Summary
CO 2 efflux from stems (CO 2_stem) accounts for a substantial fraction of tropical forest gross primary productivity, but the climate sensitivity of this flux remains poorly understood.
We present a study of tropical forest CO 2_stem from 215 trees across wet and dry seasons, at the world's longest running tropical forest drought experiment site.
We show a 27% increase in wet season CO 2_stem in the droughted forest relative to a control forest. This was driven by increasing CO 2_stem in trees 10–40 cm diameter. Furthermore, we show that drought increases the proportion of maintenance to growth respiration in trees > 20 cm diameter, including large increases in maintenance respiration in the largest droughted trees, > 40 cm diameter. However, we found no clear taxonomic influence on CO 2_stem and were unable to accurately predict how drought sensitivity altered ecosystem scale CO 2_stem, due to substantial uncertainty introduced by contrasting methods previously employed to scale CO 2_stem fluxes.
Our findings indicate that under future scenarios of elevated drought, increases in CO 2_stem may augment carbon losses, weakening or potentially reversing the tropical forest carbon sink. However, due to substantial uncertainties in scaling CO 2_stem fluxes, stand‐scale future estimates of changes in stem CO 2 emissions remain highly uncertain.
Keywords: drought, growth respiration, maintenance respiration, stem CO2 efflux, tropical rainforests, woody tissue respiration
Introduction
Aboveground woody biomass is the largest store of carbon in tropical rainforests. The respiration from the stem and branch material within this woody pool has been estimated to account for 13–25% of total ecosystem respiration (Chambers et al., 2004; Cavaleri et al., 2006; Malhi et al., 2009) and 12–27% of gross primary productivity (Ryan et al., 1994; Chambers et al., 2004; Malhi et al., 2009; Doughty et al., 2015). However estimates of stem CO2 efflux (CO2_stem) remain highly uncertain in tropical forests, as only a handful of studies of CO2_stem exist (Ryan et al., 1994; Meir & Grace, 2002; Malhi et al., 2009, 2013; Robertson et al., 2010; Angert et al., 2012; Katayama et al., 2014, 2016). Consequently, substantial inconsistency exists amongst studies concerning how CO2_stem in tropical forests changes with tree height (Cavaleri et al., 2006; Katayama et al., 2014, 2016), with season (Cavaleri et al., 2006; Stahl et al., 2011) and across environmental gradients (Robertson et al., 2010), and how CO2_stem scales with tree size and growth rate (Meir & Grace, 2002; Cavaleri et al., 2006; Katayama et al., 2016). Given the concern over tropical forests shifting from a global sink to a source of carbon as the climate changes (Lenton, 2011; Davidson et al., 2012; Brienen et al., 2015; Doughty et al., 2015), understanding how CO2_stem varies with environmental change and how we calculate fluxes at ecosystem scales is becoming increasingly important.
The CO2 efflux from tree stems is likely to be mostly comprised of respiration derived from growth of new tissue (R g) and maintenance (R m) of existing tissues (McCree, 1970; Thornley, 1970; Ryan, 1990; Damesin et al., 2002; Meir & Grace, 2002). However CO2 efflux measured on trees may under‐ or overestimate stem respiration from the immediately underlying woody tissue due to other processes occurring within the trees, for example: high concentrations of CO2 in the soil, most likely from root respired CO2, being transported up to the site of measurement in sap (McCree, 1970; Levy et al., 1999; McGuire et al., 2007; Saveyn et al., 2008; Teskey et al., 2008; Aubrey & Teskey, 2009; Trumbore et al., 2013; Hillman & Angert, 2016); the transport of CO2 from below the point of measurement upwards in sap (Angert et al., 2012; Trumbore et al., 2013; Hilman & Angert, 2016); and nonphotosynthetic CO2 fixation by phosphoenolpyruvate carboxylase (PEPC) (Berveiller & Damesin, 2008). These processes can change over time with changes in sap pH, stem temperature, sap flow velocity or changes in gas diffusivity in the stem over time, which may arise from an increase in air‐filled spaces or even cracks in the bark (Cherubini et al., 1997; Levy et al., 1999; Sorz & Hietz, 2006; Teskey et al., 2008; Trumbore et al., 2013) Within tropical trees these processes have been relatively sparsely studied, in part due to the complexities of measuring such processes (Trumbore et al., 2013), particularly in what are often remote, challenging field locations. However, a new approach recently used in tropical forests combined oxygen consumption and CO2 efflux measurements to show that the apparent respiratory quotient of O2 to CO2 (ARQ) of tropical trees was less than the expected value of 1 (0.66 ± 0.18), suggesting that up to a third of CO2 was being transported away from the site of measurement causing underestimations of stem respiration (e.g. Angert et al., 2012). These results underline the notion that stem CO2 efflux measurements are likely to comprise signals from growth and maintenance respiration in combination with other stem processes, thus requiring caution when interpreting results.
Tropical forest growth and maintenance respiration (R g and R m) components have generally been derived from linear regressions of CO2_stem on growth rate (McCree, 1970; Thornley, 1970; Meir & Grace, 2002), with the intercept interpreted to give the maintenance respiration flux at zero growth rate. Due to the potential loss or gain of CO2 from other within‐stem processes, it is unlikely that these calculations give an entirely accurate representation of R g or R m. If, however, we assume that CO2 is gained or lost equally from the CO2 produced by R g or R m, such methods may still provide a good representation of the proportion of CO2 derived from growth and respiration, even if the quantitative values are not certain. Knowing these proportions is important because as trees experience climate stress it is likely that growth rates will decline (da Costa et al., 2010; Brienen et al., 2015; Korner, 2015), whilst simultaneous investment into maintaining existing tissues may rise (Metcalfe et al., 2010; Rowland et al., 2015b). Nonetheless, no studies in tropical forest have determined how growth and maintenance respiration change as mature tropical trees experience climate‐related stress, and how this is likely to influence stand‐scale CO2 efflux from woody tissue.
One of the key future climate changes which tropical forests are expected to experience in the coming decades is water stress caused by increased seasonal, interannual and decadal‐scale drought (Fu et al., 2013; Boisier et al., 2015; Duffy et al., 2015). Relative to photosynthetic fluxes, how respiration fluxes will respond to drought stress remains poorly constrained (Meir et al., 2008; Atkin & Macherel, 2009; Rowland et al., 2014). Limited data on temperate species suggest that stem CO2 efflux declines with water stress (Saveyn et al., 2007; Rodríguez‐Calcerrada et al., 2014). These studies agree with a number of studies on leaves, which find that leaf respiration is downregulated during short‐term drought stress, due to declining substrate availability (Ayub et al., 2011; Catoni & Gratani, 2014; Chastain et al., 2014; O'Brien et al., 2015). By contrast, some studies have shown increased leaf respiration with drought stress, particularly when drought occurs over extended periods (Miranda et al., 2005; Atkin & Macherel, 2009; Metcalfe et al., 2010; Rowland et al., 2015b; Varone & Gratani, 2015). Increased respiration during drought conditions may be expected if a greater amount of substrate is required for hydraulic repair and maintenance (Brodersen & McElrone, 2013), phloem transport regulation (Mencuccini & Hölttä, 2010) or oxidation of reactive oxygen species (Atkin & Macherel, 2009). Consequently, changes in respiration following drought are likely to be controlled by tree size and genera because trees of different sizes and genera have been shown to experience different hydraulic and metabolic costs as a consequence of drought stress (Rowland et al., 2015a,b), alongside having differing stem growth and maintenance costs.
However, a paucity of studies in tropical ecosystems, and globally, means that our current understanding of how CO2_stem, one of the largest components of autotrophic respiration, will respond to future increases in water stress still remains highly uncertain. This uncertainty is amplified by the existence of various methods for scaling these fluxes to the ecosystem, including according to total stem area or sapwood volume (e.g. Levy & Jarvis 1998; Cavaleri et al., 2006; Katayama et al., 2014), which result in large differences in ecosystem‐scale estimates of stem CO2 release. In the present study, we report the results from a study of CO2_stem on 215 trees in dry and wet seasons, in a forest that has experienced 15 yr of experimental drought and in adjacent corresponding control forest. Using these data we test the following hypotheses: drought causes an increase in CO2_stem, due to increasing maintenance costs associated with low moisture availability; CO2_stem will be significantly different among genera, as metabolic processes and responses to drought are taxonomically conserved; long‐term drought increases the proportion of maintenance to growth respiration, as a consequence of increasing maintenance costs and reducing growth; and the effect of long‐term drought on stand‐scale estimates of CO2_stem changes according to whether CO2_stem rates are scaled using estimates of total stem area or of sapwood volume.
