Abstract
Aims
To assess variability in systolic blood pressure (SBP) and albuminuria (urinary albumin creatinine ratio [UACR]) responses in patients with type 2 diabetes mellitus initiating renin angiotensin aldosterone system (RAAS) inhibition, and to assess the association of response variability with cardiovascular outcomes.
Material and Methods
We performed an observational cohort study in patients with type 2 diabetes who started RAAS inhibition between 2007 and 2013 (n = 1600). Patients were identified from general practices in the Netherlands. Individual response in SBP and UACR was assessed during 15 months’ follow‐up. Patients were categorized as: good responders (∆SBP <0 mm Hg and ∆UACR <0%); intermediate responders (∆SBP <0 mm Hg and ∆UACR >0% or ∆SBP >0 mm Hg and ∆UACR <0%); or poor responders (∆SBP >0 mm Hg and ∆UACR >0%). Multivariable Cox regression was performed to test the association between initial RAAS inhibition response and subsequent cardiovascular outcomes.
Results
After starting RAAS inhibition, the mean SBP change was −13.2 mm Hg and the median UACR was −36.6%, with large between‐individual variability, both in SBP [5th to 95th percentile: 48.5‐20] and UACR [5th to 95th percentile: −87.6 to 171.4]. In all, 812 patients (51%) were good responders, 353 (22%) had a good SBP but poor UACR response, 268 (17%) had a good UACR but poor SBP response, and 167 patients (10%) were poor responders. Good responders had a lower risk of cardiovascular events than poor responders (hazard ratio 0.51, 95% confidence interval 0.30‐0.86; P = .012).
Conclusions
SBP and UACR response after RAAS inhibition initiation varied between and within individual patients with type 2 diabetes treated in primary care. Poor responders had the highest risk of cardiovascular events, therefore, more efforts are needed to develop personalized treatment plans for these patients.
Keywords: primary care, RAAS inhibition, type 2 diabetes mellitus, variability in response
1. INTRODUCTION
The cornerstone of treatment to reduce cardiovascular and kidney complications in patients with type 2 diabetes mellitus is blockade of the renin angiotensin aldosterone system (RAAS), typically with angiotensin‐converting enzyme (ACE) inhibitors or angiotensin II receptor blockers (ARBs). In accordance with clinical treatment guidelines, RAAS inhibitors are started with a focus on blood pressure reduction and not necessarily albuminuria reduction1; however, reductions in albuminuria during RAAS inhibitor treatment have been shown to be independent predictors of cardiovascular outcomes.2, 3, 4
Parallel reductions in both blood pressure and albuminuria are suggested to confer the most cardioprotection,5 but patients can also have a systolic blood pressure (SBP) reduction without a simultaneous reduction in albuminuria or vice versa.6, 7, 8 While this discordance in response to RAAS inhibition has been documented in clinical trials, it is not known whether it carries over to the population treated in clinical practice or what the effect of this individual variability in response is on cardiovascular risk. Gaining insight into these responses may help us understand why some patients in clinical practice do not benefit from guideline‐recommended therapy.
The aims of the present study, therefore, were to investigate the variability in response with regard to SBP and albuminuria after RAAS inhibition initiation in primary care patients with type 2 diabetes and to assess whether the differences in response affected cardiovascular outcomes.
2. MATERIALS AND METHODS
2.1. Study design and population
We conducted a cohort study among primary care patients with type 2 diabetes enrolled in the Groningen Initiative to Analyse Type 2 diabetes Treatment (GIANTT) cohort to assess variability in SBP and urinary albumin creatinine ratio (UACR) response to RAAS inhibition, as well as the occurrence of cardiovascular events. Only patients who had both an SBP and a UACR measurement before and after RAAS inhibition were included in the analysis. Patients were included if they initiated guideline‐recommended RAAS inhibition treatment between January 1, 2007 and July 31, 2013. Initiation with direct renin inhibitors or mineralocorticoid receptor antagonists was not included, because these are not recommended for treatment of patients with type 2 diabetes with hypertension and/or microalbuminuria. The starting date of the first RAAS inhibition prescription was defined as the index date. The follow‐up period for response in SBP and UACR was set between 1 and 15 months after the index date. Patients were followed for cardiovascular events until they left the GIANTT cohort or until the last data extraction from GIANTT, whichever came first.
