Abstract
Aim
This phase III, multicentre, randomized study (http://clinicaltrials.gov; NCT01958671) evaluated the efficacy and safety of ertugliflozin monotherapy in adults with inadequately controlled type 2 diabetes (glycated haemoglobin [HbA1c], 7.0% to 10.5% [53‐91 mmol/mol]) despite diet and exercise.
Materials and methods
The 52‐week study comprised a 26‐week, double‐blind, placebo‐controlled period (Phase A) during which 461 participants received placebo, ertugliflozin 5 mg/d or ertugliflozin 15 mg/d. This was followed by a 26‐week active‐controlled period (Phase B) during which participants in the placebo group who had not received glycaemic rescue therapy had blinded metformin added. Results to Week 52 are reported. Because of the use of metformin in Phase B, no statistical comparisons of efficacy were made between the ertugliflozin and placebo/metformin groups at Week 52.
Results
The mean (standard error) change from baseline to Week 52 in HbA1c was −0.9% (0.1) and −1.0% (0.1) in the ertugliflozin 5 and 15 mg groups, respectively. The proportions of participants with HbA1c <7.0% at Week 52 were 25.6% and 28.5%, respectively. Ertugliflozin reduced fasting plasma glucose, body weight and systolic blood pressure (SBP). The incidence of genital mycotic infections (GMIs) in females was significantly higher in both ertugliflozin groups (5 mg, 26.9%; 15 mg, 29.0%) vs the placebo/metformin group (9.9%), and in males was significantly higher in the 15 mg group (7.8%) vs the placebo/metformin group (1.2%). Ertugliflozin was not associated with increased incidence of urinary tract infections, symptomatic hypoglycaemia or hypovolaemia adverse events compared with placebo/metformin.
Conclusions
Ertugliflozin treatment over 52 weeks improved glycaemic control and reduced body weight and SBP, but increased GMIs.
Keywords: ertugliflozin, monotherapy, SGLT2 inhibitor, type 2 diabetes mellitus
1. INTRODUCTION
Inhibition of renal glucose reabsorption and reduction of the renal threshold for glucose excretion via inhibition of sodium‐glucose co‐transporter 2 (SGLT2) has been shown to improve glycaemic control in patients with type 2 diabetes mellitus (T2DM). SGLT2 inhibitors are the most recent class of oral anti‐hyperglycaemic agent (AHA) approved for the treatment of this condition.1, 2
Ertugliflozin is a highly selective SGLT2 inhibitor under clinical development for T2DM. In phase II studies in individuals with T2DM, ertugliflozin significantly reduced plasma glucose and glycated haemoglobin (HbA1c) levels, and showed beneficial effects on body weight and blood pressure.3, 4 Results from phase III studies demonstrate that ertugliflozin provided clinically meaningful reductions from baseline in HbA1c, body weight and blood pressure through 26 weeks of treatment.5, 6, 7 Several of these studies include controlled extension phases for the purpose of collecting longer‐term efficacy and safety data.
The phase III VERTIS (eValuation of ERTugliflozin effIcacy and Safety) MONO study (NCT01958671) was designed to assess the efficacy and safety of ertugliflozin monotherapy in participants with T2DM and inadequate glycaemic control despite diet and exercise. The study comprised a 26‐week, double‐blind, placebo‐controlled treatment period (Phase A), followed by a 26‐week active‐controlled treatment period (Phase B). We have previously reported the results from the Phase A treatment period, wherein ertugliflozin significantly improved glycaemic control and body weight after 26 weeks of treatment.8 Here, we report efficacy and safety results through to study completion (Week 52; Phase A + B).
2. MATERIALS AND METHODS
The study was conducted in compliance with the ethical principles derived from the Declaration of Helsinki and in compliance with all International Council on Harmonisation Good Clinical Practice Guidelines. The protocol was approved by institutional review boards or independent ethics committees, and written informed consent was obtained from all study participants. Participants were enrolled at 67 sites across 7 countries (USA, Canada, Israel, Italy, Mexico, South Africa and the UK).
