Abstract
Background and purpose
In 2011, fingolimod was approved in Switzerland for the treatment of relapsing‐remitting multiple sclerosis (RRMS). The aim of the present study was to assess the effectiveness and retention of fingolimod in a real‐life Swiss setting, in which patients can receive fingolimod as both first‐ and second‐line treatment for RRMS.
Methods
This cross‐sectional, observational study with retrospective data collection was performed at 19 sites that comprised both hospitals and office‐based physicians across Switzerland. Sites were asked to document eligible patients in consecutive chronological order to avoid selection bias. Demographic and clinical data from 274 consenting adult patients with RRMS who had received treatment with fingolimod were analyzed.
Results
Mean treatment duration with fingolimod was 32 months. Under fingolimod, 77.7% of patients remained free from relapses and 90.3% did not experience disability progression. The proportion of patients who were free from any clinical disease activity, i.e. without relapses and disability progression, was 72.1%. A total of 28.5% of patients had been RRMS treatment‐naïve prior to fingolimod therapy. High long‐term treatment retention rates ranging between 95.7% at 24 months and 87.8% at 36 months were observed.
Conclusion
In this Swiss cohort of naïve and pre‐treated subjects with RRMS, the majority of patients under fingolimod treatment showed freedom from relapses and disability progression. In addition, treatment retention rate over 2 and 3 years was high, irrespective of previous treatment.
Keywords: fingolimod, long‐term effectiveness, multiple sclerosis, real life, real world, retention, Switzerland
Introduction
Long‐term treatment objectives in relapsing‐remitting multiple sclerosis (RRMS) have come increasingly into focus, including prevention of relapses, disability progression, accumulation of brain lesions and brain atrophy 1. Therefore, the long‐term aim of RRMS therapy is to suppress disease activity.
It is now well accepted in clinical practice that effective disease‐modifying therapy (DMT) should be initiated as early as possible to better achieve these long‐term therapy goals 1, 2.
Long‐term treatment retention and adherence to therapy are also crucial for optimal outcomes 3. Therefore, careful and individualized selection of a highly effective and tolerable DMT regimen will increase treatment adherence, which is vital for long‐term treatment success.
Fingolimod (Gilenya®, Novartis Pharma AG, Basel, Switzerland) is the first approved oral DMT worldwide 4, 5, 6. In Switzerland, as opposed to the European Union and some other countries where only a second‐line label has been granted, fingolimod was also approved in 2011 for RRMS as a first‐line treatment. In the three pivotal clinical trials, fingolimod was efficacious in reducing relapse rate, progression of disability, accumulation of new brain lesions as well as progression of brain atrophy compared with placebo or intramuscular interferon beta‐1a 4, 5, 7.
Several observational studies have been published that investigated the effectiveness of fingolimod in a more heterogeneous population of patients as compared with those included in clinical trials 8. These real‐world data confirmed findings from clinical trials in terms of preventing relapses and disability progression, and highlight that fingolimod retention over longer periods of time is generally high, particularly if compared with injectable first‐line therapies 9, 10.
The aim of the present study was to provide further data on fingolimod use under real‐life conditions, particularly for naïve and pre‐treated patients with RRMS in Switzerland.
Methods
Patients and study design
This observational, multi‐center, cross‐sectional study with retrospective secondary data collection was conducted at 19 clinics and office‐based neurologists specializing in multiple sclerosis across Switzerland between August 2015 and January 2016.
Adult patients with RRMS (≥18 years of age) were eligible if they had received uninterrupted (i.e. no interruptions lasting longer than 4 weeks) treatment with commercially available fingolimod for at least 6 months. If these criteria were fulfilled, patients for whom fingolimod had already been discontinued at the time of data documentation were also included in the study. As per the observational plan, patients were included in a chronological and consecutive manner (as of fingolimod marketing authorization in Switzerland in January 2011).
The study was approved by the respective local ethics committees and written informed consent was obtained from patients prior to the start of the study.
