (3.7) | 10 (2.9) | 12 (3.6) | 7 (3.9) | 11 (3.5) |
| Headache | 17 (3.5) | 10 (2.9) | 14 (4.2) | 1 (0.6) | 16 (5.1) |
| Urinary tract infection | 17 (3.5) | 13 (3.8) | 18 (5.4) | 1 (0.6) | 16 (5.1) |
| Influenza | 14 (2.8) | 11 (3.2) | 15 (4.5) | 4 (2.2) | 10 (3.2) |
| Back pain | 13 (2.6) | 6 (1.8) | 8 (2.4) | – | – |
| Arthralgia | 12 (2.4) | 3 (0.9) | 4 (1.2) | – | – |
| Diarrhoea | 11 (2.2) | 6 (1.8) | 11 (3.3) | – | – |
| Dyslipidaemia | 11 (2.2) | 7 (2.1) | 8 (2.4) | – | – |
| Hypertriglyceridaemia | 11 (2.2) | 9 (2.6) | 13 (3.9) | – | – |
| Nausea | 8 (1.6) | 5 (1.5) | 9 (2.7) | – | – |
| Upper respiratory tract infection | 8 (1.6) | 9 (2.6) | 7 (2.1) | – | – |
| Vulvovaginal mycotic infection | 7 (1.4) | 8 (2.3) | 1 (0.3) | – | – |
| AEs of special interestb | |||||
| Urinary tract infection | 17 (3.5) | 13 (3.8) | 19 (5.7) | 1 (0.6) | 16 (5.1) |
| Genital infectionc | 8 (1.6) | 14 (4.1) | 2 (0.6) | 0 | 8 (2.6) |
| Hypotension/dehydration/hypovolaemia | 0 | 2 (0.6) | 0 | 0 | 0 |
| Renal impairment/failure | 7 (1.4) | 2 (0.6) | 6 (1.8) | 3 (1.7) | 4 (1.3) |
| Cardiac failure (confirmed adjudicated event) | 1 (0.2) | 0 | 0 | 0 | 1 (0.3) |
| Hypoglycaemiad | 6 (1.2) | 6 (1.8) | 1 (0.3) | 2 (1.1) | 4 (1.3) |
| Major | 0 | 0 | 0 | 0 | 0 |
| Minor | 2 (0.4) | 3 (0.9) | 0 | 1 (0.6) | 1 (0.3) |
| Other | 4 (0.8) | 4 (1.2) | 1 (0.3) | 1 (0.6) | 3 (1.0) |
| Confirmede | 1 (0.2) | 1 (0.3) | 0 | 0 | 1 (0.3) |
Abbreviations: AE, adverse event; DAPA, dapagliflozin; MET, metformin; SAE, serious adverse event; SAXA, saxagliptin.
Data are for patients who received ≥1 dose of the study drug during the double‐blind treatment period.
Reported in ≥2% of patients in any treatment group.
Based on a predefined list of AEs by preferred term, which includes multiple terms for a given AE. Data after receipt of rescue medication were excluded.
For triple‐therapy regimens, genital infections were reported only with sequential addition of dapagliflozin to saxagliptin plus metformin (Study 2).
Hypoglycaemia episodes were classified as minor (symptomatic or asymptomatic with plasma glucose concentration <63 mg/dL [3.5 mmol/L]), major (symptomatic requiring third party assistance, with or without a plasma glucose concentration of <54 mg/dL [3.0 mmol/L]) and other (suggestive episode not meeting the criteria for major or minor episodes).
Glucose value ≤50 mg/dL (2.8 mmol/L) with symptoms.
Common AEs across all 3 treatment groups included nasopharyngitis, headache, urinary tract infection and influenza (Table 1). The most common related AEs were reported in the system organ classes (SOCs) of renal and urinary disorders and gastrointestinal disorders. For AEs of special interest, urinary tract infections, which may be increased by SGLT‐2 and DPP‐4 inhibitors, occurred at a similar rate in the triple‐ and dual‐therapy groups and were more common in women than in men. AEs of genital infections, a common adverse event with SGLT‐2 inhibitor treatment, occurred at a low frequency across analysed groups but were more common in patients receiving dual or triple therapy that included dapagliflozin. Two cases of hypotension/dehydration/hypovolaemia occurred in patients in the dapagliflozin plus metformin group. The incidence of renal impairment/failure (based on a predefined list of events suggestive of renal impairment/failure) was 1.4%, 0.6% and 1.8% with triple therapy, dapagliflozin plus metformin and saxagliptin plus metformin, respectively. All of these events occurred in patients who had low eGFR values at baseline. In addition, AEs of decrease in GFR were reported in 1.0%, 0.6% and 0.3% of patients in the triple‐therapy, dapagliflozin plus metformin and saxagliptin plus metformin treatment groups, respectively. There were no cases of acute pancreatitis, diabetic ketoacidosis or amputation in any group.