2.1. Key eligibility criteria
The eligibility criteria and study design have been published previously.8 In brief, men and women aged ≥18 years, with a diagnosis of T2DM in accordance with American Diabetes Association guidelines, and who had inadequate glycaemic control (HbA1c, 7.0%‐10.5% [53‐91 mmol/mol]) despite diet and exercise, with a body mass index (BMI) ≥18.0 kg/m2 were included in the study. Exclusion criteria included type 1 diabetes mellitus; history of ketoacidosis; screening fasting plasma glucose (FPG) or finger‐stick glucose >15 mmol/L (270 mg/dL); estimated glomerular filtration rate (eGFR) <55 mL/min/1.73 m2; serum creatinine ≥115 μmol/L (1.3 mg/dL) in men or ≥106 μmol/L (1.2 mg/dL) in women; or history of a cardiovascular event within 3 months of screening.
2.2. Study design and treatment
The design of the trial is shown in Figure S1. This was a double‐blind, double‐dummy study. On Day 1, participants were randomized via an interactive automated system to receive placebo, ertugliflozin 5 mg, or ertugliflozin 15 mg (1:1:1) once daily, based on a computer‐generated randomization code, using the method of random permuted blocks. In Phase A, glycaemic rescue therapy with open‐label metformin was prescribed for participants who exceeded protocol‐specified glycaemic thresholds. Participants randomized to placebo who did not receive glycaemic rescue in Phase A were switched to blinded metformin in Phase B, beginning at the Week 26 visit. Participants rescued with open‐label metformin in Phase A continued to receive this in Phase B, in addition to the randomized treatment. Metformin dosing and titration was as follows: Weeks 26 and 27, 500 mg metformin twice daily; Weeks 28 and 29, 1000 mg metformin in the morning and 500 mg in the evening; Week 30 onward, 1000 mg metformin twice daily. To maintain blinding in Phase B, participants in the ertugliflozin groups received blinded metformin placebo in addition to continuing to receive ertugliflozin. Therefore, participants in the ertugliflozin groups received up to 52 weeks of treatment with ertugliflozin, and those in the control (placebo/metformin) group received 26 weeks of placebo, followed by 26 weeks of metformin therapy. During Phase B, glycaemic rescue therapy with glimepiride was initiated in participants with FPG >11.1 mmol/L (200 mg/dL) or HbA1c >8.0% (64 mmol/mol). Participants who received glycaemic rescue remained in the study and continued to receive study medication in a blinded fashion, unless they met any of the discontinuation criteria.
2.3. Study endpoints
The primary objective of the Phase B portion of the trial was to assess the safety and tolerability of ertugliflozin through Week 52. All hypotheses were tested at Week 26. The secondary objectives were to assess the change from baseline to Week 52 in efficacy endpoints (see below). There were no formal hypothesis tests at Week 52.
2.4. Efficacy assessments
A detailed description of efficacy assessments at Week 26 has been published previously.8 Change from baseline to Week 52 was calculated for HbA1c, FPG, body weight and systolic and diastolic blood pressure (SBP; DBP). The proportion of participants with HbA1c <7.0% (53 mmol/mol) and with HbA1c <6.5% (48 mmol/mol) at Week 52 was assessed, as was the proportion of participants who received glycaemic rescue therapy.
2.5. Safety assessments
A detailed description of safety assessments has been published previously.8 Here we report safety results for the combined Phase A + B treatment period, although prespecified adverse events (AEs) of special interest (termed “Tier 1 AEs”; see below) are reported for both the Phase A and Phase A + B treatment periods to enable comparison of the time course for AEs of special interest. Genital mycotic infections (GMIs), urinary tract infections (UTIs), symptomatic hypoglycaemia and hypovolaemia were prespecified “Tier 1 AEs.” AEs related to GMIs, UTIs and hypovolaemia were identified based on prespecified, sponsor‐generated, customized queries of preferred terms. Because of the mechanism of action of ertugliflozin, osmotic diuresis AEs were assessed in addition to hypovolaemia AEs. Tier 1 analysis of hypoglycaemia included the numbers and percentages of participants experiencing ≥1 AE of symptomatic hypoglycaemia, regardless of biochemical documentation. The incidence of documented hypoglycaemia, defined as episodes with a glucose level ≤3.9 mmol/L (70 mg/dL), with or without symptoms, was assessed. Severe hypoglycaemia, defined as episodes that required the assistance of a third party, was also evaluated.
