Abstract
Valbenazine (Ingrezza): the first FDA-approved treatment for tardive dyskinesia
INTRODUCTION
Tardive dyskinesia (TD) is a spectrum of hyperkinetic movement disorders associated with the use of dopamine receptor blocking agents. Among the dopamine receptor blockers, neuroleptics or antipsychotics have been the drugs most frequently associated with TD.1 The diagnosis of TD, as set forth by the Diagnostic and Statistical Manual of Mental Disorders, fourth edition, text revision, requires ruling out other potential medical conditions; exposure to an antipsychotic for at least four weeks; and the presence of physical symptomatology.2 Involuntary athetoid or choreiform movements of the tongue, lips, face, and extremities are classic characteristics of TD and may appear one to two years into treatment with dopamine receptor blockers. Routine use of the Abnormal Involuntary Movement Scale (AIMS) is widely accepted for detecting or evaluating the severity of dyskinesia in patients receiving chronic antipsychotic therapies.3 The primary pathophysiology of TD is unknown; etiologies that have been investigated include dopamine receptor super-sensitivity, gamma-aminobutyric acid hypofunction, and neurodegeneration.2,4
Antipsychotic therapy is the primary treatment for schizophrenia, and many patients may require its lifelong use. The incidence of TD is nearly 1%, with a prevalence of up to 30% in patients with schizophrenia receiving antipsychotic therapies.5 The second-generation antipsychotics have been associated with a lower risk of developing TD than first-generation antipsychotics; however, recent publications suggest that the incidence and prevalence of TD may be comparable.5,6 People at higher risk for developing TD include those receiving prolonged treatment with antipsychotics, the elderly, women, and African-Americans.7 Pharmaco genomic involvement of dopamine receptor polymorphisms (Ser9Gly and TaqA) has also been associated with increased development of TD in select ethnicities.8
Valbenazine (Ingrezza, Neurocrine Biosciences), approved by the FDA in 2017, is the first medication indicated for the treatment of adults with TD.9 Until now, there has been no well-studied treatment for TD, and management strategies have included utilizing off-label medications or supplements, such as tetrabenazine, clonazepam, or Ginkgo biloba.10 Tetrabenazine, approved for the treatment of chorea associated with Huntington’s disease, is a monoamine depleter that has shown positive results in small studies and case reports of TD.11–13 The active metabolites of tetrabenazine, alpha and beta enantiomers, function as vesicle monoamine transporter 2 (VMAT2) inhibitors and antagonists at the dopamine D2 receptor, respectively.13 This racemic combination may correspond to tetrabenazine’s antipsychotic-like adverse effect profile and the risk of suicidal ideation, depression, and neuroleptic malignant syndrome.11 Valbenazine is the purified prodrug of the (+)-alpha-isomer of tetrabenazine, the most selective enantiomer for VMAT2.13
CHEMICAL STRUCTURE
Valbenazine, a benzoquinolizidine derivative, is a highly selective VMAT2 inhibitor.14,15 Its molecular formula is C38H54N2O10S2, and its molecular weight is 762.97 g/mol; its chemical structure is shown in Figure 1. As Ingrezza, it is available as 40-mg and 80-mg capsules. Valbenazine is formulated as a tosylated salt and is slightly soluble in water. Each capsule contains 73 mg or 146 mg of valbenazine tosylate, which is equivalent to 40 mg or 80 mg of valbenazine, respectively. The capsules should be stored at temperatures between 20° and 25° C.14
Figure 1.
