Abstract
Background
Early identification of people at risk of functional decline is essential for delivering targeted preventive interventions.
Objective
The aim of this study is to identify and predict trajectories of functional decline over 9 years in males and females aged 60–70 years.
Methods
We included 403 community-dwelling participants from the InCHIANTI study and 395 from the LASA study aged 60–70 years at baseline, of whom the majority reported no functional decline at baseline (median 0, interquartile range 0–1). Participants were included if they reported data on ≥2 measurements of functional ability during a 9-year follow-up. Functional ability was scored with 6 self-reported items on activities of daily living. We performed latent class growth analysis to identify trajectories of functional decline and applied multinomial regression models to develop prediction models of identified trajectories. Analyses were stratified for sex.
Results
Three distinct trajectories were identified: no/little decline (219 males, 241 females), intermediate decline (114 males, 158 females), and severe decline (36 males, 30 females). Higher gait speed showed decreased risk of functional limitations in males (intermediate limitations, odds ratio [OR] 0.74, 95% CI 0.57–0.97; severe limitations, OR 0.42, 95% CI 0.26–0.66). The final model in males further included the predictors fear of falling and alcohol intake (no/little decline, area under the receiver operating curve [AUC] 0.68, 95% CI 0.62–0.73; intermediate decline, AUC 0.63, 95% CI 0.56–0.69; severe decline, AUC 0.79, 95% CI 0.71–0.87). In females, higher gait speed showed a decreased risk of intermediate limitations (OR 0.51, 95% CI 0.38–0.68) and severe limitations (OR 0.18, 95% CI 0.07–0.44). Other predictors in females were age, living alone, economic satisfaction, balance, physical activity, BMI, and cardiovascular disease (no/little decline, AUC 0.80, 95% CI 0.75–0.85; intermediate decline, AUC 0.74, 95% CI 0.69–0.79; severe decline, AUC 0.95, 95% CI 0.91–0.99).
Conclusion
Already in people aged 60–70 years, 3 distinct trajectories of functional decline were identified in these cohorts over a 9-year follow-up. Predictors of trajectories differed between males and females, except for gait speed. Identification of people at risk is the basis for targeting interventions.
Keywords: Decision-making, Disability, Middle age, Physical performance, Prevention, Prognosis, Successful aging
Introduction
The aging of western society has led to increased attention for healthy aging and preventing age-related diseases and decline in functioning [1]. Functional decline is characterized by an increased inability to perform basic activities of daily living (ADL), such as dressing and toileting, and jeopardizes independence [2]. Besides individual variability in the onset of limitations in functioning, progression of decline over time differs between individuals [3].
Functional decline may be postponed through enhancing or maintaining an active lifestyle at an earlier stage in life, before the decline is initiated [4]. However, prior studies on understanding the course of functional decline have been performed in older populations with a majority of people well above 70 years of age, in whom functional decline was often already present [5]. By focusing on a younger cohort of older people, we will be better able to identify distinct subgroups in the population who may be target groups for preventive interventions.
It is increasingly recognized that trajectories of functional decline over time provide more information on distinct subgroups in the population, each with their typical course, than studying merely the onset of limitations in functioning [3]. Prior studies on trajectories of functional decline during various stages of the aging process have identified up to 8 different trajectories [6, 7, 8, 9, 10]. One study in 65- to 85year-olds identified 3 distinct trajectories over 10 years: one including people with substantial decline at the age of 65 years, one including people who gradually started to decline at the age of 65 years with increased progression from the age of 75 years, and one including people who did not decline until above 80 years [8].
To identify those people at risk of functional decline who are target groups for delivering preventive interventions, we need insight into which individual characteristics predict distinct trajectories of functional decline. The aforementioned studies focused on sociodemographic characteristics and chronic diseases as predictors of the trajectories [4, 6]. Other longitudinal studies have shown that deterioration in several physical performance measures also predicts the onset of limitations in ADL in older people [11, 12, 13]. Studying a broad range of predictors, including physical performance measures, can provide clinicians insight into the most sensitive parameters for predicting change in functional performance.
Therefore, we aimed to identify and predict trajectories of self-reported functional decline over 9 years of follow-up in males and females aged 60–70 years at baseline, with potential predictors covering sociodemographic, lifestyle, physical performance, and clinical measures.
Methods
Study Sample
This study included data from 2 cohort studies. The first cohort is the Invecchiare in Chianti study (InCHIANTI), an on-going population-based study in a representative sample of 1,453 community-dwelling Italian adults [14]. Participants were sampled from 1 urban and 1 rural municipality in Tuscany, Italy, based on age strata. The first measurement cycle was completed in 1998–2000 and follow-up cycles were 3 years apart.
The second cohort is the Longitudinal Aging Study Amsterdam (LASA), an ongoing study in a representative sample of 3,107 community-dwelling Dutch older people [15]. Participants were selected from population registries in 11 municipalities (urban and rural) in the west, north-east, and south of the Netherlands, and the sample was stratified for age, sex, and urbanization level. The first measurement cycle was completed in 1992–1993 and subsequent cycles were 3 years apart.
We included data from participants aged 60–70 years at baseline who participated in the first cycle in InCHIANTI (n = 403) and the second cycle in LASA (n = 395), and who provided data on functional ability during ≥2 of 4 measurement waves (n = 572 reported data on 4 measurement waves, n = 141 on 3 measurement waves, and n = 85 on 2 measurement waves). Online supplementary Table 1 (see www.karger.com/doi/10.1159/000485135 for all online suppl. material) presents descriptive statistics of both cohorts.
Functional Decline
Longitudinal data on self-reported items on functional ability for 4 measurements (across 9 years of follow-up) were used as outcome measure. Following harmonization procedures from a prior international collaboration [16], we selected only items that overlapped in both cohorts to create a comparable assessment of functional decline. This resulted in harmonization of 2 items on basic ADL and 4 items on instrumental ADL (see Table 1 for details) [17, 18].