The following patient exclusion criteria were applied: a RAAS inhibitor prescription <730 days before the index date; insufficient medical record history to assess previous RAAS inhibitor prescription; concurrent initiation of other antihypertensive agents at index date; diagnosis with type 2 diabetes >365 days after the index date; a follow‐up period of <1.5 years; incomplete prescription data during follow‐up; insufficient medication adherence to RAAS inhibition, defined as a medication refill adherence of <75% in the first 15 months and/or a continuous measurement of gaps of >0.25 over the total follow‐up period9, 10, 11; no UACR or UACR measurement below detection limit (<0.26 mg/mmol) and/or no SBP measurement within 15 months before the index date; no UACR and/or SBP measurement between 1 and 15 months after the index date; and >365 days difference between the UACR and SBP measurements after the index date (Figure 1). Medication refill adherence was calculated as the number of days’ supply in the study period divided by the total number of days in the study period multiplied by 100. Continuous measurement of gaps was the total days of treatment gaps divided by the total days in the study period.
Figure 1.
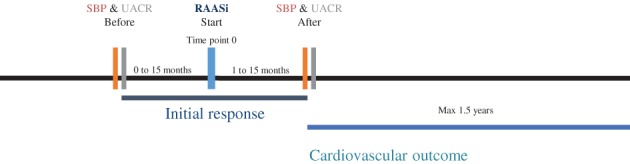
Timeline of study. RAASi, renin angiotensin aldosterone system inhibition; SBP, systolic blood pressure; UACR, urinary albumin creatinine ratio
2.2. Data collection and definitions
Data were retrieved from the GIANTT database, which contains anonymous longitudinal data collected from general practice electronic medical records of >50 000 patients with type 2 diabetes registered in ~200 general practices in the north of the Netherlands. These electronic medical records include all general practice prescriptions, patient demographics, physical examinations, laboratory measurements, diagnoses as recorded in routine practice and previous cardiovascular, cerebrovascular and peripherovascular events.12
The most recent SBP and UACR measurements before or at the index date were taken as the baseline measurements. As the outcome measurements, we used the first UACR measurement between 1 and 15 months after the index date, and the SBP measurement closest in time to this UACR measurement. This time window was used to ensure that the RAAS inhibition treatment effect was fully present and because Dutch primary practice guidelines recommend annual UACR measurements. The response in SBP was calculated as the absolute difference in mm Hg, and the response in albuminuria was defined as the percent change in UACR between baseline and outcome measurement. Four response groups were formed using previously defined thresholds.7, 8 Patients were defined as good responders if there was a reduction in both SBP and UACR (change in SBP <0 mm Hg and change in UACR <0%). Patients were defined as intermediate responders if there was decrease in only one variable (only SBP reduction: change in SBP <0 mm Hg and change in UACR ≥0%; or only UACR reduction: change in SBP ≥0 mm Hg and change in UACR <0%). If there was no decrease in SBP nor UACR, patients were defined as poor responders (change in SBP ≥0 mm Hg and change in UACR ≥0%). Variability within a patient and between patients was investigated. Within‐patient variability was defined as the patient not having a similar response to the medication in both SBP and UACR. Between‐patient variability was defined as the difference in SBP response between patients and the difference in UACR response between patients.
Differences in cardiovascular outcome between response groups were investigated using a composite of the following outcomes: occurrence of ischaemic heart disease or myocardial infarction >4 weeks previously, and percutaneous transluminal coronary angioplasty, coronary artery bypass surgery, and cerebrovascular outcome including transient ischaemic attack, or cerebrovascular accident, as documented in the medical records. Mortality data were not available for this analysis.
2.3. Statistical analysis
Descriptive data are presented as mean (SD), median (25th to 75th percentile) for skewed variables, or number (%) for proportions. Histograms and scatterplots were constructed to detect outliers. The natural logarithm of UACR was used for all analyses to account for its non‐normal distribution. Pearson's correlation between SBP and UACR response was tested. Patient characteristics were compared among the RAAS inhibition response groups using 1‐way ANOVA or the χ2 test as appropriate. For pairwise comparisons among 4 groups, 2‐tailed Bonferroni corrected P values <.01 were considered significant. In addition, stratified analyses were performed to assess the influence of covariates on the distribution in response groups. This included analyses according to: (1) initiation on an ACE inhibitor or an ARB; (2) defined daily doses <1 or ≥1 daily defined doses of the initial prescription; (3) baseline estimated glomerular filtration rate (eGFR) <60 or ≥60 mL/min/1.73 m2; (4) baseline albuminuria (UACR <3.5 or ≥3.5 mg/mmol); (5) baseline SBP level (SBP <140 or ≥140 mm Hg); and (6) time between baseline and outcome measurement (<1 year or ≥1 year).