Materials and Methods
Site
The study was performed at a through‐fall exclusion (TFE) experiment in the Caxiuanã National Forest reserve in eastern Amazonia (1°43′ S. 51°27′ W). The site is 15 m above sea level, located within terra firme forest on yellow oxisol soils (Ruivo & Cunha, 2003). It experiences a mean annual rainfall of 2000–2500 mm and a pronounced dry season in the later 6 months of the year.
The experiment comprised two 1‐ha plots, a control plot with no drought infrastructure and a TFE where plastic panels and guttering at 1–2 m in height are used to exclude 50% of the canopy through‐fall from reaching the forest floor (da Costa et al., 2010). Both plots were trenched to 1–2 m to prevent lateral flow of water in the soil. To maintain biogeochemical inputs into the soil, leaf litter on the TFE panels is relocated to the forest floor every few days. The TFE treatment has been maintained since January 2002, and therefore before this study all trees on the TFE had experienced 15–16 yr of a 50% reduction in canopy through‐fall. Further details on the experiment can be found in da Costa et al. (2010) and Meir et al. (2015).
Sample selection
Measurements were performed on 215 trees in total, 105 from the control plot and 110 from the TFE during October 2016 (mid dry season) and April 2017 (mid wet season). First, we selected trees from 12 of the most common genera found on both the control plot and the TFE (Aspidosperma, Eschweilera, Inga, Licania, Micropholis, Minqurtia, Pouteria, Protium, Swartzia, Syzygiopsis, Virola and Vouacapoua), totalling 87 and 77 trees on the control and the TFE plots, respectively. The remainder of the trees – 18 on the control plot and 33 on the TFE plot – comprised trees with a diameter at breast height (dbh) > 30 cm on the TFE and > 40 cm on the control, measured to ensure more equal division of trees amongst size classes. From October 2013 to January 2016, seven measurement campaigns were also carried out on 16–18 trees on the control and 19–20 trees on the TFE, of the genera (Eschweilera, Licania, Manilkara, Pouteria, Protium and Swartzia) previously sampled for photosynthesis measurements by Rowland et al. (2015b). A list of all of the species samples in each measurement campaign from 2013 to 2017 is presented in Supporting Information Table S1.
CO2_stem measurements
CO2_stem was measured using a transparent acrylic chamber, temporarily sealed onto the stem surface using a closed cell non‐CO2 adsorbent foam gasket and two ratcheting straps. The chamber was sealed to the stem at a constant gasket thickness and had a volume of 213 cm3 (including tubing and foam) and a surface area of 75 cm2 of the bark surface. The chamber size and construction were similar to those used for other measurements of CO2 efflux in tropical forests (Stahl et al., 2011; Rowland et al., 2013). The chamber was connected to an infrared gas analyser (EGM4, EGM5; PPSystems, Hitchen, UK) for 220 s and was used to detect an increase in CO2 concentration inside the chamber. Following Rayment & Jarvis (2001), to promote air mixing in the chamber without creating vortex effects from the operation of a fan, the chamber also contained a 15‐cm length of tube perforated with 0.5 mm diameter holes, connected to the inlet. During each measurement we tested for leaks by exposing the edges of the chamber to very high CO2 concentrations. If any increase in CO2 concentration inside the chamber was detected, the measurement was aborted. Wood temperature (T w) was measured using a type T thermocouple placed into the bark, or where this was not possible, on the bark surface. All measurements were made between 08:00 h and 14:00 h.
Measurements of the increase in CO2 concentration between 120 and 220 s were used for analysis, leaving 2 min for the chamber to stabilize post‐installation. The slope of the linear regression between time and CO2 was extracted to calculate CO2_stem (stem CO2 efflux, μmol m−2 s−1) using Eqn (Eqn 1)
| (Eqn 1) |
(ΔCO2/Δt, slope of the CO2–time relationship; V c volume (cm3) and S c the surface area (cm2) of the chamber; a, volume of a mole of CO2 (mol cm3); T w, measured wood temperature (°C)). Linear slope values with a correlation coefficient < 0.98 were discarded from the analysis and the data were temperature‐corrected to 25°C using a Q 10 of 2.0 (Cavaleri et al., 2006). After excluding measurements with leaks or with a correlation coefficient < 0.98, 97 measurements were included on the control plot and 108 on the TFE plot from the dry season; and 97 from the control and 99 from the TFE plots, respectively, were included from the wet season.
Diurnal tests
In order to test for daytime increases in stand‐scale CO2_stem (S_CO2_stem), which could result in biases according to the time CO2_stem was measured or indicate other forms of CO2 transport or consumption (Teskey et al., 2008; Angert et al., 2012), we measured CO2_stem every 15 s for 24 h on 20 trees from the control and the TFE in October 2013, using an open path respiration system similar to that used elsewhere (Rayment & Jarvis, 2001; Meir & Grace, 2002) and a CIRAS 1 IRGA (PPSystems); for further details see Methods S1). We found very limited diurnal variation in CO2_stem, indicating limited bias concerning the time of day the measurements were taken (see Fig. S1 and Methods S1).
Growth data
Quarterly mean tree‐level stem diameter increment per plot from 2010 to 2015 were taken from dendrometer measurements presented in Rowland et al. (2015a), and updated to the end of 2016 following the same methodology and converted to units of cm d−1. A long‐term annual increment then was calculated for each tree based on the 2010–2016 dataset. This interval (2010–2016) was chosen as it represented the period after which the growth rates of the small and medium trees on the TFE (10–40 cm dbh) had re‐stabilized following increased growth rates in response to elevated light intensities (see Rowland et al., 2015a for further details). We note that accurate growth measurements were not available for some of the larger trees in this study, as it was not feasible to monitor these trees on a three‐monthly basis due to their size or because a dendrometer could not be accurately fitted on the tree due to substantial trunk‐shape irregularities.
Scaling
Scaling was performed using three methods, which are described in detail in Methods S1. The three methods were used to assess the effect of different scaling assumptions on S_CO2_stem estimates. Method one (M1) involved scaling according to total stem surface area. Method two (M2) used estimated total sapwood volume as the scalar. Initially we estimated total sapwood volume to be 34% of total tree volume (an estimate of the sapwood area: basal area ratio at 1.3 m above ground level; see Methods S1 and Fig. S2) and then, given that 34% is likely to underestimate the greater percentage sapwood area in smaller diameter branches, we assessed how this calculation changed using an estimate of 50% and 80% sapwood volume. We assumed constant live‐cell fraction in all sapwood volume estimates. Method 3 (M3) involved a combination of the two scaling methods above. Following Cavaleri et al. (2006), but taking a total sapwood volume approach, we assumed that for any part of the canopy < 10 cm in diameter CO2_stem scaled with total stem surface area, and for sections > 10 cm CO2_stem was scaled with total sapwood volume. For all methods trees within 10 m of the edge of the plots were excluded from our calculations to eliminate possible long‐term effects of the trenching on the community structure and tree physiology (da Costa et al., 2010). Wet and dry season S_CO2_stem estimates from all scaling methods were averaged and converted to units of Mg C ha−1 yr−1.
