Abstract
Mg(OH)2‐ and Mg(OH)2‐containing materials can provide excellent performance as supports for AuPd nanoparticles for the oxidation of glycerol in the absence of base, which is considered to be a result of additional basic sites on the surface of the support. However, its influence on the reaction solution is not generally discussed. In this paper, we examine the relationship between the basic Mg(OH)2 support and AuPd nanoparticles in detail using four types of catalyst. For these reactions, the physical interaction between Mg(OH)2 and AuPd was adjusted. It was found that the activity of the AuPd nanoparticles increased with the amount of Mg(OH)2 added under base‐free conditions, regardless of its interaction with the noble metals. In order to investigate how Mg(OH)2 affected the glycerol oxidation, detailed information about the performance of AuPd/Mg(OH)2, physically mixed (AuPd/C+Mg(OH)2) and (AuPd/C+NaHCO3) was obtained and compared. Furthermore, NaOH and Mg(OH)2 were added during the reaction using AuPd/C. All these results indicate that the distinctive and outstanding performance of Mg(OH)2 supported catalysts in base‐free condition is in fact directly related to its ability to affect the pH during the reaction and as such, assists with the initial activation of the primary alcohol, which is considered to be the rate determining step in the reaction.
Keywords: base-free, gold, magnesium, nanoparticles, oxidation
Introduction
Biomass conversion has drawn worldwide attention in recent years because of its potential to be an alternative resource to fossil fuel. The gradually increasing production of biodiesel has generated a huge amount of by‐products, the major part of which is glycerol.1 It is reported that glycerol is comprised of approximately 10 wt % of the total product yield for biodiesel production2 and as such, it is projected that the demand for the consumption of glycerol will increase from 2000 kt in 2011 to 3070 kt by 2018.3 Therefore, transforming glycerol into valuable derivatives could be an effective method to increase the economic efficiency of biodiesel production.
In the past decades, great improvements have been made on the catalytic oxidation of glycerol. Gold‐supported catalysts were found to be efficient for the conversion of glycerol to C3 products under basic conditions.4 When compared with Pd, Pt and other noble metals, gold is more resistant to the formation of oxides5 which can poison the catalyst, making gold highly active, selective and stable. In subsequent studies, alloyed Au−Pd,6 Au−Pt7 and Au−Pt−Pd8 nanoparticles, prepared by a colloidal approach, demonstrated much better performance than corresponding monometallic catalysts. However, the requirement of base addition for gold‐based catalysts in glycerol oxidation is not favourable for industrial applications, since the products are salts of acids, which then need further treatment. Thus, considerable attention has been focused on the investigation of glycerol oxidation under base‐free conditions. Prati and co‐workers investigated the performance of H‐mordenite‐supported alloyed Au−Pt nanoparticles compared with the same particles supported on activated carbon, MgO, SiO2, ZrO2 and MCM‐41.9 Among these, MgO‐supported catalysts exhibited superior activity, however, the selectivity to C2 or C1 products was very high. By using a modified colloidal methodology and controlling the preparation temperature, the performance of the Au−Pd and Au−Pt on MgO or Mg(OH)2 could be significantly improved.10 The beneficial effect of supporting such particles on MgO and Mg(OH)2 was subsequently studied in depth and compared with other basic and acidic supports. It was reported that the quantity and strength of basic sites on MgO and Mg(OH)2 have a large influence on the product distribution.11 This influence was also observed with other basic supports such as (MgCO3)4Mg(OH)2 and CaCO3. Xu and co‐workers investigated the Au/Al2O3−MgO for glycerol oxidation.12 They found that the percentage composition of MgO could affect the acid‐base properties of the catalysts, resulting in different activities and product distributions. Similar results were also observed in the base‐free oxidation of 5‐hydroxymethylfurfural. Wang and co‐workers synthesized a mixed C‐O‐Mg support.13 It was suggested that the strong and stable basic sites of the support were the reason behind why such high activities were observed with these catalysts.