Prespecified events were subject to adjudication by an external panel of independent physicians, with medical expertise relevant to the event type, who were blinded to participant treatment. These Clinical Adjudication Committees (CACs) evaluated cardiovascular events and all‐cause deaths, fractures, pancreatitis, renal and hepatic events. Potential cases of ketoacidosis were reviewed by an internal blinded case review committee. Adjudication results from the cardiovascular committee will be reported separately as part of a programme‐wide summary.
2.6. Statistical analyses
The population for Phase A + B descriptive efficacy analyses included all randomized participants who received ≥1 dose of study medication and who had baseline data and ≥1 post‐randomization observation for the analysis endpoint subsequent to ≥1 dose of study treatment. Because of the use of metformin in Phase B, no formal efficacy comparisons between the ertugliflozin and placebo/metformin groups were made at Week 52, and raw mean changes from baseline are presented. Change from baseline in endpoints at Week 52 were also assessed, using a longitudinal data analysis (LDA) model9 for the ertugliflozin groups. Least squares (LS) mean change from baseline is presented from this analysis, which is consistent with the type conducted at Week 26, the results of which have been reported previously.8 Efficacy endpoints were summarized using the “excluding rescue approach”: efficacy data obtained after the initiation of glycaemic rescue therapy were censored (ie, treated as missing) to avoid confounding. The observed proportion of patients with HbA1c <7.0% (53 mmol/mol) and with HbA1c <6.5% (48 mmol/mol) at Week 52 was assessed.
The population for Phase A + B safety analyses included all randomized participants who received ≥1 dose of study medication, and included all data from randomization up to 14 days after the final dose of study medication for AEs and 2 days for laboratory parameters. With the exception of hypoglycaemia, safety analyses were conducted using the “including rescue approach.” GMIs (by gender), UTIs, symptomatic hypoglycaemia and hypovolaemia AEs (“Tier 1 AEs”) were subject to inferential testing without multiplicity control, for which P values and 95% CIs for between‐treatment differences were provided, using the Miettinen and Nurminen method.10 The term “significantly” higher or lower has been used when the P value was <.05. Other safety results have been described as “higher” or “lower” as part of a qualitative assessment only. Percent changes from baseline in low‐density lipoprotein cholesterol (LDL‐C) and high‐density lipoprotein cholesterol (HDL‐C) were analysed using a LDA model, which included terms for treatment, time, treatment by time interaction, prior anti‐hyperglycaemic medication (yes, no) and baseline eGFR (continuous).
3. RESULTS
3.1. Participant disposition and baseline characteristics
In Phase A, 461 participants were randomized and received ≥1 dose of study medication. A total of 384 participants entered and received ≥1 dose of study medication in Phase B (Figures S2 and S3). Baseline demographics and clinical characteristics for the Phase A + B treatment period were similar across treatment groups (Table S1). The mean (± standard deviation [SD]) overall age was 56.4 (± 11.0) years and 56.6% of participants were men. Mean (± SD) baseline HbA1c was 8.2% (± 1.0) (66.2 [± 10.7] mmol/mol), BMI was 33.0 (± 6.7) kg/m2 and mean duration of T2DM was 5.0 (± 5.1) years. Of patients who entered Phase B, mean baseline (± SD) age was 56.8 (± 11.0) years, 56.3% were men, and mean (± SD) baseline HbA1c was 8.2% (± 1.0) (65.8 [± 10.6] mmol/mol). The mean duration of exposure to study medication (“including rescue approach”) was 303.4 days for placebo/metformin, 306.4 days for ertugliflozin 5 mg and 311.7 days for ertugliflozin 15 mg.