Table 1.
Assessment of functional ability in the InCHIANTI and LASA cohorts and harmonization of data in present study
| InCHIANTI | LASA | Harmonization |
|---|---|---|
| Overlapping items for basic activities of daily living | ||
| 1. Any difficulty dressing and undressing? 2. Any difficulty getting in and out of bed? |
1. Can you dress and undress yourself? 2. Can you sit down and stand up from a chair? |
1. Dressing and undressing 2. Sitting down and standing up |
| Overlapping items for instrumental activities of daily living | ||
| 3. Any difficulty using public transportation? 4. Any difficulty walking up and down 10 steps? 5. Any difficulty walking 400 m? 6. Any difficulty cutting your toenails? |
3. Can you use your own or public transportation? 4. Can you walk up and down a staircase of 15 steps without resting? 5. Can you walk outside during 5 min without stopping? 6. Can you cut your own toenails? |
3. Using own or public transportation 4. Walking up and down a flight of stairs without resting 5. Walking outside for 400 m/during 5 min 6. Cutting own toenails |
| Scoring of the items | ||
| 1 - No difficulty/able to do but do not do 2 - With difficulty but without help 3 - With some help from another person 4 - Unable to do it |
5 - No difficulty 4 - Yes, with some difficulty 3 - Yes, with much difficulty 2 - Only with help 1 - No, I cannot |
0 - No difficulty/able to do but do not do 1 - With some/much difficulty but without help 2 - With help from another person 3 - Unable to do it |
The 6 items have shown to be well associated with fractures [19], recurrent falls [20], and dementia [21]. Items were recoded to create a score ranging from 0 to 3. We summed scores on the 6 items to a total score ranging from 0 (no decline) to 18 (maximum decline).
Potential Predictors
Variables considered as potential predictors were measured at baseline. Sociodemographic variables considered as potential predictors included age, marital status, living status, occupational status, educational level, and economic satisfaction. Physical performance measures consisted of balance, chair stands, gait speed, handgrip strength, fall history in the previous year, and fear of falling. Lifestyle variables included self-reported physical activity, smoking behavior, and alcohol intake. Clinical variables were body mass index (BMI), waist circumference, blood pressure, self-reported chronic diseases, medication use, cognitive functioning (using the Mini-Mental State Examination [MMSE]) [22], and depressive symptoms (using the Center for Epidemiologic Studies Depression Scale [CES-D]) [23]. Online supplementary Table 2 provides an overview of the measurements in both cohorts and the harmonized variables.
Statistical Analysis
The primary analysis consisted of a pooled analysis combining both cohorts. All analyses were stratified for sex, since prior research has shown that the development of functional decline and physical capacity over time differs between men and women, which may result in different trajectories and/or predictors [9]. To identify distinct trajectories of functional decline across 9 years of follow-up, we applied latent class growth modelling (LCGM). LCGM is a technique to analyze heterogeneity over time by identifying k number of distinct latent trajectories [24]. Since the scoring of functional decline showed floor effects at baseline (i.e., a majority of participants reported no/few difficulties), we assumed data to have a truncated normal distribution.
To determine the optimal number of trajectories, a common forward approach was applied starting with a model with 1 trajectory. Subsequently, one trajectory at a time was added and the shape of the trajectories was modelled by adding linear and quadratic terms. After each step, we assessed model fit by several criteria [25]: (1) Bayesian Information Criterion (BIC) with a decrease of ≥10 points recommended as an improvement in model fit [26]; (2) average posterior probabilities of trajectory membership, which should be as close to 1.00 as possible, with >0.80 recommended [27]; and (3) clinical interpretation and size of the obtained trajectories. Participants were allocated to their best fitting trajectory based on the highest posterior probability. LCGM was performed in Mplus version 7 (Mplus Development Team, Los Angeles, CA, USA) [24].
Subsequently, a clinical prediction model was developed for the identified trajectories of functional decline. Since the outcome of trajectory classification from the LCGM analyses was polytomous, we used multinomial logistic regression in which associations between predictors and each of the classes are simultaneously compared with 1 reference class [28]. First, in a complete-case analysis, analyses were performed for all potential predictors separately to check model assumptions. Considering differences in baseline values between both cohorts, the models included a dummy variable as cohort index [29].
Next, we fitted multiple regression models to identify independent predictors. Only variables with p < 0.20 on the Likelihood Ratio Test in the univariate analyses were selected. Multicollinearity was first assessed with Spearman's correlation coefficient and considered present if r ≥ 0.40. In case of multicollinearity, the variable with the highest predictive value was included in the model. We excluded the variables chair stands (multicollinear with gait speed) and marital status (multicollinear with living status) from the models. Models also included a dummy variable for cohort [29]. We applied a stepwise backward procedure to select the best reduced model in terms of number of predictors, with p < 0.05 as criterion. To assess if it was correct to pool both cohorts, we checked heterogeneity of effects across the cohorts by including an interaction term for cohort for each predictor separately in the final models (predictor*cohort). We considered heterogeneity present if the interaction term showed p < 0.10 [30].
A well-known problem in prediction research is overfitting, which may lead to overly optimistic regression coefficients when applied to a new population. We adjusted regression coefficients in the final models for possible optimism with a heuristic shrinkage factor, making the coefficients more conservative [31]. Overall model performance was assessed with adopted Nagelkerke's R2 and the Hosmer-Lemeshow test as index for goodness-of-fit [31]. We further examined calibration plots on agreement between observed and predicted outcome probabilities for each trajectory and computed the ratio between expected and observed values [32]. A ratio above 1 indicates an overestimation of the predicted risk group, while a value between 0 and 1 indicates an underestimation. The ability to discriminate between trajectories was assessed with area under the receiver operating curve (AUC), where one trajectory was compared with the others [28]. All analyses were conducted in R for Windows version 3.2, using the “nnet” package (R Development Core Team, R Foundation for Statistical Computing, Vienna, Austria).