A Cox proportional hazards regression analysis was performed to assess the association between response groups and cardiovascular outcomes, adjusting for sex, baseline age, SBP, UACR, glycated haemoglobin, eGFR and cardiovascular and peripheral vascular morbidity. For patients who experienced >1 event during follow‐up, time to the first event was used for analysis. Two‐tailed P values <.05 were considered significant. Sensitivity analyses were performed including only patients with a baseline UACR ≥3.5 mg/mmol, only patients with a baseline SBP ≥140 mm Hg, and with UACR response defined as a >30% instead of >0% decrease. All analyses were performed with stata version 13. No imputation of missing data was performed because data were missing in <5% of the included patients.
3. RESULTS
A total of 1600 patients with type 2 diabetes initiating RAAS inhibition treatment were included from the overall GIANTT cohort (Figure 2). The patients’ mean (±SD) age was 64.9 (±10.9) years and 56.4% were male (Table 1). The mean (±SD) baseline SBP was 157.1 (±20.7) mm Hg. The median (25th to 75th percentile) baseline UACR was 1.6 (0.8‐4.1) mg/mmol. When comparing characteristics of included patients (n = 1600) with all patients who initiated RAAS inhibition treatment in this cohort (n = 7755), baseline characteristics were essentially similar (Table S1).
Figure 2.
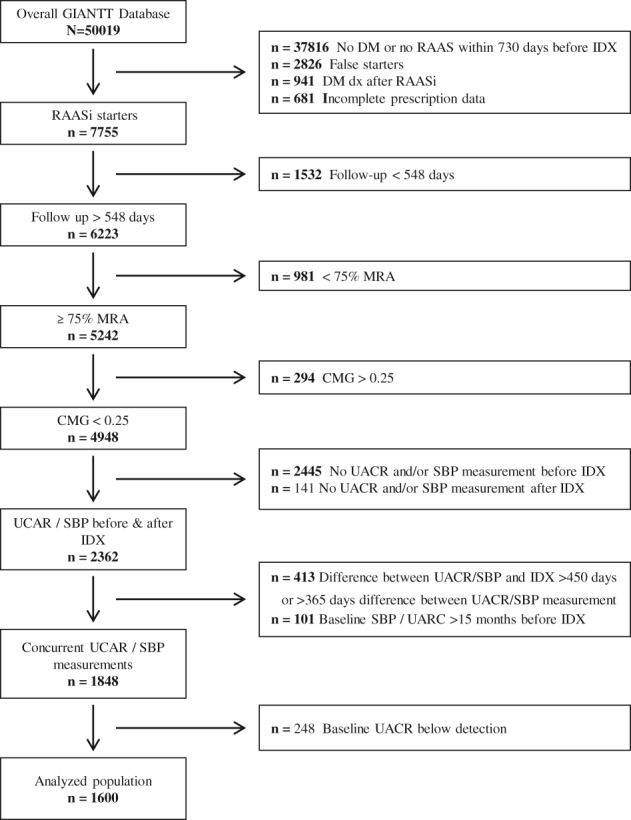
Selection of analysed population. GIANTT, Groningen Initiative to Analyse Type 2 diabetes Treatment; RAASi, renin angiotensin aldosterone system inhibition; SBP, systolic blood pressure; UACR, urinary albumin creatinine ratio
Table 1.
