Abstract
Current balloon expandable transcatheter valves have limited applicability to patients with “native” right ventricular outflow tracts (RVOT), meaning those who have had previous surgery and are left with large, compliant, irregular RVOT. The Alterra Adaptive PrestentTM is a self‐expanding, partially covered stent that was designed to internally reconfigure these types of RVOT, making them suitable for implantation of a commercially available balloon expandable heart valve, the SAPIEN 3. Herein, we describe the first human implant of this device.
Keywords: congenital heart disease, pulmonary valve disease, transcatheter valve implantation
1. INTRODUCTION
Patients who have undergone previous surgery on the right ventricular outflow tract (RVOT), such as transannular patch repair of Tetralogy of Fallot or pulmonary valvotomy, commonly experience significant pulmonary regurgitation requiring pulmonary valve placement. With time and degeneration of surgically implanted valves, multiple open‐heart surgeries are often required. While transcatheter pulmonary valve placement was first described nearly 2 decades ago by Bonhoeffer et al. 1, current balloon expandable valves are better suited to the size and geometry of surgical conduits and bioprosthetic valves 2. The “native” RVOT, usually characterized by a variable and dynamic morphology, large diameter, and unpredictable compliance often precludes transcatheter valve replacement with existing devices 3. The Alterra Adaptive PrestentTM was designed to internally remodel a wide variety of RVOT morphologies, thereby creating a suitable “landing zone” for implantation of a standard balloon expandable transcatheter heart valve (THV) in an attempt to treat a broader range of patients. Herein, we describe the first successful human implant of this device.
2. DEVICE DESCRIPTION
The Alterra Adaptive Prestent is designed to be used as a docking adaptor for the 29 mm SAPIEN 3 THV within the RVOT. It is comprised of a self‐expanding, radiopaque, nitinol frame assembly and polyethylene terephthalate (PET) fabric covering and has designated inflow and outflow ends (Figure 1). The inflow section is identifiable by the presence of two triangular tabs that are attached to the delivery system and circumferential covering of all cells. The outflow section is distinguished by open cells designed to facilitate blood flow into the branch pulmonary arteries should the device need to extend into the orifice of one or both of these structures. The PET fabric is attached by sutures to the inside surface of the frame to create a seal at the inflow section. The device has a symmetrical frame design with the inflow and outflow diameters equal to 40 mm and the central section 27 mm to provide a rigid “landing zone” for a SAPIEN 3 THV (29 mm). While the total unconstrained device length is 48 mm, the non‐fabric coated row of cells at the outflow results in a completely covered length of only 30 mm.
Figure 1.
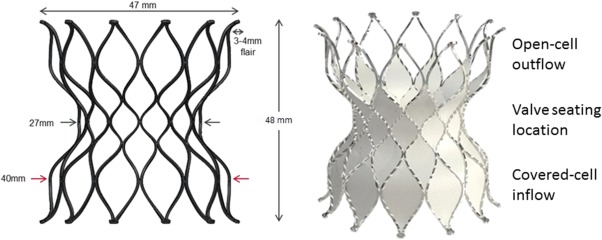
Schematic representation and photograph of the self‐expanding Alterra Adaptive Prestent. The distal and proximal flares aid in device stability. The uncovered distal row of cells allows for placement of the device into the orifice of the branch pulmonary arteries without causing obstruction to flow
2.1. Delivery system description
The Alterra Adaptive Prestent comes fully loaded in a custom delivery system consisting of a handle, retractable outer shaft, inner delivery shaft (upon which the stent sits), prestent connector, and a tapered tip meant to facilitate tracking through the vasculature (Figure 2). The delivery handle consists of a single knob that ergonomically allows for a one‐handed, slow, controlled deployment and potential recapture and a flush port to flush the guidewire lumen (consistent with a 0.035″ guide wire). The entire system fits through a 16 Fr eSheath (Edwards Life Sciences, Irvine, CA).
Figure 2.
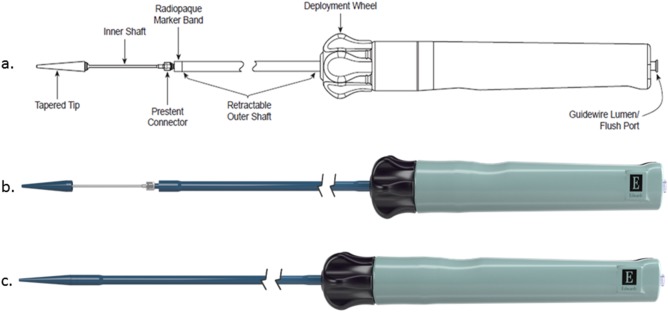
Schematic representation and photographs of the Alterra delivery system in the uncovered (A,B) and covered (C) configurations. Turning the control wheel counter‐clockwise results in slow withdrawal of the retractable outer shaft and controlled expansion and delivery of the device.