MgO is sparingly soluble in water (86 mg L−1 at 30 °C)14 and the products of glycerol oxidation are often organic acids. Some previous publications have revealed that metals from solid basic supports can leach into aqueous solution when no additional base is added to the reaction,10, 15 a phenomena which has shown to be suppressed at elevated pH′s.16 Given that it is known that these materials can partially dissolve in an aqueous media, it is somewhat surprising that no attempts have been made to correlate this with the perceived increase in catalytic performance observed with solid basic supports. This is especially important given that pH has been shown to have such a significant effect on the rate‐determining step in the oxidation of alcohols.17
It is clear that MgO or Mg(OH)2 plays a key role in the performance of Au, Au−Pd and Au−Pt nanoparticles under base‐free conditions. Most of the previous investigations were focussed on the properties of basic sites on the surface of the supports, which were correlated with the activity and selectivity of catalysts. However, no detailed information, regarding the effect of the solid base on the reaction conditions or its effect on the performance of catalysts was provided. In this work, the catalytic behaviour of Au−Pd nanoparticles on different types of support was investigated. The catalytic performance is found to be directly related to the pH of the reaction solution, which could be altered by the support. The aim of this paper is to provide a new perspective on how basic supports, such as Mg(OH)2, affect the activity and selectivity of Au based catalysts in the oxidation of glycerol by careful consideration and monitoring of the pH of the solutions during glycerol reactions. This pH change can be mimicked with the addition of a sacrificial base and the results correlated.
Results and Discussion
The performance and structure of AuPd−Mg(OH)2/C
AuPd−Mg(OH)2/C catalysts with varying quantities of Mg(OH)2 were prepared by the sol‐immobilization method using the pre‐synthesized support Mg(OH)2/C. These catalysts were observed to be effective for glycerol oxidation under “base‐free” conditions (Supporting Information Figure S1) when high amounts of Mg(OH)2 were loaded onto the carbon support. It has been previously reported that a mixed carbon/Mg(OH)2 support shows much better activity than carbon alone in 5‐hydroxymethylfurfural (HMF) oxidation with Pt nanoparticles under base‐free reaction conditions, which has been ascribed to the basic sites provided by Mg2+.13 Therefore, in view of these results we reasoned that the AuPd−Mg(OH)2/C should be present as a triple layer composite where the AuPd nanoparticles were selectively located on the Mg(OH)2, thereby enabling the interaction between basic sites and noble metals. Subsequently, to determine whether the synthesised catalyst has this triple layer structure, X‐ray diffraction (XRD) and transmission electron microscopy (TEM) characterization of the catalysts was performed.
The XRD analysis in Figure 1 for the AuPd−Mg(OH)2/C catalyst confirmed the presence of Mg(OH)2 on the carbon. No Au and Pd phase could be observed, which is ascribed to the low metal content and the effective dispersion of the AuPd nanoparticles. Figure 2 shows the TEM and energy‐dispersive X‐ray spectroscopy (EDX) results of AuPd−Mg(OH)2/C. It is clear that AuPd nanoparticles are located on the carbon without any interaction with the Mg(OH)2 (Figure 2 A). In Figure 2 B, Mg is observed as a separate phase. Thus, it could be concluded that AuPd and Mg(OH)2 were separately located on carbon for the catalyst AuPd−Mg(OH)2/C rather than forming the intended triple layer structure. As we did not form the triple layer structure, we investigated the sol‐immobilization process to compare the adsorption ability between XC‐72R and Mg(OH)2 separately and the time taken for the support to adsorb nanoparticles from the solvent. XC‐72R only needed 10 min to adsorb the AuPd nanoparticles, whereas Mg(OH)2 needed at least 30 min. Hence the carbon support has a much higher affinity for the adsorption of the nanoparticles and therefore, they will be preferentially located on the carbon support.
Figure 1.

XRD patterns of AuPd/Mg(OH)2 and AuPd−Mg(OH)2/C.
Figure 2.

TEM and EDX of AuPd−Mg(OH)2/C. Graph A and Graph B show the surface information of different parts of AuPd−Mg(OH)2/C. It is apparent that AuPd nanoparticles and Mg(OH)2 are located on different areas of the catalyst.
Previously it has been reported that the superior performance of Mg(OH)2‐containing catalysts is considered to be related to the basic sites that interact with the metal nanoparticles. However, AuPd−Mg(OH)2/C is able to achieve a very high activity without direct contact between AuPd and Mg(OH)2. Thus, we concluded that the role of the Mg(OH)2 in the oxidation of glycerol under “base‐free” conditions needed to be further considered.