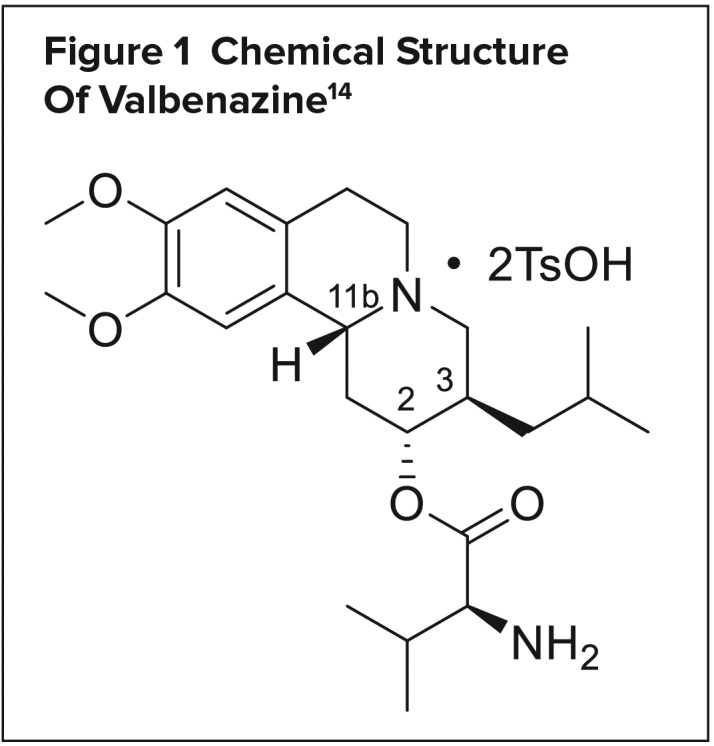
Chemical Structure Of Valbenazine14
MECHANISM OF ACTION
Valbenazine’s mechanism of action is mediated through the reversible inhibition of VMAT2 in the treatment of TD.14 VMAT2 is selective to the central nervous system and is responsible for the transport and recycling of neurotransmitters across the synapse. The inhibition of VMAT2 augments neurotransmitter degradation and results in presynaptic neurotransmitter depletion, particularly of dopamine.16 Dopamine neurotransmission is essential for motor control and its dysregulation is often associated with hyperkinetic disorders.
Valbenazine and its active metabolite (+)-alpha-dihydroxytetrabenazine (R,R,R-HTBZ) demonstrate a high degree of affinity for VMAT2 with inhibitory constants (Ki) of 150 nM and approximately 3 nM, respectively, with no appreciable binding affinity (Ki greater than 5,000 nM) for dopaminergic, serotonergic, or adrenergic receptors.14 R,R,R-HTBZ is one of the four metabolites of tetrabenazine and may be responsible for its benefit in patients with Huntington’s chorea and TD.
PHARMACOKINETICS
The selective affinity of valbenazine and R,R,R-HTBZ to VMAT2 may be responsible for the drug’s therapeutic effect in managing TD.
Absorption and Distribution
Valbenazine has moderate absorption with an absolute oral bioavailability of approximately 49%; time to maximum concentration ranges from 0.5 to 1.0 hour. Valbenazine can be taken with or without food; however, ingestion of high-fat meals decreases maximum plasma concentration (Cmax) by 47% and area under the curve (AUC) by 13%. The R,R,R-HTBZ Cmax and AUC are unaffected by high-fat meals. The mean steady-state volume of distribution is 92 L. Valbenazine and R,R,R-HTBZ exhibit protein binding of 99% and 64%, respectively.14
Metabolism and Elimination
Valbenazine is primarily metabolized via the hydrolysis of the valine ester and cytochrome P450 (CYP) 3A4/5 into R,R,R-HTBZ and a mono-oxy metabolite. The R,R,R-HTBZ is further metabolized in part by CYP2D6. Valbenazine and R,R,R-HTBZ have a half-life of 15 to 22 hours and a mean plasma clearance of 7.2 L per hour. After administration of an oral dose, approximately 60% was excreted in the urine and 30% in the feces.14
CLINICAL STUDIES
The safety and efficacy of valbenazine were established in two published randomized, double-blind, placebo-controlled trials, Kinect 2 and Kinect 3. Both used blinded movement-disorder specialists as video raters to assess AIMS scores. Unlike previous VMAT inhibitors, once-daily valbenazine demonstrated efficacy with a limited side effect profile.