3. CASE REPORT
A 48‐year‐old woman who was born with an initial diagnosis of valve and sub‐valve pulmonic stenosis underwent operative repair as a child (1978) consisting of surgical valvotomy and resection of sub‐pulmonic obstructive musculature. She was followed with chronic severe pulmonary regurgitation and recently developed worsening symptomatology (NYHA II). Pre‐procedural testing (MRI) revealed severe pulmonary regurgitation (regurgitant fraction = 29%) without RVOT obstruction and a dilated right ventricle (RV end diastolic volume = 131 mL/m2) with preserved systolic function (ejection fraction = 57%). Further work‐up involved in‐depth RVOT analysis using cardiac MRI and magnetic resonance hagiography, gated CT with creation of three‐dimensional systolic and diastolic RVOT models and the performance of virtual implants similar to that previously described 4, 5 (Figure 3). Diameter and perimeter plots of the patient's RVOT in both systole and diastole were created in 5 mm increments from the bifurcation of the branch pulmonary arteries down to contractile right ventricular tissue and analyzed in relation to the diameter and perimeter of the unconstrained device. Patients were felt to be suitable candidates when the distal and proximal portions of the device were circumferentially opposed to the RVOT wall. Cardiac catheterization entailed hemodynamic assessment, intracardiac echocardiography, three‐dimensional rotational angiography, and two‐dimensional biplane axial angiography. Findings at catheterization included a dilated RVOT with no obstruction, an anomalous circumflex artery arising from the right coronary artery coursing posterior to the aortic root and maximal angiographic diameters in the lateral projection of the proximal and distal RVOT of 30.2 and 38.5 mm, respectively. Static balloon sizing of the RVOT with simultaneous aortic angiography was performed and suggested there was no significant risk of aortic or coronary artery compression. The Alterra device was then advanced over a 0.035″ Lunderquist Extra‐Stiff Wire Guide (Cook Medical, Bloomington, IN) into position in the RVOT (Figure 4). Serial angiograms were performed during deployment to optimize positioning using a second catheter in the right ventricle. The device was deployed using a slow controlled turning of the deployment wheel until it was fixed in the intended location. Once deployed, the delivery system was removed and intra‐cardiac echocardiography and angiography performed which confirmed good apposition of the device to the RVOT wall circumferentially (Figure 4). A 29 mm SAPIEN 3 THV was prepared in the usual fashion and advanced using the Commander Delivery System through the same 16 Fr eSheath and guidewire. The valve was deployed in the intended location within the Alterra Adaptive Prestent. Post‐deployment, there continued to be normal right ventricular pressure with no gradient across the RVOT. Intra‐cardiac echocardiography and pulmonary angiography demonstrated excellent SAPIEN 3 valve function with a trivial amount of pulmonary regurgitation around the Alterra device and no central valvar regurgitation. The patient recovered uneventfully and was discharged home the next morning receiving aspirin 81 mg. Four months after implant the patient reported symptomatic improvement in terms of exercise tolerance. Echocardiography demonstrated excellent pulmonary valve function with a peak and mean Doppler estimated gradient of 7.62 and 5.00 mm Hg, respectively, and trivial para‐device leak.
Figure 3.
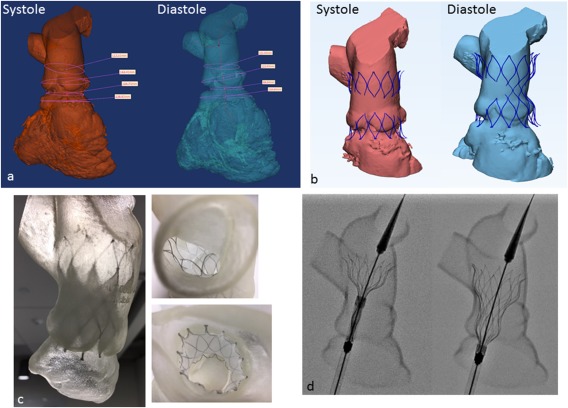
Pre‐procedural evaluation. A, Three‐dimensional CT reconstruction of the right heart in systole and diastole used to calculate RVOT dimensions including lengths, diameters, and circumference along the length of the potential deployment zone. B, Virtual “implants” of an unconstrained, fully expanded device in systole and diastole examining the extent of device contact with vessel wall. C, Deployments of the device within a three‐dimensional soft rubber model of the RVOT. D, Still fluoroscopic frames of a test deployment using the same three‐dimensional model.
Figure 4.