The primary objective of the study was to assess the long‐term effectiveness of fingolimod in patients with RRMS in terms of freedom from relapses. Secondary objectives included the collection of data on freedom from disability progression and treatment retention, defined as the proportion of patients who, at a given time, continued to receive fingolimod.
Data from 275 consecutive patients were collected and data from 274 patients were analyzed. One patient who was less than 18 years of age at the time of data collection (17 years old, off‐label use) was excluded from the analysis.
Clinical and radiological data from the patient's last available assessment prior to initiating fingolimod treatment and data from the most recent assessment under fingolimod therapy were entered into paper‐based case report forms. The data collected covered periods before and during fingolimod treatment, and included demography, treatment history and number of relapses under fingolimod and in the 24 months before starting fingolimod. Relapses were defined according to the McDonald criteria 11. Disability progression between two assessments was defined as an increase of ≥1 points in the Expanded Disability Status Score (EDSS) 12.
Statistical methods
Quantitative data were analyzed by descriptive statistical parameters. Qualitative and ordinal data were described by absolute and relative frequency distributions, and percentages were based on valid data. The annualized relapse rate was calculated as number of relapses divided by the exact (decimal) number of years on fingolimod treatment. Analyses were performed for the total sample and stratified according to the duration of treatment with fingolimod and pre‐treatment history. Wilcoxon tests for paired samples were used for statistical comparison of values before and under fingolimod treatment. All analyses were performed using the SAS® package (version 9.2; SAS Institute Inc., Cary, NC, USA).
Results
Patient characteristics
The patient characteristics are summarized in Table 1.
Table 1.
Patient characteristics
| Patients (n) | 274 | |
| Age (years) | ||
| Mean (±SD) | 39.9 (±9.5) | |
| Median (min.–max.) | 40.0 (19–69) | |
| Gender [n (%)] | ||
| Male | 95 (34.7%) | |
| Female | 179 (65.3%) | |
| RRMS disease duration (years) | ||
| Mean (±SD) | 6.3 ± 6.4 | |
| Median (min.–max.) | 4.2 (0.0–30.2) | |
| RRMS treatment status at start of fingolimod [n (%)] | ||
| Treatment naïve (first‐line) | 78 (28.5%) | |
| Pre‐treated (switch) | 196 (71.5%) | |
| Fingolimod treatment duration (months) | ||
| Mean (±SD) | 32.0 ± 11.8 | |
| Median (min.–max.) | 31.1 (7.0–57.9) | |
| EDSS at start of fingolimod | ||
| Mean (±SD) | 2.4 ± 1.3 | |
| Median (min.–max.) | 2.0 (0.0–7.5) | |
EDSS, Expanded Disability Status Score; RRMS, relapsing‐remitting multiple sclerosis.
Fingolimod was used as a first‐line therapy in 78 patients (28.5%). Patients who were switched from another DMT to fingolimod had previously most frequently received one of the interferons (Fig. 1).
Figure 1.
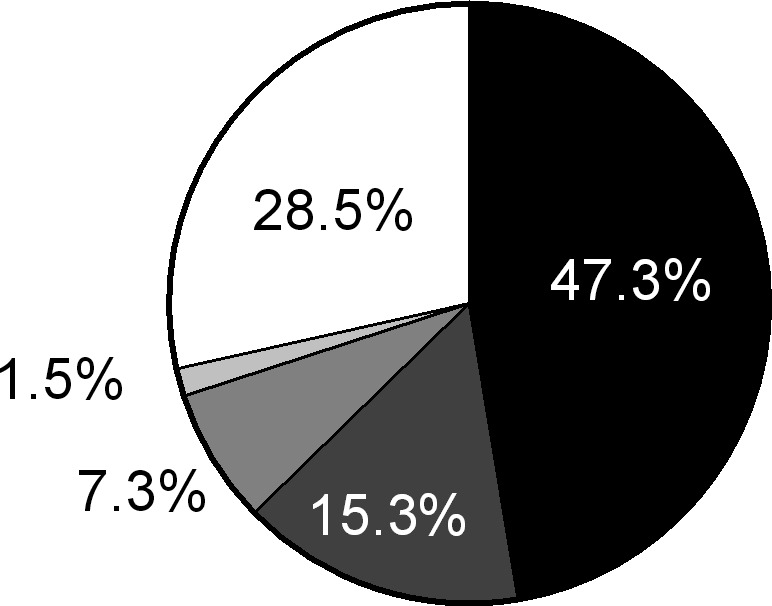
Patients’ pre‐treatment history showing the disease‐modifying therapy given before the switch to fingolimod.  , interferons;
, interferons;  , natalizumab;
, natalizumab;  , glatiramer acetate;
, glatiramer acetate;  , other treatment;
, other treatment;  , no pre‐treatment.