The performance of AuPd nanoparticles with different locations of Mg(OH)2
To further investigate the role of Mg(OH)2 in the oxidation reaction, we synthesised a series of additional catalysts. Au−Pd nanoparticles were deposited on MgO and carbon by the same sol‐immobilization method used for the preparation of the mixed‐support catalyst. From the XRD results shown in Figure 1, it is clear that MgO is converted to Mg(OH)2 during the catalyst preparation process through hydrolysis, which is consistent with our reported results.10, 15 As such, this catalyst is denoted as AuPd/Mg(OH)2.
The four types of catalyst (AuPd/Mg(OH)2, AuPd−Mg(OH)2/C, physically mixed (AuPd/C+Mg(OH)2) and physically mixed (AuPd/C+Mg(OH)2/C)) were subsequently tested for glycerol oxidation in the absence of sacrificial base. As we have shown in Figure 2, the AuPd and Mg(OH)2 are located separately on the carbon for AuPd−Mg(OH)2/C. Therefore, the interaction between metal nanoparticles and Mg(OH)2 decreases in these four catalysts, as the distance between the AuPd and Mg(OH)2 increases. However, as is shown in Figure 3, there is not a large difference among these four catalysts with respect to their performance for the oxidation of glycerol. AuPd/Mg(OH)2 even presents slightly lower activity (33 % conversion) when compared with the other carbon‐supported catalysts with Mg(OH)2 (from 36 to 40 %). With respect to the product distribution, AuPd/Mg(OH)2 gives a slightly lower selectivity towards dihydroxyacetone and tartronic acid, accompanied with a higher selectivity for glyceric acid. To demonstrate the superior activity of catalysts with Mg(OH)2; AuPd/C, AuPd/CeO2 and AuPd/TiO2 were also tested under the same conditions. They show an extremely low activity for glycerol oxidation and totally different product distributions are observed when Mg(OH)2 was not present (Figure S2). As such, we hypothesised that the effective performance of the AuPd nanoparticles could be obtained as long as Mg(OH)2 was added, regardless of its interaction with the nanoparticles and where the AuPd was located.
Figure 3.

The effect of Mg(OH)2 location on the performance of the AuPd nanoparticles in “base‐free” glycerol oxidation. Four kinds of catalyst were tested, including α (AuPd/Mg(OH)2), β (AuPd−Mg(OH)2/C), γ (physically mixed AuPd/C+Mg(OH)2), and δ (physically mixed AuPd/C+Mg(OH)2/C). The Mg(OH)2/glycerol mole ratio is 0.34, except for AuPd−Mg(OH)2/C which is 0.23. OA (oxalic acid), TA (tartronic acid), GLA (glyceric acid), GLC (glycolic acid), DHA (dihydroxyacetone). Reaction conditions: 1:1.85 mole fraction Au:Pd with 1 % metal loading by mass, water (10 mL), glycerol (0.3 m), mole fraction glycerol/metal=690, 60 °C, 4 h, 3 bar O2.
Comparison between physically mixed (AuPd/C+Mg(OH)2) and AuPd/C with the addition of NaHCO3
Having established that the location of the MgO does not affect the conversion of glycerol, we decided to compare the effect of its presence to a sacrificial base. Initially, we obtained time online data for the physically mixed (AuPd/C+Mg(OH)2) catalyst with the pH of the solution recorded at each point (Figure 4 A,B). For the purpose of facilitating the comparison, the glycerol/Mg(OH)2 mole fraction was maintained at 0.34. It is clear, as expected, that the glycerol conversion increases gradually with time. The selectivity to dihydroxyacetone (DHA) declines continuously, while the selectivity to glycolic acid and oxalic acid increases slowly, the selectivity to tartronic acid shows a similar trend while the selectivity to glyceric acid reaches a steady state after 60 min. These results are in line with the reaction mechanism reported previously,18 as it is widely accepted that glycerol could be consecutively oxidized to form glyceraldehyde, glyceric acid and then tartronic acid. DHA and glyceraldehyde are the primary oxidation products of glycerol oxidation, and the transformation between them is considered to be reversible in alkaline media.19, 20 Since the oxidation of glyceraldehyde is very facile, no glyceraldehyde could be observed. DHA and tartronic acid can be further oxidized to glycolic acid and oxalic acid, respectively. Therefore, the gradual decrease of DHA could be ascribed to the formation of glycolic acid and glyceraldehyde which can subsequently undergo further oxidation to glyceric acid. The steady state observed for glyceric acid selectivity is likely to be a result of the rate of formation being comparable with the subsequent oxidation to tartronic acid. In addition to monitoring the glycerol conversion and product distribution, the pH of the solution was also monitored with time. Interestingly, the pH of the solution appeared to remain fairly constant at pH 9 for the duration of the experiments in both systems (Figure 4). The corresponding data sets for the reactions over the AuPd/Mg(OH)2 catalyst and AuPd/C+Mg(OH)2 system were found to be comparable with respect to the observed pH and product distribution. Slightly higher conversions were observed however, with the AuPd/C+Mg(OH)2 system.