Analysis
All statistical analyses were performed in the statistical package R (v.3.4.0; R Core Team, 2017) and all errors are shown as standard errors on the mean, but do not account for the sampling error of the calibration of the gas analyser (< 1% in EGM). Following Damesin et al. (2002) and Meir & Grace (2002), we calculated averaged plot‐level maintenance respiration as the intercept of the relationship between growth and CO2_stem, but using a bootstrapping technique, to avoid assumptions about normality of distribution and to facilitate the calculation of errors. First we randomly sampled our study trees, with replacement, to create 1000 samples of the trees which had growth and CO2 efflux data on each plot (75 control, 87 TFE). Following this, we calculated 1000 estimates of: mean total CO2_stem per tree, for each plot; the y‐intercept of the woody increment– CO2_stem relationship (R m); and R g, calculated as mean CO2_stem minus R m. Mean and SE values of CO2_stem, R m and R g per tree for each plot were then calculated from the mean and SD of the bootstrapped samples. Data comparisons of the proportions of R m and R g between plots, seasons and tree size classes (small: 10–20 cm dbh, medium: 20–40 cm dbh and large: > 40 cm dbh) were then made. Given that the bootstrapping created a normal distribution, statistical comparisons of CO2_stem, R m and R g were made using a parametric paired t‐test and only percentage values of R m and R g are presented, acknowledging that absolute values are uncertain because of uncertainties in estimating woody respiration from CO2_stem (Teskey et al., 2008; Trumbore et al., 2013).
Analysis of whether CO2_stem scales with surface area or sapwood volume was performed following Levy & Jarvis (1998). Log‐transformed linear relationships were created for CO2_stem (μmol m−2 s−1) against dbh and CO2_stem (μmol m−3 s−1) against 1/dbh. A significant relationship between area‐based CO2_stem and dbh indicates that a scaling relationship with volume exists, and a significant relationship between volume‐based CO2_stem and 1/dbh indicates that a scaling relationship with area exists (Levy & Jarvis, 1998). Consequently the slopes of these relationships indicate the proportional scaling with volume or area (respectively) as, for example, a slope of 1 between dbh and CO2_stem (μmol m−2 s−1) would indicate perfect volume scaling, whilst a slope of 0 would indicate perfect area scaling (see Levy & Jarvis, 1998).
Results
Drought response of CO2_stem
The CO2_stem rates of trees on the control plot averaged 1.00 ± 0.10 μmol m−2 s−1 across both seasons, showing significantly higher CO2_stem values in the dry season (dry = 1.01 ± 0.08 μmol m−2 s−1, wet = 0.87 ± 0.07 μmol m−2 s−1; P < 0.01; Fig. 1a). By contrast, on the TFE plot there was a significant increase in CO2_stem during the wet season relative to the control plot and the dry season (P < 0.01, dry = 0.99 ± 0.06 μmol m−2 s−1, wet = 1.23 ± 0.08 μmol m−2 s−1). This represented a 27% increase in CO2_stem on the TFE during the wet season relative to the control plot, a seasonal increase on the TFE plot itself of 24% relative to the dry season, and therefore an overall 11% increase in the mean wet and dry season CO2_stem on the TFE relative to the control plot (Fig. 1a). Data from a previous analysis of 21 trees (see the Materials and Methods section, Methods S1 and Table S1) per plot measured six times between October 2013 and February 2016, also confirmed that the TFE tended to have consistently higher fluxes than the control plot during the wet season and more equal fluxes during the dry season (Fig. 1b). However, we note that the magnitude and plot differences in these latter flux values are likely to be less reliable due to a lower sample size.
Figure 1.
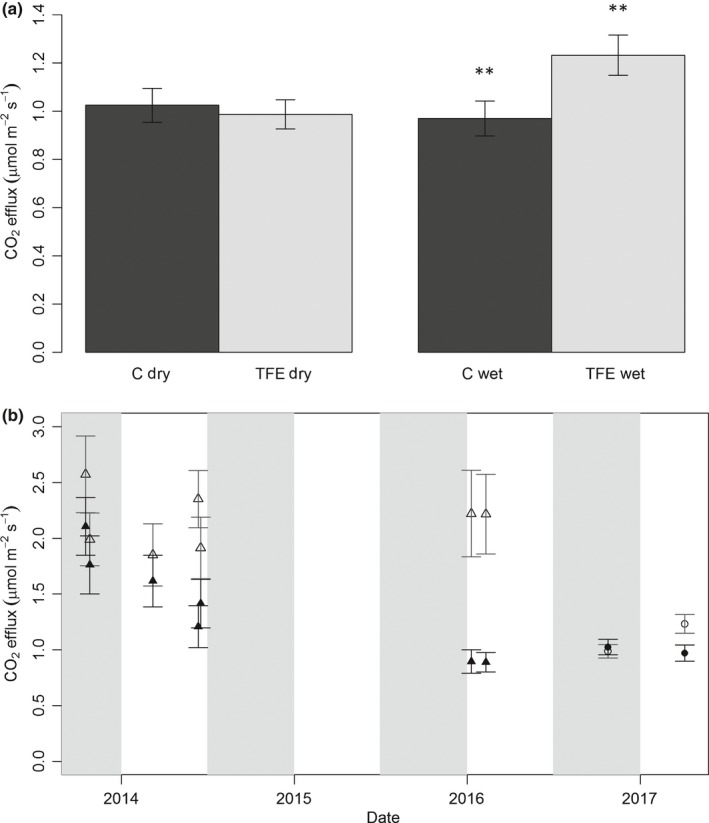
(a) Stem CO 2 efflux (μmol m−2 s−1) in mid dry season (October 2016) and mid wet season (April 2017) on the control (C, black) and through‐fall exclusion (TFE) plot (grey). Asterisks indicate significant increase at P < 0.01 between columns. (b) Stem CO 2 efflux (μmol m−2 s−1) on a time series of data from the control (closed symbols) and the TFE (open symbols) plot. Grey shaded areas show dry season months (July‐December), triangles indicate measurements taken with n = 21 individuals per plot, and circles indicate measurements taken with n = 105 on the control and n = 110 on the TFE (see the Materials and Methods section and Supporting Information Table S1). Error bars indicate ± SE.
The increase in CO2_stem on the TFE was controlled predominantly by significant increases in CO2_stem from trees smaller than 40 cm dbh, which occurred in the wet, but not the dry season (Fig. 2). Interestingly, CO2_stem increased with tree size on both plots, and this increase was more pronounced in the wet season and on the control plot (Fig. 2), where CO2_stem of the largest tree size class (> 60 cm dbh) was 3.4‐fold greater than that for the smallest (< 15 cm dbh; Fig. 2b). On the TFE, due to the elevated CO2_stem in the smallest trees, this increase in CO2_stem from the smallest to the largest trees was reduced to 2.6‐fold.
Figure 2.
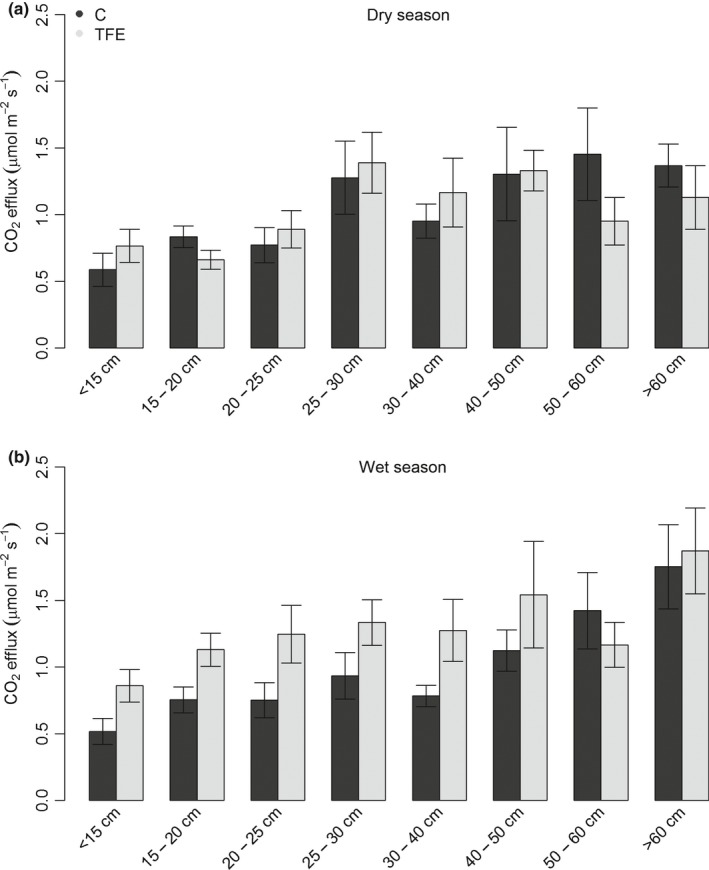
Mean stem CO 2 efflux (μmol m−2 s−1) in (a) mid dry season (October 2016) and (b) mid wet season (April 2017) on the control (C, black) and through‐fall exclusion (TFE) plot (grey) for trees divided into diameter at breast height (1.3 m; dbh) size classes of < 15, 15–20, 20–25, 25–30, 30–40, 40–50, 50–60 and > 60 cm. Error bars show ± SE.