Results
We included data from 369 males (baseline age 67.4 ± 2.1 years) and 429 females (baseline age 67.5 ± 2.1 years). Figure 1 presents the selection of participants. At baseline, a majority of the population reported no functional decline with median = 0 (interquartile range [IQR] 0–1) for males and for females, but scores ranged widely (males, range 0–17; females, range 0–12).
Fig. 1.
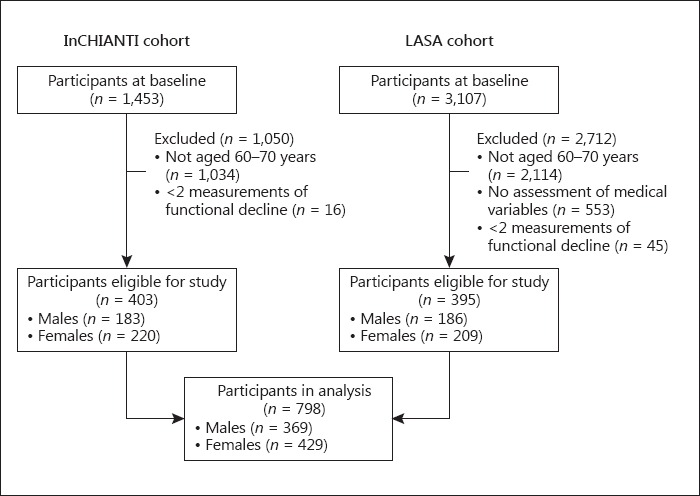
Selection of participants for the identification of trajectories of functional decline and development of the prediction model.
The optimal model from LCGM revealed 3 distinct linear trajectories as the best solution for both males and females (Fig. 2). The largest subgroup (n = 219 males; n = 241 females) was characterized by little functional decline over time and was labelled “no/little decline.” The second subgroup showed some functional decline at baseline (males, median 1, IQR 0–1; females, median 1, IQR 0–2) and showed gradual further decline and was labeled “intermediate decline” (n = 114 males; n = 158 females). The smallest subgroup consisted of people who already showed substantial functional decline at baseline (males, median 3, IQR 1–5; females, median 5, IQR 4–8) and further declined (n = 36 males; n = 30 females). This subgroup was labeled “severe decline.” Table 2 presents the baseline characteristics for each subgroup. Trajectories for the individual participants are presented in online supplementary Figure 1.
Fig. 2.
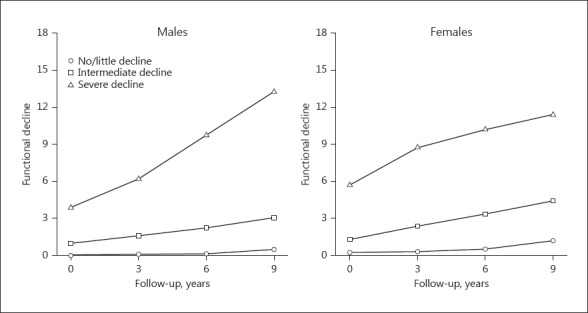
Identified trajectories of functional decline across 4 measurements over 9 years in males and females.
Table 2.
Baseline characteristics for the subgroups of young older adults classified into 3 distinct trajectories of functional decline
| Variable | Males |
Females |
|||||
|---|---|---|---|---|---|---|---|
| no/little decline (n = 219) | intermediate decline (n = 114) | severe decline (n = 36) | no/little decline (n = 241) | intermediate decline (n = 158) | severe decline (n = 30) | ||
| Sociodemographic variables | |||||||
| Age, years | 67.2 ± 2.1 | 67.9 ± 1.8 | 67.6 ± 2.0 | 67.1 ± 2.1 | 67.7 ± 2.1 | 68.7 ± 1.8 | |
| Not married | 40 (18.3) | 19 (16.7) | 10 (27.8) | 76 (31.5) | 55 (34.8) | 9 (30.0) | |
| Living alone | 24 (11.0) | 13 (11.4) | 6 (16.7) | 57 (23.7) | 41 (25.9) | 7 (23.3) | |
| Retired | 191 (87.2) | 99 (86.8) | 34 (94.4) | 228 (94.6) | 149 (94.3) | 25 (89.3) | |
| <8 years education | 105 (47.9) | 56 (49.1) | 20 (55.6) | 151 (62.7) | 105 (66.5) | 21 (70.0) | |
| Economic satisfaction | |||||||
| Good | 124 (56.6) | 64 ((56.1) | 16 (44.4) | 113 (46.9) | 70 (44.3) | 8 (26.7) | |
| Sufficient | 75 (34.2) | 43 (37.7) | 18 (50.0) | 93 (38.6) | 69 (43.7) | 14 (46.7) | |
| Bad | 16 (7.3) | 6 (5.3) | 2 (5.6) | 12 (5.0) | 17 (10.8) | 6 (20.0) | |
| Physical performance-related variables | |||||||
| Unable to perform tandem stand for 10 s | 13 (5.9) | 14 (12.3) | 7 (19.4) | 31 (12.9) | 31 (19.6) | 13 (43.3) | |
| Chair stands, s | 10.1 ± 2.6 | 11.6 ± 3.1 | 12.6 ± 4.5 | 10.7 ± 2.4 | 12.0 ± 3.5 | 16.2 ± 6.5 | |
| Gait speed, m/s | 1.36 ± 0.4 | 1.14 ± 0.4 | 0.98 ± 0.4 | 1.12 ± 0.3 | 1.06 ± 0.3 | 0.72 ± 0.2 | |
| Handgrip strength, kg | 43.7 ± 7.8 | 42.8 ± 8.3 | 37.8 ± 9.3 | 25.8 ± 5.6 | 24.7 ± 6.3 | 22.3 ± 3.9 | |
| ≥1 fall within the last 12 months | 29 (13.2) | 34 (29.8) | 8 (22.2) | 69 (28.6) | 36 (22.8) | 11 (36.6) | |
| Fear of falling | 24 (11.0) | 13 (11.4) | 12 (33.3) | 43 (17.8) | 39 (24.7) | 15 (50.0) | |
|
Lifestyle variables Physical activity | |||||||
| High level | 115 (52.5) | 50 (43.9) | 7 (19.4) | 120 (49.8) | 59 (37.3) | 7 (23.3) | |
| Moderate level | 75 (34.2) | 35 (30.7) | 16 (44.4) | 93 (38.6) | 73 (46.2) | 4 (13.3) | |