Figure 4.

Conversion/selectivity/pH/carbon balance vs. time profiles of glycerol oxidation. OA (oxalic acid), TA (tartronic acid), GLA (glyceric acid), GLC (glycolic acid), DHA (dihydroxyacetone). Graph A and B present the results of physically mixed (AuPd/C+Mg(OH)2), Mg(OH)2/glycerol mole fraction=0.34. Graph C and D present the performance of (AuPd/C+NaHCO3), mole fraction NaHCO3/glycerol=3. Reaction conditions: 1:1.85 mole fraction Au:Pd with 1 % metal loading by mass, water (35 mL), glycerol (0.3 m), mole fraction glycerol/metal=690, 60 °C, 4 h, 3 bar O2.
The K sp of Mg(OH)2 at 25 °C is 5.62×10−12, which means that the pH of water should be around 10.3 in the presence of Mg(OH)2. With the addition of glycerol and its acid products, OH− would be continuously consumed. As a result, the pH would be expected to decrease and the concentration of Mg2+ in the solution would increase according to the solubility equilibrium and acid‐base dissociation equilibrium. The pH of the solution can be calculated from the Mg2+ concentration and K sp of Mg(OH)2. The pH is estimated to be 9 for the solution with the AuPd/Mg(OH)2 catalyst after 4 h reaction on the basis of Mg concentration (1300 ppm). This value is consistent with the pH measured as shown in Figure S3 (Supporting Information). Therefore, the observed pH of the solution is consistent with the dissolution of Mg(OH)2 and the consumption of OH− by the acids produced from glycerol oxidation.
To investigate the influence of pH on the performance of the AuPd nanoparticles, Mg(OH)2 was replaced by NaHCO3. As is shown in Figure 4 C, the conversion of glycerol increases gradually, while the pH is maintained at approximately pH 9, which is in accordance with the conversion observed in the presence of Mg(OH)2. After 4 h, the conversion reaches 31 %, which is similar to the results observed for the AuPd/Mg(OH)2 and physically mixed (AuPd/C+Mg(OH)2) systems. As for the evolution of products (Figure 4 D), glyceric acid was kept steady at arpproximately 60 % and the selectivity of DHA decreased continuously from 39 to <10 %. The selectivity to glycolic acid, oxalic acid and tartronic acid increased slowly during the process. It is apparent that a similar performance is observed for both catalysts when the pH is maintained at the same level (pH 9). Thus, it is logical to correlate the high performance of the Mg(OH)2‐supported catalysts with its ability to maintain the pH at a high level through the dissolution of Mg(OH)2.
Effect of [Mg(OH)2] on the “base free” oxidation of glycerol
Additional glycerol oxidation tests were subsequently carried out using the physically mixed (AuPd/C+Mg(OH)2) catalyst with different Mg(OH)2/glycerol mole fractions and the results are shown in Figure 5 A,B. As is shown in Figure 5 A, the pH, as expected, increases gradually with the amount of Mg(OH)2. With a low Mg(OH)2/glycerol ratio (<0.23), the pH of the solution decreases in the first 15 min and then subsequently increases over the next 90 min of reaction, before starting to decrease rapidly. With a higher Mg(OH)2/glycerol molar ratio (0.23), the pH increases before 120 min reaction time and then decreases slightly in the following stages of the reaction. When the Mg(OH)2/glycerol reaches 0.34, the pH can be maintained at around pH 9 during the entire reaction. The corresponding data of the final Mg2+ concentration in the reaction solution is presented in Figure S4 A, and shows the same trend to [Mg(OH)2]. This result confirms the extent of the dissolution of Mg(OH)2 and that it can increase the pH of the solution and act to maintain the basicity of the reaction medium as the acidic products are produced.