, no pre‐treatment.
Effectiveness
The median (interquartile range) fingolimod treatment duration at the time of documentation was 31.1 (23.7–42.1) months (mean ± SD, 32.0 ± 11.8 months). Overall, 213 (77.7%) patients remained free from relapses under fingolimod treatment. Over the same period, this figure was 74.4% (58 of 78 patients) and 79.1% (155 of 196 patients) in the groups of treatment‐naïve and pre‐treated patients, respectively (Fig. 2).
Figure 2.
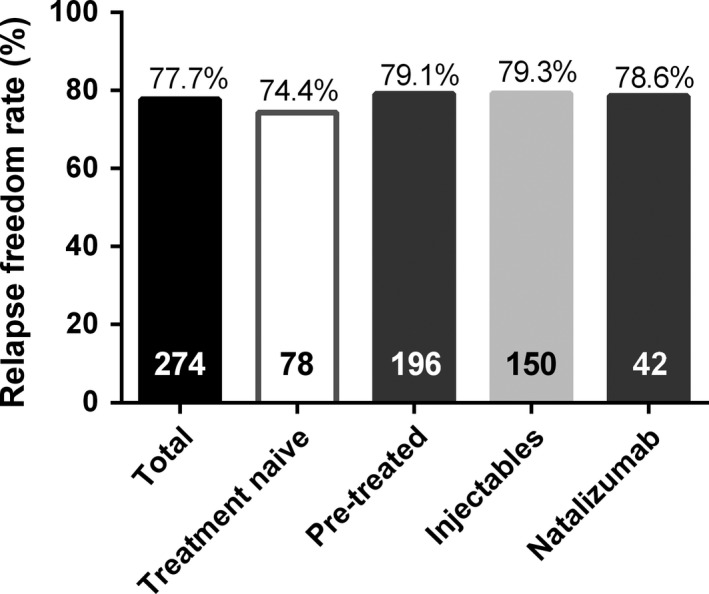
Proportion of patients free from relapse under fingolimod for the total analysis set and for subgroups according to treatment history, i.e. treatment naïve and pre‐treated. Pre‐treated patients were further stratified according to their last multiple sclerosis therapy prior to switch to fingolimod: natalizumab or injectable disease‐modifying therapy (i.e. interferons or glatiramer acetate). The number of patients for each subgroup is shown at the bottom of each bar.
The relapse freedom rate was 79.3% in patients who had last received injectable DMTs (interferons/glatiramer acetate, n = 150) and 78.6% in patients who had been switched to fingolimod after receiving natalizumab (n = 42) (Fig. 2). The mean (±SD) annual relapse rate under fingolimod was 0.12 (±0.28). The mean annual relapse rate also remained similarly low (0.12 ± 0.28) after excluding from the analysis those 12 patients who were treated with fingolimod for less than 12 months. A total of 93% of patients who were relapse‐free before fingolimod initiation (n = 78) did not show any further relapses under fingolimod treatment. Similarly, 78.8%, 64.6% and 50.0% of patients who had had one (n = 89), two (n = 42) or three or more (n = 18) relapses prior to initiation of fingolimod, respectively, were free from relapses under fingolimod treatment.