| Low level | 27 (12.3) | 29 (25.4) | 12 (33.3) | 28 (11.6) | 26 (16.5) | 17 (56.7) | |
| Smoking behavior | |||||||
| Never smoker | 77 (35.2) | 30 (26.3) | 13 (36.1) | 123 (51.0) | 84 (53.2) | 18 (60.0) | |
| Former smoker | 99 (45.2) | 60 (52.6) | 18 (50.0) | 75 (31.1) | 43 (27.2) | 7 (23.3) | |
| Current smoker | 43 (19.6) | 24 (21.1) | 5 (13.9) | 43 (17.8) | 31 (19.6) | 5 (16.7) | |
| ≥1 glasses alcohol/week | 176 (80.4) | 100 (87.7) | 26 (72.2) | 185 (76.8) | 129 (81.6) | 20 (66.7) | |
| Clinical variables | |||||||
| BMI | |||||||
| <25 | 58 (26.5) | 33 (28.9) | 6 (16.7) | 91 (37.8) | 34 (21.5) | 3 (10.0) | |
| 25 – 29.99 | 113 (51.6) | 61 (53.5) | 18 (50.0) | 97 (40.2) | 71 (44.9) | 8 (26.7) | |
| ≥30 | 43 (19.6) | 20 (17.5) | 11 (30.6) | 46 (19.1) | 44 (27.8) | 18 (60.0) | |
| Waist circumference ≥102 cm in men/≥88 cm in women | 62 (28.3) | 42 (36.8) | 14 (38.9) | 134 (55.6) | 96 (60.8) | 24 (80.0) | |
| Blood pressure, mm Hg | 114.7 ± 13.9 | 115.2 ± 15.3 | 115.6 ± 18.2 | 113.1 ± 14.2 | 115.3 ± 14.4 | 116.3 ± 16.9 | |
| Self-reported chronic diseases | |||||||
| Cardiovascular | 29 (13.2) | 28 (24.6) | 11 (30.6) | 28 (11.6) | 22 (13.9) | 10 (33.3) | |
| Peripheral artery | 19 (8.7) | 13 (11.4) | 4 (11.1) | 9 (3.7) | 18 (11.4) | 6 (20.0) | |
| Diabetes mellitus | 17 (7.8) | 11 (9.6) | 4 (11.1) | 18 (7.5) | 11 (7.0) | 6 (20.0) | |
| Stroke | 8 (3.7) | 5 (4.4) | 2 (5.6) | 3 (1.2) | 7 (4.4) | 3 (10.0) | |
| COPD | 23 (10.5) | 17 (14.9) | 4 (11.1) | 17 (7.1) | 14 (8.9) | 5 (16.7) | |
| Arthritis | 34 (15.5) | 29 (25.4) | 12 (33.3) | 72 (29.9) | 53 (33.5) | 16 (53.3) | |
| Cancer | 11 (5.0) | 6 (5.3) | 4 (11.1) | 25 (10.4) | 20 (12.7) | 6 (20.0) | |
| ≥3 medications | 51 (23.3) | 27 (23.7) | 15 (41.7) | 42 (17.4) | 43 (27.2) | 17 (56.7) | |
| Cognitive functioning, MMSE score | 28 (26 – 29) | 28 (26 – 29) | 28 (26 – 29) | 28 (27 – 29) | 27 (25 – 29) | 27.5 (26 – 28) | |
| Depressive symptoms present (>16 CES-D) | 25 (11.4) | 9 (7.9) | 9 (25.0) | 34 (14.1) | 34 (21.5) | 4 (13.3) | |
Data are presented as mean ± SD, n (%), or median (IQR). CES-D, Center for Epidemiologic Studies Depression Scale; COPD, chronic obstructive pulmonary disease; MMSE, Mini-Mental State Examination.
In males, the final model included gait speed, fear of falling, and alcohol intake as independent predictors of the trajectories of functional decline (univariate analyses of potential predictors were performed to select variables for the multiple regression models, see online suppl. Table 3). Higher gait speed was associated with lower risk of being classified in the intermediate or severe subgroup. People reporting fear of falling showed an increased risk of being classified in the unfavorable subgroups, while alcohol consumption showed an increased risk of the intermediate subgroup but decreased risk of the severe subgroup (Table 3). We found a significant interaction for gait speed with cohort in males (p = 0.002), but since gait speed showed a protective effect in both cohorts in separate analyses we did not stratify for cohort. After shrinkage, Nagelkerke's R2 for the final predictors together was 0.19 (for gait speed alone, R2 = 0.15). The AUC ranged from 0.63 to 0.79 (Table 4) and the Hosmer-Lemeshow test indicated good calibration (p = 0.343). Although 58.7% of males were correctly classified, the comparison of predicted and observed probabilities from the multinomial regression model indicated an underestimation of being classified in the intermediate and severe subgroups after shrinkage (see Table 4 for expected/observed ratios and online suppl. Fig. 2 for calibration plots).
Table 3.