The frequency of hypoglycaemia was low (<2.0%) in all groups; no major hypoglycaemic events occurred, and no patients discontinued treatment owing to hypoglycaemia. Mean clinical laboratory variables generally remained within normal ranges throughout double‐blind treatment in all treatment groups (Table S4). Heart rates, measured throughout the study, were similar across the treatment groups. There were small decreases from baseline in systolic blood pressure (SBP) and diastolic blood pressure (DBP), which were consistent over time and greatest in patients whose treatment regimen included dapagliflozin. Similar proportions of patients achieved SBP <130 mmHg and DBP < 80 mmHg at week 24 in all groups.
3.3. Safety of concomitant add‐on triple therapy vs sequential add‐on triple therapy
Overall, AEs were experienced by 48.6% and 52.1% of patients in the concomitant add‐on and sequential add‐on treatment groups, respectively; 0.6% and 2.9% of patients experienced AEs that led to treatment discontinuation with concomitant add‐on and sequential add‐on treatment, respectively (Table 1). SAE incidence was low in both treatment groups (concomitant add‐on, 1.1%; sequential add‐on, 3.2%).
Influenza and nasopharyngitis were commonly experienced AEs with both combination regimens. Urinary tract infections occurred with lower frequency in patients receiving concomitant add‐on therapy than in patients receiving sequential add‐on therapy (0.6% vs 5.1%, respectively). Genital infections were reported in 2.6% of patients receiving sequential add‐on therapy, compared with no patients in the concomitant add‐on group, and occurred only in patients receiving sequential add‐on of dapagliflozin to saxagliptin plus metformin (Study 2). No cases of hypotension, dehydration or hypovolaemia occurred in either group. Renal impairment or failure occurred in similar proportions of patients in the concomitant and sequential add‐on groups (1.7% and 1.3%, respectively). The incidences of hypoglycaemia were low and similar (<1.5%) in both groups, and there were no cases of major hypoglycaemia in either group. The incidences of laboratory abnormalities did not differ between the 2 analysis groups and vital signs were stable throughout treatment.
4. DISCUSSION
Early combination therapy may benefit patients who require additional glucose control, while avoiding the need for stepwise addition of glucose‐lowering agents to metformin.7 Despite the rational basis of this approach, a thorough assessment of safety is required when considering any combination treatment regimen.
The AEs observed in this post‐hoc, pooled safety analysis were consistent with previous reports of the safety and tolerability of dapagliflozin and saxagliptin added on to metformin from the clinical trial experience for each agent individually.4, 5, 6 The distribution of incidences of urinary tract infections, commonly reported with both dapagliflozin and saxagliptin, and genital infections, commonly reported with dapagliflozin, were similar to those of previous reports.8, 9, 10 Pooled analysis demonstrated a safety and tolerability profile of triple therapy that was similar to that of dual therapy with dapagliflozin or saxagliptin combined with metformin. Moreover, there was little difference in the safety profiles of concomitant and sequential add‐on therapeutic regimens. Urinary tract and genital infections occurred less frequently with triple therapy compared with dapagliflozin plus metformin therapy. A decreased rate of genital infections with a combination of SGLT‐2 inhibitor and DPP‐4 inhibitor has been reported previously, which could be and has been attributed to the complementary mechanisms of action of the 2 drug classes.11 These safety findings are corroborated by 52‐week data from Studies 2 and 3, which indicate that triple therapy is durable, effective and well‐tolerated, with no new safety signals detected.12, 13
The risk of hypoglycaemia, an important complication in glucose‐lowering therapy, was not increased with triple therapy compared with either dual therapy, consistent with findings from the individual studies, as well as after 52 weeks of follow‐up of Studies 2 and 3.4, 5, 6, 12, 13 There was also no difference in hypoglycaemic risk between concomitant and sequential add‐on therapeutic regimens. Additionally, while the mild osmotic diuresis seen with dapagliflozin has the potential to cause volume depletion,14 no cases of hypotension, dehydration or hypovolaemia were observed in the triple‐therapy or saxagliptin plus metformin groups, and only two events were reported with dapagliflozin plus metformin. The incidence of worsening renal function or renal impairment was similar across treatment groups, occurring in patients who already had low baseline eGFR values, and the effects of dapagliflozin‐containing regimens on blood pressure were small. Notably, no cases of diabetic ketoacidosis, a serious but rare adverse event, were reported in any of the studies in this analysis. These results should be viewed within the context of the exclusion criteria of the included studies, which prohibited enrolment of patients with poor renal function, a history of diabetic ketoacidosis or with known cardiovascular disease or hypertension.
