Abstract
This post hoc analysis assessed the effects on cardiovascular risk factors of body weight, systolic blood pressure (SBP) and triglycerides after 28 weeks’ treatment with exenatide once weekly plus dapagliflozin, as compared with exenatide once weekly or dapagliflozin, in patient subpopulations from the DURATION‐8 trial of patients with type 2 diabetes mellitus (T2DM) inadequately controlled with metformin alone. Subgroups of patients were stratified according to their baseline body weight, SBP and triglyceride levels. Body weight, SBP and triglyceride levels were reduced across most respective subgroups, with no significant subgroup‐by‐treatment interactions. For each treatment, weight loss was numerically greater as baseline body mass index increased. SBP reductions were greater among patients with SBP ≥140 vs <140 mm Hg for exenatide once weekly plus dapagliflozin and exenatide once weekly. Reductions in triglyceride levels were greater among patients with baseline triglycerides <1.69 vs ≥1.69 mmol/L for each treatment. The combination of exenatide once weekly plus dapagliflozin reduced cardiovascular risk factors across baseline subgroups for each variable to a greater extent than did either individual drug; the greatest effects were observed in the high baseline subgroups for body weight and SBP.
Keywords: dapagliflozin, exenatide, GLP‐1 analogue, SGLT2 inhibitor, type 2 diabetes
1. INTRODUCTION
Type 2 diabetes mellitus (T2DM) is associated with an increased risk of developing cardiovascular disease (CVD), the leading cause of morbidity and mortality in people with this disease. Risk factors associated with CVD include comorbid conditions such as obesity, hypertension and dyslipidaemia, while diabetes independently increases the risk of CVD.1 The control of cardiovascular risk factors has been shown to prevent or slow the progression of CVD, with the greatest benefit observed for simultaneous control of multiple cardiovascular risk factors.2
The glucagon‐like peptide‐1 receptor agonist (GLP‐1RA) exenatide3, 4 and the sodium‐glucose co‐transporter‐2 (SGLT2) inhibitor dapagliflozin5 have both independently been shown to lower blood glucose and improve some cardiovascular risk factors, with a low risk of hypoglycaemia. The DURATION‐8 (Diabetes Therapy Utilization: Researching Changes in A1C, Weight and Other Factors Through Intervention With Exenatide Once Weekly) trial assessed the efficacy and safety of exenatide once weekly plus dapagliflozin vs either exenatide once weekly or dapagliflozin in patients with T2DM inadequately controlled with metformin alone.6 After 28 weeks, exenatide once weekly plus dapagliflozin significantly improved glycaemic control and the cardiovascular risk factors of body weight and systolic blood pressure (SBP) vs exenatide once weekly or dapagliflozin.
The goal of the present post hoc analysis was to determine whether effects on cardiovascular risk factors with exenatide once weekly plus dapagliflozin, as compared with exenatide once weekly or dapagliflozin, differed across patient subpopulations. This analysis used data from DURATION‐8 to assess the impact of each cardiovascular risk factor by its respective baseline cardiovascular subgroup and the treatment effects for changes in body weight, SBP and triglycerides at week 28.
2. MATERIALS AND METHODS
This post hoc analysis used data from the DURATION‐8 study (http://clinicaltrials.gov identifier: NCT02229396), a multicentre, randomized, double‐blind, active‐controlled phase III trial. The study design and methodology of the primary phase of the trial (28 weeks) were previously published.6 Briefly, adult patients (aged ≥18 years) with T2DM and inadequate glycaemic control (glycated haemoglobin [HbA1c] 64–108 mmol/mol [8.0%–12.0%]) despite treatment with a stable metformin dose (≥1500 mg/day) were randomized to receive exenatide once weekly (2 mg, subcutaneous injection) plus dapagliflozin (10 mg, oral tablet), exenatide once weekly with oral placebo, or dapagliflozin with injected placebo for 28 weeks. From weeks 8 to 28, patients could receive rescue therapy with added basal insulin based on progressively stricter fasting plasma glucose criteria (>15.0 to >11.1 mmol/L).
The original study protocol was developed in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines and approved by the ethics and regulatory committees and institutional review boards of the participating institutions.6 All participants provided written informed consent.