Figure 5.

Conversion/pH vs. time profiles of glycerol oxidation over physically mixed (AuPd/C+Mg(OH)2). Graph A presents the pH evolution, while graph B presents the glycerol conversion with the increasing amount of Mg(OH)2. Reaction conditions: 1:1.85 mole fraction Au:Pd with 1 % metal loading by mass, water (35 mL), glycerol (0.3 m), mole fraction glycerol/metal=690, 60 °C, 4 h, 3 bar O2.
The glycerol conversion as a function of time on line is presented in Figure 5 B. It is clear that the activity of the catalyst increases with increasing [Mg(OH)2], which corresponds to the pH observed. Davis and co‐workers showed that the oxygen source in the oxidation of glycerol over Au based catalysts is OH−.21 As such, it is rational to suggest that the concentration of OH− could be directly related to the activity of AuPd nanoparticles, which is consistent with the observed phenomenon. As for the product distribution (Figure S4 B), the selectivity for DHA goes down gradually with the amount of Mg(OH)2, which is accompanied by an increasing selectivity towards acid products.
Glycerol oxidation in the presence of NaOH and Mg(OH)2
We have shown that the pH evolution which occurs upon the addition of Mg(OH)2 is important for the performance of AuPd nanoparticles. To further explain the importance of the maintenance of pH on the catalytic performance, NaOH and Mg(OH)2 were added into the reaction solution during the reaction with the AuPd/C catalyst.
Figure 6 shows the effect of NaOH addition during the reaction. During the first 15 min of reaction, only <1 % glycerol was converted and the pH decreased to 3.6. Once NaOH was added, an immediate increase of pH was observed (reaching 10), which resulted in a rapid increase in the oxidation of glycerol for the second 15 min (5.5 %). However, such small quantities of NaOH are not sufficient to stabilize and maintaing this elevated pH, owing to the increasing amount of acid products produced. The pH decreased to 4.2 at the end of the second 15 min period of reaction. In the following 15 min, the pH was kept at a very low value (4.2 to 3.7), resulting in only 0.4 % glycerol conversion. The same phenomenon could be observed as the second and third additions of NaOH were made. With regards to the product selectivity, it is clear that DHA is extremely sensitive to the increases in the pH, which is consistent with the previous observation that DHA is not stable at high pH.18 A marked decrease of DHA selectivity was observed after the addition of NaOH, leading to the rapid increase in the selectivity to glyceric acid.
Figure 6.

Glycerol oxidation in the presence of NaOH. TA (tartronic acid), GLA (glyceric acid), GLD (glyceraldehyde), GLC (glycolic acid), DHA (dihydroxyacetone). Reaction conditions: 1:1.85 mol fraction Au:Pd with 1 % metal loading by mass, water (35 mL), glycerol (0.3 m), mole fraction of glycerol/meta l=690, 60 °C, 4 h, 3 bar O2. NaOH (3 m) was added at the 15th, 45th and 75th min. The amount of NaOH added was 0.05, 0.06 and 0.05 mL, respectively, which correspond to 0.15, 0.18 and 0.15 mmol OH−.
Figure 7 shows the performance of Mg(OH)2 addition with a concentration that corresponds to the same amount of OH− provided by NaOH in the previous experiment. In contrast to the results observed with NaOH addition, the pH went up slowly and was maintained at around pH 6 during the 2 h reaction. However, with such a small amount of Mg(OH)2 at each time of addition (Mg(OH)2/glycerol<0.02), the pH is restricted by the rate of Mg(OH)2 dissolution. Consequently, the glycerol conversion increases gradually to 9 %, which is slightly lower than that observed with NaOH addition. Since the pH is maintained at a low level, a relatively high selectivity for DHA and low selectivity for glyceric acid is also observed.
Figure 7.

Glycerol oxidation in the presence of Mg(OH)2. TA (tartronic acid), GLA (glyceric acid), GLD (glyceraldehyde), GLC (glycolic acid), DHA (dihydroxyacetone). Reaction conditions: 1:1.85 mole fraction Au:Pd with 1 % metal loading by mass, water (35 mL), glycerol (0.3 m), mole fraction glycerol/metal=690, 60 °C, 3 bar O2, 4 h. Mg(OH)2 was added at the 15th, 45th and 75th min. The amount of Mg(OH)2 added was 4.4, 5.2 and 4.4 mg, respectively, which correspond to 0.15, 0.18 and 0.15 mmol OH−.