A key strength of this analysis was the amalgamation of data from 3 randomized trials, which provided sufficient patient numbers for a large‐scale safety analysis and increased the precision of AE incidence estimates. However, the limitations of pooling post‐hoc data from separately conducted trials include differences in the characteristics of included patients. Although large, the patient population was somewhat limited by the predefined study exclusion criteria and known contraindications for each drug (eg, renal impairment). Therefore, the side effect profiles associated with the drug combinations used in these analyses are likely to differ somewhat from those observed in routine clinical practice.
In conclusion, the findings from this post‐hoc pooled analysis suggest that triple therapy with dapagliflozin plus saxagliptin added on to metformin is a well‐tolerated treatment option for patients with type 2 diabetes who require an intensified treatment approach to control elevated HbA1c levels.
Supporting information
Text S1. Details of the data pooling methodology used in this analysis.
Table S1. Participant demographics and baseline characteristics for each trial.
Table S2. Participant demographics and baseline characteristics for dapagliflozin plus saxagliptin plus metformin triple therapy and saxagliptin plus metformin or dapagliflozin plus metformin dual therapy.
Table S3. HbA1c changes in each study.
Table S4. Changes in selected clinical laboratory parameters from baseline to week 24.
ACKNOWLEDGMENTS
The authors would like to acknowledge the substantial contributions of our colleague Stephan Matthaei, deceased, who was active as a clinical investigator in clinical trials and in the design and interpretation of this pooled safety analysis.
Medical writing support was provided by Lucy Ambrose, DPhil of Oxford PharmaGenesis, Oxford, UK, with funding from AstraZeneca.
Conflict of interest
R. G. S., N. I., E. J. and H. C. are employees of AstraZeneca and L. H. is an employee of Bristol‐Myers Squibb. S. D. P. serves or has served on advisory boards for AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, GlaxoSmithKline, Hanmi Pharmaceuticals, Intarcia, Janssen Pharmaceutics, Merck Sharp & Dohme Ltd, Novartis, Novo Nordisk, Sanofi, Servier and Takeda and serves or has served on speakers' bureaus for AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, Janssen Pharmaceutics, Merck Sharp & Dohme Ltd, Novartis, Novo Nordisk, Sanofi and Takeda; he has received research support from Boehringer Ingelheim, Merck Sharp & Dohme Ltd and Novartis. J. R. has served on scientific advisory boards and received honoraria or consulting fees from companies involved in development of SGLT‐2 and DPP‐4 inhibitors, including AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, Janssen, Lexicon and Sanofi, and has received grants/research support from AstraZeneca, Boehringer Ingelheim, Bristol‐Myers Squibb, Eli Lilly and company, Janssen, Lexicon, Merck, Pfizer and Sanofi. C. M. serves or has served on advisory panels for AstraZeneca, Boehringer Ingelheim, Bristol‐Myers Squibb, Eli Lilly and Company, Hanmi Pharmaceuticals, Intrexon, Janssen Pharmaceuticals, Mannkind, Medtronic, Merck Sharp & Dohme Ltd, Novartis, Novo Nordisk, Pfizer, Roche Diagnostics, Sanofi and UCB and serves or has served on speakers' bureaus for AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, Merck Sharp and Dohme, Novartis, Novo Nordisk and Sanofi. University of Leuven has received research support for C. M. from Abbott, Eli Lilly and Company, Intrexon, Merck Sharp and Dohme Ltd, Novartis, Novo Nordisk, Roche Diagnostics and Sanofi.
Author contributions
S. D. P., J. R., R. G. S., L. H., E. J. and C. M. contributed to the study design; all authors contributed to the analysis and interpretation of the data, provided critical input during the development of the manuscript and approved the final version for submission.
Del Prato S, Rosenstock J, Garcia‐Sanchez R, et al. Safety and tolerability of dapagliflozin, saxagliptin and metformin in combination: Post‐hoc analysis of concomitant add‐on versus sequential add‐on to metformin and of triple versus dual therapy with metformin. Diabetes Obes Metab. 2018;20:1542–1546. https://doi.org/10.1111/dom.13258
Funding information This study was sponsored by AstraZeneca, Mölndal, Gothenburg, Sweden.