A total of 127 participants (27.5%) discontinued study medication by Week 52 (Figures S2 and S3). The proportion of participants who discontinued study medication in the Phase A + B treatment period was higher in the placebo/metformin group (33.3%) compared with the ertugliflozin 5 mg and ertugliflozin 15 mg groups (26.9% and 22.4%, respectively). In Phase B, 50 participants (13.0%) discontinued study medication (14.3%, 14.9% and 9.9% in the placebo/metformin, ertugliflozin 5 mg and ertugliflozin 15 mg groups, respectively).
3.2. Efficacy outcomes
Reductions from baseline in HbA1c were observed in both ertugliflozin groups at the first post‐randomization visit (Week 6) and thereafter. Mean HbA1c levels at Week 26 were 7.8% (61.3 mmol/mol), 7.3% (56.4 mmol/mol) and 7.3% (56.1 mmol/mol) in the placebo/metformin, ertugliflozin 5 mg and ertugliflozin 15 mg groups, respectively. The reductions in HbA1c observed at Week 26 with ertugliflozin were maintained through Week 52 (Figure 1A). At Week 52, the mean (standard error [SE]) change from baseline in HbA1c was −1.0% (0.1), −0.9% (0.1) and −1.0% (0.1) in the placebo/metformin and ertugliflozin 5 mg and ertugliflozin 15 mg groups, respectively. In Phase B, the median metformin dose was 1790.0 mg/d. The last dose of metformin was 2000 mg/d for 67.4% of participants in the placebo/metformin group (who were not rescued in Phase A). At Week 52, 27.5%, 25.6% and 28.5% of patients in the placebo/metformin, ertugliflozin 5 mg and ertugliflozin 15 mg groups, respectively, had HbA1c <7%. The proportions with HbA1c <6.5% at Week 52 were 18.3%, 9.0% and 9.9%, respectively. The percentage of participants who received glycaemic rescue medication through Week 52 was 31.4% in the placebo/metformin group, 19.9% in the ertugliflozin 5 mg group and 15.1% in the ertugliflozin 15 mg group. Reductions from baseline to Week 52 in both ertugliflozin groups were also observed for FPG, body weight and SBP. The mean (SE) change from baseline to Week 52 in FPG was −1.6 mmol/L (0.2), −1.6 mmol/L (0.2) and −1.8 mmol/L (0.2) in the placebo/metformin, ertugliflozin 5 mg and ertugliflozin 15 mg groups, respectively (Figure 1B). The mean (SE) change from baseline to Week 52 in body weight was −3.3 kg (0.5), −3.6 kg (0.4) and −3.7 kg (0.4), respectively (Figure 2A), and that for SBP was −2.4 (1.3), −3.7 (1.3) and −1.8 (1.2) mm Hg, respectively (Figure 2B). Results for DBP are shown in Figure 2C. LDA model‐based LS mean changes from baseline for the ertugliflozin groups are provided in Table S2. These results are consistent with the change from baseline in raw means at Week 52.
Figure 1.