Taxonomic patterns in CO2_stem
Strong changes in CO2_stem with tree size resulted in high variation in CO2_stem within each genus (Fig. 3). Consequently, no significant differences were found among genera on each plot in dry season (Fig. 3). Protium was found to have significantly elevated CO2_stem on the TFE, relative to the control during the wet season, although it did not demonstrate a significant increase from dry to wet season on the TFE (Fig. 3b,d). It is also noteworthy that, excluding Protium on the control and Aspidosperma and Inga on the TFE, the mean values per genus are largely similar within and between plots, as well as between seasons. These results suggest that CO2_stem drought responses are not strongly taxonomically conserved.
Figure 3.
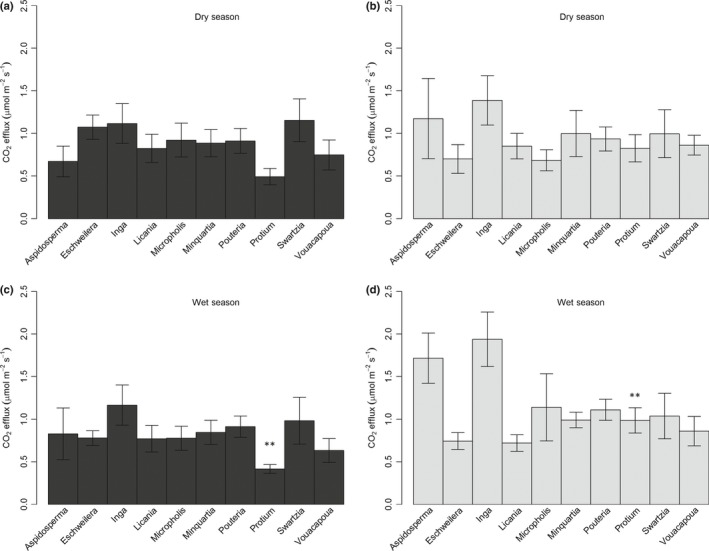
Mean stem CO 2 efflux (μmol m−2 s−1) in (a, b) mid dry season (October 2016) and (c, d) mid wet season (April 2017) on (a, c) the control (C, black) and (b, d) through‐fall exclusion (TFE) plot (grey) for trees divided into genus groups, with greater than two individuals per group (see Supporting Information Table S1). Error bars show ± SE. Matching symbols indicate that columns are different at P < 0.05.
Growth and maintenance respiration
Relationships between CO2_stem and mean woody increment for 2010–2016 were performed on a per tree basis separately for mean annual total (wet and dry season), wet season and dry season CO2_stem, and for mean annual CO2_stem divided into small, medium and large size classes. On both plots CO2_stem by season or size class always had a positive and significant (at least P < 0.01) relationship with mean wood increment (Fig. 4; Table 1). These relationships had a larger r 2 values on the control plot (e.g. r 2 control plot annual mean = 0.61, TFE plot annual mean = 0.37; Table 1); however, there were also consistently greater r 2 values in larger trees compared to small trees on both plots (Fig. 4; Table 1). When the percentage R m and R g values were estimated from these relationships, we find that on an annual basis the CO2 efflux associated with R m accounts for 58 ± 10% and 67 ± 10% of total respiration on the control and TFE plot, respectively (Table 1; Fig. 5a). Furthermore, we find limited seasonal change in these values when averaging across trees of all size classes (Fig. 5a; Table 1). When trees were divided into size classes there were, however, strong shifts in the percentage division of R m and R g. On the control plot in the small trees 80 ± 10% of the respiration was R m, and this declined to 60 ± 22% and 43 ± 27% in the medium and large trees, respectively (Fig. 5b; Table 1). By contrast, on the TFE the small trees had a lower percentage R m, 62 ± 14%, and this increased in the medium and large trees to 75 ± 20% and 78 ± 21%, respectively (Fig. 5b; Table 1). This suggests that R m increases substantially in larger trees as a consequence of drought.
Figure 4.
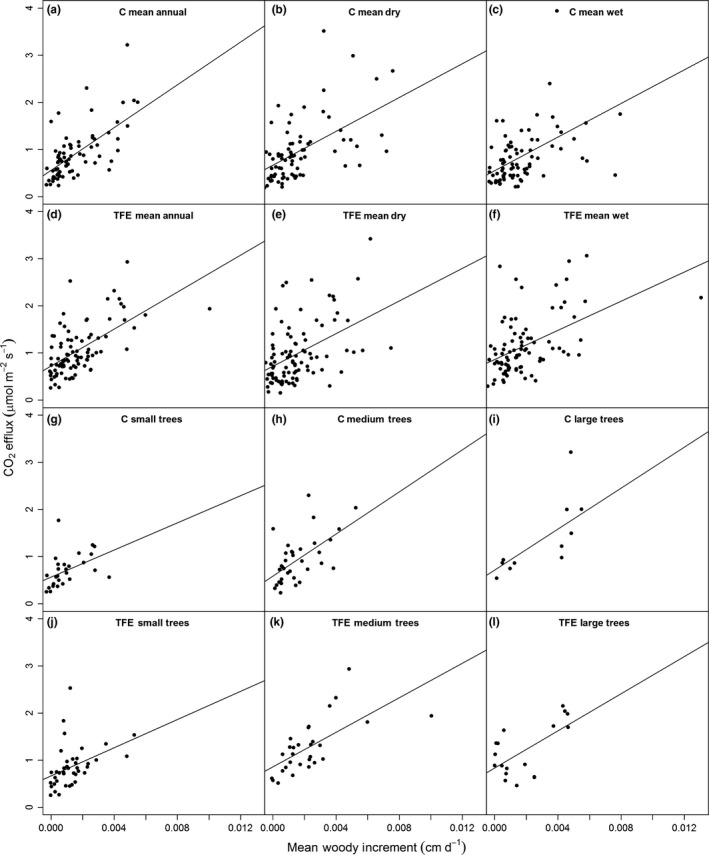
Relationships between mean stem CO 2 efflux (μmol m−2 s−1) and the 2010–2015 mean annual woody increment (cm d−1) for the control (C) and through‐fall exclusion (TFE) plots for (a, d) mean annual stem CO 2 efflux, (b, e) dry season CO 2 efflux, (c, f) wet season stem CO 2 efflux, and mean annual CO 2 efflux split into (g, j) small (10–20 cm), (h, k) medium (20–40 cm) and (i, l) large trees (> 40 cm). Linear fit lines indicate significant (P < 0.05) linear relationships. Correlation coefficients, P‐values and intercepts are shown in Table 1.
Table 1.
Intercept (Int.), correlation coefficient (r 2) and P‐values (P), mean total stem CO2 efflux (CO2_stem; standard error given as CO2_stem_se) and the percentage (%) of total CO2 efflux associated with R m and R g for the panels in Fig. 5 (pan.) representing CO2_stem values on the control (C) and through‐fall exclusion (TFE) averaged annually, for the wet and dry seasons, and average annual values separated by tree size (small, 10–20 cm diameter at breast height (dbh); medium, 20–40 cm dbh; large > 40 cm dbh)
| Panel | Variable | r 2 | P | Int. | CO2_stem | CO2_stem_se | % R m | % R g |
|---|---|---|---|---|---|---|---|---|
| (a) | C annual | 0.61 | 0.00 | 0.55 | 0.95 | 0.07 | 58 | 42 |
| (b) | C dry | 0.44 | 0.00 | 0.68 | 1.02 | 0.08 | 66 | 34 |
| (c) | C wet | 0.44 | 0.00 | 0.53 | 0.89 | 0.08 | 60 | 40 |
| (d) | TFE annual | 0.37 | 0.00 | 0.72 | 1.07 | 0.06 | 67 | 33 |
| (e) | TFE dry | 0.17 | 0.00 | 0.75 | 0.99 | 0.07 | 76 | 24 |
| (f) | TFE wet | 0.25 | 0.00 | 0.85 | 1.15 | 0.07 | 74 | 26 |
| (g) | C small | 0.19 | 0.01 | 0.56 | 0.70 | 0.06 | 80 | 20 |
| (h) | C medium | 0.41 | 0.00 | 0.58 | 0.98 | 0.11 | 60 | 40 |
| (i) | C large | 0.73 | 0.00 | 0.68 | 1.59 | 0.31 | 43 | 57 |
| (j) | TFE small | 0.14 | 0.01 | 0.67 | 1.07 | 0.10 | 62 | 38 |
| (k) | TFE medium | 0.46 | 0.00 | 0.80 | 1.07 | 0.10 | 75 | 25 |
| (l) | TFE large | 0.36 | 0.01 | 0.83 | 1.07 | 0.16 | 78 | 22 |
Figure 5.
