Abstract
Aims
The metabolic state of human adults is associated with their gut microbiome. The symbiosis between host and microbiome is initiated at birth, and early life microbiome perturbation can disturb health throughout life. Here, we determined how beneficial microbiome interventions in early life affect metabolic health in adulthood.
Methods
Postnatal diets were supplemented with either prebiotics (scGOS/lcFOS) or synbiotics (scGOS/lcFOS with Bifidobacterium breve M‐16 V) until post‐natal (PN) day 42 in a well‐established rodent model for nutritional programming. Mice were subsequently challenged with a high‐fat Western‐style diet (WSD) for 8 weeks. Body weight and composition were monitored, as was gut microbiota composition at PN21, 42 and 98. Markers of glucose homeostasis, lipid metabolism and host transcriptomics of 6 target tissues were determined in adulthood (PN98).
Results
Early life synbiotics protected mice against WSD‐induced excessive fat accumulation throughout life, replicable in 2 independent European animal facilities. Adult insulin sensitivity and dyslipidaemia were improved and most pronounced changes in gene expression were observed in the ileum. We observed subtle changes in faecal microbiota composition, both in early life and in adulthood, including increased abundance of Bifidobacterium. Microbiota transplantation using samples collected from synbiotics‐supplemented adolescent mice at PN42 to age‐matched germ‐free recipients did not transfer the beneficial phenotype, indicating that synbiotics‐modified microbiota at PN42 is not sufficient to transfer long‐lasting protection of metabolic health status.
Conclusion
Together, these findings show the potential and importance of timing of synbiotic interventions in early life during crucial microbiota development as a preventive measure to lower the risk of obesity and improve metabolic health throughout life.
Keywords: body composition, dietary intervention, insulin resistance, liver, mouse model, obesity therapy
1. INTRODUCTION
Obesity is a multifactorial condition of pandemic dimensions,1 which is often accompanied by perturbation of glucose and lipid homeostasis, together increasing the risk of developing type 2 diabetes mellitus, cardiovascular disease and fatty liver disease.2, 3 In addition to diet and other environmental factors, gut microbiota has been linked to the metabolic health status of an individual.4, 5 Concurrent with host physiology, the gut microbiota develops dynamically in the first years of life and is shaped by intrinsic and extrinsic factors, with nutrition playing a significant role.6 It is now commonly accepted that nutrition in early postnatal life has a pronounced impact on lifelong metabolic health,7, 8, 9 often referred to as nutritional programming. For instance, breastfed children have a higher abundance of bifidobacteria and a lower risk of developing obesity, glucose intolerance and hypercholesterolaemia as adults compared with formula‐fed infants.10, 11, 12, 13 Exposure to antibiotics in early life perturbs development of the gut microbiota, reduces Bifidobacterium abundance14 and predisposes infants to developing obesity later in life.15, 16 These studies unlock the potential of altering gut microbiota beneficially in early life to counteract the development of obesity and metabolic disease later in life.
Pre‐, pro‐ and synbiotics are known to affect gut microbiota composition beneficially.17, 18, 19, 20 For example, short‐chain galacto‐oligosaccharides (scGOS), long‐chain fructo‐oligosaccharides (lcFOS) as prebiotics or in combination with Bifidobacterium breve M16 V as a synbiotic increase bifidobacteria abundance in infants.21, 22, 23, 24 Furthermore, pre‐, pro‐ and synbiotics are indicated to improve the host's growth and metabolic health by microbiota‐induced mechanisms,25 but their long‐term effects on the host's metabolic health beyond intervention have not been well studied. Therefore, we tested whether nutritional intervention with these components early in life lessens the subsequent development of diet‐induced obesity and affects long‐term metabolic health in the host by supplementing with scGOS/lcFOS alone as a prebiotic or in combination with Bifidobacterium breve M16 V as a synbiotic in a well‐established nutritional programming rodent model.26, 27
2. METHODS
All experimental procedures in this study were in accordance with the principles of good laboratory animal care and practice, and complied with national legislation, following the EU‐guidelines for the protection of animals used for scientific purposes, and were reviewed and approved by the Animal Experimental Committee DEC‐Consult in Bilthoven, The Netherlands (Study 1) and the Ethics Committee on Animal Care and Use in Gothenburg, Sweden (Study 2).
At 2 independent European locations, we investigated whether nutritional pre‐ and synbiotics protect mice against diet‐induced obesity in our nutritional programming model, as depicted in Figure 1A (Study 1) and Figure S2A (Study 2). On postnatal day (PN) 2, offspring from C57BL/6 J dams were randomly culled to 4 male and 2 female pups per litter, and lactating dams were assigned AIN‐93G‐based intervention diets supplemented with prebiotics (PRE: 2% w/w scGOS/lcFOS; Study 1 only), synbiotics (SYN: 2% w/w scGOS/lcFOS+109 CFU/g Bifidobacterium breve M‐16 V [Morinaga Milk Industries Ltd., Tokyo, Japan]) or a non‐active control component (REF and CTRL: 2% w/w maltodextrin). In addition to dietary supplementation, pups received daily (PN10–15) an oral dose of approximately 10 to 15 mg/day maltodextrin or scGOS/lcFOS, with or without 1 × 109 cfu B. breve M‐16V of their respective supplement. After weaning (PN21), male offspring were housed in pairs and continued respective intervention diets until PN42. Thereafter CTRL, PRE and SYN animals received a Western‐style diet (WSD, 40% energy from fat) as a challenge until sacrifice on PN98. A non‐supplemented, non‐WSD‐challenged group (REF) served as a normal healthy reference. Throughout the experiments, body weight and composition were determined, using either DEXA (Study 1) or MRI (Study 2), and faecal samples were collected on PN21, 42 and 98. On PN98, animal length was determined and blood plasma, white adipose tissue (WAT) depots, back‐hind muscles, liver and intestinal segments were collected, weighed, snap frozen and stored at −80 °C until further analysis.
Figure 1.

Early life synbiotics prevent excessive fat accumulation under WSD challenge. A, Schematic overview of Study1. Litters were culled at postnatal day (PN) 2 and were randomly divided into 4 diet groups: Reference (REF) and Control (CTRL) groups receiving AIN‐G (standard semi‐synthetic diet appropriate for breeding) plus control component (maltodextrin); the PRE group receiving AIN‐G supplemented with prebiotics (scGOS/lcFOS in ratio 9:1); and the SYN group receiving AIN‐G, supplemented with synbiotics (scGOS/lcFOS in a ratio 9:1 + B. breve M‐16V) until PN42. At PN42, the REF group was maintained on AIN‐M (semi‐synthetic diet appropriate for maintenance) and the CTRL, PRE and SYN groups were challenged with Western‐style diet (40% energy from fat) until PN98. Body weight (B), fat mass (C) and lean body mass (D) in the REF (n = 8), CTRL (n = 7), PRE (n = 9) and SYN (n = 11) groups of mice at PN42, PN70 and PN98. Sum of fat pads (E) and plasma leptin level (F) in the REF (n = 8), CTRL (n = 7), PRE (n = 9) and SYN (n = 11) groups of mice at PN98. Data are given as mean ± SEM. In (B) to (D), repeated‐measures two‐way ANOVA, followed by Sidak's multiple comparisons test for (1) REF vs CTRL (2), PRE vs SYN and (3) CTRL vs SYN. && P ≤ .01, &&& P ≤ .001 indicates significance for REF vs CTRL. †† P ≤ .01, ††† P ≤ .001 indicate significance for PRE vs SYN. $ P ≤ .05, $$ P ≤ .01; $$$ P ≤ .001 indicate significance for CTRL vs SYN. In (E) and (F), one‐way ANOVA, followed by Tukey's multiple comparisons test. *P ≤ .05
Cecal contents of CTRL and SYN mice collected at PN42 in Study 2 were transplanted to age‐matched germ‐free C57BL/6 male recipients that were maintained on WSD for 8 weeks, and their body weight and composition were determined (Figure 5A).