KINECT 2
In this phase 2 trial, O’Brien et al. observed a mean reduction of 2.4 points on the AIMS at six weeks in patients with moderate-to-severe TD taking valbenazine. This dose-titration study randomized 102 medically stable patients 18 to 85 years of age with clinical diagnoses of schizophrenia, schizoaffective disorder, or mood disorder. Patients had to remain on a stable dose of a dopamine antagonist for at least 30 days before baseline. Tetrabenazine and the use of as-needed benzodiazepines, amantadine, or anticholinergic medications were prohibited.17
Patients were randomized 1:1 to valbenazine or placebo. The valbenazine group was initiated at 25 mg and titrated 25 mg every two weeks to a maximum of 75 mg per day as tolerable. Fifty-seven percent of the patients were men, mean age was 56 years, and the mean AIMS score was 8 at baseline. At six weeks, the reduction in AIMS score from baseline was 1.1 for placebo and 3.6 for valbenazine (P = 0.0005). Secondary assessments noted statistical improvement in the Clinical Global Impression of Change Scale for Tardive Dyskinesia (CGI-TD) of 0.8 (P < 0.0001) at six weeks.17
KINECT 3
The Kinect 3 trial was a phase 3, parallel-group trial of valbenazine in patients with TD. The six-week study randomized 225 participants in a 1:1:1 fixed-sequence ratio to once-daily placebo, valbenazine 40 mg per day, or valbenazine 80 mg per day. The primary efficacy endpoint was change from baseline at week 6 between placebo and valbenazine 80 mg per day on the AIMS score. Nearly 65% of patients were diagnosed with schizophrenia or schizoaffective disorder, with an average baseline AIMS of 10, and 85.5% of the patients were receiving antipsychotics.18
At six weeks, valbenazine 80 mg per day demonstrated a mean reduction in AIMS score of 3.2 (P < 0.001) versus placebo.18
ADVERSE EFFECTS
In three six-week, randomized, double-blind, placebo-controlled clinical trials, 262 patients received 25 mg to 100 mg of valbenazine per day. Based on pooled data analysis, the most common adverse reactions were somnolence (10.9%) and anticholinergic effects (5.4%). Other adverse effects included balance disorders and falls (4.1%), headache (3.4%), akathisia (2.7%), vomiting (2.6%), nausea (2.3%), and arthralgia (2.3%). During the controlled studies, there were dose-related increases in prolactin, alkaline phosphatase, and bilirubin.14,17,18
DOSAGE AND ADMINISTRATION
Valbenazine is intended only for oral administration in adults and is dosed once daily, with or without food. The initial dose of 40 mg once daily is administered for one week and then increased to the recommended dose of 80 mg once daily.14
WARNING AND PRECAUTIONS
Somnolence and QT prolongation have been associated with valbenazine. Patients should not perform activities requiring mental alertness, such as operating heavy machinery, until they know how they will be affected. Based on two healthy volunteer studies, valbenazine resulted in a mean QT prolongation of 6.7 msec in healthy individuals and 11.7 msec in poor metabolizers of CYP2D6 or patients taking strong CYP2D6 or CYP3A4 inhibitors. A baseline electrocardiogram may be warranted in patients with these risk factors.14
Special Populations
Pediatric and Geriatric Use
No controlled trials have been conducted to establish the safety and efficacy of valbenazine in the pediatric population. In the geriatric population, no dose adjustment is recommended. In the Kinect 3 trial, only 16% of patients included in the efficacy and safety analysis were 65 years of age and older.14
Hepatic and Renal Impairment
The recommended dose for patients with moderate or severe hepatic impairment (Child–Pugh score 7–15) is 40 mg once daily. Valbenazine does not undergo primary renal metabolism, and no dose reduction is needed in patients with mild-to-moderate renal impairment (creatinine clearance, 30 to 90 mL/min). Valbenazine is not recommended in patients with severe renal impairment.14
Pregnancy and Lactation
Limited data exist to inform prescribers of any drug-associated risk in pregnant or lactating women. In animal studies, a delay in fetal development in pregnant rabbits and an increased incidence of stillbirths and postnatal pup mortality in pregnant rats were noted at the maximum recommended human dose of 80 mg per day. No malformation or effect on neurobehavioral functioning was observed. Valbenazine and its metabolite were found in rat milk at 0.1 to 1.2 times the maximum recommended human dose. Due to a lack of information, women should be cautioned about valbenazine use in pregnancy. Breastfeeding is not recommended during treatment and for five days after the final dose.14
Drug–Drug Interactions
Several drug classes have clinically important interactions with valbenazine, including monoamine oxidase inhibitors, strong CYP3A4 inhibitors, strong CYP2D6 inhibitors, strong CYP3A4 inducers, and digoxin (Table 1).14
Table 1.