These results explain the difference between Mg(OH)2 and NaOH with respect to the ability to adjust the pH. The dissolution of Mg(OH)2 can increase the pH of the solution, but its effect is restricted by the amount added. The rate of Mg(OH)2 dissolution, which is affected by the amount of Mg(OH)2 and the rate of OH− consumption, which is controlled by the acid products, compete with each other and form the observed pH evolution. Unlike with the NaOH however, the highest pH for the solution in the presence of enough Mg(OH)2 will always be lower than 10.4, according to the solubility equilibrium at 25 °C.
Effect of support on glycerol oxidation with NaHCO3
Mg(OH)2 when employed as a sacrificial support can maintain the pH at a relatively high level through its dissolution, while other supports (carbon, TiO2) could not stabilize the OH− concentration once acidic products are produced. This is likely to be the main reason why Mg(OH)2 can attain such an outstanding performance under “base‐free” conditions (Figure S1). As such, it is not rational to compare the support effect, as the pH of the reaction media are different. Therefore, similar reactions were conducted with the addition of NaHCO3, to keep the solution at approximately pH 9. The results are shown in Figure 8.
Figure 8.
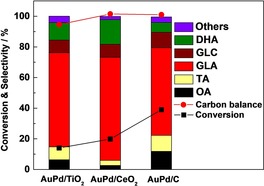
Effect of support on the performance of AuPd NPs in glycerol oxidation. Three kinds of catalysts were tested, AuPd/C, AuPd/TiO2, AuPd/CeO2. OA (oxalic acid), TA (tartronic acid), GLA (glyceric acid), GLC (glycolic acid), DHA (dihydroxyacetone). Conditions: 1:1.85 mol fraction Au:Pd with 1 % metal loading by mass, water (10 mL), glycerol (0.3 m), mole fraction glycerol/metal=690, 60 °C, 4 h, 3 bar O2, mole fraction NaHCO3/glycerol=3.
For CeO2, TiO2 and carbon‐supported catalysts, a marked improvement in the catalytic activity occurs. The carbon‐supported catalyst gives the best activity in these conditions, which is approximately 39 % of glycerol conversion and even higher than that of catalyst Mg(OH)2. The activity of CeO2‐containing catalyst (20 % conversion) is better than AuPd supported on TiO2 (14 % conversion). However, without NaHCO3, they only convert 0.3 % and 1 % glycerol, respectively.
The product distribution varies among these supports. According to the reaction mechanism, high conversion should favour the highly oxidized products but CeO2‐supported catalyst shows lower selectivity than AuPd/TiO2 for tartronic acid, which is probably ascribed to the acid‐base nature of the support which has been discussed previously.11
Conclusion
For a long time, the investigation into basic supports for the oxidation of alcohols has focused on correlating catalytic performance with their acid–base properties. Given that solid base supports are often sparingly soluble in an aqueous medium, it seems surprising that catalytic performance is often not associated with the formation of homogeneous base in solution. We have attempted to demonstrate that catalytic performance is exceptionally dependant on the pH of the aqueous medium. We have shown in this study that the activity of such catalysts is simply an effect of the dissolution of the basic support. We have discussed this in detail with respect to the influence of Mg(OH)2 on the pH of the solution during glycerol oxidation and compared it to the use of standard bases in similar quantities. We have shown the level of Mg(OH)2 dissolution by MP‐AES, and we have found that this can control the pH of the reaction solution, which can influence both the activity and selectivity of the reaction products. The rate of Mg(OH)2 dissolution, which is controlled by the amount of Mg(OH)2, competes with the rate of OH− consumption from acid products. As a result, the pH will increase with the amount of Mg(OH)2, leading to the rise of AuPd activity and selectivity for acids. With enough Mg(OH)2, as the pH is not restricted by the Mg(OH)2 dissolution rate, the pH could be stabilized at a relatively high level. But, increasing Mg2+ concentration in turn affects the OH− concentration according to the solubility equilibrium, leading to a gradual decline of the pH. These pH changes that Mg(OH)2 bring to the reaction system are in fact the main reason for the outstanding performance of AuPd NPs with Mg(OH)2 in the oxidation of glycerol. We believe that this is an important consideration when designing “base‐free” oxidation systems. We have shown that in reactions in water, especially when acid products are formed, magnesium based catalysts can never truly be described as base free.