Figure 5.
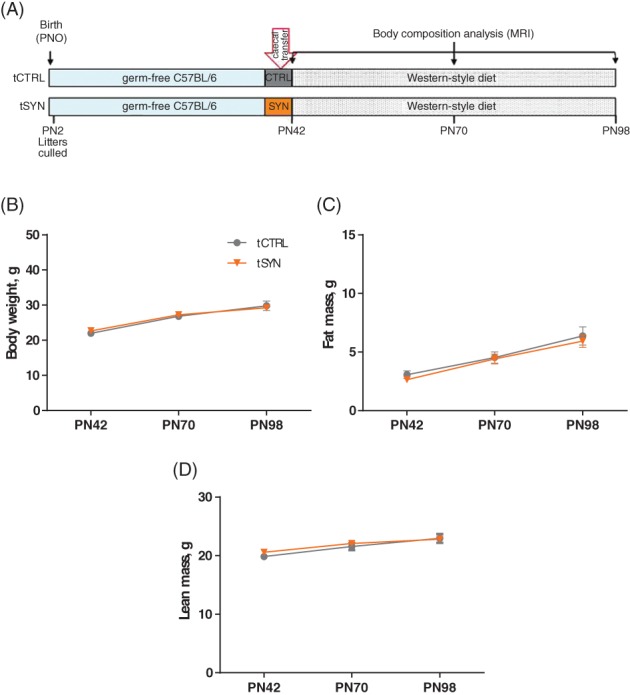
Beneficial phenotype induced by synbiotics is not transferred by microbiota transplant in early adulthood. A, Schematic overview of Study. Six‐weeks‐old germ‐free (GF) mice maintained on standard diet were transplanted with cecal contents collected from CTRL or SYN mice at PN42. Both transplanted groups (ie,. tCTRL and tSYN) were challenged by Western‐style diet until PN98. Body weight (B), fat mass (C) and lean body mass (D) in tCTRL (n = 8) and tSYN (n = 9) groups of mice at PN42, PN70 and PN98. Data are given as mean ± SEM. Repeated‐measures two‐way ANOVA, followed by Sidak's multiple comparisons test
Fasting total cholesterol, triglycerides, beta‐hydroxybutyrate, leptin, insulin and glucose were measured in plasma. Total triglyceride and cholesterol content were also measured in liver. RNA from duodenum, jejunum, ileum, colon, liver and WAT was extracted and hybridized to Affymetrix GeneChip Mouse Gene 1.1 ST microarrays. Gene expression was analysed using a Bioconductor software package‐based pipeline.28 Faecal microbiota composition was profiled by 16S rRNA gene sequencing using an Illumina MiSeq platform and data were analysed using the QIIME pipeline. Differentially abundant genera were identified based on negative binomial distribution (Wald test) using DESeq2 package in the R.
Detailed information related to study conduct, diets, sample collection and data analysis are available online in Appendix S1.
3. RESULTS
3.1. Early life synbiotics induce long‐term protection against diet‐induced obesity
To explore whether modulating the gut microbiota in early life may have beneficial effects on metabolism in adulthood, we supplemented mouse diets with prebiotics (PRE; short chain galacto‐oligosaccharides [scGOS], long chain fructo‐oligosaccharides [lcFOS], ratio 9:1) or synbiotics (SYN; same as PRE plus Bifidobacterium breve M‐16V) (Study1) (Figure 1A) after birth until PN42. Subsequently, mice were fed a Western‐style diet (WSD) until PN98 and compared to mice that did not receive early life nutritional supplementation (CTRL). Early life nutritional supplementation did not affect developmental growth during the intervention period; no significant difference in body weight (P = .174), fat mass (P = .059), lean body mass (P = .097) (Figure 1B‐D) or adiposity (P = .324) (Figure S1) was observed between any of the groups at PN42. There were also no differences between groups in lean body mass development under WSD challenge (diet*time interaction, P = .541) (Figure 1D) or in animal length at PN98 (P = .109) (Table S1), indicating a normal growth trajectory. The effectiveness of the WSD challenge was confirmed by accelerated weight gain (diet*time interaction P < .001) and increased fat disposition (diet*time interaction P < .001) in the CTRL group compared with non‐supplemented, non‐challenged healthy reference mice (REF) (Figure 1B,C).
We observed that early life synbiotics supplementation protected mice against diet‐induced obesity, as the SYN group had significantly reduced body weight and fat mass compared with the CTRL and PRE groups, both at PN70 (SYN vs CTRL, P BW = .012, P FM < .001; SYN vs PRE, P BW = .007, P FM = .014) and PN98 (SYN vs CTRL, P BW = .004, P FM < .001; SYN vs PRE, P BW = .002, P FM < .001) (Figure 1B,C). In accordance with this observation, average fat mass percentage was reduced in the SYN group compared with the CTRL group at PN70 (P = .001) and PN98 (P = .002) (Figures S1B and S1C). Food intake did not differ between groups (data not shown).
Synbiotic supplementation also reduced individual fat pad weights (Table S1) and the sum of fat pad weights (P = .036) at PN98 in the SYN group compared with the CTRL group (Figure 1E). In agreement with reduced adiposity, we observed that plasma leptin was reduced in the SYN group compared with the CTRL group (Figure 1F). In contrast, body weight, fat mass, fat pad weights and leptin level did not differ significantly at any time point in the PRE group compared with the CTRL group, suggesting that PRE is more effective in combination with the probiotic component in protecting against diet‐induced obesity.
To test the robustness of the findings, the study was repeated in a second animal facility in another European country, and the results on body weight and adipose phenotype (Study 2) (Figure S2A) were reproduced. Synbiotic supplementation reduced both body weight (P = .031) and fat mass (P = .026) compared with the CTRL group at PN98 (Figures S2B and S2C). Lean body mass remained similar in the SYN and CTRL groups throughout the experiment (P PN70 = .652, P PN98 = .914), reflecting normal growth (Figure S2D). Thus, it seems that the beneficial long‐term effects of synbiotic supplementation in early life are independent of environmental conditions that may differ between animal facilities.
3.2. Early life synbiotics improve glucose and lipid metabolism in adulthood
To address whether protection from obesity was also associated with improved metabolic parameters, we determined the HOMA‐IR index as a surrogate marker for insulin resistance and found that the SYN group showed a trend towards reduced HOMA‐IR (P = .067) compared with the CTRL group (Figure 2A), suggesting improved insulin sensitivity. This effect was driven mainly by reduced insulin levels (P = .039) in the SYN group compared with the CTRL group (Figure 2B), whereas fasting glucose levels were similar between groups (Figure 2C). While plasma levels of triglycerides (TG) were similar in all WSD‐challenged groups (P = .986) (Figure 2D), we observed that total plasma cholesterol was significantly reduced in the SYN group compared with the CTRL group (P = .003), resembling REF group levels (Figure 2E). In addition, we detected increased plasma levels of beta‐hydroxybutyrate (P = .047) in the SYN group compared with the CTRL group (Figure 2F), indicating higher fatty acid oxidation, which might contribute to protection against obesity and improved glucose metabolism.
Figure 2.