Clinically Significant Drug Interactions With Valbenazine14
| Monoamine Oxidase Inhibitors | |
|---|---|
| Clinical implication | Concomitant use of valbenazine with MAOIs may increase the concentration of monoamine neurotransmitters in synapses, potentially leading to increased risk of adverse reactions, such as serotonin syndrome, or attenuated treatment effect of valbenazine. |
| Prevention or management | Avoid concomitant use of valbenazine with MAOIs. |
| Examples | Isocarboxazid (Marplan, Validus Pharmaceuticals), phenelzine, selegiline |
| Strong CYP3A4 Inhibitors | |
| Clinical implication | Concomitant use of valbenazine with strong CYP3A4 inhibitors increased the exposure (Cmax and AUC) to valbenazine and its active metabolite compared with the use of valbenazine alone. Increased exposure of valbenazine and its active metabolite may increase the risk of exposure-related adverse reactions. |
| Prevention or management | Reduce valbenazine dose when valbenazine is coadministered with a strong CYP3A4 inhibitor. |
| Examples | Itraconazole, ketoconazole, clarithromycin |
| Strong CYP2D6 Inhibitors | |
| Clinical implication | Concomitant use of valbenazine with strong CYP2D6 inhibitors may increase the exposure (Cmax and AUC) to valbenazine’s active metabolite compared with the use of valbenazine alone. Increased exposure of active metabolite may increase the risk of exposure-related adverse reactions. |
| Prevention or management | Consider reducing valbenazine dose based on tolerability when valbenazine is coadministered with a strong CYP2D6 inhibitor. |
| Examples | Paroxetine, fluoxetine, quinidine |
| Strong CYP3A4 Inducers | |
| Clinical implication | Concomitant use of valbenazine with a strong CYP3A4 inducer decreased the exposure of valbenazine and its active metabolite compared with the use of valbenazine alone. Reduced exposure of valbenazine and its active metabolite may reduce efficacy. |
| Prevention or management | Concomitant use of strong CYP3A4 inducers with valbenazine is not recommended. |
| Examples | Rifampin, carbamazepine, phenytoin, St. John’s worta |
| Digoxin | |
| Clinical implication | Concomitant use of valbenazine with digoxin increased digoxin levels because of inhibition of intestinal P-glycoprotein. |
| Prevention or management | Digoxin concentrations should be monitored when coadministering valbenazine with digoxin. Increased digoxin exposure may increase the risk of exposure-related adverse reactions. Dosage adjustment of digoxin may be necessary. |
The induction potency of St. John’s wort may vary widely based on preparation.
AUC = area under the curve; Cmax = maximum plasma concentration; CYP = cytochrome P450; MAOIs = monoamine oxidase inhibitors.
In contrast, based on in vitro study results, substrates of CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2E1, or CYP3A4/5 have no clinically important interactions with valbenazine, and dosage adjustment for valbenazine is not needed.14
COST
The average wholesale price for 30 capsules of valbenazine (a one-month supply for most patients) is $5,750 for the 40-mg strength and $6,225 for the 80-mg strength.19 Neurocrine, the maker of Ingrezza, offers a patient-assistance program and a patient copay coupon that will cover up to a maximum of 35% of annual wholesale acquisition costs for Ingrezza. This program is not available for uninsured patients or those insured under any federal, state, or governmental program. Valbenazine can only be purchased from specialty pharmacies.20
P&T COMMITTEE CONSIDERATIONS
Over the last decade, antipsychotics have become ubiquitous, gaining indications and off-label uses in many psychiatric disorders. Despite the increased use of second-generation antipsychotics, the foreseeable risks for TD have apparently not changed, and its prevalence may continue to remain stable or even rise. Unlike its predecessors, valbenazine has demonstrated efficacy without a reported risk of worsening psychiatric conditions. In patients who develop TD from chronic antipsychotic therapies, valbenazine could be considered as the first-line option to manage their dyskinesia.
The high cost of valbenazine may limit its use and will be the major obstacle for access to this medication. Nearly 80% of patients with schizophrenia receive insurance through the government, and a large population is treated within state hospital systems where resources and budgets may be limited.21
CONCLUSION
Valbenazine is the first medication approved by the FDA for the treatment of adults with TD, a spectrum of hyperkinetic movement disorders often associated with antipsychotic therapy. Once-daily valbenazine has demonstrated efficacy with a limited side effect profile, but the extent of its utilization remains to be seen. Clinicians will have to consider the implications on the cost of health care if this novel treatment is to be implemented to manage TD.
Footnotes
Disclosures: Dr. Rey serves on the Advisory Board and Speakers’ Bureau at Neurocrine Biosciences. Dr. Uhlyar reports no commercial or financial interests in regard to this article.
