Abstract
Aim
Our aim was to perform an in‐depth analysis of the composition of fatty acids in milk from mothers delivering extremely preterm babies. We investigated longitudinal changes in milk fatty acid profiles and the relationship between several types of fatty acids, including omega‐3 and omega‐6.
Methods
Milk samples were collected at three stages of lactation from 78 mothers who delivered at less than 28 weeks of pregnancy at the Sahlgrenska University Hospital, Gothenburg, Sweden, from April 2013 to September 2015. Fatty acid composition was analysed by gas chromatography–mass spectrometry.
Results
A reduction in long‐chain polyunsaturated fatty acids (LCPUFAs) was observed during the lactation period. The concentrations of arachidonic acid and docosahexaenoic acid declined from medians of 0.34 to 0.22 mol% and 0.29 to 0.15 mol%, respectively, between postnatal day 7 and a postmenstrual age of 40 weeks. Strong correlations were found between the intermediates of several classes of fatty acids, including omega‐3, omega‐6 and omega‐9.
Conclusion
A rapid reduction in LCPUFA content in the mother's milk during the lactation period emphasises the importance of fatty acid supplementation to infants born extremely preterm, at least during the period corresponding to the third trimester, when rapid development of the brain and adipose tissue requires high levels of LCPUFAs.
Keywords: Arachidonic acid, Docosahexaenoic acid, Extremely preterm infants, Human milk, Long‐chain polyunsaturated fatty acids
Abbreviations
- LCPUFA
Long‐chain polyunsaturated fatty acid
- LCSFA
Long‐chain saturated fatty acid
- MCSFA
Medium‐chain saturated fatty acid
- MUFA
Monounsaturated fatty acid
- PMA
Postmenstrual age
- SFA
Saturated fatty acid
Key notes.
This study examined the changes in the fatty acid composition of breastmilk collected from 78 lactating mothers at three time points after they delivered extremely preterm infants.
Nearly all the long‐chain polyunsaturated fatty acids (LCPUFA) we analysed using gas chromatography–mass spectrometry declined significantly during lactation.
The rapid decline in LCPUFAs warrants prolonged fatty acid supplementation, as these are important for infant growth and brain development.
Introduction
Human breastmilk contains a complex mix of micronutrients and macronutrients. One of the key elements of milk is fat, predominately in the form of triglycerides comprising of fatty acid esters of glycerol. Milk fatty acids play multiple roles for the growing infant: as an energy source, as precursors for bioactive molecules and directly as integral components of cell membranes 1.
During pregnancy, long‐chain polyunsaturated fatty acids (LCPUFAs), including docosahexaenoic acid and arachidonic acid, are selectively transferred across the placenta to the growing foetus at increasing rates as the pregnancy progresses. This fatty acid transfer culminates in the last trimester, when the foetus builds up adipose tissue and extensive brain growth occurs 2. In the case of preterm birth, this transfer is disrupted and infants must rely instead on fatty acids supplied in the diet and/or intravenously through parenteral lipid emulsions. LCPUFA deficiency following moderately to extremely preterm birth has been associated with long‐term effects on cognition and visual acuity 3, 4, 5, 6. There is compelling evidence that the comparably low LCPUFA content of breastmilk is not sufficient to meet the high demand of the preterm infant 7. To meet infants’ needs for LCPUFAs in the early perinatal life, appropriate supplementation strategies must be established. One aspect that must be taken into consideration when such strategies are developed is the fatty acid composition of the mother's milk and potential qualitative and quantitative changes during lactation.
The fatty acid composition of human milk is influenced by a number of factors, including maternal nutrition 8, 9, genetic background 10 and stage of lactation 11. The main determinant of the milk fatty acid profile is the mother's diet, resulting in great variations between geographic regions and sociocultural contexts. Consequently, the highest docosahexaenoic acid content in human milk is found in coastal populations, where the intake of fish and other marine products rich in docosahexaenoic acid is high 12, and lowest in societies with modern Western diets 13. Several studies have also found that pregnancy duration impacts milk fatty acid composition 11, 14, 15, 16, 17, 18, 19, 20. One of the main findings of these studies is that LCPUFA levels, including docosahexaenoic acid, are significantly elevated in preterm milk compared to milk from mothers delivering at full term 21. However, other studies have noted no effect of pregnancy duration on milk LCPUFA content 22, 23.
The aim of this study was to analyse longitudinal changes in the fatty acid composition of breastmilk from mothers delivering extremely preterm in a 2013–2015 Swedish cohort. We also analysed how pregnancy duration influenced milk fatty acid composition, and how different fatty acid species were quantitatively related.
Materials and methods
Milk collection
Human milk samples were collected from 78 mothers delivering at less than 28 weeks of pregnancy who were admitted to the neonatal intensive care unit at Sahlgrenska University Hospital in Gothenburg, Sweden, between April 4, 2013, and September 22, 2015. The mean and standard deviation (SD) age of the mothers at delivery were 30.5 ± 6.6 (range 16.4–48.9) years. Seven mothers gave birth to twins. The mean (SD) duration of pregnancy at delivery was 25.5 ± 1.4 (range 22.7–27.8) weeks and infant birthweight 797 ± 223 (range 414–1260) grams. The study cohort has already been described in detail 24. Milk was collected on postnatal day 7 (n = 78), a postmenstrual age (PMA) of 32 weeks (n = 61) and a PMA of 40 weeks (n = 24).