Predictors in final multivariable models for trajectories of functional decline in young older males and females
| Variable | Males (n = 312) |
Females (n = 360) |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| intermediate vs. no/little decline |
severe vs. no/little decline |
p value LRT | intermediate vs. no/little decline |
severe vs. no/little decline |
p value LRT | |||||||||||||||
| beta1 | OR (95% CI) | beta1 | OR (95% CI) | beta1 | OR (95% CI)1 | beta | OR (95% CI) | |||||||||||||
| intercept | ||||||||||||||||||||
| inCHIANTI | −1.20 | −2.14 | −8.13 | −34.47 | ||||||||||||||||
| LASA | −0.56 | −1.09 | −9.04 | −34.00 | ||||||||||||||||
| Age (years) | - | - | 0.11 | 1.17 (1.03 – 1.32) | 0.43 | 1.83 (1.22 – 2.75) | 0.001 | |||||||||||||
| Living alone | - | - | 0.28 | 1.48 (0.80 – 2.72) | −1.11 | 0.21 (0.05 – 0.97) | 0.015 | |||||||||||||
| Economic satisfaction | ||||||||||||||||||||
| Good | - | - | 0.0 | 1.0 | 0.0 | 1.0 | 0.008 | |||||||||||||
| Sufficient | - | - | 0.05 | 1.07 (0.62 – 1.87) | 1.47 | 7.78 (1.56 – 38.87) | ||||||||||||||
| Bad | - | - | 0.68 | 2.59 (0.93 – 7.18) | 2.01 | 16.65 (2.50– 110.92) | ||||||||||||||
| Unable to perform tandem stand for 10 s | - | - | 0.61 | 2.34 (1.20 – 4.58) | 0.68 | 2.58 (0.66 – 10.13) | 0.037 | |||||||||||||
| Gait speed2 (m/s) | −0.21 | 0.74 (0.57 – 0.97) | −0.63 | 0.42 (0.26 – 0.66) | 0.000 | −0.48 | 0.51 (0.38 – 0.68) | −1.24 | 0.18 (0.07 – 0.44) | 0.000 | ||||||||||
| Fear of falling | 0.36 | 1.65 (0.69 – 3.94) | 1.24 | 5.67 (1.94 – 16.57) | 0.008 | - | - | |||||||||||||
| Physical activity | ||||||||||||||||||||
| High level | - | - | 0.0 | 1.0 | 0.0 | 1.0 | 0.023 | |||||||||||||
| Moderate level | - | - | 0.06 | 1.09 (0.61 – 1.94) | −0.29 | 0.67 (0.11 – 4.17) | ||||||||||||||
| Low level | - | - | 0.14 | 1.23 (0.56 – 2.70) | 1.45 | 7.60 (1.77 – 32.52) | ||||||||||||||
| ≥1 glasses alcohol/week | 0.26 | 1.43 (0.66 – 3.08) | −0.77 | 0.34 (0.13 – 0.90) | 0.029 | - | - | |||||||||||||
| BMI | ||||||||||||||||||||
| <25 | - | - | 0.0 | 1.0 | 0.0 | 1.0 | 0.005 | |||||||||||||
| 25 – 29.99 | - | - | 0.45 | 1.88 (1.02 – 3.48) | 0.31 | 1.54 (0.21 – 11.18) | ||||||||||||||
| ≥30 | - | - | 0.72 | 2.74 (1.38 – 5.43) | 1.49 | 7.99 (1.40 – 45.62) | ||||||||||||||
| Cardiovascular disease | - | - | 0.21 | 1.35 (0.63 – 2.90) | 1.42 | 7.27 (1.75 – 30.21) | 0.023 | |||||||||||||
CI, confidence interval; LRT, likelihood ratio test; OR, odds ratio. The OR in multinomial regression analysis indicates the risk of the trajectory compared to the reference group (i.e., no/little decline trajectory). For example, females with sufficient economic satisfaction had a 7% higher risk (OR 1.07, 95% CI 0.62 – 1.87) of being in the intermediate trajectory than females with good economic satisfaction, but they had a 8 times higher risk of being in the severe trajectory (OR 7.78, 95% CI 1.56 – 38.87) than females with good economic satisfaction.
Shrunken, shrinkage factor 0.711 for males, 0.716 for females.
OR for gait speed refer to standardized Z scores since different tests were applied in the two cohorts to assess gait speed. Z scores were calculated per cohort: Zmales inCHIANTI = (m/s − 1.570)/0.256; Zmales lasa = (m/s − 0.959)/0.257. Zfemales Inschianti = (m/s − 1.271)/0.249; Zfemales lasa = (m/s − 0.876)/0.242.
Table 4.
Performance of optimism-corrected prediction models for trajectories of functional decline in males and females
| Performance measure | Final model in males | Final model in females |
|---|---|---|
| Nagelkerke's R2 | 19% | 46% |
| Hosmer-Lemeshow test (p value) | 0.343 | 0.328 |
| C statistic (95% CI) | ||
| No/little decline vs. rest | 0.68 (0.62 – 0.73) | 0.80 (0.75 – 0.85) |
| intermediate decline vs. rest | 0.63 (0.56 – 0.69) | 0.74 (0.69 – 0.79) |
| Severe decline vs. rest | 0.79 (0.71 – 0.87) | 0.95 (0.91 – 0.99) |
| E/O ratio (95% CI) | ||
| No/little decline vs. rest | 1.62 (1.48 – 1.77) | 1.24 (1.10 – 1.37) |
| intermediate decline vs. rest | 0.10 (0.00 – 0.29) | 0.65 (0.48 – 0.83) |
| Severe decline vs. rest | 0.27 (0.00 – 0.63) | 0.71 (0.31 – 1.11) |
E/O, expected/observed. E/O ratio >1 indicates an overestimation and E/O ratio <1 an underestimation.