REFERENCES
- 1.Waln O, Jankovic J. An update on tardive dyskinesia: from phenomenology to treatment. Tremor Other Hyperkinet Mov (N Y) 2013;12:3. doi: 10.7916/D88P5Z71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, D.C.: American Psychiatric Association; 2000. text revision. [Google Scholar]
- 3.Lane RD, Glazer WM, Hansen TE, et al. Assessment of tardive dyskinesia using the Abnormal Involuntary Movement Scale. J Nerv Ment Dis. 1985;173(6):353–357. doi: 10.1097/00005053-198506000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Tamminga CA, Woerner MG. Clinical course and cellular pathology of tardive dyskinesia. In: Davis KL, Charney D, Coyle JT, Nemeroff C, editors. Neuropsychopharmacology: The Fifth Generation of Progress. Philadelphia, Pennsylvania: Lippincott, Williams and Wilkins; 2002. pp. 1831–1841. [Google Scholar]
- 5.Woods SW, Morgenstern H, Saksa JR, et al. Incidence of tardive dyskinesia with atypical and conventional antipsychotic medications: prospective cohort study. J Clin Psychiatry. 2010;71(4):463–474. doi: 10.4088/JCP.07m03890yel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Correll CU, Leucht S, Kane JM. Lower risk for tardive dyskinesia associated with second-generation antipsychotics: a systemic review of 1-year studies. Am J Psychiatry. 2004;161:414–425. doi: 10.1176/appi.ajp.161.3.414. [DOI] [PubMed] [Google Scholar]
- 7.Woerner MG, Correll CU, Alvir JM, et al. Incidence of tardive dyskinesia with risperidone or olanzapine in the elderly: results from a 2-year, prospective study in antipsychotic-naïve patients. Neuropsychopharmacology. 2011;36(8):1738–1746. doi: 10.1038/npp.2011.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang F, Fung V. Clinical significance of pharmacogenomic studies in tardive dyskinesia associated with patients with psychiatric disorders. Pharmgenomics Pers Med. 2014;7:317–328. doi: 10.2147/PGPM.S52806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Food and Drug Administration. FDA approves first drug to treat tardive dyskinesia. Apr 11, 2017. [Accessed February 28, 2018]. Available at: www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm552418.htm.
- 10.Bhidayasiri R, Fahn S, Weiner GS. Evidence-based guidelines: treatment of tardive syndromes. Neurology. 2013;81(5):463–469. doi: 10.1212/WNL.0b013e31829d86b6. [DOI] [PubMed] [Google Scholar]
- 11.Xenazine (tetrabenazine) prescribing information. Deerfield, Illinois: Ovation Pharmaceuticals, Inc.; 2008. [Google Scholar]
- 12.Leung JG, Breden EL. Tetrabenazine for the treatment of tardive dyskinesia. Ann Pharmacother. 2011;45(4):525–531. doi: 10.1345/aph.1P312. [DOI] [PubMed] [Google Scholar]
- 13.Muller T. Valbenazine granted breakthrough drug status for treating tardive dyskinesia. Expert Opin Investig Drugs. 2015;24(6):737–742. doi: 10.1517/13543784.2015.1029573. [DOI] [PubMed] [Google Scholar]
- 14.Ingrezza (valbenazine) prescribing information. San Diego, California: Neurocrine Biosciences, Inc.; 2017. [Google Scholar]
- 15.Grigoriadis DE, Smith E, Hoare SR, et al. Pharmacological characterization of valbenazine (NBI-98854) and its metabolites. J Pharmacol Exp Ther. 2017;361(3):454–461. doi: 10.1124/jpet.116.239160. [DOI] [PubMed] [Google Scholar]
- 16.Stahl SM, Grady MM. Stahl’s Essential Psychopharmacology: Neuroscientific Basis and Practical Applications. Cambridge, United Kingdom: Cambridge University Press; 2011. [Google Scholar]
- 17.O’Brien CF, Jimenez R, Hauser RA, et al. NBI-98854, a selective monoamine transport inhibitor for the treatment of tardive dyskinesia: a randomized, double-blind, placebo-controlled study. Mov Disrd. 2015;30(12):1681–1687. doi: 10.1002/mds.26330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hauser RA, Factor SA, Marder SR, et al. KINECT 3: A phase 3 randomized, double-blind, placebo-controlled trial of valbenazine for tardive dyskinesia. Am J Psychiatry. 2017;174(5):476–484. doi: 10.1176/appi.ajp.2017.16091037. [DOI] [PubMed] [Google Scholar]
- 19.Red Book Online. Ann Arbor, Michigan: Truven Health Analytics; [Accessed May 2, 2018]. [Google Scholar]
- 20.Neurocrine. Introducing the INBRACE support program. 2018. [Accessed March 1, 2018]. Available at: http://inbracesupportprogram.com.
- 21.Khaykin E, Eaton W, Ford D, et al. Health insurance coverage among persons with schizophrenia in the United States. Psychiatr Serv. 2010;61(8):830–834. doi: 10.1176/ps.2010.61.8.830. [DOI] [PMC free article] [PubMed] [Google Scholar]