Lipid extraction and GC‐MS analysis
Total lipids were extracted from 50 μL of lyophilised milk and fatty acid methyl esters prepared from 1/15 of the extract 25. Milk fatty acid methyl esters were analysed by gas chromatography–mass spectrometry as previously described 24, but using a different oven temperature gradient and selected ion monitoring mode for quantification. Briefly, the column temperature was maintained at 135°C, increased 1°C/min to 150°C, 2°C/min to 175°C and 70°C/min to 250°C and then held for two minutes. The detector was operated in scan (m/z 50–400)/selected ion monitoring data mode at 70 eV with the transfer line heated to 220°C. Fatty acid methyl esters were identified by comparing retention time and mass spectra to authentic standards, notably ME‐100 and individual fatty acids methyl esters (Larodan, Solna, Sweden) and Supelco PUFA No 3 from menhaden oil (Sigma‐Aldrich, St. Louis, MN, USA). Quantification was performed using MassHunter Workstation Quantitative Software for GC‐MS, version B.06.00 (Agilent Technologies, Santa Clara, CA, USA) using selected ion monitoring data and calibration curves made from authentic standards of known amounts with a few exceptions. For branched fatty acids, the straight chain equivalent fatty acid was used for quantification and 20:4n‐6 was used as a surrogate standard for quantification of 20:4n‐3. Mass fragments used for selected ion monitoring for the quantification of fatty acid methyl esters are listed in Table S1.
Statistical analysis
Statistical analyses were performed using SPSS Statistics, version 24 (IBM Corp, Armonk NY, USA). Differences were considered significant if p ≤ 0.05. As many of the fatty acids were not normally distributed, the nonparametric Spearman's rank‐order test was used for correlation analyses and the Wilcoxon signed‐rank test was used to determine different distributions depending on the time of sampling. Multiple linear models were validated according to common practices: the error terms were normally distributed, residuals showed homoscedasticity, error terms were independent, there were no or few outlier observations, and no multicollinearity was found.
Ethics
The study was approved by the Regional Ethical Board, Gothenburg (Dnr 303‐11) and registered at ClinicalTrials.gov (NCT 02760472).
Results
The fatty acid composition of preterm milk changes substantially over the lactation period
A total of 52 fatty acids were detected in the milk samples, and a true identity could be assigned to 41 of these by comparisons with authentic standards. The unresolved fatty acids comprised <1 mol% of all the analysed fatty acids. Fatty acids with acyl chain lengths shorter than 12 carbon atoms were not included in the analysis. Of the 41 fatty acids quantified in the mothers’ milk, 34 showed a change in the relative concentration (mol%) from postnatal day 7 to a PMA of 32 weeks (Table 1). Overall, there was great variability in the milk fatty acid composition between mothers, particularly in the LCPUFA species (Fig. 1). The total levels of both n‐3 and n‐6 LCPUFAs were significantly (p < 0.001) reduced over the lactation period. Arachidonic acid decreased from a median of 0.35 mol% to 0.22 mol% (Fig. 1A, p < 0.001) and docosahexaenoic acid from 0.29 mol% to 0.15 mol% (Fig. 1B, p < 0.001) from postnatal day 7 to a PMA of 40 weeks. Of the LCPUFAs, only mead acid (20:3n‐9) and eicosapentaenoic acid (20:5n‐3) (Fig. 1C) remained unchanged during the study period. The monounsaturated fatty acid nervonic acid (24:1n‐9) presented the greatest proportional change, as it was reduced from a median of 0.09 mol% to 0.02 mol% (p < 0.001) between the first and last milk sampling. Fatty acids that increased in relative concentration included linoleic acid (18:2n‐6, p < 0.001), γ‐linolenic acid (18:3n‐6, p < 0.001) and α‐linolenic acid (18:3n‐3, p = 0.02). No change in the n‐6/n‐3 fatty acid ratio was observed over the lactation period. Similar results were also obtained when only data from the 22 women providing milk at all three stages of lactation were included in the analysis (data not shown).
Table 1.
Proportions of individual fatty acid species in milk samples collected at three stages of lactation
| Postnatal day 7 (n = 78) | PMA 32 weeks (n = 61) | PMA 40 weeks (n = 24) | ||
|---|---|---|---|---|
| Fatty acid | Median (25th–75th perc.) | Median (25 th –75 th perc.) | Median (25th–75th perc.) | |
| Branched‐chain FA (BCFA) | ||||
| 13‐methyltetradecanoic acid | iso‐15:0 | 0.02 (0.02–0.03) | 0.02 (0.02–0.03) | 0.03 (0.02–0.04) |
| 12‐methyltetradecanoic acid | anteiso‐15:0 | 0.04 (0.03–0.06) | 0.04 (0.03–0.06) | 0.05 (0.03–0.07) |
| 14‐methylhexadecanoic acid | iso‐16:0 | 0.04 (0.03–0.05) | 0.04 (0.03–0.05) | 0.05 (0.03–0.06) |
| 15‐methylheptadecanoic acid | iso‐17:0 | 0.07 (0.06–0.09) | 0.08 (0.06–0.10) | 0.08 (0.05–0.11) |
| 14‐methylheptadecanoic acid | anteiso‐17:0 | 0.07 (0.06–0.09) | 0.08 (0.06–0.09) | 0.09 (0.07–0.11) |
| ΣBCFA | 0.25 (0.19–0.32) | 0.27 (0.21–0.34) | 0.29 (0.16–0.41) | |
| Saturated FA (SFA) | ||||
| Lauric acid | 12:0 | 4.50 (3.19–5.98) | 4.61 (3.13–6.13) | 5.13 (3.36–5.85) |
| Tridecanoic acid | 13:0 | 0.02 (0.02–0.03)a , b | 0.02 (0.01–0.02) | 0.02 (0.01–0.02) |
| Myristic acid | 14:0 | 9.40 (7.54–11.25)a | 8.26 (6.69–9.92) | 9.25 (7.76–10.98) |
| Pentadecanoic acid | 15:0 | 0.28 (0.24–0.33) | 0.29 (0.22–0.34) | 0.29 (0.21–0.36) |
| Palmitic acid (PA) | 16:0 | 24.55 (22.62–26.34)a , b | 23.34 (20.41–25.15) | 24.45 (21.59–26.14) |
| Margaric acid | 17:0 | 0.25 (0.21–0.30) | 0.24 (0.21–0.29) | 0.26 (0.20–0.31) |
| Stearic acid (SA) | 18:0 | 6.04 (5.11–6.72) | 6.08 (5.29–7.07) | 6.34 (5.08–7.64) |
| Nonadecanoic acid | 19:0 | 0.01 (0.01–0.01)a , b | 0.01 (0.01–0.01) | 0.01 (0.01–0.02) |
| Arachidic acid (ArA) | 20:0 | 0.18 (0.15–0.20)b | 0.18 (0.15–0.20) | 0.15 (0.12–0.18) |
| Heneicosanoic acid | 21:0 | 0.00 (0.00–0.00)a | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) |
| Behenic acid (BA) | 22:0 | 0.05 (0.04–0.06)b | 0.05 (0.04–0.07) | 0.04 (0.03–0.05) |
| Tricosanoic acid | 23:0 | 0.01 (0.01–0.01)a , b | 0.00 (0.00–0.01) | 0.00 (0.00–0.01) |
| Lignoceric acid (LigA) | 24:0 | 0.06 (0.05–0.08)a , b | 0.04 (0.03–0.05) | 0.03 (0.02–0.03) |
| ΣMCSFA (C12‐16) | 38.94 (36.22–43.12)a | 36.25 (33.31–38.82) | 38.94 (35.77–41.29) | |
| ΣLCSFA (≥C17) | 6.61 (5.68–7.41) | 6.72 (5.84–7.71) | 6.85 (5.44–8.34) | |
| ΣSFA | 46.14 (42.25–49.77)a | 43.52 (40.04–45.83) | 45.48 (42.77–48.73) | |
| Monounsaturated FA (MUFA) | ||||
| Myristoleic acid | 14:1n‐5 | 0.22 (0.17–0.28) | 0.21 (0.17–0.28) | 0.25 (0.20–0.29) |
| Palmitoleic acid | 16:1n‐7 | 1.73 (1.47–2.28) | 1.75 (1.42–2.21) | 1.64 (1.48–2.01) |
| Oleic acid (OA) | 18:1n‐9 | 36.15 (33.35–38.97)a | 38.47 (35.85–40.71) | 36.33 (33.15–38.11) |
| cis‐Vaccenic acid | 18:1n‐7 | 2.85 (2.57–3.13)a , b | 2.68 (2.39–2.90) | 2.36 (2.13–2.68) |
| Gondoic acid (GA) | 20:1n‐9 | 0.47 (0.39–0.55)a , b | 0.41 (0.36–0.46) | 0.33 (0.30–0.37) |
| Erucic acid (EA) | 22:1n‐9 | 0.09 (0.07–0.10)a , b | 0.06 (0.05–0.07) | 0.04 (0.04–0.05) |
| Nervonic acid (NA) | 24:1n‐9 | 0.09 (0.06–0.12)a , b | 0.03 (0.03–0.04) | 0.02 (0.02–0.03) |
| ΣMUFA | 41.78 (37.96–45.33)a | 43.90 (41.11–45.86) | 41.26 (37.88–43.37) | |
| Polyunsaturated FA n‐9 | ||||
| Mead acid | 20:3n‐9 | 0.01 (0.01–0.01) | 0.01 (0.00–0.01) | 0.01 (0.01–0.01) |
| Polyunsaturated FA n‐6 (PUFA n‐6) | ||||
| Linoleic acid (LA) | 18:2n‐6 | 8.33 (7.43–9.49)a , b | 10.08 (8.32–11.17) | 10.11 (8.27–12.07) |
| γ‐linolenic acid (GLA) | 18:3n‐6 | 0.04 (0.03–0.07)a , b | 0.05 (0.04–0.07) | 0.05 (0.04–0.06) |
| Eicosadienoic acid | 20:2n‐6 | 0.24 (0.21–0.30)a , b | 0.16 (0.13–0.19) | 0.14 (0.12–0.17) |
| Dihomo‐γ‐linolenic acid (DGLA) | 20:3n‐6 | 0.36 (0.27–0.47)a , b | 0.23 (0.18–0.26) | 0.17 (0.15–0.2) |
| Arachidonic acid | 20:4n‐6 | 0.34 (0.27–0.39)a , b | 0.23 (0.20–0.26) | 0.22 (0.19–0.24) |
| Docosadienoic acid | 22:2n‐6 | 0.03 (0.03–0.04)a , b | 0.01 (0.01–0.02) | 0.01 (0.01–0.01) |
| Adrenic acid (AdrA) | 22:4n‐6 | 0.14 (0.09–0.18)a , b | 0.06 (0.05–0.07) | 0.05 (0.05–0.06) |
| Docosapentaenoic acid (DPAn‐6) | 22:5n‐6 | 0.03 (0.02–0.04)a , b | 0.02 (0.01–0.02) | 0.02 (0.01–0.02) |
| ΣPUFAn‐6 | 9.44 (8.63–10.93)a | 10.80 (9.1–12.18) | 10.78 (8.54–12.79) | |
| ΣLCPUFA n‐6 (≥C20) | 1.15 (0.97–1.45)a , b | 0.71 (0.62–0.8) | 0.60 (0.56–0.69) | |
| Polyunsaturated FA n‐3 (PUFA n‐3) | ||||
| α‐linolenic acid (ALA) | 18:3n‐3 | 1.12 (0.85–1.33)a , b | 1.39 (1.12–1.88) | 1.35 (0.97–2.21) |
| Stearidonic acid (SDA) | 18:4n‐3 | 0.02 (0.01–0.02)a , b | 0.02 (0.01–0.03) | 0.02 (0.01–0.02) |
| Eicosatrienoic acid | 20:3n‐3 | 0.06 (0.05–0.08)a , b | 0.04 (0.03–0.05) | 0.03 (0.03–0.05) |
| Juniperonic acid (JA) | 20:4n‐3 | 0.08 (0.06–0.10)a , b | 0.06 (0.04–0.08) | 0.04 (0.04–0.05) |
| Timnodonic acid/eicosapentaenoic acid | 20:5n‐3 | 0.05 (0.03–0.06) | 0.05 (0.04–0.07) | 0.04 (0.04–0.07) |
| Docosapentaenoic acid (DPAn‐3) | 22:5n‐3 | 0.17 (0.13–0.22)a , b | 0.13 (0.10–0.16) | 0.13 (0.10–0.15) |
| Docosahexaenoic acid | 22:6n‐3 | 0.29 (0.24–0.38)a , b | 0.20 (0.15–0.27) | 0.15 (0.13–0.22) |
| ΣPUFA n‐3 | 1.79 (1.58–2.1)a | 1.91 (1.6–2.59) | 1.93 (1.44–2.59) | |
| ΣLCPUFA n‐3 (≥C20) | 0.67 (0.55–0.84)a , b | 0.48 (0.38–0.65) | 0.39 (0.35–0.56) | |
Data are expressed as the molar per cent (mol%) of all analysed fatty acids.
Significantly different between postnatal day 7 and PMA 32 weeks.
Significantly different between postnatal day 7 and PMA 40 weeks according to the Wilcoxon signed‐rank test (p < 0.05).
Figure 1.
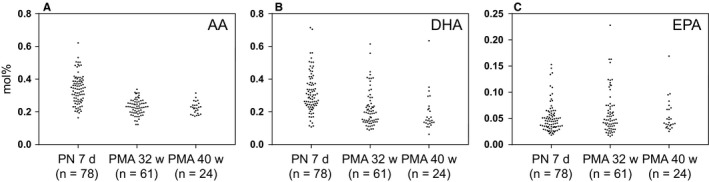
LCPUFA fractions in milk collected at three different stages of lactation. (A) Arachidonic acid (arachidonic acid, 20:4n‐6), (B) docosahexaenoic acid (docosahexaenoic acid, 22:6n‐3) and (C) eicosapentaenoic acid (eicosapentaenoic acid, 20:5n‐3). PN = Postnatal; PMA = Postmenstrual age.
Pregnancy duration influences milk fatty acid composition
To investigate how pregnancy duration influences milk FA composition, the length of the pregnancy (23–28 weeks) was correlated with the different fatty acid fractions in milk collected on postnatal day 7. Medium‐chain saturated fatty acids (MCSFAs), namely C12‐16, and monounsaturated FAs (MUFAs) significantly correlated with pregnancy duration (p < 0.001). Detailed analysis of the MCSFAs revealed an inverse correlation between lauric acid (12:0) and myristic acid (14:0, Fig. 2A) with pregnancy duration (r = −0.347, p = 0.002 and r = −0.470, p < 0.001, respectively). Of the MUFAs, myristoleic acid (14:1n‐5) inversely correlated with pregnancy length (r = −0.354, p = 0.001), whereas oleic acid (18:1n‐9, Fig. 2B) and cis‐vaccenic acid (18:1n‐7, Fig. 2C) positively correlated with pregnancy duration (r = 0.339, p = 0.003 and r = 0.317, p = 0.005, respectively). The only LCPUFA fraction that correlated with pregnancy duration was mead acid (20:3n‐9; r = 0.355, p = 0.001).
Figure 2.
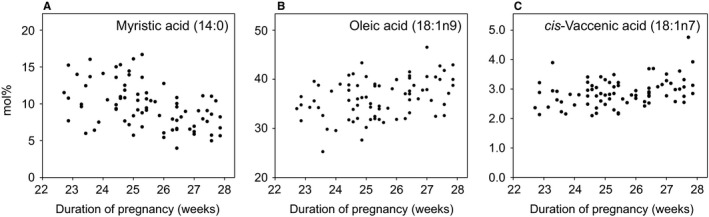
Influence of pregnancy duration on levels of fatty acids in milk collected postnatal day 7. (A) Myristic acid (14:0), (B) oleic acid (OA, 18:1n9) and (C) cis‐vaccenic acid (cVA, 18:1n7).
Relationships between milk fatty acids from different biosynthesis pathways
Figure 3 shows the pairwise associations between products of the n‐3, n‐6, n‐7, n‐9 and saturated fatty acid (SFA) biosynthesis pathways in milk collected on postnatal day 7. There was no correlation between the levels of the first precursor and the end product in any of the investigated pathways, namely between the precursor‐product pair α‐linolenic acid and docosahexaenoic acid of the n‐3 family, linoleic acid and docosapentaenoic acid (DPAn‐6, 22:5n‐6) of the n‐6 family, oleic acid and nervonic acid of the n‐9 family or palmitic acid (16:0) and lignoceric acid (LigA, 24:0) among the SFA species. However, significant correlations were found between immediate precursors and products within the biosynthesis pathways at almost all individual steps (Fig. 3A,B). An exception was the desaturation of SFAs to MUFAs catalysed by stearoyl‐CoA desaturase, in other words Δ9 desaturation of palmitic acid and stearic acid (18:0) to their respective MUFA analogue.
Figure 3.
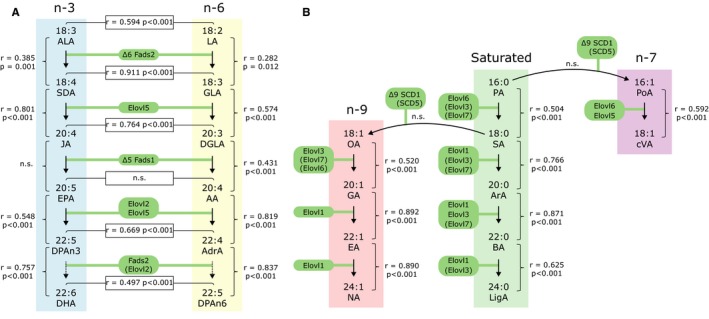
Correlations between fatty acid species in milk collected postnatal day 7. (A) Fatty acids of the n‐3 and n‐6 series. (B) Fatty acids of the n‐7 and n‐9 series and saturated fatty acids. Enzymes responsible for desaturation (Fads and SCD) and elongation (Elovl) are shown in green boxes. The Spearman correlation (r) and significance (p) between fatty acids are shown. n.s., not significant (p > 0.05). Full names of acronyms can be found in Table 1.
When assessing associations between fatty acids of the n‐3 and n‐6 pathways, those found at parallel biosynthesis steps demonstrated strong positive correlations, with the exception of eicosapentaenoic acid and arachidonic acid (Fig. 3A). Docosahexaenoic acid and arachidonic acid positively correlated (r = 0.576, p < 0.001, not shown in figure).
Modelling of fatty acid synthesis in milk by multiple linear regression in a multiple linear
In a multiple linear regression model with milk docosahexaenoic acid on postnatal day 7 as the dependent variable and other fatty acids of the n‐3 family as independent variables, stearidonic acid (18:4n‐3) negatively contributed to the amount of docosahexaenoic acid, whereas eicosapentaenoic acid and docosapentaenoic acid (n‐3) were positively related (stearidonic acid β = −2.88, p = 0.05; eicosapentaenoic acid β = 2.65, p < 0.001; docosapentaenoic acid (n‐3) β = 0.78, p < 0.001; R = 0.837, model R2 = 0.700). α‐Linolenic acid and juniperonic acid (20:4n‐3) were nonsignificant contributors to the docosahexaenoic acid level in the model. We found that 70% of the variance in docosahexaenoic acid could be explained by the levels of stearidonic acid, eicosapentaenoic acid and docosapentaenoic acid (n‐3).
In a multiple linear regression analysis with arachidonic acid as the dependent variable and n‐6 precursor fatty acids as independent variables, γ‐linolenic acid (18:3n‐6) negatively contributed to the amount of arachidonic acid, whereas linoleic acid and dihomo‐γ‐linolenic acid (20:3n‐6) were positively related (γ‐linolenic acid β = −1.61, p < 0.001; linoleic acid β = 0.009, p < 0.05; dihomo‐γ‐linolenic acid β = 0.38, p < 0.001; R = 0.559, model R2 = 0.312). The low R2 value indicates that factors not included in the model were important for arachidonic acid levels.