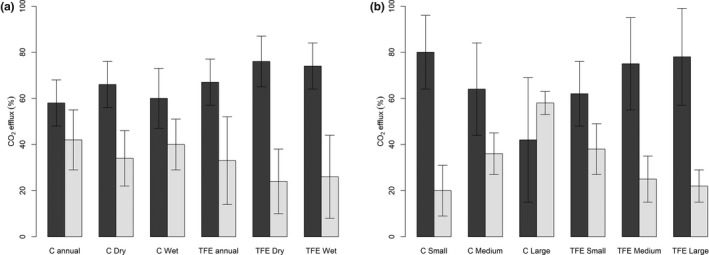
Estimated percentage of maintenance respiration (black) and growth respiration (grey) for the control plot (C) and through‐fall exclusion (TFE) plot, divided by (a) plot and season and (b) by tree size, averaging respiration across seasons. Error bars show ± SE.
Scaling CO2_stem
On the control plot in both the wet and dry season data, there was a stronger correlation between log‐transformed CO2_stem on an area basis (r 2 = 0.20–0.28) and dbh, than on a volume basis and 1/dbh (r 2 = 0.08; Fig. 6a,b,e,f). On the TFE plot, the relationships with dbh and 1/dbh were generally weaker than on the control plot (r 2 = 0.10–0.18; Fig. 6). However, on both the control and the TFE such low r 2 values created substantial uncertainty concerning whether area or volume is a better scalar; for example, the slope values for CO2_stem by area against dbh and CO2_stem by volume against 1/dbh in the dry season indicated a range of the percentage of the data which scaled with area from 50 ± 10% to 70 ± 10% on the control plot and of 39 ± 10% to 62 ± 10% on the TFE.
Figure 6.
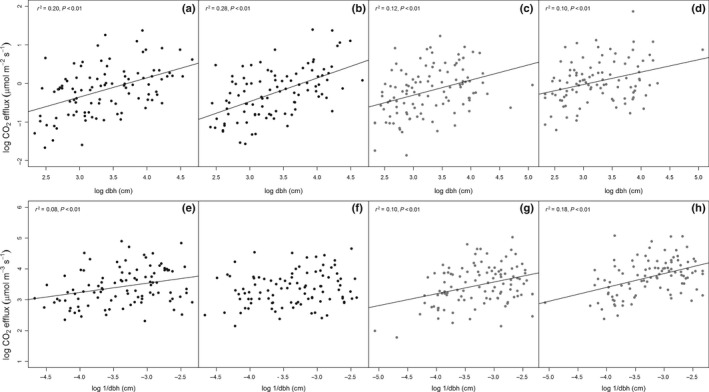
Relationships between log stem CO 2 efflux by area (μmol m−2 s−1) and log diameter at breast height (dbh, cm) for the control (black) and through‐fall exclusion (TFE) (grey) plot in (a, c) dry and (b, d) wet. Relationships between log stem CO 2 efflux by volume (μmol m−3 s−1) and log1/diameter are also shown for the control and TFE plot in (e, g) dry and (f, h) wet season. Linear regression fits, r 2 and P‐values are shown for significant (P < 0.05) relationships.
When we scaled up the CO2_stem values to S_CO2_stem for each plot, the various estimates for the stand‐scale flux of the control ranged by 4.7 Mg C ha−1 yr−1 and those of the TFE plot by 5.1 Mg C ha−1 yr−1 (Table 2). Furthermore, the percentage reduction in the S_CO2_stem on the TFE relative to the control ranged from 0.7–22.9%, depending on the method of scaling (Table 2). The highest estimates of S_CO2_stem came from using surface area as the scalar; however, these values were similar to the scaling outcome using the method of assuming volume as the scalar for wood < 10 cm diameter and area as the scalar for bole diameters > 10 cm. The area and the area–volume scaling methods both produced very small percentage differences between the control and the TFE S_CO2_stem. By contrast, scaling by sapwood volume alone produced substantially larger differences between the plots (in both absolute and relative terms), which were well‐conserved across the range of percentage sapwood volume used (34–80%). Scaling by sapwood volume produced far lower S_CO2_stem values, which were also highly sensitive to the percentage value of sapwood volume used (Table 2).
Table 2.
StemCO2 efflux (CO2_stem) values scaled to plot level (Mg C ha−1 yr−1) for the control and the through‐fall exclusion (TFE) plots, calculated according to: surface area scaling; volume scaling assuming 34%, 50% and 80% of the volume is sapwood (SW); scaling assuming CO2_stem scales with volume for tree boles < 10 cm and with area for all woody sections > 10 cm diameter at breast height (dbh)
| Control | TFE | Change (%) | |
|---|---|---|---|
| Surface area | 7.07 ± 0.72 | 6.94 ± 0.63 | 1.8 |
| Volume, 80% SW | 5.53 ± 0.56 | 4.26 ± 0.39 | 22.9 |
| Volume, 50% SW | 3.46 ± 0.35 | 2.67 ± 0.24 | 22.8 |
| Volume, 34% SW | 2.40 ± 0.24 | 1.86 ± 0.17 | 22.5 |
| Volume bole > 10 cm, area < 10 cm | 6.81 ± 0.69 | 6.76 ± 0.61 | 0.7 |
Error term shows the ± SE propagated from the error on the measured CO2 efflux values only. The final column demonstrates the percentage change of the TFE relative to the control. The frequency distribution of trees across size categories for each plot can be seen in Supporting Information Table S2.
Discussion
Using the world's longest‐running drought experiment in tropical forest and measurements of CO2 efflux from 215 stems in the wet and dry seasons, we demonstrated that the efflux of CO2 from stems (CO2_stem) increased by 27% on drought‐treated TFE (through‐fall exclusion) trees relative to control trees in the wet season. The increases in CO2_stem were caused by large increases, of up to 40%, in the efflux rate of CO2 released from trees < 40 cm diameter at breast height (dbh) in the wet season, increases which were absent in the dry season. Furthermore, we found that there was a substantial increase in the percentage of total respiration that is associated with respiration resulting from maintenance (R m) on the TFE relative to the control, driven by reduced efflux associated with respiration resulting from growth (R g) and increased efflux associated with R m in the medium and large trees. Finally we show that the stand‐scale CO2_stem (S_CO2_stem) estimates, as well as the differences in S_CO2_stem between plots are highly sensitive to the scaling method used, with absolute values varying by > 300% within plots and the percentage change between the plots varying by up to 22%.
Following 15 yr of rainfall exclusion, wet season CO2_stem rates on the TFE plot were 27% higher (Figs 1, 3). This result contrasts with findings in temperate forests, where CO2_stem declined, but with short‐term water stress (Saveyn et al., 2007; Rodríguez‐Calcerrada et al., 2014). However, our result is consistent with several reports elsewhere of drought‐related increases in respiration (Miranda et al., 2005; Varone & Gratani, 2015) and corroborates previous results from this site which showed substantial increases in leaf dark respiration on the TFE plot following extended periods of reduced soil moisture availability (Metcalfe et al., 2010; Rowland et al., 2015b), and evidence of coupled increases in root respiration (Metcalfe et al., 2007; Meir et al., 2008). Given that the elevated CO2_stem occurs only in the wet season, we speculate that this could be caused by increased growth rates in the small and medium trees found to occur on the TFE (Rowland et al., 2015a) or potentially because the xylem tissue is undergoing hydraulic recovery (Brodersen & McElrone, 2013), following high hydraulic stress which is likely to occur during periods of extreme vapour pressure deficit (VPD) and low rainfall during the dry season on the TFE (Rowland et al., 2015a). This hypothesis is supported further by the significant increase in percentage of R m on the TFE relative to the control during the wet season (Fig. 1a; Table 1), suggesting that the cost of maintaining existing tissues may be substantially higher on the TFE plot, especially in the largest trees.