In the present analysis, changes in cardiovascular risk factors were assessed in subgroups of patients categorized by their respective baseline cardiovascular risk factor status. The change in body weight was assessed in the body mass index (BMI) subgroups: <25 kg/m2 (normal body weight); ≥25 to <30 kg/m2 (overweight); and ≥30 kg/m2 (obese). The change in SBP was assessed in patients with normal SBP vs systolic hypertension: <140 mm Hg vs ≥140 mm Hg. The change in triglyceride levels was assessed in patients with normal vs elevated triglyceride levels: <1.69 mmol/L vs ≥1.69 mmol/L.
2.1. Statistical analysis
Efficacy analyses excluded measurements after the initiation of rescue therapy or discontinuation of the study drug. Adjusted least‐squares means in the change from baseline values at week 28 were modelled using a mixed model with repeated measures including treatment, region (North America, Europe or South Africa), baseline HbA1c stratum (<75 or ≥75 mmol/mol [<9.0% or ≥9.0%]), week, subgroup, treatment‐by‐week, subgroup‐by‐week, subgroup‐by‐treatment, and subgroup‐by‐week‐by‐treatment interactions as fixed factors and the baseline value as a covariate. Nominal P values for the subgroup‐by‐treatment interaction were calculated. Baseline values are reported as mean ± SD, and change values are reported as least‐squares mean ± SE.
3. RESULTS
3.1. Patients
Demographics and patient characteristics at baseline were generally similar across treatment groups, as previously reported.6 Patient subgroups were based on clinically relevant thresholds for each cardiovascular risk factor at baseline; the repartition of patients in each subgroup is shown in Table 1.
Table 1.
Distribution of patients in the cardiovascular subgroup analyses
| Baseline subgroup | Exenatide once weekly + dapagliflozin | Exenatide once weekly + placebo | Dapagliflozin + placebo |
|---|---|---|---|
| BMI, n (%) | n = 194 | n = 184 | n = 196 |
| <25 kg/m2 | 14 (7.2) | 12 (6.5) | 12 (6.1) |
| ≥ 25 to <30 kg/m2 | 63 (32.5) | 65 (35.3) | 49 (25.0) |
| ≥ 30 kg/m2 | 117 (60.3) | 107 (58.2) | 135 (68.9) |
| SBP, n (%) | n = 201 | n = 190 | n = 207 |
| < 140 mm Hg | 151 (75.1) | 153 (80.5) | 162 (78.3) |
| ≥ 140 mm Hg | 50 (24.9) | 37 (19.5) | 45 (21.7) |
| Triglycerides, n (%) | n = 196 | n = 187 | n = 200 |
| < 1.69 mmol/L | 84 (42.9) | 72 (38.5) | 86 (43.0) |
| ≥ 1.69 mmol/L | 112 (57.1) | 115 (61.5) | 114 (57.0) |
Abbreviations: BMI, body mass index; SBP, systolic blood pressure.
3.2. Body weight
At baseline, mean body weight in the exenatide once weekly plus dapagliflozin, exenatide once weekly, and dapagliflozin groups was 91.8 ± 22.2 kg, 89.8 ± 20.2 kg, and 91.1 ± 19.7 kg, respectively; mean BMI was 33.2 ± 6.8 kg/m2, 32.0 ± 5.9 kg/m2, and 33.0 ± 6.1 kg/m2, respectively. Among the overall population, weight loss was significantly greater after 28 weeks for patients receiving exenatide once weekly plus dapagliflozin (−3.55 ± 0.29 kg), compared with patients receiving exenatide once weekly (−1.56 ± 0.29 kg; P < .001) or dapagliflozin (−2.22 ± 0.28 kg; P < .001).6 In the baseline BMI subgroup analyses, patients in the overweight and obese subgroups lost weight with all tested treatments (Figure 1A). Within each treatment group, weight loss was numerically greater among patients with a baseline BMI ≥30 kg/m2 vs those with a BMI ≥25 to <30 kg/m2. Similar to the results in the overall population, exenatide once weekly plus dapagliflozin resulted in numerically greater reductions in body weight vs each individual drug across all baseline BMI subgroups, although there were too few patients in the <25 kg/m2 BMI category for reliable conclusions to be drawn for this subgroup. No potential subgroup‐by‐treatment interactions for changes in body weight were observed.