Discussion
In this study, the levels of arachidonic acid and docosahexaenoic acid in breast milk were 0.34 mol% and 0.29 mol% of the total fat at postnatal day 7 and declined to almost half the initial concentration by a PMA of 40 weeks. Assuming a fat content of 3.6% and daily milk intake of 160 mL/kg/day, the milk analysed in this study would have provided the infant with approximately 20 mg/kg/day of both arachidonic acid and docosahexaenoic acid on average at the beginning of lactation and 15 mg/kg/day at a PMA of 40 weeks. This represents approximately 10% of arachidonic acid and 50% of docosahexaenoic acid of the normal daily foetal accretion during the third trimester of pregnancy, which is estimated to be 212 and 43 mg/kg/day for arachidonic acid and docosahexaenoic acid, respectively 26. We previously reported that infant plasma levels of arachidonic acid and docosahexaenoic acid decrease significantly during the first weeks of life in this cohort 24. LCPUFA deprivation during this sensitive time, when organogenesis and neural development occur, may have lifelong health consequences. The majority of LCPUFAs transferred from mother to foetus during normal pregnancy are deposited into adipose tissue, serving as a reservoir that may be mobilised to other tissues, such as the brain and retina, as the infant grows. Thus, the daily requirement for dietary arachidonic acid and docosahexaenoic acid is higher for extremely preterm infants compared to full‐term infants, as they almost completely lack stored fat. Infants are capable of de novo synthesis of arachidonic acid and docosahexaenoic acid from precursor essential fatty acids, namely linoleic acid and α‐linolenic acid, adding to the total available amount of these LCPUFAs, but the conversion rate is limited 27. There is a growing body of evidence suggesting that LCPUFAs provided solely via breastmilk are not sufficient to meet the needs of very preterm infants 7.
Arachidonic acid and docosahexaenoic acid were not the only long‐chained fatty acids to decline during lactation. The proportions of nearly all quantified fatty acids >20 carbons in length decreased during the lactation period concomitant with an increase in linoleic acid and α‐linolenic acid. The concentration of nervonic acid changed the most, decreasing by 77% from postnatal day 7 to a PMA of 40 weeks. Such a large difference in concentration in nervonic acid has been noted previously between preterm milk and donor milk, probably due to the donated milk being collected from later lactation stages 18. This finding has been suggested to be of special importance for preterm infants, because nervonic acid is essential for myelination and thus brain development and function. Therefore, in instances when infants receive donors’ milk, there is a risk that the intake of several fatty acids that are important for normal development is considerably lower than when the mother′s milk is the sole dietary source. Overall, the fatty acid profiles of breast milk from mothers delivering extremely preterm reported here agree with previously published data on similar cohorts, demonstrating that low milk LCFUFA levels are a widespread concern 11, 16, 19, 20, 28, 29.
When assessing the role of pregnancy length on milk lipid composition, we correlated fatty acid fractions with pregnancy duration. The SFAs lauric acid and myristic acid were found at higher proportions in milk from mothers with shorter pregnancies, whereas the two main MUFAs linoleic acid and cis‐vaccenic acid followed an opposite pattern. These observations were in line with other reports that showed an association between preterm delivery and increased levels of short‐chain SFAs and MCSFAs together with reduced levels of MUFAs 11, 19, 20, 23. Short‐chain SFAs and MCSFAs are easily absorbed by the digestive system and readily accessible as an energy source. Thus, these classes of fatty acids have been suggested to be of particularly high importance for very preterm infants with immature gastrointestinal tracts 20. However, considering the rather small differences between full‐term and preterm milk, the physiological relevance of these findings is likely to be negligible. In contrast to the elevated fraction of MCSFAs in milk from mothers with shorter pregnancies, these FAs are known to increase with progressing stages of lactation. MCSFAs are found in lower proportions in colostrum than in transitional and mature milk due to maturation of the mammary gland and de novo fatty acid synthesis from acetyl‐CoA 11. Genzel‐Boroviczeny et al. 23 proposed that the immature mammary gland after preterm delivery has a reduced capacity for the uptake and conversion of long‐chain fatty acids from circulation to milk lipids and that this may stimulate de novo fatty acid synthesis as a compensatory mechanism. Milk LCPUFA abundance has been reported in some studies to be influenced by pregnancy duration (reviewed in 21). Among the quantified LCPUFAs in this study, only mead acid levels correlated with pregnancy length. Mead acid was found at higher levels in milk from women with relatively longer pregnancies. However, the mead acid content was low in all investigated samples, only representing about 0.01 mol% of the total quantified fatty acids. Notably, the distribution of several LCPUFAs within the study population, including arachidonic acid, was considerably larger in samples collected on postnatal day 7 compared to PMA of 32 and 40 weeks. This indicates that the interindividual variation in certain milk LCPUFAs was greater closer to delivery than at later stages of lactation. If this variation was due to differences in pregnancy duration or depended on some other factor remains to be investigated.