Experimental Section
Materials: KOH (laboratory reagent grade) and NaOH (>97 %) were purchased from Fisher Scientific UK Limited. PdCl2 and HAuCl3⋅3 H2O were purchased from Johnson Matthey. Polyvinyl alcohol (PVA) (Mw=10 000, 80 % hydrolyzed), NaBH4, CeO2 (nanopowder, <25 nm particle size (BET)), and MgCl2 (>98 %, anhydrous) were purchased from Sigma–Aldrich. MgO was purchased from BDH Limited. TiO2 (Degussa P25) was purchased from Evonik. Carbon black (XC‐72R) is purchased from Cabot Corporation. The deionized water was purified by a Millipore water purification system.
Mg(OH)2/C preparation: Mg(OH)2 was deposited on the carbon via a precipitation method. Carbon black (4 g) was added to the solution of MgCl2 in deionized water (250 mL). After vigorous stirring for 2 h, aqueous solution of KOH (2 m) was added to adjust the pH to 12. The solid formed was recovered by filtration and washed with water until the pH of the filtrate decreased to 7, the solid was then dried (110 °C, 16 h).
Catalyst preparation: Au‐Pd nanoparticles were prepared and deposited on the supports using a sol‐immobilization method. Typically, the solution of HAuCl3⋅3 H2O (12.25 g in 1000 ml) and PdCl2 (1 g in 100 mL), polyvinyl alcohol (PVA) (1 wt % solution), NaBH4 (0.1 m) were prepared. Then, PVA (10 mg) was added into the aqueous solution of Au and Pd (Au/Pd mole fraction=1:1.85). After 5 min, NaBH4 (NaBH4/Metal mole fraction=4) solution was added to form a dark‐brown sol. After 30 min, support (1 g) was added to make the 1 wt % catalyst. The supports include Mg(OH)2, MgO, TiO2, Carbon black, CeO2 and mixed support Mg(OH)2/C (pre‐synthesized). After 2 h, the slurry was filtered and washed with water (250 mL) and then dried in 110 °C oven for 16 h.
Glycerol oxidation: The glycerol oxidation was carried out in a glass reactor (50 mL). Aqueous glycerol (0.3 m), and catalyst were added (mol fraction glycerol/metal=690). Then, pure oxygen was used to purge the reactor three times and then the oxygen pressure was maintained at 3 bar during the reaction. The reaction mixture was heated to 60 °C for 4 h and then cooled down in ice bath. The products were analyzed by high‐performance liquid chromatography (HPLC) with ultraviolet and refractive index detectors. A Metacarb 67H column was used to separate the products with 0.01 m aqueous H3PO4 at the rate of 0.8 mL min−1. The concentration of magnesium was measured by microwave plasma atomic emission spectroscopy (Agilent 4100 MP‐AES). The pH of the solution were all measured at room temperature with Mettler Toledo FE20‐Basic FiveEasyTM Benchtop pH Meter.
Characterization: Powder X‐ray diffraction (XRD) was performed using a Panalytical X′pert Pro diffractometer using CuKα radiation at 40 kV and 40 mA. Scans were in the range of 10–80°. Transmission electron microscopy (TEM) was carried out using a Jeol 2100 with a LaB6 filament operating at 2000 kV. Ultra‐high spatial resolution EDX microanalysis was performed in a VG HB601‐UX scanning transmission electron microscope, operating at 100 kV.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
Acknowledgements
UK Catalysis Hub is kindly thanked for resources and support provided via our membership of the UK Catalysis Hub Consortium and funded by EPSRC (grants EP/K014706/2, EP/K014668/1, EP/K014854/1, EP/K014714/1 and EP/M013219/1). Information on the data underpinning the results presented here can be found in the Cardiff University data catalogue at: [http://doi.org/10.17035/d.2017.0040286171].
J. Fu, Q. He, P. J. Miedziak, G. L. Brett, X. Huang, S. Pattisson, M. Douthwaite, G. J. Hutchings, Chem. Eur. J. 2018, 24, 2396.