When stratifying patients according to the duration of fingolimod treatment, the proportion of those free from relapses was 91.7%, 82.5%, 76.9% and 74.1% during year 1, 2, 3 and over 3 years of therapy (Table 2).
Table 2.
Number of relapses under fingolimod by treatment duration
| Fingolimod treatment duration | No. of relapses under fingolimod | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| None | 1 | 2 | 3 | |||||||
| n | % | n | % | n | % | n | % | n | % | |
| <1 year | 11 | 91.7 | 1 | 8.3 | 0 | 0.0 | 0 | 0.0 | 12 | 100.0 |
| 1 to <2 years | 52 | 82.5 | 8 | 12.7 | 2 | 3.2 | 1 | 1.6 | 63 | 100.0 |
| 2 to <3 years | 70 | 76.9 | 15 | 16.5 | 5 | 5.5 | 1 | 1.1 | 91 | 100.0 |
| ≥3 years | 80 | 74.1 | 20 | 18.5 | 6 | 5.6 | 2 | 1.9 | 108 | 100.0 |
A total of 243 (90.3%) patients remained free from disability progression with fingolimod treatment. When patients were stratified according to four EDSS categories, i.e. <1, 1 to <4, 4 to <7 and 7 to <8 as defined in 13, 214 (78.1%) patients belonged to the category ‘1 to < 4’ (i.e. minimal to moderate disability 13) at fingolimod initiation. Under fingolimod treatment, 257 (94.4%) patients remained in the same or moved to a lower grade disability category (Fig. 3a).
Figure 3.
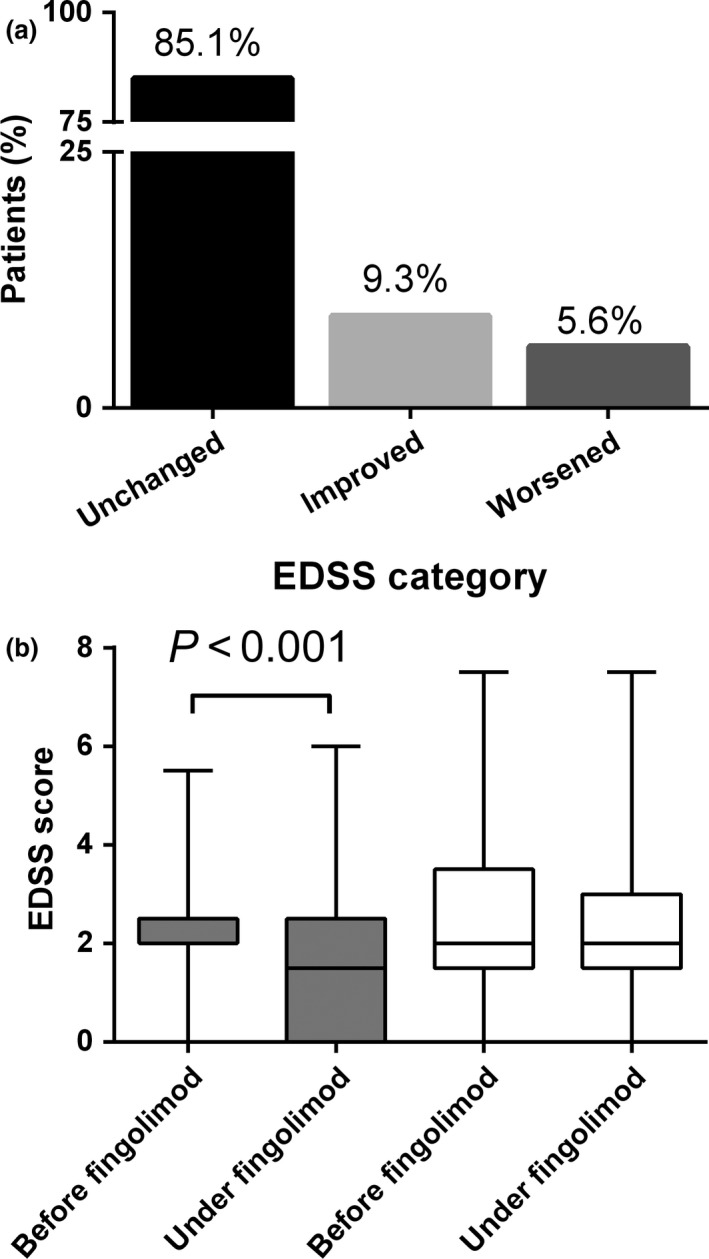
(a) Transition between pre‐defined Expanded Disability Status Score (EDSS) categories (<1, 1 to <4, 4 to < 7 or 7 to <8). Rate of patients without change ( ) and with a transition to a lower (
) and with a transition to a lower ( ) or higher (
) or higher ( ) disability category. (b) Median EDSS before and under fingolimod therapy, stratified according to treatment history. For treatment‐naïve patients (
) disability category. (b) Median EDSS before and under fingolimod therapy, stratified according to treatment history. For treatment‐naïve patients ( ), a significant improvement of EDSS was seen (P < 0.001), whereas for pre‐treated patients (