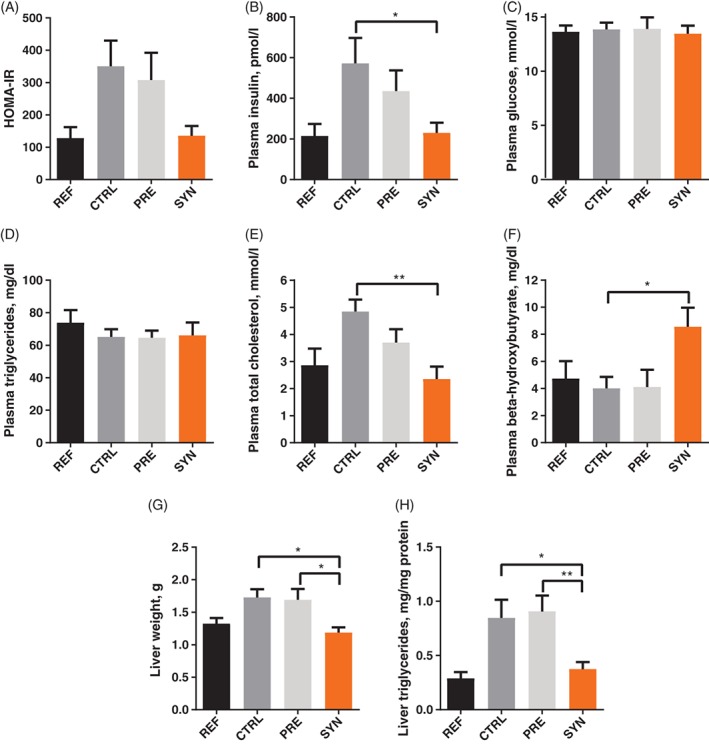
Early life synbiotics improve markers for glucose and lipid homeostasis in adult mice. HOMA‐IR (A) was calculated from fasted plasma insulin (B) and fasted plasma glucose levels (C) at PN98. Plasma triglycerides (D), total cholesterol (E) and beta‐hydroxybutyrate (F), liver weight (G) and hepatic triglycerides (H) at PN98 in the REF (n = 8), CTRL (n = 5‐7), PRE (n = 7‐9) and SYN (n = 10‐11) groups. Data are given as mean ± SEM. One‐way ANOVA, followed by Tukey's multiple comparisons test. *P ≤ .05; **P ≤ .01
The improvement in adult plasma parameters following early life synbiotic supplementation was also associated with reduced liver weight (SYN vs CTRL, P = .017; SYN vs PRE, P = .016) and decreased hepatic TG content (SYN vs CTRL, P = .034; SYN vs PRE, P = .009) (Figure 2G and H). These data suggest that the protective effect of synbiotic supplementation against obesity is accompanied by improvements in metabolism, including parameters of glucose and lipid homeostasis.
3.3. Early life synbiotics alter cholesterol metabolism in the ileum in adulthood
To explore potential molecular mechanisms underlying the beneficial long‐term effects of early life synbiotic supplementation compared with the CTRL group, we performed microarray analysis of 6 relevant tissues harvested on PN98: duodenum, jejunum, ileum and colon, liver and white adipose tissue. We observed that 144 genes were differentially regulated (q ≤ .05) in ileum in the SYN vs CTRL comparison. Of these 144 genes, 57% were relatively less expressed in the SYN group compared with the CTRL group. In contrast, other tissues did not show differentially expressed genes after FDR adjustment in the SYN vs CTRL comparison, indicating that synbiotic supplementation in early life has the most pronounced effects on ileal gene expression in adulthood. Additional information on all differentially expressed genes and their functions in the ileum, as defined by the Gene Ontology (GO) Database, is provided online in Table S2.
To identify regulated biological functions and canonical pathways in the ileum for SYN vs CTRL comparison, we performed Ingenuity Pathway Analysis (IPA) on differentially expressed genes (q ≤ .05). “Lipid metabolism” was identified as one of the strongest regulated biological functions in the ileum of adult mice supplemented with synbiotics in early life compared with the CTRL group (Figure 3A). In agreement with this, the 4 most strongly regulated canonical pathways (Figure 3B) were: “LPS/IL‐1 mediated inhibition of RXR,” related to the regulation of lipid, cholesterol and xenobiotic metabolism, “Superpathway of cholesterol biosynthesis,” “Zymosterol Biosynthesis,” feeding into cholesterol biosynthesis and “LXR/RXR activation,” which is also related to lipid and cholesterol metabolism. This suggests that lipid and cholesterol metabolism are the most strongly regulated functions in adult mice following synbiotic supplementation in early life. Following this lead, we selected gene sets related to cholesterol metabolism and fate, including cholesterol biosynthesis, esterification, transport, distribution and regulation. The expression level of respective genes (n = 31), displayed as signal intensity per sample normalized to the mean signal of the CTRL group, is shown in the heatmap in Figure 3C, highlighting that the effects are strongest in SYN samples compared to the CTRL group. We observed that 6 of the selected 31 genes were significantly (q ≤ .05) regulated in SYN vs CTRL comparison, while other genes followed the same trend of regulation as indicated in the heatmap (Figure 3C). Gene sets related to cholesterol biosynthesis, that is, mevalonate pathway and steroid biosynthesis (Hmgcs1, Pmvk, Tm7sf2, Msmo1 and Nsdhl with q ≤ 0.05) and cholesterol uptake into the cell were up‐regulated in adult mice with synbiotic supplementation in early life compared with the CTRL group (Figure 3C,D and Table S3). In contrast, genes related to cholesterol storage, distribution and excretion, including Abca1 (q = 0.038), were down‐regulated in the SYN group vs the CTRL group (Figure 3C,D and Table S3). Taken together, synbiotic supplementation in early life induced the expression of genes involved in intestinal cholesterol synthesis and uptake in ileal cells of adult mice compared with CTRL mice receiving WSD. These observations for the SYN vs CTRL groups were largely in line with the effects in the REF vs CTRL groups (Figure 3C).
Figure 3.