Patient characteristics by response groups
| Good responders (n = 812) | SBP only (n = 353) | UACR only (n = 268) | Poor responders (n = 167) | ||
|---|---|---|---|---|---|
| Total analysed populationN = 1600 | ΔUACR <0% ΔSBP <0 mm Hg | ΔUACR ≥0% ΔSBP <0 mm Hg | ΔUACR <0% ΔSBP ≥0 mm Hg | ΔUACR ≥0% ΔSBP ≥0 mm Hg | |
| Age, years | 64.9 ± 10.9 | 64.3 ± 10.7 | 64.7 ± 11.3 | 66.1 ± 11.0 | 66.3 ± 10.1 |
| Men, n (%) | 903 (56.4) | 446 (54.9) | 196 (55.5) | 157 (58.6) | 104 (62.3) |
| HbA1c, mmol/mol | 52.1 ± 11.3 | 52.6 ± 12.5 | 51.6 ± 10.3 | 52.1 ± 10.3 | 51.0 ± 8.36 |
| SBP, mm Hg | 157.1 ± 20.7 | 161.9 ± 19.6ab | 162.8 ± 18.6ce | 143.0 ± 17.8ae | 144.4 ± 18.9bc |
| DBP, mm Hg | 85.8 ± 11.0 | 87.7 ± 10.8ab | 87.1 ± 10.3ce | 81.2 ± 10.9ae | 81.1 ± 10.4bc |
| UACR, mg/mmol | 1.6 [0.8‐4.1] | 1.8 [0.9‐4.8]abd | 0.9 [0.5‐2.1]cde | 2.7 [1.2‐7.3]aef | 1.2 [0.6‐3.4]bcf |
| Normoalbuminuria, n (%) | 1141 (71.3) | 560 (69.0) | 297 (84.1) | 158 (59.0) | 126 (75.4) |
| Microalbuminuria, n (%) | 390 (24.4) | 211 (26.0) | 52 (14.7) | 91 (33.9) | 36 (21.6) |
| Macroalbuminuria, n (%) | 69 (4.3) | 41 (5.0) | 4 (1.1) | 19 (7.1) | 5 (3.0) |
| eGFR, mL/min/1.73 m2 | 78.5 ± 18.3 | 79.2 ± 17.9 | 78.5 ± 18.5 | 77.4 ± 18.8 | 76.8 ± 18.6 |
| Total cholesterol, mmol/L | 4.6 ± 1.1 | 4.6 ± 1.1 | 4.5 ± 1.1 | 4.6 ± 1.0 | 4.5 ± 1.1 |
| HDL cholesterol, mmol/L | 1.2 ± 0.3 | 1.2 ± 0.3 | 1.2 ± 0.4 | 1.2 ± 0.3 | 1.2 ± 0.3 |
| BMI, kg/m2 | 30.1 ± 5.5 | 30.3 ± 5.7 | 29.9 ± 5.6 | 29.4 ± 4.6 | 30.1 ± 5.5 |
| ACE inhibitor treatment, n (%) | 1307 (81.7) | 664 (81.8) | 289 (81.9) | 223 (83.2) | 131 (78.4) |
| ARB treatment, n (%) | 293 (18.3) | 148 (18.2) | 64 (18.1) | 45 (16.8) | 36 (21.6) |
| Cardiovascular morbidity, n (%) | 252 (15.8) | 99 (12.2)ab | 52 (14.7)ce | 64 (23.9)ae | 37 (22.2)bc |
| Peripheral vascular morbidity, n (%) | 232 (14.5) | 113 (13.9)a | 42 (11.9)e | 53 (19.8)ae | 24 (14.4) |
| Nephropathy, n (%) | 71 (4.4) | 38 (4.7) | 11 (3.1) | 14 (5.2) | 8 (4.8) |
| Retinopathy, n (%) | 44 (2.8) | 24 (3.0) | 12 (3.4) | 7 (2.6) | 1 (0.6) |
| Diabetes duration, years | 5.0 ± 4.9 | 4.9 ± 5.0 | 5.0 ± 4.6 | 5.3 ± 5.2 | 5.3 ± 4.6 |
Abbreviations: ACE, angiotensin‐converting enzyme; ARB, angiotensin II receptor blocker; BMI, body mass index; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HbA1c, glycated haemoglobin; SBP, systolic blood pressure; UACR, urinary albumin creatinine ratio.
P < .01 with Bonferroni correction between: aonly UACR and good, bpoor and good, cpoor and only SBP, donly SBP and good, eonly SBP and only UACR, fpoor and only UACR.
3.1. Response and response groups
The mean SBP decrease after a median of 6.7 months after RAAS inhibition initiation was −13.2 mm Hg and the median UACR reduction during the same time interval after RAAS inhibition initiation was −36.6%. There was a large between‐individual variability in response in both SBP (5th to 95th percentile −48.5 to 20) and UACR (5th to 95th percentile −87.6 to 171.4). With regard to within‐individual SBP and UACR responses, a response in both SBP and UACR (good responders) was observed in 51% of patients, while 10% had no reduction in both SBP and UACR (Table 2). A total of 22.1% patients had a reduction in SBP without UACR reduction, and 16.8% of patients had a reduction in UACR without SBP reduction (Table 2). SBP response showed a weak correlation (r = 0.06) with UACR response (Figure 3).
Table 2.