REFERENCES
- 1. Turner RC, Cull CA, Frighi V, Holman RR. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. JAMA. 1999;281:2005‐2012. [DOI] [PubMed] [Google Scholar]
- 2. American Diabetes Association . Standards of medical care in diabetes‐2016. Diabetes Care. 2016;39(suppl):S1‐S119.26696671 [Google Scholar]
- 3. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes, 2015: a patient‐centred approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia. 2015;58:429‐442. [DOI] [PubMed] [Google Scholar]
- 4. Rosenstock J, Hansen L, Zee P, et al. Dual add‐on therapy in type 2 diabetes poorly controlled with metformin monotherapy: a randomized double‐blind trial of saxagliptin plus dapagliflozin addition versus single addition of saxagliptin or dapagliflozin to metformin. Diabetes Care. 2015;38:376‐383. [DOI] [PubMed] [Google Scholar]
- 5. Mathieu C, Ranetti AE, Li D, et al. Randomized, double‐blind, phase 3 trial of triple therapy with dapagliflozin add‐on to saxagliptin plus metformin in type 2 diabetes. Diabetes Care. 2015;38:2009‐2017. [DOI] [PubMed] [Google Scholar]
- 6. Matthaei S, Catrinoiu D, Celinski A, et al. Randomized, double‐blind trial of triple therapy with saxagliptin add‐on to dapagliflozin plus metformin in patients with type 2 diabetes. Diabetes Care. 2015;38:2018‐2024. [DOI] [PubMed] [Google Scholar]
- 7. Bianchi C, Daniele G, Dardano A, Miccoli R, Del Prato S. Early combination therapy with oral glucose‐lowering agents in type 2 diabetes. Drugs. 2017;77:247‐264. [DOI] [PubMed] [Google Scholar]
- 8. Johnsson KM, Ptaszynska A, Schmitz B, Sugg J, Parikh SJ, List JF. Urinary tract infections in patients with diabetes treated with dapagliflozin. J Diabetes Complications. 2013;27:473‐478. [DOI] [PubMed] [Google Scholar]
- 9. Johnsson KM, Ptaszynska A, Schmitz B, Sugg J, Parikh SJ, List JF. Vulvovaginitis and balanitis in patients with diabetes treated with dapagliflozin. J Diabetes Complications. 2013;27:479‐484. [DOI] [PubMed] [Google Scholar]
- 10. Hirshberg B, Parker A, Edelberg H, Donovan M, Iqbal N. Safety of saxagliptin: events of special interest in 9156 patients with type 2 diabetes mellitus. Diabetes Metab Res Rev. 2014;30:556‐569. [DOI] [PubMed] [Google Scholar]
- 11. Scheen AJ. DPP‐4 inhibitor plus SGLT‐2 inhibitor as combination therapy for type 2 diabetes: from rationale to clinical aspects. Expert Opin Drug Metab Toxicol. 2016;12:1407‐1417. [DOI] [PubMed] [Google Scholar]
- 12. Mathieu C, Herrera Marmolejo M, Gonzalez Gonzalez JG, et al. Efficacy and safety of triple therapy with dapagliflozin add‐on to saxagliptin plus metformin over 52 weeks in patients with type 2 diabetes. Diabetes Obes Metab. 2016;18:1134‐1137. [DOI] [PubMed] [Google Scholar]
- 13. Matthaei S, Aggarwal N, Garcia‐Hernandez P, et al. One‐year efficacy and safety of saxagliptin add‐on in patients receiving dapagliflozin and metformin. Diabetes Obes Metab. 2016;18:1128‐1133. [DOI] [PubMed] [Google Scholar]
- 14. Ptaszynska A, Johnsson KM, Parikh SJ, de Bruin TW, Apanovitch AM, List JF. Safety profile of dapagliflozin for type 2 diabetes: pooled analysis of clinical studies for overall safety and rare events. Drug Saf. 2014;37:815‐829. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Text S1. Details of the data pooling methodology used in this analysis.
Table S1. Participant demographics and baseline characteristics for each trial.
Table S2. Participant demographics and baseline characteristics for dapagliflozin plus saxagliptin plus metformin triple therapy and saxagliptin plus metformin or dapagliflozin plus metformin dual therapy.
Table S3. HbA1c changes in each study.
Table S4. Changes in selected clinical laboratory parameters from baseline to week 24.