Figure 1.
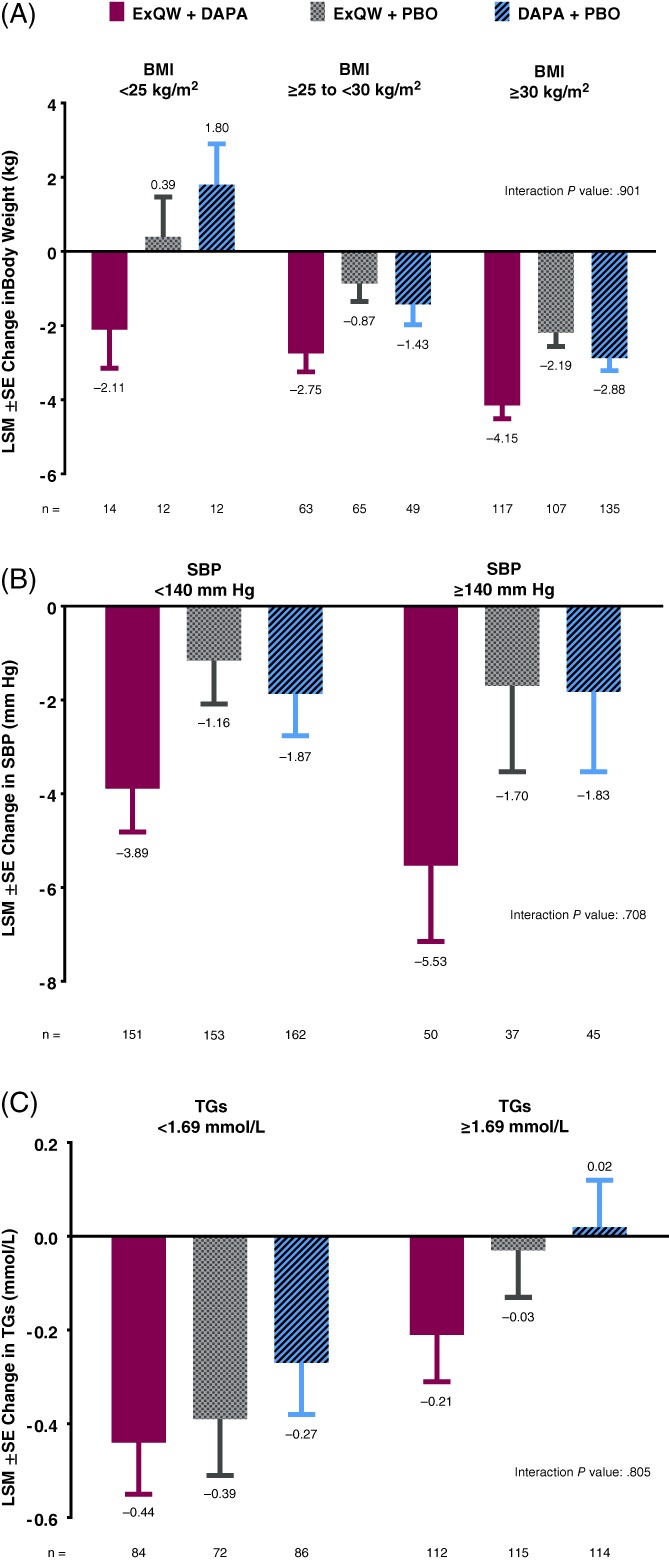
A, Changes in body weight by treatment in baseline body mass index (BMI) subgroups (<25 kg/m2; ≥25 to <30 kg/m2; and ≥30 kg/m2)* at week 28. B, Changes in systolic blood pressure (SBP) by treatment in baseline SBP subgroups (<140 vs ≥140 mm Hg) at week 28. C, Changes in triglycerides (TGs) by treatment in baseline TG subgroups (<1.69 vs ≥1.69 mmol/L) at week 28. To convert mmol/L to mg/dL for TGs, multiply by 88.5. Changes from baseline are summarized as the least‐squares mean (LSM) ± SE in the intention‐to‐treat population. *There were too few patients with BMI <25 kg/m2 for reliable conclusions to be drawn for this subgroup. DAPA, dapagliflozin; ExQW, exenatide once weekly; PBO, placebo
3.3. Systolic blood pressure
At baseline, mean SBP in the exenatide once weekly plus dapagliflozin, exenatide once weekly, and dapagliflozin groups was 130.1 ± 12.7 mm Hg, 129.1 ± 13.1 mm Hg, and 130.0 ± 12.9 mm Hg, respectively. Treatment with exenatide once weekly plus dapagliflozin resulted in a significantly greater reduction in SBP (−4.3 ± 0.8 mm Hg), compared with exenatide once weekly (−1.2 ± 0.8 mm Hg; P = .005) or dapagliflozin (−1.8 ± 0.8 mm Hg; P = .022) in the overall population at week 28.6 For the baseline SBP subgroups, exenatide once weekly plus dapagliflozin and exenatide once weekly resulted in numerically greater reductions in SBP in the ≥140 mm Hg vs <140 mm Hg subgroup, whereas SBP reductions with dapagliflozin were similar in the <140 mm Hg vs ≥140 mm Hg subgroup (Figure 1B). Combination treatment was most effective, resulting in numerically greater reductions in SBP vs either individual treatment in both SBP subgroups. No potential subgroup‐by‐treatment interactions for changes in SBP were observed.
3.4. Triglycerides