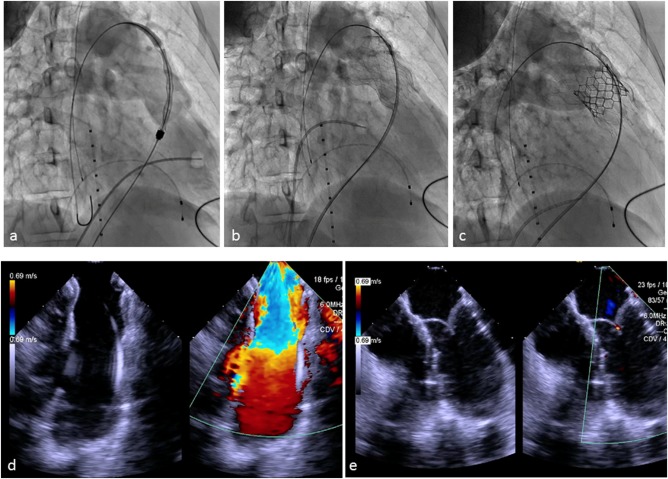
Implantation of the Alterra Adaptive Prestent and SAPIEN 3 valve. Top row, fluoroscopic images. Bottom row, intracardiac echocardiographic images (ICE). A, Right ventricular angiogram with the device in position prior to deployment. B, Following deployment of the Alterra and removal of the delivery system, a pulmonary angiogram demonstrates free pulmonary regurgitation with good apposition of the device to the vessel wall as also seen on the corresponding ICE image (D). C, Pulmonary angiogram following delivery of the SAPIEN 3 demonstrating excellent valve function with trivial pulmonary regurgitation, corresponding to the ICE image below (E).
4. DISCUSSION
Patients who have undergone surgical rehabilitative procedures of their RVOT (e.g., transannular patch, surgical valvotomy, etc.) commonly develop significant pulmonary regurgitation. As the long‐term outcomes of these patients become more clearly defined, it appears that the majority will ultimately benefit from placement of a competent, non‐obstructive pulmonary valve 6. While surgical valve placement remains a viable option, it comes with all of the attendant risks, morbidities, and discomfort associated with repeat open‐heart surgical procedures 7. Currently available balloon expandable THVs have been successfully placed in selected patients with “native” RVOT, however, the vast majority are not suitable anatomic candidates due to the large size and compliant nature of their RVOT 8, 9.
Several centers have recently reported unique approaches to reconfiguring the RVOT in this patient population so as to make them candidates for transcatheter pulmonary valve therapy 10, 11. Our group recently published our experience with a hybrid approach designed to remodel the RVOT using an intravascular technique 10. In that study, a subxiphoid approach was used to simultaneously place a “landing‐zone” stent alongside 1–3 covered stents filled with vascular plugs in an effort to reduce the volume of the RVOT, thereby allowing for implantation of a balloon expandable transcatheter valve. Other centers have used off‐pump surgical approaches to plicate or band the RVOT in an effort to create a “landing zone” for a balloon expandable valve 11. The Alterra Adaptive Prestent was designed to facilitate internal RVOT remodeling providing a predictable rigid “landing zone” for a balloon expandable valve through a simple catheter‐based approach. The concept of preparing the RVOT for a transcatheter valve implant with a prestent is well ingrained in the congenital interventional community and has been shown to have numerous benefits including: providing a stable and safe “landing zone” for valve placement, protection of valve components from extrinsic forces which could lead to valve dysfunction and longer freedom from re‐intervention 12. In the case presented, the ability of this new device to successfully reduce the effective diameter of a large “native RVOT” and create a suitable “landing zone” for successful implantation of a commercially available transcatheter valve provided a simple method to extend this proven therapy (balloon expandable transcatheter valve) to a patient who otherwise would not have been a suitable transcatheter valve candidate. As shown in this initial case, delivery appears to be straight forward and finely controlled with the simple turning of a control knob allowing for precise placement. The fact that the Alterra is designed to provide a “landing zone” for a 29 mm SAPIEN 3 THV has several potential advantages including: (1) use of valve technology with a proven track record and (2) the potential to perform multiple, future re‐valving procedures, an important issue in young patients. Additionally, the generous dimensions of the inflow and outflow portions of this prestent (40 mm) coupled with its relatively short length, particularly when the true covered length (30 mm) is considered, along with its symmetric design may allow for treatment of an expanded range of RVOT anatomies in this population.
As described by others 13, the tremendous variability of RVOT anatomy in this population makes pre‐procedural cross‐sectional imaging a vital tool in selecting patients and procedural planning. Furthermore, the ability to create systolic and diastolic three‐dimensional models in which to perform pre‐procedural virtual “implants” was felt to have added greatly to the success of this initial implant and will likely be of value going forward, particularly early in one's learning curve.
5. CONCLUSION
The patient described in this report was implanted as part of an early feasibility study in the United States. While this initial report is encouraging, much more remains to be evaluated about this novel approach to this complex group of patients.
CONFLICT OF INTEREST
Dr. Zahn is a consultant for Edwards Lifesciences, and is the National Principal Investigator for this trial.