References
- 1. Hutchings G. J., Kiely C. J., Acc. Chem. Res. 2013, 46, 1759–1772. [DOI] [PubMed] [Google Scholar]
- 2. Kong P. S., Aroua M. K., Daud W., Renewable Sustainable Energy Rev. 2016, 63, 533–555. [Google Scholar]
- 3. Villa A., Dimitratos N., Chan-Thaw C. E., Hammond C., Prati L., Hutchings G. J., Acc. Chem. Res. 2015, 48, 1403–1412. [DOI] [PubMed] [Google Scholar]
- 4. Carrettin S., McMorn P., Johnston P., Griffin K., Hutchings G. J., Chem. Commun. 2002, 696–697. [DOI] [PubMed] [Google Scholar]
- 5. Prati L., Rossi M., J. Catal. 1998, 176, 552–560. [Google Scholar]
- 6. Dimitratos N., Lopez-Sanchez J. A., Lennon D., Porta F., Prati L., Villa A., Catal. Lett. 2006, 108, 147–153. [Google Scholar]
- 7. Villa A., Veith G. M., Prati L., Angew. Chem. Int. Ed. 2010, 49, 4499–4502; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2010, 122, 4601–4604. [Google Scholar]
- 8. Kondrat S. A., Miedziak P. J., Douthwaite M., Brett G. L., Davies T. E., Morgan D. J., Edwards J. K., Knight D. W., Kiely C. J., Taylor S. H., Hutchings G. J., ChemSusChem 2014, 7, 1326–1334. [DOI] [PubMed] [Google Scholar]
- 9. Villa A., Campisi S., Mohammed K. M. H., Dimitratos N., Vindigni F., Manzoli M., Jones W., Bowker M., Hutchings G. J., Prati L., Catal. Sci. Technol. 2015, 5, 1126–1132. [Google Scholar]
- 10. Brett G. L., He Q., Hammond C., Miedziak P. J., Dimitratos N., Sankar M., Herzing A. A., Conte M., Lopez-Sanchez J. A., Kiely C. J., Knight D. W., Taylor S. H., Hutchings G. J., Angew. Chem. Int. Ed. 2011, 50, 10136–10321; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2011, 123, 10318–10321. [Google Scholar]
- 11. Xu C. L., Du Y. Q., Li C., Yang J., Yang G., Appl. Catal. B 2015, 164, 334–343. [Google Scholar]
- 12. Yuan Z. F., Gao Z. K., Xu B. Q., Chin. J. Catal. 2015, 36, 1543–1551. [Google Scholar]
- 13. Han X. W., Geng L., Guo Y., Jia R., Liu X. H., Zhang Y. G., Wang Y. Q., Green Chem. 2016, 18, 1597–1604. [Google Scholar]
- 14. Patnaik P., Handbook of Inorganic Chemicals, McGraw-Hill, New York, 2003. [Google Scholar]
- 15. Miedziak P. J., Alshammari H., Kondrat S. A., Clarke T. J., Davies T. E., Morad M., Morgan D. J., Willock D. J., Knight D. W., Taylor S. H., Hutchings G. J., Green Chem. 2014, 16, 3132–3141. [Google Scholar]
- 16. Douthwaite M., Huang X., Iqbal S., Miedziak P. J., Brett G. L., Kondrat S. A., Edwards J. K., Sankar M., Knight D. W., Bethell D., Hutchings G. J., Catal. Sci. Technol. 2017, 22, 5284–5293. [Google Scholar]
- 17. Ide M. S., Davis R. J., Acc. Chem. Res. 2014, 47, 825–833. [DOI] [PubMed] [Google Scholar]
- 18. Rodrigues E. G., Carabineiro S. A. C., Delgado J. J., Chen X., Pereira M. F. R., Orfao J. J. M., J. Catal. 2012, 285, 83–91. [Google Scholar]
- 19. Appayee C., Breslow R., J. Am. Chem. Soc. 2014, 136, 3720–3723. [DOI] [PubMed] [Google Scholar]
- 20. Sharninghausen L. S., Campos J., Manas M. G., Crabtree R. H., Nat. Commun. 2014, 5, 5084. [DOI] [PubMed] [Google Scholar]
- 21. Zope B. N., Hibbitts D. D., Neurock M., Davis R. J., Science 2010, 330, 74–78. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary