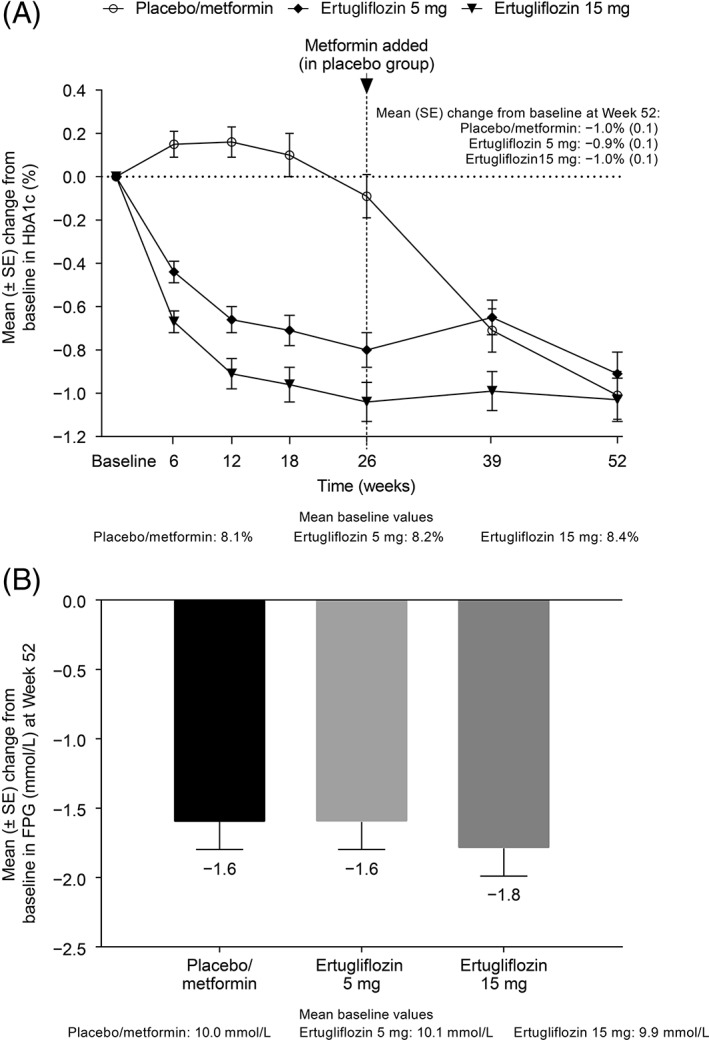
A, Change from baseline over time in HbA1c and B, change from baseline at Week 52 in fasting plasma glucose. Abbreviations: FPG, fasting plasma glucose; HbA1c, glycated haemoglobin; SE, standard error
Figure 2.
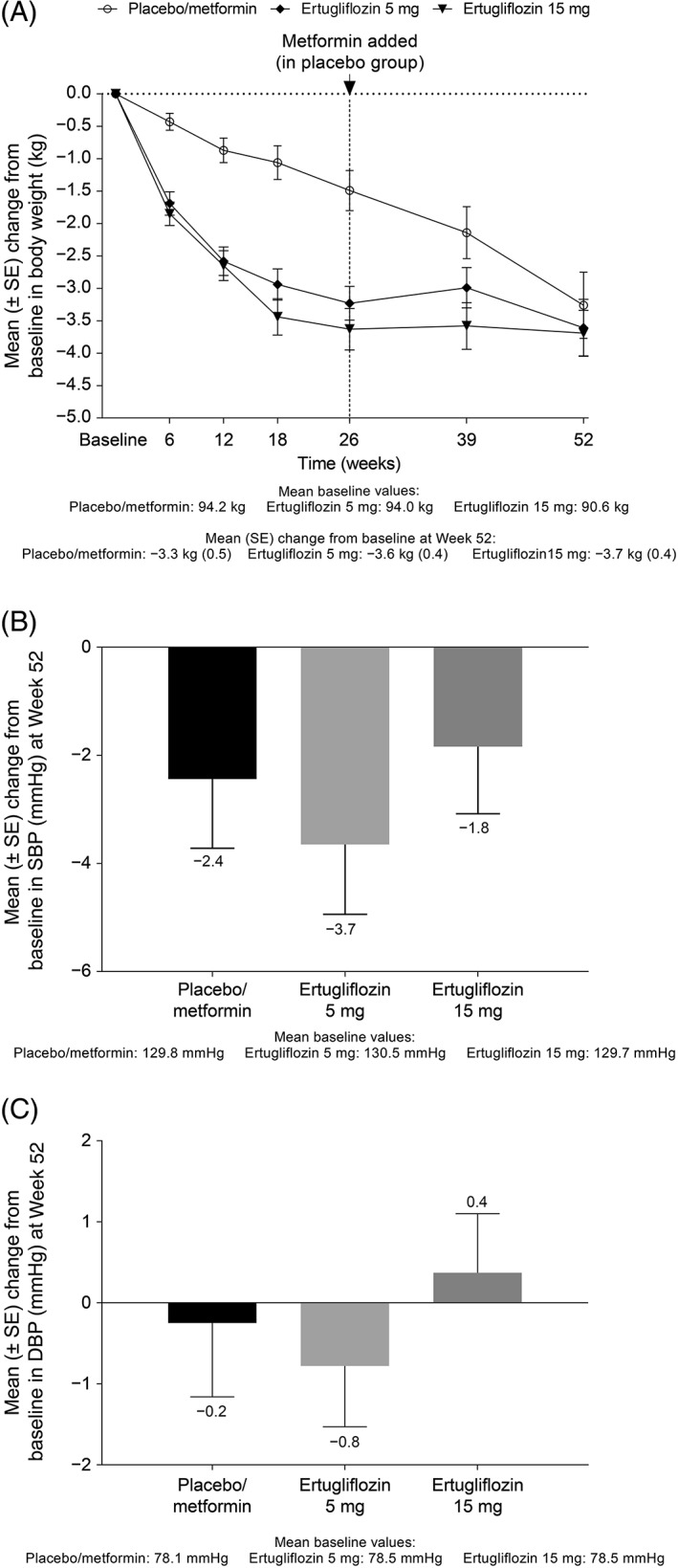
A, Change from baseline over time in body weight and change from baseline at Week 52 in B, systolic blood pressure and C, diastolic blood pressure. Abbreviations: DBP, diastolic blood pressure; SBP, systolic blood pressure; SE, standard error
3.3. Safety outcomes
3.3.1. Overall safety
The overall incidence of AEs, serious AEs (SAEs) and drug‐related AEs was similar between the ertugliflozin and placebo/metformin groups (Table 1). The incidences of AEs leading to discontinuation of study medication were not notably different across treatment groups (placebo/metformin, 6.5%; ertugliflozin 5 mg, 4.5%; ertugliflozin 15 mg, 3.9%). The most commonly reported drug‐related AEs in the ertugliflozin groups were associated with GMIs; the AEs with the highest incidence in the ertugliflozin groups were vulvovaginal candidiasis and vulvovaginal mycotic infection. The incidence of drug‐related AEs in Phase B was lower in the ertugliflozin groups compared with the placebo/metformin group, and was largely the result of an increased incidence of gastrointestinal‐related AEs in Phase B in the placebo/metformin group. There was 1 death (ruptured cerebral aneurysm), which occurred in a participant in the ertugliflozin 5 mg group on Day 362, and which the investigator assessed as not related to study medication.
Table 1.
Summary of adverse events (Phase A + B)
| Event | Placebo/metformin n = 153 | Ertugliflozin 5 mg n = 156 | Ertugliflozin 15 mg n = 152 |
|---|---|---|---|
| ≥1 AE(s) | 102 (66.7) | 100 (64.1) | 95 (62.5) |
| Drug‐related AEsa | 45 (29.4) | 42 (26.9) | 37 (24.3) |
| ≥1 SAE(s) | 7 (4.6) | 10 (6.4) | 6 (3.9) |
| Deaths | 0 | 1b | 0 |
| AEs resulting in discontinuation of study medication | 10 (6.5) | 7 (4.5) | 6 (3.9) |
| AEs associated with osmotic diuresis | |||
| Pollakiuria | 3 (2.0) | 5 (3.2) | 3 (2.0) |
| Polyuria | 0 | 3 (1.9) | 2 (1.3) |
| Nocturia | 3 (2.0) | 1 (0.6) | 0 |
Abbreviations: AE, adverse event; SAE, serious adverse event.
Data are presented as n (%), including rescue therapy.
Determined by the investigator to be related to the drug.
One participant in the ERTU 5‐mg group died during the post‐randomization follow‐up period (ruptured cerebral aneurysm, which the investigator assessed as not related to study medication). For all other AEs, this table contains events that occurred between the first dose and 14 d after the final dose of study medication.