In females, the final model included age, living alone, economic satisfaction, tandem stands, gait speed, physical activity, BMI, and cardiovascular disease as independent predictors of trajectories of functional decline. Table 3 shows that all predictors were associated with increased risk of being classified in the intermediate or severe subgroups, except for living alone, moderate physical activity levels, and higher gait speed. Risk of being classified in the severe subgroup was lower for females living alone and those having moderate physical activity levels. Higher gait speed was associated with a lower risk of being classified in the unfavorable subgroups. None of the predictors showed a significant interaction with cohort. After shrinkage, the final model in females showed Nagelkerke's R2 of 0.46 (for gait speed alone, R2 = 0.24), and the AUC ranged from 0.74 to 0.95. The Hosmer-Lemeshow test showed good calibration with a p value of 0.328 (Table 4), and 71.7% of females were correctly classified. The calibration plots and expected/observed ratios indicated an underestimation of females classified in the intermediate and severe subgroups (Table 4; online suppl. Fig. 2).
Discussion
Based on data from 2 population-based cohorts, we identified 3 distinct trajectories of functional decline across 9 years in young older males and females: 1 subgroup with no/little decline over time, 1 with intermediate decline, and 1 with severe decline. Prediction models identified different predictors in males and females for being classified to the unfavorable subgroups, except for gait speed. The prediction models showed satisfactory discrimination between the 3 subgroups, this was similar in males and females.
Most prior research on trajectories of functional decline has focused on older populations, in which a substantial proportion of people with decline is expected [6, 7, 8]. We showed that even in a relatively young older population, distinct trajectories for risk of functional decline can be identified. Despite classification of the majority of our younger population in the subgroup with no/little predicted functional decline over time, a fair proportion of people already suffered limitations in functioning at baseline which progressed over time. The characteristics of these subgroups resemble the findings of Martin et al. [8] in 9,471 people aged 65–85 years and those of Liang et al. [33] in 18,486 people aged 50 years and older, who found comparable low, intermediate, and severe subgroups. In contrast, Kok et al. [10], who analyzed trajectories of functional decline across 16 years in 2,185 LASA participants aged 55–85 years, identified 4 trajectories for males and 5 for females [10]. Inclusion of more participants, a wider age range, and development of functional decline over a longer timespan may result in different intermediate trajectories, compared to the intermediate trajectory we observed. Participants in the subgroup of the severe trajectory in our study already started with limitations and showed a steeper slope of the trajectory than the other subgroups, indicating a quicker deterioration. This finding was also observed in the older participants in the study by Martin et al. [8] and may suggest that once people experience some limitations in their functional ability, the decline will continue to progress at a faster rate.
Although the trajectories that we identified in males and females showed similar patterns, predictors differed across both sexes. In males, gait speed, fear of falling, and alcohol intake were independent predictors of the trajectories, while predictors for females were age, living alone, economic satisfaction, tandem stands, gait speed, physical activity, BMI, and cardiovascular disease. In their sample of 65- to 85-year-olds, Martin et al. [8] identified clinical conditions as important predictors (i.e., chronic diseases, BMI, cognition) rather than sociodemographic variables. The older population is a likely explanation for this discrepancy with our findings. Most prior prediction models focused on predicting the onset of functional decline and consistently revealed age as an important predictor [34, 35, 36]. In our study, age was only a predictor in females and not in males, which is probably due to our restricted age range. Further comparison is difficult, since predicting the onset of functional decline at one point in time differs markedly from predicting the course of functional decline. Distinguishing different trajectories of functional decline over time is particularly relevant, since people developing more severe functional decline may particularly benefit from preventive interventions to postpone the progression.
Predictors differed for males and females, but interestingly, higher gait speed was a protective predictor in males and females. In both sexes, higher gait speed was associated with the favorable subgroups of no/little functional decline. Low gait speed is a well-established predictor of disability at later age [11] and mortality in people older than 65 years [37]. Our findings indicate that this predictive effect of gait speed is consistent across both sexes, thereby confirming the results of the Health ABC study [38], where a low gait speed (<1.0 m/s) predicted incident mobility limitations in males and females alike. Moreover, in our cohort of people aged 60–70 years, gait speed was shown to be an important predictor for functional decline already at a younger age. A recent study in a late-mid-life population showed that the predictive effect of gait speed on mortality is also irrespective of age [39]. Since gait speed accounted for the largest part of explained variance in our prediction models, our study confirms the importance of gait speed compared to other variables in predicting clinical outcomes in young older people [11]. It has been shown that changes in gait speed can already be detected from the age of 40 years, if more challenging tasks such as the fast speed task are used [40]. This underscores the potential of gait speed assessments for targeting preventive strategies, even in a young older population.
To our knowledge, this study was the first to identify latent subgroups of increased risk of functional decline in a young older population confined to people aged 60–70 years, a primary target group to initiate preventive interventions. We were able to harmonize two complex databases of ongoing population-based cohort studies and, using sophisticated statistical approaches of LCGM and prediction modeling, we could classify young older people in subgroups of risk for developing functional decline. Nevertheless, several limitations deserve further discussion. First, modeling subgroups with LCGM depends on the variation within the data. Although trajectories were comparable across InCHIANTI and LASA, the number of participants in the severe subgroups was rather low. Therefore, replication of the identified trajectories in other cohorts is needed for validation. Second, our included sample might not be representative of the general population aged 60–70 years due to a comparatively low number of people aged 60–64 years. Third, availability of data for harmonization restricted the number of items we could use to assess functional decline. Although the summary score we computed has shown to be a valid measure of functional performance in prior studies [19, 20, 21], comparison of the summary score with validated instruments to assess (instrumental) ADL is warranted. Fourth, with the low number of participants classified in the severe subgroups and the high number of potential predictors, there is a substantial chance of overfitting in our prediction models [41]. Finally, the calibration plots revealed that the applied shrinkage to correct for optimistic estimates might have been too restrictive. External validation in this specific age group of young older people is needed to confirm our findings in a representative sample and estimate the calibration and discrimination in new populations, before applying the prediction models in clinical practice for early identification of those at risk of functional decline in later life. Although we consider it too early to present a risk score to estimate individual risks, our presentation models do provide valuable insight for clinicians on predictive factors of functional decline in young older people, underscoring the importance of gait speed as a predictor in both males and females.