Distribution of patients according to blood pressure and albuminuria response
| ΔUACR | ΔSBP | <−15 mm Hg n (%) | −15 to 0 mm Hg n (%) | Total (%) | 0 to 15 mm Hg n (%) | ≥15 mm Hg n (%) | Total (%) |
|---|---|---|---|---|---|---|
| >30% decrease | 373 (45.9) | 274 (33.7) | 647 | 136 (50.7) | 42 (15.7) | 178 |
| 30% to 0% decrease | 95 (11.7) | 70 (8.6) | 165 | 62 (23.1) | 28 (10.4) | 90 |
| Total (%) | 468 (57.6) | 344 (42.4) | 812 (50.8) | 198 (73.9) | 70 (26.1) | 268 (16.8) |
| 0 to 30% increase | 77 (21.8) | 46 (13.0) | 123 | 38 (22.7) | 17 (10.2) | 55 |
| ≥ 30% increase | 138 (39.1) | 92 (26.1) | 230 | 74 (44.3) | 38 (22.8) | 112 |
| Total (%) | 215 (60.9) | 138 (39.1) | 353 (22.1) | 112 (67.1) | 55 (32.9) | 167 (10.4) |
Abbreviations: SBP, systolic blood pressure; UACR, urinary albumin creatinine ratio.
ΔUACR: difference in UACR between before and after renin angiotensin aldosterone system (RAAS) inhibition measurement; ΔSBP: difference in SBP between before and after RAAS inhibition measurement.
Figure 3.
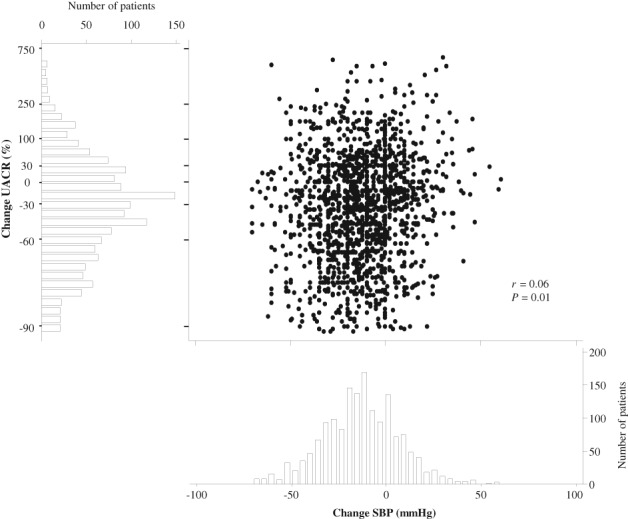
Scatterplot: Correlation between UACR change and systolic blood pressure (SBP) change before and after start of renin angiotensin aldosterone system inhibition. Histograms: Distribution of patients according to urinary albumin creatinine ratio (UACR) change (left) and SBP change (bottom)
Good responders and SBP‐only responders had significantly higher blood pressure compared with UACR‐only and poor responders at baseline (Table 1). Good responders and UACR‐only responders had higher albuminuria at baseline compared with SBP‐only and poor responders (Table 1). More poor responders and UACR‐only responders had a history of cardiovascular and peripheral vascular morbidity. The stratified analysis also showed that the proportion of good responders in both SBP and UACR was higher in patients with a baseline SBP ≥140 mm Hg and baseline UACR ≥3.5 mg/mmol, respectively (Table S2). The distribution in response groups remained similar when stratified for ACE inhibitor or ARB use, dose <1 or ≥1 defined daily doses, eGFR <60 or ≥60 mL/min/1.73 m2, and time between measurement <1 year or ≥1 year (Table S2).
3.2. Cardiovascular outcomes
During a median (25th to 75th percentile) follow‐up time of 4.4 (3.2‐5.7) years, 128 cardiovascular events occurred. After adjustment for multiple cardiovascular risk markers, being a good responder to both SBP and UACR was associated with a significantly lower risk of cardiovascular events compared with being a poor responder in both variables (adjusted hazard ratio [HR] 0.51, 95% CI 0.30 to 0.86; P = .012 [Table 3]). SBP‐only responders also had significantly lower risk of cardiovascular events compared with poor responders (adjusted HR 0.48, 95% CI 0.26‐0.88; P = .018 [Table 3]).
Table 3.