Previously, maintenance respiration was estimated to comprise c. 80% of total respiration in mature trees in closed tropical rainforests (Ryan et al., 1994; Meir & Grace, 2002). Our analysis indicates that the division of CO2_stem associated with R g and R m varies substantially by tree size class and with drought in tropical forest. On the control plot there was a strong trend toward decreases in percentage R m with increasing tree size and increasing percentage R g (Fig. 5b). This strong percentage decline in R m with tree size was absent from the TFE plot trees, where percentage R g declined with tree size (Table 1; Fig. 5b). Instead, on the TFE we observed a substantial increase in R m in the largest trees relative to the control plot (Fig. 5b). As the largest trees are mostly likely to suffer damage, particular hydraulic damage, following drought stress (Bennett et al., 2015; McDowell & Allen, 2015; Rowland et al., 2015a), these results may suggest that these trees are unable to invest as much carbohydrate resource into R g. This may be driven by elevated maintenance costs associated with repairing drought‐damaged cells, removing reactive oxygen species, elevated phloem transport regulation or repair and/or replacement of hydraulically damaged xylem tissue. However, we note that the errors on our estimates of maintenance respiration are large for certain tree size classes (Table 1), due to smaller proportions of variance in CO2_stem being explained by growth in some size classes than others. This may suggest that other unmeasured interaction variables are necessary to quantify the proportions of growth and maintenance respiration with greater accuracy.
In our analysis, we find no clear evidence of whether scaling by surface area or sapwood volume is more appropriate (Fig. 6). However we note that having used a relationship to estimate sapwood volume, we have estimates of sapwood volume, rather than a direct measurement and CO2_stem may be more prone to error when calculated on a sapwood volume basis, than when calculated on a surface area to CO2_stem. Consequently we tested a variety of scaling methods to estimate our fluxes at the plot level. Competitive release of smaller trees on the TFE plot following a 40% loss of biomass from the mortality of the largest trees (da Costa et al., 2010; Rowland et al., 2015a) enhanced the growth and recruitment of the smallest size‐class trees, which also have the largest surface area to volume ratio. This shift in size distribution caused the TFE plot to have S_CO2_stem that was almost equal to the S_CO2_stem for the control plot when surface area, or mostly surface area‐based scaling was used, but substantially lower S_CO2_stem when volume was used as the scalar.
Scaling by area is the most common form of scaling of CO2_stem to the canopy (Chambers et al., 2004; Malhi et al., 2013). Given the radial live‐cell distribution in woody tissue it is unlikely, particularly in large diameter woody sections, that CO2_stem scales directly with area, because CO2 production occurs in the living sapwood and phloem tissue (Fig. 5; Meir & Grace, 2002; Cavaleri et al., 2006; Levy & Jarvis, 1998). Scaling by sapwood volume does, however, introduce very large uncertainties into S_CO2_stem estimates (Table 2), because the proportion of tree volume that is sapwood remains uncertain, as does the fraction of sapwood cells that are metabolically active. How sapwood volume scales with diameter within trees and between species in tropical forests is very sparsely studied (Meir et al., 2017), with no current estimates on how to calculate the sapwood volume of a tree (including the canopy), or its variation among species. In addition, the allometric scaling equations used for calculating tree volume and surface area (Methods S1) are also likely to introduce large errors into S_CO2_stem estimates, the magnitudes of which are hard to estimate. Biomass studies have shown this may be particularly true for the largest trees (Calders et al., 2015), and this may suggest that greater unknown error exists in the S_CO2_stem value for the control plot, where there are more large trees.
Throughout this study we present all absolute measured values as CO2_stem while acknowledging that there are likely to be many other processes occurring within the stem, which may result in raw chamber‐based measurements of CO2 efflux from the stem, leading to over‐ or underestimates of the actually woody stem respiration underlying the measurement chamber (McCree, 1970; Levy et al., 1999, McGuire et al., 2007; Berveiller & Damesin, 2008; Saveyn et al., 2008; Teskey et al., 2008; Aubrey & Teskey, 2009; Angert et al., 2012; Trumbore et al., 2013; Hilman & Angert, 2016). However, we do note that we found limited diurnal changes in CO2_stem (Fig. S1), suggesting, as found elsewhere (Ubierna et al., 2009; Stahl et al., 2011), that the upward transport of ‘excess’ CO2 from the soil or roots or the upward transport of CO2 from the point of measurement may be limited in this forest, or compensated for by other processes. Measurements of woody tissue respiration using techniques for measuring oxygen absorption were not feasible at our remote study site, nor on the number of trees presented here. However, given the number of trees sampled, the limited evidence of diurnal variation in CO2_stem, and the good replication of tree genera and tree sizes between the plots, we believe that our study does give as accurate a representation as is currently possible of the changes in stem CO2 efflux and the proportions of associated R m and R g which occur as a result of long‐term drought.
Our results suggest that under prolonged periods of drought stress, increasing CO2_stem, particularly from small and medium trees, is likely to augment carbon losses from vegetation to atmosphere, which are already likely from drought‐induced mortality. At large scales this response will either further weaken or potentially reverse the tropical forest carbon sink. However, we demonstrate that scaling CO2_stem values to the stand‐scale is currently subject to very high levels of uncertainty, limiting predictions of both the absolute values of stand‐scale CO2_stem and their proportional variation. This will be especially relevant when ecosystems are subject to climatic stresses, such as drought, which are likely to alter ecosystem size structure and related growth, and related physiological‐response regimens.
Author contributions
The research was designed by L.R., P.M., M.M., A.C.L.d.C., R.S.O., L.V.F. and S.S.V.; data collection, interpretation and analysis was carried out by L.R., A.C.L.d.C., A.A.R.O., P.L.B., P.B.C., A.L.G., A.I.S., I.C., J.L.G., J.A.S.J., M.M. and P.M.; and the manuscript was written by L.R. with contributions from all other authors.
Supporting information
Please note: Wiley Blackwell are not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Fig. S1 Diurnal variation of stem CO2 efflux from trees on the control and TFE plots.
Fig. S2 Relationships between sapwood depth and tree diameter and basal area.
Table S1 List of the tree diameter and species of all trees sampled in this study
Table S2 Distribution of trees across size classes for all trees >10 cm diameter at 1.3 m above ground on the control and TFE plots
Methods S1 Additional methods relating to the measurement and scaling of stem CO2 efflux data.
Acknowledgements
This work is a product of a UK NERC independent fellowship grant NE/N014022/1 to L.R., a UK NERC grant NE/J011002/1 to P.M. and M.M., CNPQ grant 457914/2013‐0/MCTI/CNPq/FNDCT/LBA/ESECAFLOR to A.C.L.d.C., an ARC grant FT110100457 to P.M. It was previously supported by NERC NER/A/S/2002/00487, NERC GR3/11706, EU FP5‐Carbonsink and EU FP7‐Amazalert to P.M. L.R. would also like to acknowledge the support of Dr Robert Clement, University of Edinburgh and Dr Timothy Hill, University of Exeter, alongside the contribution of three anonymous reviewers.