Zahn EM, Chang JC, Armer D, Garg R. First human implant of the Alterra Adaptive PrestentTM: A new self‐expanding device designed to remodel the right ventricular outflow tract. Catheter Cardiovasc Interv. 2018;91:1125–1129. https://doi.org/10.1002/ccd.27581
REFERENCES
- 1. Bonhoeffer P, Boudjemline Y, Saliba Z, Merckx J, Aggoun Y, Bonnet D, Acar P, Le Bidois J, Sidi D, Kachaner J. Percutaneous replacement of pulmonary valve in a right‐ventricle to pulmonary‐artery prosthetic conduit with valve dysfunction. Lancet 2000;356:1403–1405. [DOI] [PubMed] [Google Scholar]
- 2. O'Byrne ML, Gillespie MJ. Will catheter interventions replace surgery for valve abnormalities? Curr Opin Cardiol 2014;29:83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schievano S, Coats L, Migliavacca F, Norman W, Frigiola A, Deanfield J, Bonhoeffer P, Taylor AM. Variations in right ventricular outflow tract morphology following repair of congenital heart disease: Implications for percutaneous pulmonary valve implantation. J Cardiovasc Magn Reson 2007;9:687–695. [DOI] [PubMed] [Google Scholar]
- 4. Capelli C, Taylor AM, Migliavacca F, Bonhoeffer P, Schievano S. Patient‐specific reconstructed anatomies and computer simulations are fundamental for selecting medical device treatment: Application to a new percutaneous pulmonary valve. Philos Trans A Math Phys Eng Sci 2010;368:3027–3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gillespie MJ, Benson LN, Bergersen L, Bacha EA, Cheatham SL, Crean AM, Eicken A, Ewert P, Geva T, Hellenbrand WE, Hor KN, Horlick EM, Jones TK, Mayer J, McHenry BT, Lsten MD, Powell AJ, Zahn EM, Cheatham JP. Patient selection process for the Harmony transcatheter pulmonary valve early feasibility study. Am J Cardiol 2017;120:1387–1392. [DOI] [PubMed] [Google Scholar]
- 6. Geva T. Indications and timing of pulmonary valve replacement after tetralogy of Fallot repair. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2006;9:11–22. [DOI] [PubMed] [Google Scholar]
- 7. Bhagra CJ, Hickey EJ, Van De Bruaene A, Roche SL, Horlick EM, Wald RM. Pulmonary valve procedures late after repair of Tetralogy of Fallot: Current perspectives and contemporary approaches to management. Can J Cardiol 2017;33:1138–1149. [DOI] [PubMed] [Google Scholar]
- 8. Meadows JJ, Moore PM, Berman DP, Cheatham JP, Cheatham SL, Porras D, Gillespie MJ, Rome JJ, Zahn EM, McElhinney DB. Use and performance of the Melody Transcatheter Pulmonary Valve in native and postsurgical, nonconduit right ventricular outflow tracts. Circ Cardiovasc Interv 2014;7:374–380. [DOI] [PubMed] [Google Scholar]
- 9. Boudjemline Y, Brugada G, Van‐Aerschot I, Patel M, Basquin A, Bonnet C, Legendre A, Bonnet D, Iserin L. Outcomes and safety of transcatheter pulmonary valve replacement in patients with large patched right ventricular outflow tracts. Arch Cardiovasc Dis 2012;105:404–413. [DOI] [PubMed] [Google Scholar]
- 10. Phillips AB, Nevin P, Shah A, Olshove V, Garg R, Zahn EM. Development of a novel hybrid strategy for transcatheter pulmonary valve replacement in patients following transannular patch repair of tetralogy of fallot. Catheter Cardiovasc Interv 2016;87:403–410. [DOI] [PubMed] [Google Scholar]
- 11. Sosnowski C, Matella T, Fogg L, Ilbawi M, Nagaraj H, Kavinsky C, Wolf AR, Diab K, Caputo M, Kenny D. Hybrid pulmonary artery plication followed by transcatheter pulmonary valve replacement: Comparison with surgical PVR. Catheter Cardiovasc Interv 2016;88:804–810. [DOI] [PubMed] [Google Scholar]
- 12. McElhinney DB, Cheatham JP, Jones TK, Lock JE, Vincent JA, Zahn EM, Hellenbrand WE. Stent fracture, valve dysfunction, and right ventricular outflow tract reintervention after transcatheter pulmonary valve implantation patient‐related and procedural risk factors in the US Melody Valve Trial. Circ Cardiovasc Interv 2011;4:602–614. [DOI] [PubMed] [Google Scholar]
- 13. Chung R, Taylor AM. Imaging for preintervention planning: Trancatheter pulmonary valve therapy. Circ Cardiovasc Imaging 2014;7:182–189. [DOI] [PubMed] [Google Scholar]