Association between response groups and cardiovascular events (N = 1600)
| Unadjusted | Adjusteda | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Response group | ΔSBP, mm Hg Mean (SD) | ΔUACR (%) Median (25th to 75th) | Event n (%) | HR | 95% CI | P | HR | 95% CI | P | |
|
Poor responders
(n = 167) |
SBP ≥ 0 mm Hg / UACR ≥ 0% | 12.1 (12.4) | 50.0 (20‐125) | 23 (13.8) | ref | ref | ref | ref | ref | ref |
|
SBP‐only responders
(n = 353) |
SBP < 0 mm Hg / UACR ≥ 0% | −22.7 (14.9) | 46.9 (18‐127) | 21 (6.0) | 0.42 | 0.23–0.76 | 0.004 | 0.48 | 0.26–0.88 | 0.018 |
|
UACR‐only responders
(n = 268) |
SBP ≥ 0 mm Hg / UACR < 0% | 10.0 (10.0) | −47.4 (−74 to −23) | 30 (11.9) | 0.82 | 0.47–1.41 | 0.46 | 0.64 | 0.36–1.13 | 0.121 |
|
Good responders
(n = 812) |
SBP < 0 mm Hg / UACR < 0% | −21.9 (15.4) | −56.7 (−75 to −35) | 54 (6.7) | 0.48 | 0.30–0.78 | 0.003 | 0.51 | 0.30–0.86 | 0.012 |
Abbreviations: HR, hazard ratio; SBP, systolic blood pressure; UACR, urinary albumin creatinine ratio.
Adjusted for sex, baseline age, SBP, logUACR, glycated haemoglobin, diastolic blood pressure, estimated glomerular filtration rate, and cardiovascular and peripherovascular morbidity.
Sensitivity analysis including only patients with a baseline SBP ≥140 mm Hg, showed similar results (Table S3). Including only patients with a baseline UACR ≥3.5 mg/mmol, also showed that good responders had fewer cardiovascular events than poor responders (Table S3). Although the point estimate of the HR for the SBP‐only responders remained similar, the association lost significance because of the smaller number of included patients. Also no changes were seen regarding the HR for the UACR‐only responders (Table S3). Finally, a sensitivity analysis in which UACR response was defined as a reduction in UACR ≥30% had similar results to those of our main analysis (Table S3).
4. DISCUSSION
The present study showed that there was large between‐ and within‐patient variability in blood pressure and albuminuria response after RAAS inhibition initiation in patients with type 2 diabetes treated in primary care. In the present cohort, who mostly had elevated blood pressure levels and normoalbuminuria, a good response in both SBP and UACR was observed in 51% of patients, while 10% were poor responders. An intermediate response (response in either SBP or UACR) was observed in 39% of the patients. Despite having poorer baseline SBP and UACR levels, good responders and SBP‐only responders had a lower risk of cardiovascular outcomes compared with poor responders.
Clinical trial data have shown that there is large variability in response to RAAS inhibition, and that SBP and UACR response may not always run in parallel within a patient.7, 8 The present study is the first to show similar discordance between SBP and UACR in a “real‐world” population, which is highly relevant for clinical practice. Randomized controlled trials have included patients with nephropathy and microalbuminuria or worse, but in our “real‐life” cohort the majority of the patients with type 2 diabetes had normoalbuminuria. While SBP and UACR are independent predictors of cardiovascular and renal outcomes,2, 3, 4 a parallel good response in both SBP and UACR appears best for cardiovascular and renal protection.5 Our finding that 39% of patients respond in only one variable is similar to the results in the clinical trial populations, including the 39% in the RENAAL trial and 44% in the IRMA‐2 trial.7, 8 This indicates that the variability in response to RAAS inhibition seen in clinical trials is also relevant in primary care.
Previously, it has been suggested that differences in tissue penetration and RAAS activity could explain the difference in albuminuria and SBP response within individuals.5 The albuminuria response is thought to depend on intra‐renal RAAS inhibition, while the SBP response may depend on systemic vascular RAAS blockade.13 Aside from different pharmacological pathways, it is possible that there are other explanations for the observed response variation. One relevant finding was the difference in baseline variables and drug use between response groups. In particular, there were significant differences in baseline SBP and UACR, which could suggest that the observed responses were partly attributable to differences in room for improvement or possibly to regression to the mean. Our stratified analyses, however, showed that there were also substantial groups with good and intermediate response among patients with low SBP or low UACR baseline levels. Moreover, approximately 1 in 5 patients with high SBP or high UACR baseline levels showed no improvement in these respective measures, suggesting that other, as yet undetermined, factors could be involved.