In conclusion, even in young older people aged 60–70 years, 3 distinct trajectories of functional decline across 9 years of follow-up can already be identified. Although the courses of the trajectories indicated similar subgroups in males and females, gait speed at baseline was the only consistent predictor of the subgroups in both sexes. Validation of the predictors in a larger cohort of young older people is needed to confirm the associations observed in our study.
Disclosure Statement
The authors declare that they have no competing interests.
Funding Sources
This work was supported by funding from the European Union's Horizon 2020 research and innovation programme (grant agreement number 689238). The InCHIANTI baseline study (1998–2000) was supported as a “targeted project” by the Italian Ministry of Health (ICS110.1/RF97.71) and by the US National Institute on Aging (Contracts 263 MD 9164, 263 MD 8213360); the InCHIANTI Follow-Up 1 (2001–2003) was funded by the US National Institute on Aging (Contracts N.1-AG-1-1, N.1-AG-1-2111); and the InCHIANTI Follow-Ups 2 and 3 (2004–2010) were financed by the US National Institute on Aging (Contract N01-AG-5-0002). The Longitudinal Aging Study Amsterdam was supported by a grant from the Netherlands Ministry of Health Welfare and Sports, Directorate of Long-Term Care. The funding agencies had no role in the design, execution, analysis and interpretation of data, or writing of the study.
Supplementary Material
Supplementary data
References
- 1.World Health Organization . Good Health Adds Life to Years: Global Brief for World Health Day 2012. Geneva: WHO; 2012. [Google Scholar]
- 2.Stenholm S, Westerlund H, Salo P, Hyde M, Pentti J, Head J, Kivimaki M, Vahtera J. Age-related trajectories of physical functioning in work and retirement: the role of sociodemographic factors, lifestyle and disease. J Epidemiol Community Health. 2014;68:503–509. doi: 10.1136/jech-2013-203555. [DOI] [PubMed] [Google Scholar]
- 3.Hardy SE, Dubin JA, Holford TR, Gill TM. Transitions between states of disability and independence among older persons. Am J Epidemiol. 2005;161:575–584. doi: 10.1093/aje/kwi083. [DOI] [PubMed] [Google Scholar]
- 4.Tak E, Kuiper R, Chorus A, Hopman-Rock M. Prevention of onset and progression of basic ADL disability by physical activity in community dwelling older adults: a meta-analysis. Ageing Res Rev. 2013;12:329–338. doi: 10.1016/j.arr.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 5.O'Caoimh R, Cornally N, Weathers E, O'Sullivan R, Fitzgerald C, Orfila F, Clarnette R, Paul C, Molloy DW. Risk prediction in the community: a systematic review of case-finding instruments that predict adverse healthcare outcomes in community-dwelling older adults. Maturitas. 2015;82:3–21. doi: 10.1016/j.maturitas.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 6.Deeg DJ. Longitudinal characterization of course types of functional limitations. Disabil Rehabil. 2005;27:253–261. doi: 10.1080/09638280400006507. [DOI] [PubMed] [Google Scholar]
- 7.Gill TM, Gahbauer EA, Han L, Allore HG. Trajectories of disability in the last year of life. N Engl J Med. 2010;362:1173–1180. doi: 10.1056/NEJMoa0909087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin LG, Zimmer Z, Lee J. Foundations of activity of daily living trajectories of older Americans. J Gerontol B Psychol Sci Soc Sci. 2017;72:129–139. doi: 10.1093/geronb/gbv074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Botoseneanu A, Allore HG, Mendes de Leon CF, Gahbauer EA, Gill TM. Sex differences in concomitant trajectories of self-reported disability and measured physical capacity in older adults. J Gerontol A Biol Sci Med Sci. 2016;71:1056–1062. doi: 10.1093/gerona/glw038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kok AA, Aartsen MJ, Deeg DJ, Huisman M. Capturing the diversity of successful aging: An operational definition based on 16-year trajectories of functioning. Gerontologist. 2017;57:240–251. doi: 10.1093/geront/gnv127. [DOI] [PubMed] [Google Scholar]
- 11.Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, Studenski S, Berkman LF, Wallace RB. Lower extremity function and subsequent disability: Consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–M231. doi: 10.1093/gerona/55.4.m221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Terhorst L, Holm MB, Toto PE, Rogers JC. Performance-based impairment measures as predictors of early-stage activity limitations in community-dwelling older adults. J Aging Health. 2016 doi: 10.1177/0898264316648113. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 13.Wennie Huang WN, Perera S, Van Swearingen J, Studenski S. Performance measures predict onset of activity of daily living difficulty in community-dwelling older adults. J Am Geriatr Soc. 2010;58:844–852. doi: 10.1111/j.1532-5415.2010.02820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferrucci L, Bandinelli S, Benvenuti E, Di Iorio A, Macchi C, Harris TB, Guralnik JM. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 15.Huisman M, Poppelaars J, van der Horst M, Beekman AT, Brug J, van Tilburg TG, Deeg DJ. Cohort profile: the Longitudinal Aging Study Amsterdam. Int J Epidemiol. 2011;40:868–876. doi: 10.1093/ije/dyq219. [DOI] [PubMed] [Google Scholar]