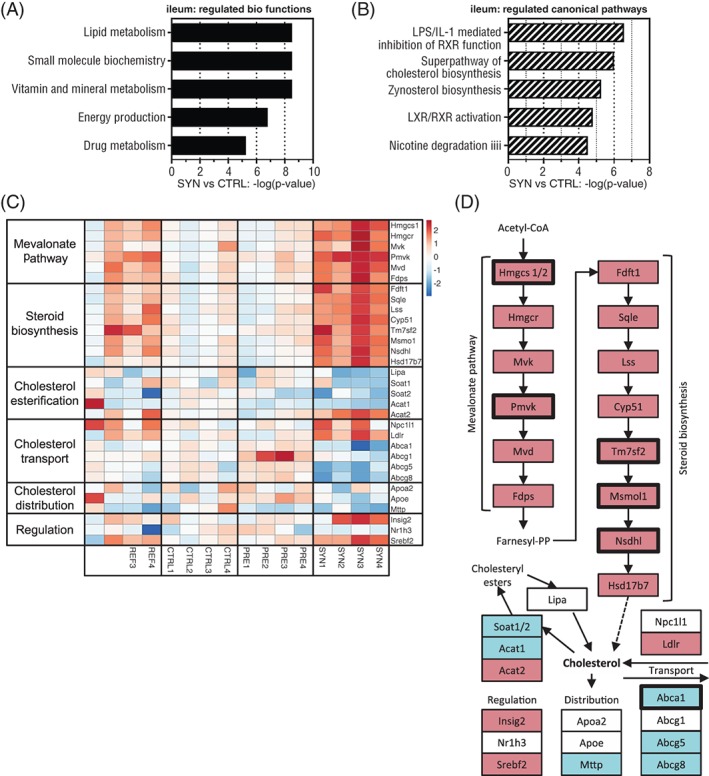
Early life synbiotics affect cholesterol metabolism in the ileum on PN98. The 5 most strongly regulated regulated biological functions (A) and canonical pathways (B) in the SYN vs CTRL comparison in the ileum, determined by Ingenuity Pathway Analysis (IPA) of differential expressed genes (q ≤ .05). (C) Heatmap of selected gene sets related to cholesterol biosynthesis, esterification, transport, distribution and regulation, indicating relative gene expression (signal intensities per sample normalized to the mean of the CTRL group in the REF, CTRL, PRE and SYN groups as color gradient: red, higher expression than mean of CTRL; blue, lower expression than mean of CTRL). (D) Schematic overview of cholesterol biosynthesis pathway, transport, distribution and regulation. Up‐regulation (red) and down‐regulation (blue) are indicated for genes with P ≤ .05 in SYN vs CTRL comparison. Bold frames indicate significantly altered genes after FDR adjustment (q ≤ .05). White boxes indicate non‐significant genes (P > .05). For more information see Table S3
3.4. Early life synbiotics intervention induces subtle microbiota changes
The microbiota composition of faecal samples collected at PN21, PN42 and PN98 was determined using Illumina MiSeq platform. In total, we generated 4 176 221 sequences with a mean ± SD of 42 184 ± 6391 sequences per sample. To investigate the variance within and between groups, weighted unifrac distances (sensitive to abundance of taxa) were obtained. We observed no significant difference in distances within the individual groups or between groups, or between distances within and between groups (Figure 4A). Alpha diversity, which reflects within sample diversity, measured by “observed species,” was significantly higher in the SYN group compared with the PRE (P = .042) and CTRL (P = .006) groups at PN21 and PN42, respectively (Figure 4B), indicating that synbiotic supplementation increased early life faecal bacterial diversity.
Figure 4.
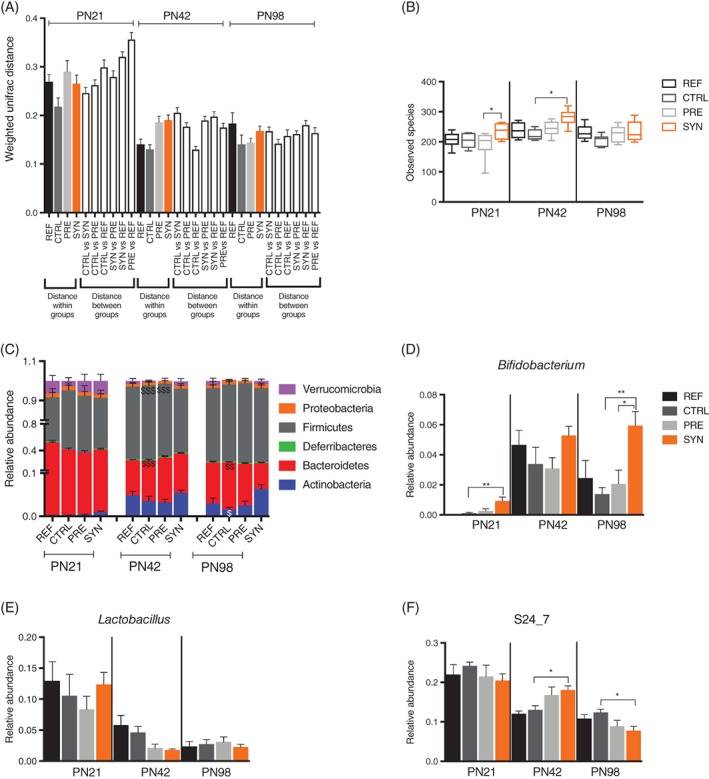
Early life synbiotics modulate early life microbiota. A, Weighted Unifrac distances showing distance within and between groups in the REF (n = 7), CTRL (n = 7), PRE (n = 8) and SYN (n = 11) groups of mice. B, Bacterial diversity as shown by diversity box plots obtained from alpha rarefaction measured by observed species. Data are shown as box and whiskers plots, indicating the median (thick line in the box), the 25th to 75th percentile range (boxes), and the 1st to 99th percentile range (whiskers). Relative abundance of major phyla (C), Bifidobacterium (D) Lactobacillus (E) and S24_7 (F) in the REF (n = 7), CTRL (n = 7), PRE (n = 8) and SYN (n = 11) groups of mice. Data are given as mean ± SEM. In (C), two‐way analysis of variance, followed by Tukey's multiple comparisons test. $ P < .05, $$ P < .01, $$$ P < .001 indicate significance between the CTRL and SYN groups. In (D) to (F), non‐parametric the Kruskal‐Wallis test, followed by Dunn's multiple comparisons test. *P < .05, **P < .01 as indicated
To determine the overall microbiota structure over time, we analysed the microbiota composition at phylum level. The abundance of Firmicutes reduced in the SYN group compared with the CTRL and PRE groups at PN42 (Figure 4C). Synbiotic supplementation trended towards increasing the abundance of Actinobacteria at PN42 and reached statistical significance at PN98 compared with the CTRL group (Figure 4C). At PN98, the abundance of Bacteroidetes was significantly reduced in the SYN group compared with the CTRL group, while the level of Firmicutes did not differ between these groups (Figure 4C). Levels of abundant genera that differed across different time points were plotted in Figure 4D‐F to explain observed phenotypic differences between the groups. The abundance of Bifidobacterium was highest in the synbiotic‐supplemented group compared with the CTRL group at PN21 (Figure 4D). Moreover, a higher proportion of Bifidobacterium in the SYN group, compared with all other groups including the PRE group, was maintained until PN98 (Figure 4D). The level of Lactobacillus fluctuated at different time points and was not significantly altered in the SYN group compared with the CTRL group at any time point (Figure 4E). The relative abundance of unidentified genus from family S24_7, belonging to phylum Bacteroidetes, increased in the SYN group compared with the CTRL group at PN42, but decreased at PN98 (Figure 4F). We also identified differentially abundant genera that were altered in the SYN vs CTRL comparison at different time points, using negative binomial generalized linear models (Figure S3). Interestingly, a low‐abundance unidentified genus from Alphaproteobacteria (order RF32) was identified as the most differentially abundant genus, with higher levels in the SYN group compared with the CTRL group at all 3 time points (Figure S3A). At PN42, Lactobacillus was identified with logFC = −1.40 (Figure S3A), consistent with its trend of lower abundance in the SYN group compared with the CTRL group (Figure 4E). In addition, Dorea (logFC = 4.31) and Parabacteroides (logFC = −1.67) were also identified at PN42 (Figure S3A).
Next, we performed sequencing of the samples obtained in the second facility (Study 2) at PN21, PN42 and PN98 (Figure S3), using the same protocol. Weighted unifrac distances revealed that distance within the SYN group was significantly reduced from that in the CTRL vs SYN and SYN vs REF comparison at PN21, and the SYN vs REF comparison at PN98 (Figure S4A), indicating altered bacterial composition. Alpha diversity did not differ between groups (Figure S4B). At phylum level, there was a reduction in abundance of Bacteroidetes with no difference in levels of Firmicutes in SYN mice compared with CTRL mice at PN98 (Figure S4C). The relative abundance of Bifidobacterium was higher in the SYN group vs the CTRL group at PN21 (similar to Figure 4D), but it was not maintained at PN42 and PN98 (Figure S4D). The level of Lactobacillus was reduced in the SYN group compared with the CTRL group at PN21 (Figure S4E). The relative abundance of unidentified genus from family S24_7 increased following SYN supplementation at PN21, but was not maintained at later time points (Figure S4F). DESeq2 analysis revealed Bifidobacterium (log FC = 1.35) and an unidentified genus from family S24_7 (logFC = 1.30) that showed higher levels in the SYN group compared with the CTRL group at PN21 (Figure S3B), consistent with data presented in Figure S4D and S4F. Interestingly, we also identified the low‐abundance genus Akkermansia that showed lower levels at PN21 (logFC = −2.34) and then increased (logFC = 6.58) at PN98 in the SYN group vs the CTRL group, probably coinciding with shifts in diet (Figure S3B). Thus, we observed different taxonomic changes within the microbiota, despite similar metabolic outcomes of nutritional intervention.