When examining the associations between response groups and cardiovascular outcomes, we adjusted for baseline SBP and UACR levels, history of cardiovascular morbidity, as well as other possible confounders. These adjustments slightly attenuated the risk estimates but did not affect the finding that good responders for both SBP and UACR were at lower risk of cardiovascular events than poor responders. This finding suggests that SBP and UACR response should be monitored after ACE inhibitor or ARB initiation to predict cardiovascular risk. Accordingly, clinical practice guidelines recommend that blood pressure be monitored after initiation of RAAS inhibition. With respect to albuminuria however, primary practice guideline recommendations state that albuminuria response should not be monitored in adults with or without diabetes.14 According to the guidelines, the variability in albuminuria may introduce measurement error which may obscure the true variation in response to treatment.14 Indeed, day‐to‐day variation in SBP and UACR is present in particular when single measurements are used, as was the case in the present study. Despite this limitation, however, we were still able to detect a significant association between UACR and SBP response and cardiovascular risk, highlighting the importance of monitoring responses in both variables after RAAS inhibition initiation.
The present study has several strengths. To our knowledge, it is the first real‐life study in primary care assessing variability in response in SBP and UACR in patients with type 2 diabetes. We performed rigorous checks to ensure that only new users of RAAS inhibition treatment were included in the study in order to assess changes in SBP and UACR after RAAS inhibition. We tried to control for non‐adherence to RAAS inhibition treatment by excluding patients with low levels of refill adherence during the study period (<75% medication refill adherence or >0.25 continuous measurement of gaps). We do not know, however, whether the patients actually took the medication they refilled. Some between‐patient variation may be attributable to variation in medication‐taking behaviour. We also excluded patients who concurrently initiated other antihypertensive treatment. We adjusted our models for several possible confounders, and conducted stratified and sensitivity analyses to check for possible biases. In particular, we found no impact of difference in initial dose or response period; however, the observational design precludes any causal interpretations.
Limitations of the present study include the fact that a substantial number of patients were excluded because they did not have a UACR measurement before RAAS inhibition or because they had too short a follow‐up. This may have led to selection of patients with more advanced disease not representative of the general type 2 diabetes population treated in Dutch primary practice. However, there appeared to be no relevant differences in baseline characteristics between patients included for analysis and all patients who initiated RAAS inhibition in the GIANTT cohort. Furthermore, the included patients form a dynamic cohort with variation in time and follow‐up period. Because more intensive monitoring may occur in more diseased patients, this could result in a shorter initial response period for such patients. We therefore conducted a stratified analysis to see whether the response groups would differ between patients with a response period >1 and <1 year, which yielded similar results. Finally, the response thresholds were somewhat arbitrary. We therefore performed sensitivity analysis for UACR ≥30% reduction from baseline, and similar results were obtained. Unfortunately, we were unable to assess the impact of variability in response on mortality.
In conclusion, variability in blood pressure and albuminuria response after RAAS inhibition initiation occurs between and within patients with type 2 diabetes in primary care. Patients without improvement in SBP and UACR were found to have a higher risk of cardiovascular events than patients with improved SBP and UACR. Further research is necessary to identify why these patients with type 2 diabetes do not respond to RAAS inhibition, in order to develop personalized treatment plans.
Supporting information
Table S1. Patient characteristics analyzed population & RAAS inhibition starters.
Table S2. Stratified analyses by response groups.
Table S3. Sensitivity analysis including only patients with baseline UACR ≥3.5 mg/mmol or baseline SBP ≥ 140 mm Hg.
ACKNOWLEDGMENTS
H.J.L.H. is supported by a VIDI grant from the Netherlands Organization for Scientific Research (917.15.306). This project received funding from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement No 115974. This Joint Undertaking receives support from the European Union's Horizon 2020 research and innovation programme and EFPIA.
Conflict of interest
D.d.Z. has consultancy agreements with the following companies: Abbvie, Astellas, Bristol‐Meyers Squibb, Hemocue, Johnson & Johnson, Merck Sharpe & Dohme, Novartis, Reata Pharmaceuticals and Vitae. All honoraria are paid to his institution. H.J.L.H. has consultancy agreements with the following companies: Abbvie, Astellas, AstraZeneca, Boehringer Ingelheim, Fresenius, Janssen, Merck. He has a policy that all honoraria are paid to his institution. E.M.A., M.J.P. and P.D. report no conflicts.
Author contributions
E.M.A. and H.J.L.H. developed the study proposal and design. E.M.A. and M.J.P. are responsible for data analysis, interpretation, and manuscript preparation. H.J.L.H., D.d.Z. and P.D. contributed to data interpretation. All authors participated in the writing, review and approval of this manuscript.