- 16.Schaap LA, Peeters GM, Dennison EM, Zambon S, Nikolaus T, Sanchez-Martinez M, Musacchio E, van Schoor NM, Deeg DJ, ESPOSA research group European Project on OSteoArthritis (EPOSA): methodological challenges in harmonization of existing data from five European population-based cohorts on aging. BMC Musculoskelet Disord. 2011;12:272. doi: 10.1186/1471-2474-12-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katz S, Downs TD, Cash HR, Grotz RC. Progress in development of the index of ADL. Gerontologist. 1970;10:20–30. doi: 10.1093/geront/10.1_part_1.20. [DOI] [PubMed] [Google Scholar]
- 18.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 19.Stel VS, Pluijm SM, Deeg DJ, Smit JH, Bouter LM, Lips P. Functional limitations and poor physical performance as independent risk factors for self-reported fractures in older persons. Osteoporos Int. 2004;15:742–750. doi: 10.1007/s00198-004-1604-7. [DOI] [PubMed] [Google Scholar]
- 20.Stel VS, Pluijm SM, Deeg DJ, Smit JH, Bouter LM, Lips P. A classification tree for predicting recurrent falling in community-dwelling older persons. J Am Geriatr Soc. 2003;51:1356–1364. doi: 10.1046/j.1532-5415.2003.51452.x. [DOI] [PubMed] [Google Scholar]
- 21.Comijs HC, Dik MG, Rijmen F, Jonker C, Van den Kommer TN, Deeg DJ. Predictors of dementia, the construction of classification trees (in Dutch) Tijdschr Gerontol Geriatr. 2006;37:237–242. [PubMed] [Google Scholar]
- 22.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 23.Radloff LS, Teri L. Use of the CES-D with older adults. Clin Gerontol. 1986;5:119–136. [Google Scholar]
- 24.Muthén B, Muthén LK. Integrating person-centered and variable-centered analyses: Growth mixture modeling with latent trajectory classes. Alcohol Clin Exp Res. 2000;24:882–891. [PubMed] [Google Scholar]
- 25.Nylund KL, Asparouhov T, Muthén BO. Deciding on the number of classes in latent class analysis and growth mixture modeling: a Monte Carlo simulation study. Struct Equ Modeling. 2007;14:535–569. [Google Scholar]
- 26.Raftery A. Bayesian model selection in social research. Sociol Methodol. 1995;25:111–163. [Google Scholar]
- 27.Nagin DS. Group-Based Modeling of Development. Cambridge/London: Harvard University Press; 2005. [Google Scholar]
- 28.Biesheuvel CJ, Vergouwe Y, Steyerberg EW, Grobbee DE, Moons KG. Polytomous logistic regression analysis could be applied more often in diagnostic research. J Clin Epidemiol. 2008;61:125–134. doi: 10.1016/j.jclinepi.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 29.Debray TP, Moons KG, Ahmed I, Koffijberg H, Riley RD. A framework for developing, implementing, and evaluating clinical prediction models in an individual participant data meta-analysis. Stat Med. 2013;32:3158–3180. doi: 10.1002/sim.5732. [DOI] [PubMed] [Google Scholar]
- 30.Aiken LS, West SG. Multiple Regression: Testing and Interpreting Interactions. Newbury Park: Sage; 1991. [Google Scholar]
- 31.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 32.Boggs DA, Rosenberg L, Pencina MJ, Adams-Campbell LL, Palmer JR. Validation of a breast cancer risk prediction model developed for Black women. J Natl Cancer Inst. 2013;105:361–367. doi: 10.1093/jnci/djt008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liang J, Xu X, Bennett JM, Ye W, Quinones AR. Ethnicity and changing functional health in middle and late life: a person-centered approach. J Gerontol B Psychol Sci Soc Sci. 2010;65:470–481. doi: 10.1093/geronb/gbp114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tas U, Steyerberg EW, Bierma-Zeinstra SM, Hofman A, Koes BW, Verhagen AP. Age, gender and disability predict future disability in older people: the Rotterdam Study. BMC Geriatr. 2011;11:22. doi: 10.1186/1471-2318-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.den Ouden ME, Schuurmans MJ, Mueller-Schotte S, van der Schouw YT. Identification of high-risk individuals for the development of disability in activities of daily living. A ten-year follow-up study. Exp Gerontol. 2013;48:437–443. doi: 10.1016/j.exger.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 36.Nuesch E, Pablo P, Dale CE, Prieto-Merino D, Kumari M, Bowling A, Ebrahim S, Casas JP. Incident disability in older adults: prediction models based on two British prospective cohort studies. Age Ageing. 2015;44:275–282. doi: 10.1093/ageing/afu159. [DOI] [PubMed] [Google Scholar]
- 37.Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, Brach J, Chandler J, Cawthon P, Connor EB, Nevitt M, Visser M, Kritchevsky S, Badinelli S, Harris T, Newman AB, Cauley J, Ferrucci L, Guralnik J. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simonsick EM, Newman AB, Visser M, Goodpaster B, Kritchevsky SB, Rubin S, Nevitt MC, Harris TB, Health, Aging and Body Composition Study Mobility limitation in self-described well-functioning older adults: importance of endurance walk testing. J Gerontol A Biol Sci Med Sci. 2008;63:841–847. doi: 10.1093/gerona/63.8.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elbaz A, Sabia S, Brunner E, Shipley M, Marmot M, Kivimaki M, Singh-Manoux A. Association of walking speed in late midlife with mortality: results from the Whitehall II cohort study. Age (Dordr) 2013;35:943–952. doi: 10.1007/s11357-012-9387-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferrucci L, Cooper R, Shardell M, Simonsick EM, Schrack JA, Kuh D. Age-related change in mobility: perspectives from life course epidemiology and geroscience. J Gerontol A Biol Sci Med Sci. 2016;71:1184–1194. doi: 10.1093/gerona/glw043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steyerberg EW. Clinical Prediction Models: A Practical Approach to Development, Validation, and Updating. New York: Springer; 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data