), a significant improvement of EDSS was seen (P < 0.001), whereas for pre‐treated patients ( ), EDSS remained stable under fingolimod. Box plots represent median EDSS, interquartile range (box margins) and minimum and maximum range (whiskers).
), EDSS remained stable under fingolimod. Box plots represent median EDSS, interquartile range (box margins) and minimum and maximum range (whiskers).
At fingolimod start, the median EDSS (interquartile range) of the total population was 2.0 (1.5–3.0). At the last available follow‐up, EDSS was 2.0 (1.0–3.0) (P = 0.001).
Naïve patients prescribed with fingolimod as a first‐line therapy showed a significant reduction of median EDSS [from 2.0 (2.0–2.5) to 1.5 (0.0–2.5), P < 0.001), whereas pre‐treated patients who were switched to fingolimod showed stable EDSS values [2.0 (1.5–3.5) and 2.0 (1.5–3.0), respectively, P = 0.659] at these two time points (Fig. 3b). A total of 194 (72.1%) patients were free from any clinical disease activity.
Fingolimod retention
A total of 262 (95.6%) and 199 (72.6%) patients were treated with fingolimod for more than 12 and 24 months, respectively. The treatment retention rates over different treatment periods are displayed in Fig. 4. At the time of data documentation, 29 patients (10.6%) had discontinued fingolimod use. Reasons for discontinuation were lack of efficacy in 11 (4.0%) patients and adverse events in 11 (4.0%) patients, including viral infections (e.g. herpes zoster and human papilloma virus, all rated as ‘non‐serious’ by the respective physician) in five patients and basal cell carcinoma in two patients; nausea, cholecystitis and lymphoid papulosis were reported as discontinuation reasons for one patient each. Two patients discontinued fingolimod due to pregnancy, three due to a planned pregnancy and five due to further undefined patient decision. No cardiovascular adverse events were reported by the physicians as reasons for fingolimod discontinuation.
Figure 4.