Apperloo EM, Pena MJ, de Zeeuw D, Denig P, Heerspink HJL. Individual variability in response to renin angiotensin aldosterone system inhibition predicts cardiovascular outcome in patients with type 2 diabetes: A primary care cohort study. Diabetes Obes Metab. 2018;20:1377–1383. https://doi.org/10.1111/dom.13226
Funding information Innovative Medicines Initiative 2 Joint Undertaking, Grant/Award number: 115974; Nederlandse Organisatie voor Wetenschappelijk Onderzoek, Grant/Award number: 917.15.306
REFERENCES
- 1. Ilyas Z, Chaiban JT, Krikorian A. Novel insights into the pathophysiology and clinical aspects of diabetic nephropathy. Rev Endocrine Metab Disord. 2017;18(1):21‐28. [DOI] [PubMed] [Google Scholar]
- 2. De Zeeuw D, Remuzzi G, Parving HH, et al. Albuminuria, a therapeutic target for cardiovascular protection in type 2 diabetic patients with nephropathy. Circulation. 2004;110:921‐927. [DOI] [PubMed] [Google Scholar]
- 3. Heerspink HJL, Ninomiya T, Persson F, et al. Is a reduction in albuminuria associated with renal and cardiovascular protection? A post hoc analysis of the ALTITUDE trial. Diabetes Obes Metab. 2016;18(2):169‐177. [DOI] [PubMed] [Google Scholar]
- 4. Zandbergen A, Vogt L, de Zeeuw D, et al. Change in albuminuria is predictive of cardiovascular outcome in normotensive patients with type 2 diabetes and microalbuminuria. Diabetes Care. 2007;30(12):3119‐3121. [DOI] [PubMed] [Google Scholar]
- 5. Holtkamp FA, De Zeeuw D, De Graeff PA, et al. Albuminuria and blood pressure, independent targets for cardioprotective therapy in patients with diabetes and nephropathy: a post hoc analysis of the combined RENAAL and IDNT trials. Eur Heart J. 2011;32:1493‐1499. [DOI] [PubMed] [Google Scholar]
- 6. Laverman GD, de Zeeuw D, Navis G. Between‐patient differences in the renal response to renin‐angiotensin system intervention: clue to optimising renoprotective therapy? J Renin Angiotensin Aldosterone Syst. 2002;3:205‐213. [DOI] [PubMed] [Google Scholar]
- 7. Eijkelkamp WB, Zhang Z, Remuzzi G, et al. Albuminuria is a target for renoprotective therapy independent from blood pressure in patients with type 2 diabetic nephropathy: post hoc analysis from the Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (RENAAL) trial. J Am Soc Nephrol. 2007;18(5):1540‐1546. [DOI] [PubMed] [Google Scholar]
- 8. Hellemons ME, Persson F, Bakker SJL, et al. Initial angiotensin receptor blockade‐induced decrease in albuminuria is associated with long‐term renal outcome in type 2 diabetic patients with microalbuminuria: a post hoc analysis of the IRMA‐2 trial. Diabetes Care. 2011;34(9):2078‐2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Andrade SE, Kahler KH, Frech F, Chan KA. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf. 2006;15(8):565‐574. [DOI] [PubMed] [Google Scholar]
- 10. Karve S, Cleves MA, Helm M, Hudson TJ, West DS, Martin BC. An empirical basis for standardizing adherence measures derived from administrative claims data among diabetic patients. Med Care. 2008;46:1125‐1133. [DOI] [PubMed] [Google Scholar]
- 11. Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: methods, validity, and applications. J Clin Epidemiol. 1997;50(1):105‐116. [DOI] [PubMed] [Google Scholar]
- 12.Groningen Initiative to Analyse Type 2 diabetes Treatment (GIANTT). GIANTT website. https://www.giantt.nl/en/. Accessed February 6, 2018.
- 13. Crowley SD, Gurley SB, Oliverio MI, et al. Distinct roles for the kidney and systemic tissues in blood pressure regulation by the renin‐angiotensin system. J Clin Invest. 2005;115(4):1092‐1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Qaseem A, Hopkins RH Jr, Sweet DE, Starkey M, Shekelle P. Clinical Guidelines Committee of the American College of Physicians. Screening, monitoring, and treatment of stage 1 to 3 chronic kidney disease: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2013;159(12):835‐847. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Patient characteristics analyzed population & RAAS inhibition starters.
Table S2. Stratified analyses by response groups.
Table S3. Sensitivity analysis including only patients with baseline UACR ≥3.5 mg/mmol or baseline SBP ≥ 140 mm Hg.