References
- Angert A, Muhr J, Negron Juarez R, Alegria Muñoz W, Kraemer G, Ramirez Santillan J, Barkan E, Mazeh S, Chambers JQ, Trumbore SE. 2012. Internal respiration of Amazon tree stems greatly exceeds external CO2 efflux. Biogeosciences 9: 4979–4991. [Google Scholar]
- Atkin OK, Macherel D. 2009. The crucial role of plant mitochondria in orchestrating drought tolerance. Annals of Botany 103: 581–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubrey DP, Teskey RO. 2009. Root‐derived CO2 efflux via xylem stream rivals soil CO2 efflux. New Phytologist 184: 35–40. [DOI] [PubMed] [Google Scholar]
- Ayub G, Smith RA, Tissue DT, Atkin OK. 2011. Impacts of drought on leaf respiration in darkness and light in Eucalyptus saligna exposed to industrial‐age atmospheric CO2 and growth temperature. New Phytologist 190: 1003–1018. [DOI] [PubMed] [Google Scholar]
- Bennett AC, McDowell NG, Allen CD, Anderson‐Teixeira KJ. 2015. Larger trees suffer most during drought in forests worldwide. Nature Plants 1: Article number: 15139. [DOI] [PubMed] [Google Scholar]
- Berveiller D, Damesin C. 2008. Carbon assimilation by tree stems: potential involvement of phosphoenolpyruvate carboxylase. Trees ‐ Structure and Function 22: 149–157. [Google Scholar]
- Boisier JP, Ciais P, Ducharne A, Guimberteau M. 2015. Projected strengthening of Amazonian dry season by constrained climate model simulations. Nature Climate Change 5: 656–660. [Google Scholar]
- Brienen RJW, Phillips OL, Feldpausch TR, Gloor E, Baker TR, Lloyd J, Lopez‐Gonzalez G, Monteagudo‐Mendoza A, Malhi Y, Lewis SL et al 2015. Long‐term decline of the Amazon carbon sink. Nature 519: 344–348. [DOI] [PubMed] [Google Scholar]
- Brodersen CR, McElrone AJ. 2013. Maintenance of xylem network transport capacity: a review of embolism repair in vascular plants. Frontiers in Plant Science 4: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calders K, Newnham G, Burt A, Murphy S, Raumonen P, Herold M, Culvenor D, Avitabile V, Disney M, Armston J et al 2015. Nondestructive estimates of above‐ground biomass using terrestrial laser scanning. Methods in Ecology and Evolution 6: 198–208. [Google Scholar]
- Catoni R, Gratani L. 2014. Variations in leaf respiration and photosynthesis ratio in response to air temperature and water availability among Mediterranean evergreen species. Journal of Arid Environments 102: 82–88. [Google Scholar]
- Cavaleri MA, Oberbauer SF, Ryan MG. 2006. Wood CO2 efflux in a primary tropical rain forest. Global Change Biology 12: 2442–2458. [Google Scholar]
- Chambers JQ, Tribuzy ES, Toledo LC, Crispim BF, Higuchi N, dos Santos J, Araujo AC, Kruijt B, Nobre AD, Trumbore SE. 2004. Respiration from a tropical forest ecosystem: partitioning of sources and low carbon use efficiency. Ecological Applications 14: S72–S88. [Google Scholar]
- Chastain DR, Snider JL, Collins GD, Perry CD, Whitaker J, Byrd SA. 2014. Water deficit in field‐grown Gossypium hirsutum primarily limits net photosynthesis by decreasing stomatal conductance, increasing photorespiration, and increasing the ratio of dark respiration to gross photosynthesis. Journal of Plant Physiology 171: 1576–1585. [DOI] [PubMed] [Google Scholar]
- Cherubini P, Schweingruber FH, Forster T. 1997. Morphology and ecological significance of intra‐annual radial cracks in living conifers. Trees 11: 216–222. [Google Scholar]
- da Costa ACL, Galbraith D, Almeida S, Portela BTT, da Costa M, Silva JD, Braga AP, de Goncalves PHL, de Oliveira AAR, Fisher R et al 2010. Effect of 7 yr of experimental drought on vegetation dynamics and biomass storage of an eastern Amazonian rainforest. New Phytologist 187: 579–591. [DOI] [PubMed] [Google Scholar]
- Damesin C, Ceschia E, Le Goff N, Ottorini JM, Dufrene E. 2002. Stem and branch respiration of beech: from tree measurements to estimations at the stand level. New Phytologist 153: 159–172. [Google Scholar]
- Davidson EA, de Araujo AC, Artaxo P, Balch JK, Brown IF, Bustamante MMC, Coe MT, DeFries RS, Keller M, Longo M et al 2012. The Amazon basin in transition. Nature 481: 321–328. [DOI] [PubMed] [Google Scholar]
- Doughty CE, Metcalfe DB, Girardin CAJ, Amezquita FF, Cabrera DG, Huasco WH, Silva‐Espejo JE, Araujo‐Murakami A, da Costa MC, Rocha W et al 2015. Drought impact on forest carbon dynamics and fluxes in Amazonia. Nature 519: 78–82. [DOI] [PubMed] [Google Scholar]
- Duffy PB, Brando P, Asner GP, Field CB. 2015. Projections of future meteorological drought and wet periods in the Amazon. Proceedings of the National Academy of Sciences, USA 112: 13172–13177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu R, Yin L, Li WH, Arias PA, Dickinson RE, Huang L, Chakraborty S, Fernandes K, Liebmann B, Fisher R et al 2013. Increased dry‐season length over southern Amazonia in recent decades and its implication for future climate projection. Proceedings of the National Academy of Sciences, USA 110: 18110–18115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilman B, Angert A. 2016. Measuring the ratio of CO2 efflux to O2 influx in tree stem respiration. Tree Physiology 36: 1422–1431. [DOI] [PubMed] [Google Scholar]
- Katayama A, Kume T, Komatsu H, Ohashi M, Matsumoto K, Ichihashi R, Kumagai T, Otsuki K. 2014. Vertical variations in wood CO2 efflux for live emergent trees in a Bornean tropical rainforest. Tree Physiology 34: 503–512. [DOI] [PubMed] [Google Scholar]
- Katayama A, Kume T, Ohashi M, Matsumoto K, Nakagawa M, Saito T, Kumagai To, Otsuki K. 2016. Characteristics of wood CO2 efflux in a Bornean tropical rainforest. Agricultural and Forest Meteorology 220: 190–199. [Google Scholar]
- Korner C. 2015. Paradigm shift in plant growth control. Current Opinion in Plant Biology 25: 107–114. [DOI] [PubMed] [Google Scholar]
- Lenton TM. 2011. Early warning of climate tipping points. Nature Climate Change 1: 201–209. [Google Scholar]
- Levy PE, Jarvis PG. 1998. Stem CO2 fluxes in two Sahelian shrub species (Guiera senegalensis and Combretum micranthum). Functional Ecology 12: 107–116. [Google Scholar]
- Levy PE, Meir P, Allen SJ, Jarvis PG. 1999. The effect of aqueous transport of CO2 in xylem sap on gas exchange in woody plants. Tree Physiology 19: 53–58. [DOI] [PubMed] [Google Scholar]
- Malhi Y, Aragão LEOC, Metcalfe DB, Paiva R, Quesada CA, Almeida S, Anderson L, Brando P, Chambers JQ, da Costa ACL et al 2009. Comprehensive assessment of carbon productivity, allocation and storage in three Amazonian forests. Global Change Biology 15: 1255–1274. [Google Scholar]
- Malhi Y, Farfán Amézquita F, Doughty CE, Silva‐Espejo JE, Girardin CAJ, Metcalfe DB, Aragão LEOC, Huaraca‐Quispe LP, Alzamora‐Taype I, Eguiluz‐Mora L et al 2013. The productivity, metabolism and carbon cycle of two lowland tropical forest plots in south‐western Amazonia, Peru. Plant Ecology & Diversity 7: 85–105. [Google Scholar]
- McCree KJ. 1970. An equation for the rate of dark respiration of white cover plants grown under controlled conditions In: Setlik I, ed. Prediction and measurement of photosynthetic productivity. Wageningen, the Netherlands: Pudoc, 221–229. [Google Scholar]
- McDowell NG, Allen CD. 2015. Darcy's law predicts widespread forest mortality under climate warming. Nature Climate Change 5: 669–672. [Google Scholar]
- McGuire MA, Cerasoli S, Teskey RO. 2007. CO2 fluxes and respiration of branch segments of sycamore (Platanus occidentalis L.) examined at different sap velocities, branch diameters, and temperatures. Journal of Experimental Botany 58: 2159–2168. [DOI] [PubMed] [Google Scholar]