3.5. Microbiota transplant in adolescence does not transfer beneficial phenotype of synbiotics
To determine whether the improved adult metabolic phenotype of SYN mice was mediated by altered microbiota, we colonized 6‐week‐old germ‐free (GF) mice with cecal content from age‐matched CTRL or SYN donor mice and determined the development of body weight and body composition under WSD challenge over 8 weeks (Figure 5A). At the time of transfer (PN42), there were no differences in body weight, fat mass and lean body mass (P = .996; P > .999; P = .604, respectively) between recipient GF mice (Figure 5B‐D). Following microbiota transfer, we observed no significant differences in development of body weight (P = .310), fat mass (P = .902) or lean body mass (P = 0.302) between the CTRL and SYN groups (Figure 5B‐D). Thus, transferring the modified microbiota after synbiotic supplementation to age‐matched adolescent recipients is not sufficient to transfer the protected phenotype of synbiotic‐supplemented mice.
4. DISCUSSION
Perturbation of the gut microbiota in early life by antibiotics has been shown to promote development of obesity and an adverse metabolic phenotype later in life.15, 16, 29 In the present study, we showed that beneficial modulation of the gut microbiota by early life synbiotics, consisting of scGOS/lcFOS plus Bifidobacterium breve M16V, but not by the prebiotic component alone, protected against later‐life development of diet‐induced obesity, reproducibly in 2 different locations. Early life synbiotics also improved the adult metabolic phenotype and induced long‐term changes in gene expression patterns, specifically in the ileum, related to lipid and cholesterol metabolism in adult mice. Although we observed similar metabolic improvements in the 2 vivaria following synbiotic supplementation, the taxonomic configuration of the microbiota differed, apart from the similarly increased Bifidobacterium abundance in early life. Furthermore, cecal microbiota transplantation from adolescent donors did not transfer the protective phenotype against diet‐induced obesity in age‐matched GF recipients, indicating a critical window of opportunity for the effectiveness of microbiota modulation. Thus, early synbiotic supplementation improves metabolic health later in life and affects the gut microbiota.
We did not detect any direct effects of prebiotics or synbiotics on developmental growth and body composition during the intervention period itself. This is in accordance with previous clinical intervention studies that showed that applied prebiotics and synbiotic blend support normal growth in healthy full‐term infants.30, 31, 32, 33, 34, 35 Under moderate WSD challenge following the intervention, we found that the SYN group was protected against diet‐induced excessive fat accumulation and related accelerated weight gain compared with the CTRL group. In contrast, the prebiotic group did not show protection against diet‐induced obesity. As we did not investigate the effects of B. breve M‐16V alone, we cannot exclude that the beneficial effects observed in the SYN group are mainly mediated by the bacterium. Apart from the knowledge that human milk, containing naturally occurring pre‐ and probiotics, is protective against metabolic disturbances later in life,,11, 12, 25, 36, 37 there is only 1 study with a prevention paradigm, showing that early probiotic supplementation in pregnant mothers and infants for 6 months supports healthy growth in infants up to 4 years of age,38 which is consistent with our findings. Another probiotic intervention in infants during weaning (at 4‐13 months of age) resulted in no beneficial effect on growth and metabolic parameters,39 indicating the importance of the timing for effectiveness of dietary supplements. In a treatment setting, however, there is substantial evidence that pre‐ and synbiotics can improve metabolic disorders and support weight loss.40, 41
The protection against adult obesity via early life synbiotics was associated with improved glucose metabolism, decreased hepatic lipid deposition and attenuated hypercholesterolaemia in adulthood. The reduced steatosis was associated with increased serum levels of beta‐hydroxybutyrate, suggesting that SYN intervention, in part, acts by increasing fatty acid oxidation. It is known that pro‐ and synbiotics are successful for treatment of fatty liver disease,40, 42, 43 with intestinal microbiota being indicated in the mode of action.44, 45
Our transcriptomic analyses for exploration of molecular long‐term changes induced by early life synbiotic supplementation revealed the strongest effects on gene expression related to lipid metabolism in the small intestine in adult mice, which is a major contributor to the cholesterol pool.46, 47 In synbiotic‐supplemented mice compared with CTRL mice, we detected a collective activation of genes related to cholesterol biosynthesis upon WSD challenge in the ileum, while the expression of genes related to cholesterol excretion, such as Abca1 was down‐regulated. In type 2 diabetic rats, Abca1 is increased in intestinal epithelial cells.48 Thus, down‐regulation of intestinal Abca1, plus higher intestinal cholesterol synthesis in SYN‐supplemented mice, concurrent with beneficially decreased blood cholesterol levels, might be an adaptive mechanism to cope with the higher cholesterol requirement in ileal enterocytes.
Pre‐, pro‐ and synbiotic supplementation is associated with beneficial changes in the gut microbiome in mice49 and humans.22, 23, 50, 51 We observed that early life synbiotics altered the microbiome, with a significant increase in genus Bifidobacterium at PN21 in both independent experiments. This is in line with previous studies advocating addition of pre‐ and synbiotic supplements to infant formula.21, 22, 23, 24 The bifidogenic effect of our early life synbiotic supplementation may drive the observed anti‐obesity effects, as childhood obesity has been linked to depletion of the early life bifidobacteria abundance.52, 53 For the prebiotic component alone, whose effect depends on already‐present resident microbes at the time of intervention,17 we did not observe a bifidogenic effect. It is interesting to note that other taxonomic changes were not similar in the 2 independent locations, but metabolic phenotypes were reproducible. Furthermore, we showed with our transplant experiment that transferring the synbiotics‐modified microbial community from adolescent donors (at PN42) to age‐matched GF mice does not confer protection against diet‐induced obesity in recipients. This implicates the importance of the timing of microbiota modulation; that is, exposure to synbiotics in early life is relevant for achieving long‐lasting beneficial and protective metabolic effects. Provided that the gut microbiota differed between P21 and P42, we cannot exclude that the microbiota transplant from PN21 donors might have produced a different result. Nevertheless, our data strengthen the notion of the importance of breastfeeding and synbiotic supplementation of infant formulas in early life to promote the bifidogenic effect in infants, thereby improving metabolism and protecting against metabolic disorders later in life.
In conclusion, this study adds evidence that early colonization of the digestive tract by symbiotic microbes may be critical for healthy metabolic development, and maintenance of a healthy metabolism in adulthood. With nutritional interventions in a relevant rodent model, we show that early life synbiotic supplementation can protect against the development of obesity and metabolic disease later in life. Obesity in adults and children is on the rise globally and there is no powerful cure, other than bariatric surgery, for this worldwide epidemic and its associated metabolic impairments. Our study provides important insights into the beneficial effects of supplementation with synbiotics in early life, which may lead to the development of preventive strategies; however, validation in humans is warranted.
Supporting information
Appendix S1. Material & Methods.
Table S1. Animal length and tissue weights at PN98.