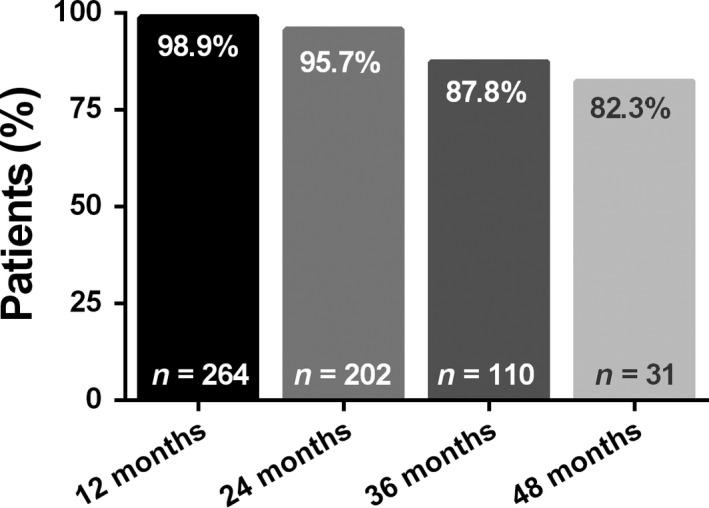
Fingolimod treatment retention, i.e. the proportion of patients who, at a given time point, had retained fingolimod treatment. The number at the bottom of each bar represents the number of patients available for the respective period and the number at the top of each bar represents the percentage of patients who were still receiving fingolimod treatment at that time.
Discussion
The important role of data obtained from real‐world observational studies in complementing evidence from clinical trials is increasingly acknowledged. Specifically in the field of multiple sclerosis, a recent publication endorsed the benefits of using data from multiple study types and data sources 14, particularly including patient cohorts that would not be classically eligible for randomized clinical trials, and thus, reflecting a real‐world patient population.
Over 90% of our study population, including Swiss patients with RRMS treated with fingolimod under real‐life conditions, remained free from relapses over a median treatment period of 31 months. The proportion of patients free from relapses was similar when stratifying patients according to first‐line (naïve patients) or second‐line indication, independent of the treatments received before fingolimod start. It is of note that these results are confirmed even for natalizumab treatment, which is known to be associated with a potential risk of disease activity rebound in the months following its discontinuation 15, 16. Interestingly, we did not observe this phenomenon. The majority of patients switching from natalizumab had been discontinued within 3 months prior to fingolimod start. Thus, it is also unlikely that the low relapse rate observed in this subgroup represents a regression to the mean effect after relapses occurred during the wash‐out period after natalizumab discontinuation.
The majority of patients also achieved a long‐term stabilization of EDSS, supporting the findings from clinical trials and other long‐term observational clinical studies 5, 6, 8. In addition, treatment‐naïve patients showed a significant improvement of their EDSS, probably reflecting a recovery from the relapse that motivated fingolimod start and the larger compensatory capacity known to characterize younger patients 17, 18. At first glance, the size of this effect may appear too small to be of clinical relevance 13. It should be noted, however, that the majority of treatment‐naïve patients presented a priori with low EDSS. Thus, the attenuation of disability progression observed here in very early stages of the disease, even if small, might represent a particular benefit for these patients – a finding well in line with current treatment guidance stipulating an early initiation of effective DMT to achieve better long‐term outcomes in patients with RRMS 1, 2.
The rate of patients who were treated with fingolimod as a first‐line therapy in our sample was 28.5%. In another Swiss study that was performed within only 1 year of the Swiss market launch of fingolimod, the proportion of treatment‐naïve patients was approximately 12% 19. It seems plausible that adoption of fingolimod use depends not only on the physicians’ familiarity with the drug, but also on the availability of real‐life data supporting effectiveness and a good tolerability profile.
Treatment retention, satisfaction, tolerability and adherence are prerequisites for treatment effectiveness and long‐term therapeutic success 3. In comparison to other real‐world studies, the mean treatment duration of 32 months in our study is relatively long, and the retention rates, e.g. 95.7% at 24 months and 87.8% at 36 months, are mostly higher than previously reported treatment retention rates for fingolimod under real‐life conditions (reviewed by Ziemssen et al. 8). For instance, in other real‐world studies, Alroughani et al. 10 showed a fingolimod discontinuation rate of 5.3% after a mean treatment duration of 18.5 months, whereas Yamout et al. saw a discontinuation rate of 28.7% after a mean treatment duration of 19.2 months 9. Indeed, in our study, only patients receiving continuous fingolimod treatment for at least 6 months were included, which might have led to a selection bias towards patients who were more likely to continue fingolimod in the long term. Nonetheless, we believe that the high retention rates at 24 and 36 months observed in our sample suggest a good long‐term acceptance of and satisfaction with fingolimod treatment under real‐life conditions. Adverse events and/or lack of efficacy were given as the most frequent reasons for discontinuation.
Real‐world evidence studies like ours have inherent limitations. Firstly, we have no magnetic resonance imaging data supporting our clinical results. Available data on magnetic resonance imaging lesion load were indeed collected but, in the course of evaluation, it became apparent that the time windows defined in the observational plan were often violated and important data were incompletely documented. Thus, these data were not analyzed in detail. Second, the observational (i.e. uncontrolled) and retrospective study design exposes the study to the risk of selection and reporting bias, which cannot be ruled out entirely.
Finally, due to the study design, safety data were collected only upon drug discontinuation, these remaining, however, within the known safety profile of fingolimod. Despite these limitations, the population in the present study represents approximately 5% of the entire patient population with RRMS in Switzerland receiving a DMT 20. The data obtained in this population therefore probably reflect the current use of fingolimod in Swiss patients with RRMS. Moreover, our results are of interest not only for Switzerland, where fingolimod is also approved as a first‐line multiple sclerosis therapy, but also for other countries such as the USA and Australia sharing similar indications.
In conclusion, our data show that under first‐ as well as second‐line real‐life use of fingolimod:
a high proportion of patients with RRMS achieve or maintain relapse freedom and/or freedom from disability progression over a mean follow‐up of 32 months, irrespective of previous treatment history and
over this time interval, fingolimod is continued by the majority of patients.
Disclosure of conflicts of interest
The employer of C.Z. received financial support from Teva, Merck Serono, Roche, Biogen Idec, Genzyme, Novartis, Bayer and Celgene. The submitted work is not related to these agreements. S.R. received compensation as a consultant from Biogen, Merck, Novartis, Sanofi‐Aventis and Teva Pharma. O.F. received compensation as a consultant from Novartis, Biogen, Bayer, Teva Pharma, Merck, Genzyme and Allmiral. M.L.P. received compensation from Bayer, Biogen, Merck, Novartis, Sanofi Genzyme and Teva Pharma. A.B. received personal compensation for activities with Abbvie AG, Bayer Pharmaceuticals Corporation, Biogen Idec, Merck & Co. Inc., Novartis, Genzyme Corporation, UCB Pharma, Teva Neuroscience, CSL Behring, Pangas, Eli Lilly & Co., Boehringer Ingelheim Pharmaceuticals Inc. and Merz Pharma. C.P.K. received honoraria for lectures as well as research support from Biogen Idec, Novartis, Almirall, Bayer Schweiz AG, Teva Pharma, Merck Serono, Genzyme and the Swiss MS Society (SMSG). P.H.L. received honoraria for speaking from Biogen Idec, CSL Bering, Merck Serono, Novartis, Sanofi‐Aventis and Teva Pharma; consulting fees from Biogen Idec, Geneuro, Genzyme, Merck Serono, Novartis, Roche, Sanofi‐Aventis and Teva Pharma; and research grants from Biogen Idec, Merck Serono and Novartis. A.C. received honoraria and consulting fees from Bayer, Merck Serono, Novartis, Sanofi‐Aventis, Roche, Teva Pharma, Biogen Idec, Genzyme. G.P. and V.B. are employees of Novartis. The study was funded by Novartis Pharma AG, Switzerland.
Acknowledgements
The authors would like to thank all patients who participated for granting permission for use of their personal data for this study as well as all study personnel who participated in the collection of these data. The study was conducted with CRO support by GKM Gesellschaft für Therapieforschung mbH, Munich, Germany.
References
- 1. Freedman MS. Long‐term follow‐up of clinical trials of multiple sclerosis therapies. Neurology 2011; 76(1 Suppl. 1): S26–S34. [DOI] [PubMed] [Google Scholar]
- 2. McGraw CA, Lublin FD. Interferon beta and glatiramer acetate therapy. Neurotherapeutics 2013; 10: 2–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Patti F. Optimizing the benefit of multiple sclerosis therapy: the importance of treatment adherence. Patient Prefer Adherence 2010; 4: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cohen JA, Barkhof F, Comi G, et al Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 2010; 362: 402–415. [DOI] [PubMed] [Google Scholar]
- 5. Kappos L, Radue EW, O'Connor P, et al A placebo‐controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 2010; 362: 387–401. [DOI] [PubMed] [Google Scholar]
- 6. Calabresi PA, Radue EW, Goodin D, et al Safety and efficacy of fingolimod in patients with relapsing‐remitting multiple sclerosis (FREEDOMS II): a double‐blind, randomised, placebo‐controlled, phase 3 trial. Lancet Neurol 2014; 13: 545–556. [DOI] [PubMed] [Google Scholar]
- 7. Cohen JA, Barkhof F, Comi G, et al Fingolimod versus intramuscular interferon in patient subgroups from TRANSFORMS. J Neurol 2013; 260: 2023–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ziemssen T, Medin J, Couto CA‐M, Mitchell CR. Multiple sclerosis in the real world: a systematic review of fingolimod as a case study. Autoimmun Rev 2017; 16: 355–376. [DOI] [PubMed] [Google Scholar]
- 9. Yamout BI, Zeineddine MM, Sawaya RA, Khoury SJ. Safety and efficacy of reduced fingolimod dosage treatment. J Neuroimmunol 2015; 285: 13–15. [DOI] [PubMed] [Google Scholar]
- 10. Alroughani R, Ahmed S, Behbehani R, Al‐Hashel J. Use of fingolimod in patients with relapsing remitting multiple sclerosis in Kuwait. Clin Neurol Neurosurg 2014; 119: 17–20. [DOI] [PubMed] [Google Scholar]
- 11. Polman CH, Reingold SC, Banwell B, et al Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011; 69: 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983; 33: 1444–1452. [DOI] [PubMed] [Google Scholar]
- 13. Confavreux C, Vukusic S, Moreau T, Adeleine P. Relapses and progression of disability in multiple sclerosis. N Engl J Med 2000; 343: 1430–1438. [DOI] [PubMed] [Google Scholar]
- 14. Rothenbacher D, Capkun G, Uenal H, Tumani H, Geissbühler Y, Tilson H. New opportunities of real‐world data from clinical routine settings in life‐cycle management of drugs: example of an integrative approach in multiple sclerosis. Current Med Res Opin 2015; 31: 953–965. [DOI] [PubMed] [Google Scholar]
- 15. Rasenack M, Derfuss T. Disease activity return after natalizumab cessation in multiple sclerosis. Expert Rev Neurother 2016; 16: 587–594. [DOI] [PubMed] [Google Scholar]
- 16. Sorensen PS, Koch‐Henriksen N, Petersen T, Ravnborg M, Oturai A, Sellebjerg F. Recurrence or rebound of clinical relapses after discontinuation of natalizumab therapy in highly active MS patients. J Neurol 2014; 261: 1170–1177. [DOI] [PubMed] [Google Scholar]
- 17. Mowry EM, Pesic M, Grimes B, Deen S, Bacchetti P, Waubant E. Demyelinating events in early multiple sclerosis have inherent severity and recovery. Neurology 2009; 72: 602–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vercellino M, Romagnolo A, Mattioda A, et al Multiple sclerosis relapses: a multivariable analysis of residual disability determinants. Acta Neurol Scand 2009; 119: 126–130. [DOI] [PubMed] [Google Scholar]
- 19. Rasenack M, Rychen J, Andelova M, et al Efficacy and safety of fingolimod in an unselected patient population. PLoS One 2016; 11: e0146190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schweizerische Multiple Sklerose Gesellschaft . https://www.multiplesklerose.ch/de/ueber-ms/(accessed 13/02/2017)