At baseline, mean triglyceride concentration in the exenatide once weekly plus dapagliflozin, exenatide once weekly, and dapagliflozin groups was 2.12 ± 1.25 mmol/L, 2.24 ± 1.31 mmol/L, and 2.14 ± 1.26 mmol/L, respectively. Among the overall population, exenatide once weekly plus dapagliflozin resulted in a numerically greater reduction in triglycerides at week 28 (−0.31 ± 0.08 mmol/L) vs exenatide once weekly (−0.18 ± 0.08 mmol/L; P = .181) and a greater reduction vs dapagliflozin (−0.11 ± 0.08 mmol/L; P = .036).6 Across all treatments, triglyceride reductions at week 28 were numerically greater among patients with a baseline triglyceride level < 1.69 mmol/L vs ≥1.69 mmol/L (Figure 1C). Exenatide once weekly plus dapagliflozin resulted in greater reductions in triglycerides vs either exenatide once weekly or dapagliflozin in both subgroups. No potential subgroup‐by‐treatment interactions for changes in triglycerides were observed.
4. DISCUSSION
The results of this post hoc analysis showed that, among patients with T2DM who had inadequate glycaemic control with metformin monotherapy, exenatide once weekly plus dapagliflozin lowered body weight, SBP and triglycerides across their respective baseline categorical subgroups, with numerically greater reductions in all cardiovascular risk factors compared with either treatment. Greater improvements with exenatide once weekly plus dapagliflozin may be explained in part by their different, and potentially complementary, mechanisms of action.7 Reductions in body weight and SBP were generally greater among patients with higher baseline BMI and SBP, respectively. Triglycerides were reduced to a greater extent among patients with normal vs elevated triglycerides with all treatments; dapagliflozin did not reduce triglycerides in patients with elevated triglycerides at baseline. These findings remain unexplained as greater reductions are typically observed with higher baseline values with most variables, attributable at least in part to regression to the mean.