Table S2. List of all differentially expressed genes (q < 0.05) in ileum of SYN compared with CTRL on PN98 and annotation. Data is based on microarrays of ileal tissue on PN98 in CTRL and SYN mice; n = 4 per group; Intensity‐based moderate t‐statistics (IBMT) with empirical Bayes correction of SYN vs. CTRL; annotated with Gene Ontology (GO) database.
Table S3. Gene expression fold changes, p‐values and q‐values of genes related to cholesterol metabolism in ileum of REF, CTRL, PRE and SYN groups on PN98. Data is based on microarrays of ileal tissue on PN98 in REF, CTRL, PRE and SYN groups; n = 4 per group; Intensity‐based moderate t‐statistics (IBMT) with empirical Bayes correction of (i) SYN vs. CTRL; (ii) PRE vs. CTRL and (iii) CTRL‐REF.
Figure S1. Early life synbiotics reduce fat mass percentage.
Fat mass (expressed as fat mass %) at PN42 (A), PN70 (B) and PN98 (C) in REF (n=8), CTRL (n=7), PRE (n=9) and SYN (n=11) groups of mice. Data are mean ± SEM. One‐way ANOVA followed by Tukey's multiple comparisons test. *p≤0.05; ***p≤0.001.
Figure S2. Prevention of excessive fat accumulation by early life synbiotics under WSD challenge is reproducible.
(A) Schematic overview of Study2. Litters were culled at postnatal day (PN) 2 and were divided into 3 diet groups: Reference (REF) and Control (CTRL) on AIN‐G (standard semi‐synthetic diet appropriate for breeding) plus control component (maltodextrin), and SYN on AIN‐G supplemented with synbiotics (scGOS/lcFOS in ratio 9:1 + B.breve M‐16V) until PN42. At PN42, REF was maintained on AIN‐M (semi‐synthetic diet appropriate for maintenance) and CTRL and SYN groups were challenged with Western‐style diet (40% energy from fat) until PN98.
Body weight (B), fat mass (C) and lean body mass (D) in REF (n=6), CTRL (n=9) and SYN (n=8) groups of mice at PN42, PN70 and PN98. Data are mean ± SEM. Repeated‐measures two‐way ANOVA followed by Sidak's multiple comparisons test for (i) REF vs. CTRL and (ii) CTRL vs. SYN. &&&p≤0.001 indicates significance for REF vs. CTRL. $p≤0.05 indicates significance for CTRL vs. SYN.
Figure S3. Differentially abundant genera identified using negative binomial distribution method (DESEq2).
Differentially abundant genera identified in CTRL versus SYN comparison at PN21, PN42 and PN98 in study 1 and study 2. LogFC indicates the log fold change in the SYN versus CTRL comparison; negative values indicate lower while positive values indicate higher abundance in synbiotic (SYN) compared with WSD fed group (CTRL). All genera identified are significant at padj≤0.05. The genera names are italicized; unidentified genera are indicated as ‘g’ with their known closest rank.
Figure S4. Early life microbiota modulation by early life synbiotics in study 2.
Weighted Unifrac distances showing distance within and between groups in REF (n=7), CTRL (n=12) and SYN (n=11) groups of mice. Significance between the groups was analyzed by default 2 sample t‐test in Qiime, *p<0.05. (B) Bacterial diversity as shown by diversity box plots obtained from alpha rarefaction measured by observed species. Data are shown as box and whiskers plots, indicating the median (thick line in the box), the 25‐75th percentile range (boxes), and the 1‐99th percentile range (whiskers). Relative abundance of (C) major phyla (D) Bifidobacterium (E) Lactobacillus and (F) S24_7 in REF (n=7), CTRL (n=12) and SYN (n=11) groups of mice. Data are mean ± SEM. In C, two‐way analysis of variance followed by Tuckey's multiple comparisons test. $p≤0.05, $$$p≤0.001 indicates significance between CTRL and SYN. In D‐F, non‐parametric Kruskal‐Wallis test followed by Dunn's multiple comparisons test. **p≤0.01; ***p≤0.001 as indicated.
ACKNOWLEDGMENTS
We would like to thank Tiemen van Eijndthoven and Valentina Tremaroli for assistance with gut microbiota analysis. We would also like to thank Carina Arvidsson, Louise Mannerås Holm, Sara Nordin‐Larsson, Ulrica Enqvist, Caroline Wennberg, Anna Hallén and Zakarias Gulic for excellent animal husbandry.
Conflict of interest
M. M., S. T., E. E., K. vL., A. B., R. O., A. O. and J. K. are employees of Nutricia Research. F. B. is the recipient of an ERC Consolidator Grant (European Research Council, Consolidator grant 615 362 ‐ METABASE).
Author contributions
A. O., E. E., F. B., J. K., K. vL., N. So. and R. O. conceived and designed the experiments. A. Ba., E. E., K. vL., N. So. and T. A. performed the experiments. A. Ba., E. E., K. vL., M. M., N. So., S. T. and T. A. analysed the data. A. O., A. Ba., E. E., F. B., J. K., K. vL., M. M., S. T. and T. A. interpreted the data. M. M. and T. A. wrote the manuscript. A. O., A. Ba., F. B., J. K., N. So. and S. T. provided valuable feedback on the manuscript. All authors read and approved the final manuscript.
Mischke M, Arora T, Tims S, et al. Specific synbiotics in early life protect against diet‐induced obesity in adult mice. Diabetes Obes Metab. 2018;20:1408–1418. https://doi.org/10.1111/dom.13240
Funding information This study was funded by Nutricia Research and supported by the Swedish Research Council, the Torsten Söderberg Foundations, the IngaBritt and Arne Lundberg Foundation, the Swedish Foundation for Strategic Research, the Knut and Alice Wallenberg Foundation and the regional agreement on medical training and clinical research (ALF) between Region Västra Götaland and Sahlgrenska University Hospital.
REFERENCES
- 1. WHO . Obesity and overweight fact sheet. June 2016; http://www.who.int/mediacentre/factsheets/fs311/en/. Accessed October 18, 2017.