- Meir P, Grace J. 2002. Scaling relationships for woody tissue respiration in two tropical rain forests. Plant, Cell & Environment 25: 963–973. [Google Scholar]
- Meir P, Metcalfe DB, da Costa ACL, Fisher RA. 2008. The fate of assimilated carbon during drought: impacts on respiration in Amazon rain forests. Philosophical Transactions of the Royal Society B 363: 1849–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meir P, Shenkin A, Disney M, Rowland L, Malhi Y, Herold M, da Costa ACL. 2017. Plant structure‐function relationships and woody tissue respiration: upscaling to forests from laser‐derived measurements In: Ghashghaie J, Tcherkez G, eds. Plant respiration: metabolic fluxes and carbon balance. In: Govindjee, Sharkey TD, eds. Series: Advances in Photosynthesis and Respiration Dordrecht, the Netherlands: Springer; Series 43, 91–108. [Google Scholar]
- Meir P, Wood TE, Galbraith DR, Brando PM, Da Costa ACL, Rowland L, Ferreira LV. 2015. Threshold responses to soil moisture deficit by trees and soil in tropical rain forests: insights from field experiments. BioScience 65: 882–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mencuccini M, Hölttä T. 2010. The significance of phloem transport for the speed with which canopy photosynthesis and belowground respiration are linked. New Phytologist 185: 189–203. [DOI] [PubMed] [Google Scholar]
- Metcalfe DB, Lobo‐do‐Vale R, Chaves MM, Maroco JP, Aragao LEOC, Malhi Y, Da Costa AL, Braga AP, Goncalves PL, De Athaydes J et al 2010. Impacts of experimentally imposed drought on leaf respiration and morphology in an Amazon rain forest. Functional Ecology 24: 524–533. [Google Scholar]
- Metcalfe DB, Meir P, Aragão LEO, Malhi Y, da Costa ACL, Braga A, Gonçalves PHL, Athaydes J, Almeida SS, Williams M. 2007. Factors controlling spatio‐temporal variation in carbon dioxide efflux from surface litter, roots and soil organic matter at four rain forest sites in the eastern Amazon. Journal of Geophysical Research Biogeosciences 112: G04001. [Google Scholar]
- Miranda EJ, Vourlitis GL, Priante N, Priante PC, Campelo JH, Suli GS, Fritzen CL, Lobo FDA, Shiraiwa S. 2005. Seasonal variation in the leaf gas exchange of tropical forest trees in the rain forest‐savanna transition of the southern Amazon Basin. Journal of Tropical Ecology 21: 451–460. [Google Scholar]
- O'Brien MJ, Burslem DFRP, Caduff A, Tay J, Hector A. 2015. Contrasting nonstructural carbohydrate dynamics of tropical tree seedlings under water deficit and variability. New Phytologist 205: 1083–1094. [DOI] [PubMed] [Google Scholar]
- R Core Team . 2017. R: a language and environment for statistical computing. Version 3.4.0. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Rayment MB, Jarvis PG. 2001. Photosynthesis and respiration of black spruce at three organisational scales. shoot, branch and canopy. Tree Physiology 22: 219–229. [DOI] [PubMed] [Google Scholar]
- Robertson AL, Malhi Y, Farfan‐Amezquita F, Aragão LEOC, Silva Espejo JE, Robertson MA. 2010. Stem respiration in tropical forests along an elevation gradient in the Amazon and Andes. Global Change Biology 16: 3193–3204. [Google Scholar]
- Rodríguez‐Calcerrada J, Martin‐StPaul NK, Lempereur M, Ourcival J‐M, Rey MdCd, Joffre R, Rambal S. 2014. Stem CO2 efflux and its contribution to ecosystem CO2 efflux decrease with drought in a Mediterranean forest stand. Agricultural and Forest Meteorology, 195–196: 61–72. [Google Scholar]
- Rowland L, da Costa ACL, Galbraith DR, Oliveira RS, Binks OJ, Oliveira AAR, Pullen AM, Doughty CE, Metcalfe DB, Vasconcelos SS et al 2015a. Death from drought in tropical forests is triggered by hydraulics not carbon starvation. Nature 528: 119–122. [DOI] [PubMed] [Google Scholar]
- Rowland L, Hill TC, Stahl C, Siebicke L, Burban B, Zaragoza‐Castells J, Ponton S, Bonal D, Meir P, Williams M. 2014. Evidence for strong seasonality in the carbon storage and carbon use efficiency of an Amazonian forest. Global Change Biology 20: 979–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland L, Lobo‐do‐Vale RL, Christoffersen BO, Melem EA, Kruijt B, Vasconcelos SS, Domingues T, Binks OJ, Oliveira AAR, Metcalfe D et al 2015b. After more than a decade of soil moisture deficit, tropical rainforest trees maintain photosynthetic capacity, despite increased leaf respiration. Global Change Biology 21: 4662–4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland L, Stahl C, Bonal D, Siebicke L, Williams M, Meir P. 2013. The response of tropical rainforest dead wood respiration to seasonal drought. Ecosystems 16: 1294–1309. [Google Scholar]
- Ruivo M, Cunha E. 2003. Mineral and organic components in archaeological black earth and yellow latosol in Caxiuanã, Amazon, Brazil In: Tiezzi E, Brebbia CA, Uso JL, eds. Ecosystems and sustainable development. Southampton, UK: WIT Press, 1113–1121. [Google Scholar]
- Ryan MG. 1990. Growth and maintenance respiration in stems of Pinus contorta and Picea engelmannii . Canadian Journal of Forest Research–Revue Canadienne De Recherche Forestiere 20: 48–57. [Google Scholar]
- Ryan MG, Hubbard RM, Clark DA, Sanford RL. 1994. Woody‐tissue respiration for Simarouba amara and Minquartia guianensis, two tropical wet forest trees with different growth habits. Oecologia 100: 213–220. [DOI] [PubMed] [Google Scholar]
- Saveyn A, Steppe K, Lemeur R. 2007. Drought and the diurnal patterns of stem CO2 efflux and xylem CO2 concentration in young oak (Quercus robur). Tree Physiology 27: 365–374. [DOI] [PubMed] [Google Scholar]
- Saveyn A, Steppe K, McGuire MA, Lemeur R, Teskey RO. 2008. Stem respiration and carbon dioxide efflux of young Populus deltoides trees in relation to temperature and xylem carbon dioxide concentration. Oecologia 154: 637–649. [DOI] [PubMed] [Google Scholar]
- Sorz J, Hietz P. 2006. Gas diffusion through wood: implications for oxygen supply. Trees ‐ Structure and Function 20: 34–41. [Google Scholar]
- Stahl C, Burban B, Goret J‐Y, Bonal D. 2011. Seasonal variations in stem CO2 efflux in the Neotropical rainforest of French Guiana. Annals of Forest Science 68: 771–782. [Google Scholar]
- Teskey RO, Saveyn A, Steppe K, McGuire MA. 2008. Origin, fate and significance of CO2 in tree stems. New Phytologist 177: 17–32. [DOI] [PubMed] [Google Scholar]
- Thornley JHM. 1970. Respiration, growth, and maintenance in plants. Nature 227: 304–305. [DOI] [PubMed] [Google Scholar]
- Trumbore SE, Angert A, Kunert N, Muhr J, Chambers JQ. 2013. What's the flux? Unraveling how CO2 fluxes from trees reflect underlying physiological processes. New Phytologist 197: 353–355. [DOI] [PubMed] [Google Scholar]
- Ubierna N, Kumar AS, Cernusak LA, Pangle RE, Gag PJ, Marshall JD. 2009. Storage and transpiration have negligible effects on δ13C of stem CO2 efflux in large conifer trees. Tree Physiology 29: 1563–1574. [DOI] [PubMed] [Google Scholar]
- Varone L, Gratani L. 2015. Leaf respiration responsiveness to induced water stress in Mediterranean species. Environmental and Experimental Botany 109: 141–150. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: Wiley Blackwell are not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Fig. S1 Diurnal variation of stem CO2 efflux from trees on the control and TFE plots.
Fig. S2 Relationships between sapwood depth and tree diameter and basal area.
Table S1 List of the tree diameter and species of all trees sampled in this study
Table S2 Distribution of trees across size classes for all trees >10 cm diameter at 1.3 m above ground on the control and TFE plots
Methods S1 Additional methods relating to the measurement and scaling of stem CO2 efflux data.