Several studies have investigated the effects of exenatide once weekly on cardiovascular risk factors among patient subgroups. In a post hoc analysis of pooled data from 8 studies of exenatide once weekly, patient data were assessed according to weight‐loss quartiles.8 While patients in most weight‐loss quartiles showed improvements in cardiovascular risk factors, including SBP, total cholesterol, LDL cholesterol and triglycerides, patients with the most weight loss at study end showed the greatest trend of improvement in these cardiovascular risk factors. Another pooled analysis of 4 trials including 52 weeks of treatment with exenatide once weekly showed that patients with elevated SBP (≥130 mm Hg) or triglycerides (≥1.69 mmol/L) at baseline, as compared with all patients, had greater reductions in SBP (−8.5 vs −3.6 mm Hg) and triglycerides (−21% vs −7%).9
Dapagliflozin has also been shown to improve cardiovascular risk factors in patients with increased cardiovascular risk. In a pooled analysis of 2 trials investigating initial combination therapy with dapagliflozin and metformin for 24 weeks, the proportion of patients achieving a composite endpoint of HbA1c <53 mmol/mol (<7.0%) and SBP/diastolic blood pressure < 140/90 mm Hg increased as baseline BMI increased (<25 kg/m2, 21%; 25 to <30 kg/m2, 29%; ≥30 kg/m2, 48%).10 Consistent with the results reported in this analysis, a pooled analysis of 13 studies found that patients with T2DM and systolic hypertension (SBP ≥140 mm Hg) had a greater placebo‐subtracted decrease in SBP (−3.6 mm Hg) vs those without hypertension (−2.6 mm Hg) over 24 weeks of treatment.11
While the present analysis focused on changes in cardiovascular risk factors with exenatide once weekly plus dapagliflozin in combination, several large‐scale randomized trials have investigated cardiovascular outcomes with GLP‐1RAs and SGLT2 inhibitors separately among patients with T2DM and high cardiovascular risk. Liraglutide and semaglutide each demonstrated a significantly lower risk of major adverse cardiovascular events vs placebo in their respective cardiovascular outcomes trials.12, 13 Recently, the Exenatide Study of Cardiovascular Event Lowering (EXSCEL), which examined the cardiovascular effects of exenatide once‐weekly treatment vs usual care, found that exenatide once weekly did not increase cardiovascular risk, while also demonstrating a consistent safety profile14; however, the recorded 9% decrease in cardiovascular events did not reach statistical significance.
Among the SGLT2 inhibitors, empagliflozin and canagliflozin have shown reduced risk of major adverse cardiovascular events vs placebo in their respective cardiovascular outcomes trials.15, 16 The results of a large cardiovascular outcomes trial investigating the effects of dapagliflozin (Dapagliflozin Effect on Cardiovascular Events [DECLARE‐TIMI 58]) on cardiovascular events are also expected.
A limitation of the present analysis is the small sample size of some of the subpopulations, which may limit the interpretation of the results. Additional limitations include the post hoc nature of the assessment and the lack of correction for multiplicity.
In conclusion, in patients with T2DM with inadequate glycaemic control on metformin alone, adding a combination of exenatide once weekly plus dapagliflozin reduced cardiovascular risk factors across baseline subgroups for each variable to a greater extent than adding either individual drug. The greatest effects were observed in the high baseline subgroups for body weight and SBP and the lower baseline subgroup for triglycerides.
ACKNOWLEDGMENTS
Amanda Sheldon, PhD, CMPP, of inScience Communications, Springer Healthcare (Philadelphia, PA, USA) provided medical writing support, funded by AstraZeneca.
Conflict of interest
S.A.J. has worked as a consultant for AstraZeneca, Eli Lilly, and Janssen. J.P.F. has received research support from AbbVie, AstraZeneca, Boehringer Ingelheim, Bristol‐Myers Squibb, Eli Lilly, IONIS, Janssen, Johnson and Johnson, Ligand, Merck, Mylan, Novartis, Novo Nordisk, Pfizer, Sanofi, Theracos, and vTv Therapeutics, and has participated in scientific advisory boards and received consulting fees from AstraZeneca, Bristol‐Myers Squibb, Novo Nordisk, Sanofi, and Theracos. C.G. has participated in scientific advisory boards and received consulting fees from Alfa Wasserman, AstraZeneca, Bayer AG, Berlin‐Chemie Menarini, Eli Lilly, Merck Sharp & Dohme, Novo Nordisk, and Sanofi. A.A. has received research grants from AbbVie, AstraZeneca, Kowa Pharmaceuticals, Novo Nordisk, and Sanofi‐Aventis. E.H. and P.Ö. are employees of AstraZeneca.