- 2. Bayard M, Holt J, Boroughs E. Nonalcoholic fatty liver disease. Am Fam Physician. 2006;73:1961‐1968. [PubMed] [Google Scholar]
- 3. Wilson PW, D'Agostino RB, Parise H, Sullivan L, Meigs JB. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005;112:3066‐3072. [DOI] [PubMed] [Google Scholar]
- 4. Tremaroli V, Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489:242‐249. [DOI] [PubMed] [Google Scholar]
- 5. Le Chatelier E, Nielsen T, Qin J, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541‐546. [DOI] [PubMed] [Google Scholar]
- 6. Wopereis H, Oozeer R, Knipping K, Belzer C, Knol J. The first thousand days ‐ intestinal microbiology of early life: establishing a symbiosis. Pediatr Allergy Immunol. 2014;25:428‐438. [DOI] [PubMed] [Google Scholar]
- 7. Tarry‐Adkins JL, Ozanne SE. Mechanisms of early life programming: current knowledge and future directions. Am J Clin Nutr. 2011;94(suppl 6):1765S‐1771S. [DOI] [PubMed] [Google Scholar]
- 8. Okubo H, Crozier SR, Harvey NC, et al. Diet quality across early childhood and adiposity at 6 years: the Southampton Women's Survey. Int J Obes (Lond). 2015;39:1456‐1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Benyshek DC. The developmental origins of obesity and related health disorders‐‐prenatal and perinatal factors. Coll Antropol. 2007;31:11‐17. [PubMed] [Google Scholar]
- 10. Nauta AJ, Ben Amor K, Knol J, Garssen J, van der Beek EM. Relevance of pre‐ and postnatal nutrition to development and interplay between the microbiota and metabolic and immune systems. Am J Clin Nutr. 2013;98(suppl):586S‐593S. [DOI] [PubMed] [Google Scholar]
- 11. Owen CG, Martin RM, Whincup PH, Smith GD, Cook DG. Effect of infant feeding on the risk of obesity across the life course: a quantitative review of published evidence. Pediatrics. 2005;115:1367‐1377. [DOI] [PubMed] [Google Scholar]
- 12. Arenz S, Ruckerl R, Koletzko B, von Kries R. Breast‐feeding and childhood obesity‐‐a systematic review. Int J Obes Relat Metab Disord. 2004;28:1247‐1256. [DOI] [PubMed] [Google Scholar]
- 13. Mott GE, Jackson EM, McMahan CA, McGill HC Jr. Cholesterol metabolism in adult baboons is influenced by infant diet. J Nutr. 1990;120:243‐251. [DOI] [PubMed] [Google Scholar]
- 14. Penders J, Thijs C, Vink C, et al. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006;118:511‐521. [DOI] [PubMed] [Google Scholar]
- 15. Ajslev TA, Andersen CS, Gamborg M, Sorensen TI, Jess T. Childhood overweight after establishment of the gut microbiota: the role of delivery mode, pre‐pregnancy weight and early administration of antibiotics. Int J Obes (Lond). 2011;35:522‐529. [DOI] [PubMed] [Google Scholar]
- 16. Azad MB, Bridgman SL, Becker AB, Kozyrskyj AL. Infant antibiotic exposure and the development of childhood overweight and central adiposity. Int J Obes (Lond). 2014;38:1290‐1298. [DOI] [PubMed] [Google Scholar]
- 17. Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125:1401‐1412. [DOI] [PubMed] [Google Scholar]
- 18. Roberfroid M, Gibson GR, Hoyles L, et al. Prebiotic effects: metabolic and health benefits. Br J Nutr. 2010;104(suppl 2):S1‐S63. [DOI] [PubMed] [Google Scholar]
- 19. Floch MH, Walker WA, Madsen K, et al. Recommendations for probiotic use‐2011 update. J Clin Gastroenterol. 2011;45(suppl):S168‐S171. [DOI] [PubMed] [Google Scholar]
- 20. Kolida S, Gibson GR. Synbiotics in health and disease. Annu Rev Food Sci Technol. 2011;2:373‐393. [DOI] [PubMed] [Google Scholar]
- 21. Chua MC, Ben‐Amor K, Lay C, et al. Effect of synbiotic on the gut microbiota of cesarean delivered infants: a randomized, double‐blind, multicenter study. J Pediatr Gastroenterol Nutr. 2017;65:102‐106. [DOI] [PubMed] [Google Scholar]
- 22. Bakker‐Zierikzee AM, Alles MS, Knol J, Kok FJ, Tolboom JJ, Bindels JG. Effects of infant formula containing a mixture of galacto‐ and fructo‐oligosaccharides or viable Bifidobacterium animalis on the intestinal microflora during the first 4 months of life. Br J Nutr. 2005;94:783‐790. [DOI] [PubMed] [Google Scholar]
- 23. Knol J, Scholtens P, Kafka C, et al. Colon microflora in infants fed formula with galacto‐ and fructo‐oligosaccharides: more like breast‐fed infants. J Pediatr Gastroenterol Nutr. 2005;40:36‐42. [DOI] [PubMed] [Google Scholar]
- 24. Haarman M, Knol J. Quantitative real‐time PCR assays to identify and quantify fecal Bifidobacterium species in infants receiving a prebiotic infant formula. Appl Environ Microbiol. 2005;71:2318‐2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Koleva PT, Bridgman SL, Kozyrskyj AL. The infant gut microbiome: evidence for obesity risk and dietary intervention. Nutrients. 2015;7:2237‐2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Oosting A, Kegler D, Boehm G, Jansen HT, van de Heijning BJ, van der Beek EM. N‐3 long‐chain polyunsaturated fatty acids prevent excessive fat deposition in adulthood in a mouse model of postnatal nutritional programming. Pediatr Res. 2010;68:494‐499. [DOI] [PubMed] [Google Scholar]
- 27. Oosting A, van Vlies N, Kegler D, et al. Effect of dietary lipid structure in early postnatal life on mouse adipose tissue development and function in adulthood. Br J Nutr. 2014;111:215‐226. [DOI] [PubMed] [Google Scholar]
- 28. Lin K, Kools H, de Groot PJ, et al. MADMAX ‐ Management and analysis database for multiple ~omics experiments. J Integr Bioinform. 2011;8:160. [DOI] [PubMed] [Google Scholar]
- 29. Cox LM, Yamanishi S, Sohn J, et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell. 2014;158:705‐721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Harvey BM, Langford JE, Harthoorn LF, et al. Effects on growth and tolerance and hypoallergenicity of an amino acid‐based formula with synbiotics. Pediatr Res. 2014;75:343‐351. [DOI] [PubMed] [Google Scholar]
- 31. Abrahamse‐Berkeveld M, Alles M, Franke‐Beckmann E, et al. Infant formula containing galacto‐and fructo‐oligosaccharides and Bifidobacterium breve M‐16V supports adequate growth and tolerance in healthy infants in a randomised, controlled, double‐blind, prospective, multicentre study. J Nutr Sci. 2016;5:e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Huet F, Abrahamse‐Berkeveld M, Tims S, et al. Partly fermented infant formulae with specific oligosaccharides support adequate infant growth and are well‐tolerated. J Pediatr Gastroenterol Nutr. 2016;63:e43‐e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Decsi T, Arato A, Balogh M, et al. Randomised placebo controlled double blind study on the effect of prebiotic oligosaccharides on intestinal flora in healthy infants. Orv Hetil. 2005;146:2445‐2450. [PubMed] [Google Scholar]
- 34. Schmelzle H, Wirth S, Skopnik H, et al. Randomized double‐blind study of the nutritional efficacy and bifidogenicity of a new infant formula containing partially hydrolyzed protein, a high beta‐palmitic acid level, and nondigestible oligosaccharides. J Pediatr Gastroenterol Nutr. 2003;36:343‐351. [DOI] [PubMed] [Google Scholar]
- 35. Costalos C, Kapiki A, Apostolou M, Papathoma E. The effect of a prebiotic supplemented formula on growth and stool microbiology of term infants. Early Hum Dev. 2008;84:45‐49. [DOI] [PubMed] [Google Scholar]
- 36. Horta BL, Loret de Mola C, Victora CG. Long‐term consequences of breastfeeding on cholesterol, obesity, systolic blood pressure and type 2 diabetes: a systematic review and meta‐analysis. Acta Paediatr. 2015;104:30‐37. [DOI] [PubMed] [Google Scholar]