Not unexpectedly, we found strong associations between levels of intermediates in milk for all investigated fatty acid biosynthesis pathways. Furthermore, the fatty acid species found at parallel steps of the n‐3 and n‐6 pathways demonstrated high positive correlations. As the intermediates of the n‐3 and n‐6 pathways share common modifying enzymes, there is also competition between substrates for elongation and desaturation. However, milk arachidonic acid and docosahexaenoic acid positively correlated in our material, as well as their shorter chain precursors, linoleic acid and α‐linolenic acid.
Human milk is the recommended food for very preterm infants. Under standard care, this patient group does not generally receive any supplementation with LCPUFAs. If the supply of mother′s milk is inadequate or the infant is unable to breastfeed, human milk is complemented or substituted with preterm formulas. Most commercial premature formulas used today are fortified with arachidonic acid and docosahexaenoic acid in various ratios. The levels of these LCPUFAs in preterm formulas are based on the content found in breast milk from full‐term mothers and the amounts of dietary arachidonic acid and docosahexaenoic acid are similar if the infant is fed human milk and/or formula 30. Independent of whether the preterm infant receives human milk or formula, arachidonic acid and docosahexaenoic acid are not provided at levels that recreate the in utero transfer or that meet the most recent recommended intake 2.
Conclusions
This study provides high‐resolution data on the fatty acid composition of milk from a relatively large cohort of women delivering extremely preterm. We show that many of the long‐chain fatty acids that are associated with health benefits in infants decline rapidly during the lactation period. We also corroborate previous findings that pregnancy duration has an impact on milk fatty acid composition. The low arachidonic acid and docosahexaenoic acid content of the milk stresses the importance of early supplementation of these vital LCPUFAs to infants born extremely prematurely. Supplementation strategies may target both the infant and the mother to improve milk quality.
Funding
This study was supported by the Swedish Research Council (DNR# 2016‐01131) and De Blindas Vänner.
Conflict of interests
The authors have no conflict of interests to declare.
Supporting information
Table S1 Ions used for selected ion monitoring (SIM) of fatty acids by mass spectrometry analysis.
Acknowledgements
The authors thank all of the participating mothers and their families and the study team led by Carola Pfeiffer‐Mosesson, and Berit Holmberg who extracted and prepared the fatty acids from milk.
References
- 1. Delplanque B, Gibson R, Koletzko B, Lapillonne A, Strandvik B. Lipid quality in infant nutrition: current knowledge and future opportunities. J Pediatr Gastroenterol Nutr 2015; 61: 8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Robinson DT, Martin CR. Fatty acid requirements for the preterm infant. Semin Fetal Neonatal Med 2017; 22: 8–14. [DOI] [PubMed] [Google Scholar]
- 3. Qawasmi A, Landeros‐Weisenberger A, Bloch MH. Meta‐analysis of LCPUFA supplementation of infant formula and visual acuity. Pediatrics 2013; 131: e262–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fang PC, Kuo HK, Huang CB, Ko TY, Chen CC, Chung MY. The effect of supplementation of docosahexaenoic acid and arachidonic acid on visual acuity and neurodevelopment in larger preterm infants. Chang Gung Med J 2005; 28: 708–15. [PubMed] [Google Scholar]
- 5. Wang Q, Cui Q, Yan C. The effect of supplementation of long‐chain polyunsaturated fatty acids during lactation on neurodevelopmental outcomes of preterm infant from infancy to school age: a systematic review and meta‐analysis. Pediatr Neurol 2016; 59: e1. [DOI] [PubMed] [Google Scholar]
- 6. Tam EW, Chau V, Barkovich AJ, Ferriero DM, Miller SP, Rogers EE, et al. Early postnatal docosahexaenoic acid levels and improved preterm brain development. Pediatr Res 2016; 79: 723–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lapillonne A, Groh‐Wargo S, Gonzalez CH, Uauy R. Lipid needs of preterm infants: updated recommendations. J Pediatr 2013; 162: S37–47. [DOI] [PubMed] [Google Scholar]
- 8. Innis SM. Impact of maternal diet on human milk composition and neurological development of infants. Am J Clin Nutr 2014; 99: 734s–41s. [DOI] [PubMed] [Google Scholar]
- 9. Insull W, Hirsch J, James T, Ahrens EH. The fatty acids of human milk. II. Alterations produced by manipulation of caloric balance and exchange of dietary fats. J Clin Invest 1959; 38: 443–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xie L, Innis SM. Genetic variants of the FADS1 FADS2 gene cluster are associated with altered (n‐6) and (n‐3) Essential fatty acids in plasma and erythrocyte phospholipids in women during pregnancy and in breast milk during lactation. J Nutr 2008; 138: 2222–8. [DOI] [PubMed] [Google Scholar]