Author contributions
S.A.J. contributed to the conception and design of the study, acquisition and interpretation of the data, and critical revisions to the manuscript. J.P.F., C.G. and A.A. contributed to the acquisition and interpretation of the data and provided critical revisions to the manuscript. E.H. and P.Ö. contributed to the conception and design of the study, acquisition, analysis and interpretation of the data, and critical revisions to the manuscript. All authors read and approved the final version.
Jabbour SA, Frías JP, Guja C, Hardy E, Ahmed A, Öhman P. Effects of exenatide once weekly plus dapagliflozin, exenatide once weekly, or dapagliflozin, added to metformin monotherapy, on body weight, systolic blood pressure, and triglycerides in patients with type 2 diabetes in the DURATION‐8 study. Diabetes Obes Metab. 2018;20:1515–1519. https://doi.org/10.1111/dom.13206
Funding information AstraZeneca
REFERENCES
- 1. Bertoluci MC, Rocha VZ. Cardiovascular risk assessment in patients with diabetes. Diabetol Metab Syndr. 2017;9:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gaede P, Oellgaard J, Carstensen B, et al. Years of life gained by multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: 21 years follow‐up on the Steno‐2 randomised trial. Diabetologia. 2016;59:2298‐2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Karagiannis T, Liakos A, Bekiari E, et al. Efficacy and safety of once‐weekly glucagon‐like peptide 1 receptor agonists for the management of type 2 diabetes: a systematic review and meta‐analysis of randomized controlled trials. Diabetes Obes Metab. 2015;17:1065‐1074. [DOI] [PubMed] [Google Scholar]
- 4. Genovese S, Mannucci E, Ceriello A. A review of the long‐term efficacy, tolerability, and safety of exenatide once weekly for type 2 diabetes. Adv Ther. 2017;34:1791‐1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Storgaard H, Gluud LL, Bennett C, et al. Benefits and harms of sodium‐glucose co‐transporter 2 inhibitors in patients with type 2 diabetes: a systematic review and meta‐analysis. PLoS One. 2016;11:e0166125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Frías JP, Guja C, Hardy E, et al. Exenatide once weekly plus dapagliflozin once daily versus exenatide or dapagliflozin alone in patients with type 2 diabetes inadequately controlled with metformin monotherapy (DURATION‐8): a 28 week, multicentre, double‐blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol. 2016;4:1004‐1016. [DOI] [PubMed] [Google Scholar]
- 7. Busch RS, Kane MP. Combination SGLT2 inhibitor and GLP‐1 receptor agonist therapy: a complementary approach to the treatment of type 2 diabetes. Postgrad Med. 2017;129:686‐697. [DOI] [PubMed] [Google Scholar]
- 8. Blonde L, Pencek R, MacConell L. Association among weight change, glycemic control, and markers of cardiovascular risk with exenatide once weekly: a pooled analysis of patients with type 2 diabetes. Cardiovasc Diabetol. 2015;14:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bergenstal RM, Li Y, Porter TK, Weaver C, Han J. Exenatide once weekly improved glycaemic control, cardiometabolic risk factors and a composite index of an HbA1c < 7%, without weight gain or hypoglycaemia, over 52 weeks. Diabetes Obes Metab. 2013;15:264‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bell KF, Katz A, Sheehan JJ. Quality measure attainment with dapagliflozin plus metformin extended‐release as initial combination therapy in patients with type 2 diabetes: a post hoc pooled analysis of two clinical studies. Risk Manag Healthc Policy. 2016;9:231‐241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sjöström CD, Johansson P, Ptaszynska A, List J, Johnsson E. Dapagliflozin lowers blood pressure in hypertensive and non‐hypertensive patients with type 2 diabetes. Diab Vasc Dis Res. 2015;12:352‐358. [DOI] [PubMed] [Google Scholar]
- 12. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834‐1844. [DOI] [PubMed] [Google Scholar]
- 13. Marso SP, Daniels GH, Brown‐Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Holman RR, Bethel MA, Mentz RJ, et al. Effects of once‐weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377:1228‐1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644‐657. [DOI] [PubMed] [Google Scholar]
- 16. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117‐2128. [DOI] [PubMed] [Google Scholar]