- 37. Owen CG, Whincup PH, Odoki K, Gilg JA, Cook DG. Infant feeding and blood cholesterol: a study in adolescents and a systematic review. Pediatrics. 2002;110:597‐608. [DOI] [PubMed] [Google Scholar]
- 38. Luoto R, Kalliomaki M, Laitinen K, Isolauri E. The impact of perinatal probiotic intervention on the development of overweight and obesity: follow‐up study from birth to 10 years. Int J Obes (Lond). 2010;34:1531‐1537. [DOI] [PubMed] [Google Scholar]
- 39. Karlsson Videhult F, Ohlund I, Stenlund H, Hernell O, West CE. Probiotics during weaning: a follow‐up study on effects on body composition and metabolic markers at school age. Eur J Nutr. 2015;54:355‐363. [DOI] [PubMed] [Google Scholar]
- 40. Saez‐Lara MJ, Robles‐Sanchez C, Ruiz‐Ojeda FJ, Plaza‐Diaz J, Gil A. Effects of Probiotics and Synbiotics on Obesity, Insulin Resistance Syndrome, Type 2 Diabetes and Non‐Alcoholic Fatty Liver Disease: A Review of Human Clinical Trials. Int J Mol Sci. 2016;17:pii:E928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Delzenne NM, Cani PD. Nutritional modulation of gut microbiota in the context of obesity and insulin resistance: potential interest of prebiotics. Int Dairy J. 2010;20:277‐280. [Google Scholar]
- 42. Ishimwe N, Daliri EB, Lee BH, Fang F, Du G. The perspective on cholesterol‐lowering mechanisms of probiotics. Mol Nutr Food Res. 2015;59:94‐105. [DOI] [PubMed] [Google Scholar]
- 43. Aggarwal J, Swami G, Kumar M. Probiotics and their effects on metabolic diseases: an update. J Clin Diagn Res. 2013;7:173‐177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rabot S, Membrez M, Bruneau A, et al. Germ‐free C57BL/6J mice are resistant to high‐fat‐diet‐induced insulin resistance and have altered cholesterol metabolism. FASEB J. 2010;24:4948‐4959. [DOI] [PubMed] [Google Scholar]
- 45. Pugalendhi KV, Sudhakaran PR, Ramakrishnan S. Effect of antimicrobials on cholesterol synthesis and content in liver and small intestines. Indian J Exp Biol. 1992;30:152‐154. [PubMed] [Google Scholar]
- 46. Dietschy JM, Weis HJ. Cholesterol synthesis by the gastrointestinal tract. Am J Clin Nutr. 1971;24:70‐76. [DOI] [PubMed] [Google Scholar]
- 47. Kruit JK, Groen AK, van Berkel TJ, Kuipers F. Emerging roles of the intestine in control of cholesterol metabolism. World J Gastroenterol. 2006;12:6429‐6439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lou J, Zhou H, Li C, et al. ABCA1 and ABCG1 expression in the small intestine of type 2 diabetic rats. Lab Med. 2014;45:17‐24. [DOI] [PubMed] [Google Scholar]
- 49. Bomhof MR, Saha DC, Reid DT, Paul HA, Reimer RA. Combined effects of oligofructose and Bifidobacterium animalis on gut microbiota and glycemia in obese rats. Obesity (Silver Spring). 2014;22:763‐771. [DOI] [PubMed] [Google Scholar]
- 50. Moro G, Arslanoglu S, Stahl B, Jelinek J, Wahn U, Boehm G. A mixture of prebiotic oligosaccharides reduces the incidence of atopic dermatitis during the first six months of age. Arch Dis Child. 2006;91:814‐819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Scholtens PA, Alles MS, Bindels JG, van der Linde EG, Tolboom JJ, Knol J. Bifidogenic effects of solid weaning foods with added prebiotic oligosaccharides: a randomised controlled clinical trial. J Pediatr Gastroenterol Nutr. 2006;42:553‐559. [DOI] [PubMed] [Google Scholar]
- 52. Kalliomaki M, Collado MC, Salminen S, Isolauri E. Early differences in fecal microbiota composition in children may predict overweight. Am J Clin Nutr. 2008;87:534‐538. [DOI] [PubMed] [Google Scholar]
- 53. Korpela K, Zijlmans MA, Kuitunen M, et al. Childhood BMI in relation to microbiota in infancy and lifetime antibiotic use. Microbiome. 2017;5:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Material & Methods.
Table S1. Animal length and tissue weights at PN98.
Table S2. List of all differentially expressed genes (q < 0.05) in ileum of SYN compared with CTRL on PN98 and annotation. Data is based on microarrays of ileal tissue on PN98 in CTRL and SYN mice; n = 4 per group; Intensity‐based moderate t‐statistics (IBMT) with empirical Bayes correction of SYN vs. CTRL; annotated with Gene Ontology (GO) database.
Table S3. Gene expression fold changes, p‐values and q‐values of genes related to cholesterol metabolism in ileum of REF, CTRL, PRE and SYN groups on PN98. Data is based on microarrays of ileal tissue on PN98 in REF, CTRL, PRE and SYN groups; n = 4 per group; Intensity‐based moderate t‐statistics (IBMT) with empirical Bayes correction of (i) SYN vs. CTRL; (ii) PRE vs. CTRL and (iii) CTRL‐REF.
Figure S1. Early life synbiotics reduce fat mass percentage.
Fat mass (expressed as fat mass %) at PN42 (A), PN70 (B) and PN98 (C) in REF (n=8), CTRL (n=7), PRE (n=9) and SYN (n=11) groups of mice. Data are mean ± SEM. One‐way ANOVA followed by Tukey's multiple comparisons test. *p≤0.05; ***p≤0.001.
Figure S2. Prevention of excessive fat accumulation by early life synbiotics under WSD challenge is reproducible.
(A) Schematic overview of Study2. Litters were culled at postnatal day (PN) 2 and were divided into 3 diet groups: Reference (REF) and Control (CTRL) on AIN‐G (standard semi‐synthetic diet appropriate for breeding) plus control component (maltodextrin), and SYN on AIN‐G supplemented with synbiotics (scGOS/lcFOS in ratio 9:1 + B.breve M‐16V) until PN42. At PN42, REF was maintained on AIN‐M (semi‐synthetic diet appropriate for maintenance) and CTRL and SYN groups were challenged with Western‐style diet (40% energy from fat) until PN98.
Body weight (B), fat mass (C) and lean body mass (D) in REF (n=6), CTRL (n=9) and SYN (n=8) groups of mice at PN42, PN70 and PN98. Data are mean ± SEM. Repeated‐measures two‐way ANOVA followed by Sidak's multiple comparisons test for (i) REF vs. CTRL and (ii) CTRL vs. SYN. &&&p≤0.001 indicates significance for REF vs. CTRL. $p≤0.05 indicates significance for CTRL vs. SYN.
Figure S3. Differentially abundant genera identified using negative binomial distribution method (DESEq2).
Differentially abundant genera identified in CTRL versus SYN comparison at PN21, PN42 and PN98 in study 1 and study 2. LogFC indicates the log fold change in the SYN versus CTRL comparison; negative values indicate lower while positive values indicate higher abundance in synbiotic (SYN) compared with WSD fed group (CTRL). All genera identified are significant at padj≤0.05. The genera names are italicized; unidentified genera are indicated as ‘g’ with their known closest rank.
Figure S4. Early life microbiota modulation by early life synbiotics in study 2.
Weighted Unifrac distances showing distance within and between groups in REF (n=7), CTRL (n=12) and SYN (n=11) groups of mice. Significance between the groups was analyzed by default 2 sample t‐test in Qiime, *p<0.05. (B) Bacterial diversity as shown by diversity box plots obtained from alpha rarefaction measured by observed species. Data are shown as box and whiskers plots, indicating the median (thick line in the box), the 25‐75th percentile range (boxes), and the 1‐99th percentile range (whiskers). Relative abundance of (C) major phyla (D) Bifidobacterium (E) Lactobacillus and (F) S24_7 in REF (n=7), CTRL (n=12) and SYN (n=11) groups of mice. Data are mean ± SEM. In C, two‐way analysis of variance followed by Tuckey's multiple comparisons test. $p≤0.05, $$$p≤0.001 indicates significance between CTRL and SYN. In D‐F, non‐parametric Kruskal‐Wallis test followed by Dunn's multiple comparisons test. **p≤0.01; ***p≤0.001 as indicated.