- 11. Molto‐Puigmarti C, Castellote AI, Carbonell‐Estrany X, Lopez‐Sabater MC. Differences in fat content and fatty acid proportions among colostrum, transitional, and mature milk from women delivering very preterm, preterm, and term infants. Clin Nutr 2011; 30: 116–23. [DOI] [PubMed] [Google Scholar]
- 12. Brenna JT, Varamini B, Jensen RG, Diersen‐Schade DA, Boettcher JA, Arterburn LM. Docosahexaenoic and arachidonic acid concentrations in human breast milk worldwide. Am J Clin Nutr 2007; 85: 1457–64. [DOI] [PubMed] [Google Scholar]
- 13. Juber BA, Jackson KH, Johnson KB, Harris WS, Baack ML. Breast milk DHA levels may increase after informing women: a community‐based cohort study from South Dakota USA. Int Breastfeed J 2016; 12: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bobinski R, Mikulska M, Mojska H, Simon M. Comparison of the fatty acid composition of transitional and mature milk of mothers who delivered healthy full‐term babies, preterm babies and full‐term small for gestational age infants. Eur J Clin Nutr 2013; 67: 966–71. [DOI] [PubMed] [Google Scholar]
- 15. Luukkainen P, Salo MK, Nikkari T. Changes in the fatty acid composition of preterm and term human milk from 1 week to 6 months of lactation. J Pediatr Gastroenterol Nutr 1994; 18: 355–60. [DOI] [PubMed] [Google Scholar]
- 16. Maas C, Franz AR, Shunova A, Mathes M, Bleeker C, Poets CF, et al. Choline and polyunsaturated fatty acids in preterm infants’ maternal milk. Eur J Nutr 2017; 56: 1733–42. [DOI] [PubMed] [Google Scholar]
- 17. Berenhauser AC, Pinheiro do Prado AC, da Silva RC, Gioielli LA, Block JM. Fatty acid composition in preterm and term breast milk. Int J Food Sci Nutr 2012; 63: 318–25. [DOI] [PubMed] [Google Scholar]
- 18. Ntoumani E, Strandvik B, Sabel KG. Nervonic acid is much lower in donor milk than in milk from mothers delivering premature infants–of neglected importance? Prostaglandins Leukot Essent Fatty Acids 2013; 89: 241–4. [DOI] [PubMed] [Google Scholar]
- 19. Bitman J, Wood L, Hamosh M, Hamosh P, Mehta NR. Comparison of the lipid composition of breast milk from mothers of term and preterm infants. Am J Clin Nutr 1983; 38: 300–12. [DOI] [PubMed] [Google Scholar]
- 20. Guerra E, Downey E, O'Mahony JA, Caboni MF, O'Shea C‐A, Ryan AC, et al. Influence of duration of gestation on fatty acid profiles of human milk. Eur J Lipid Sci Technol 2016; 118: 1775–87. [Google Scholar]
- 21. Bokor S, Koletzko B, Decsi T. Systematic review of fatty acid composition of human milk from mothers of preterm compared to full‐term infants. Ann Nutr Metab 2007; 51: 550–6. [DOI] [PubMed] [Google Scholar]
- 22. Granot E, Ishay‐Gigi K, Malaach L, Flidel‐Rimon O. Is there a difference in breast milk fatty acid composition of mothers of preterm and term infants? J Matern Fetal Neonatal Med 2016; 29: 832–5. [DOI] [PubMed] [Google Scholar]
- 23. Genzel‐Boroviczeny O, Wahle J, Koletzko B. Fatty acid composition of human milk during the 1st month after term and preterm delivery. Eur J Pediatr 1997; 156: 142–7. [DOI] [PubMed] [Google Scholar]
- 24. Najm S, Löfqvist C, Hellgren G, Engström E, Lundgren P, Hård A‐L, et al. Effects of a lipid emulsion containing fish oil on polyunsaturated fatty acid profiles, growth and morbidities in extremely premature infants: a randomized controlled trial. Clin Nutr ESPEN 2017; 20: 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sabel KG, Lundqvist‐Persson C, Bona E, Petzold M, Strandvik B. Fatty acid patterns early after premature birth, simultaneously analysed in mothers’ food, breast milk and serum phospholipids of mothers and infants. Lipids Health Dis 2009; 8: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lapillonne A, Jensen CL. Reevaluation of the DHA requirement for the premature infant. Prostaglandins Leukot Essent Fatty Acids 2009; 81: 143–50. [DOI] [PubMed] [Google Scholar]
- 27. Carnielli VP, Simonato M, Verlato G, Luijendijk I, De Curtis M, Sauer PJ, et al. Synthesis of long‐chain polyunsaturated fatty acids in preterm newborns fed formula with long‐chain polyunsaturated fatty acids. Am J Clin Nutr 2007; 86: 1323–30. [DOI] [PubMed] [Google Scholar]
- 28. De Rooy L, Hamdallah H, Dyall SC. Extremely preterm infants receiving standard care receive very low levels of arachidonic and docosahexaenoic acids. Clin Nutr 2016; 36: 1593–600. [DOI] [PubMed] [Google Scholar]
- 29. Beijers RJ, Schaafsma A. Long‐chain polyunsaturated fatty acid content in Dutch preterm breast milk; differences in the concentrations of docosahexaenoic acid and arachidonic acid due to length of gestation. Early Hum Dev 1996; 44: 215–23. [DOI] [PubMed] [Google Scholar]
- 30. Collins CT, Gillis J, McPhee AJ, Suganuma H, Makrides M. Avoidance of bottles during the establishment of breast feeds in preterm infants. Cochrane Database Syst Rev 2016; 9: Cd005252. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Ions used for selected ion monitoring (SIM) of fatty acids by mass spectrometry analysis.