3.3.2. “Tier 1” and other adverse events
Over 52 weeks, the incidence of GMIs in women was significantly higher in the ertugliflozin 5 mg (26.9%; P = .010) and ertugliflozin 15 mg (29.0%; P = .005) groups compared with the placebo/metformin (9.9%) group (Figure 3). GMI AEs led to discontinuation of study medication in 3 female participants (2, ertugliflozin 15 mg; 1, ertugliflozin 5 mg). In men, the incidence of GMIs was higher in the ertugliflozin 5 mg group (3.4%) and significantly higher in the ertugliflozin 15 mg group (7.8%, P = .042) compared with the placebo/metformin group (1.2%) (Figure 3). The majority of GMIs occurred in the first 6 months of treatment (Phase A) (Figure 3). The incidence of UTIs was 10.9% in the ertugliflozin 5 mg group and 6.6% in the ertugliflozin 15 mg group (P = .039 compared with the placebo/metformin group [13.7%]) (Figure 3). The incidence of participants with symptomatic hypoglycaemia was low across all groups, although lower in the ertugliflozin groups (5 mg, 1.3%; 15 mg, 2.6%) compared with the placebo/metformin group (4.6%). The incidence of hypovolaemia was 4.6%, 1.9% and 2.0%, in the placebo/metformin, ertugliflozin 5 mg and ertugliflozin 15 mg groups, respectively (Figure 3). AEs associated with osmotic diuresis occurred infrequently across groups, without an evident pattern of increased incidence in the ertugliflozin groups relative to the placebo/metformin group (Table 1).
Figure 3.
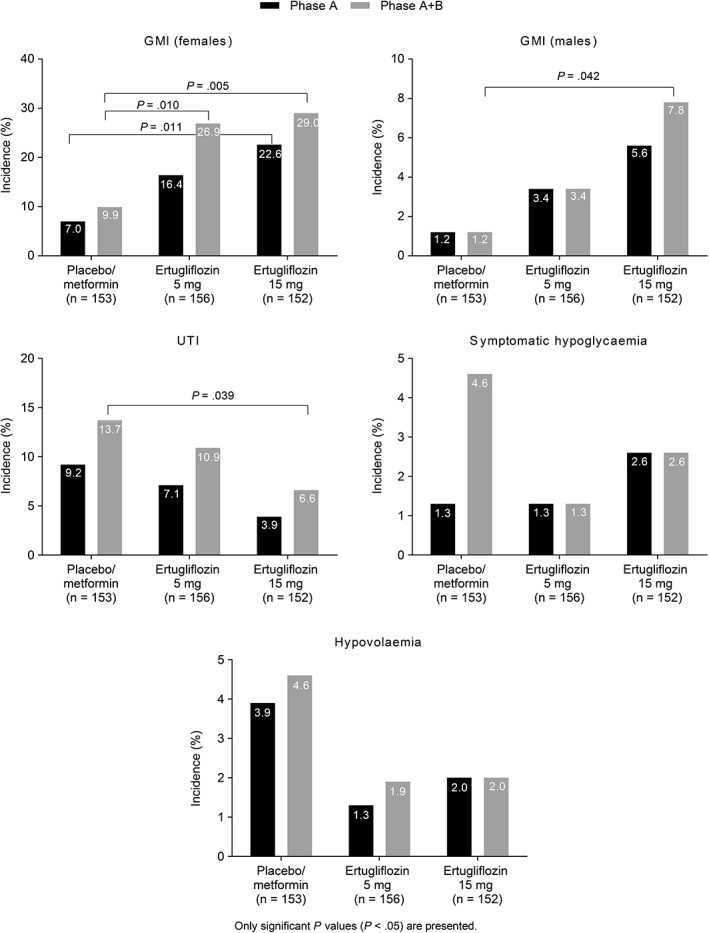
Incidence of Tier 1 AEs in the Phase A and Phase A + B treatment periods. Abbreviations: AE, adverse event; GMI, genital mycotic infection; UTI, urinary tract infection
The incidence of documented hypoglycaemia was similar across the 3 treatment groups (placebo/metformin, 5.2%; ertugliflozin 5 mg, 3.8%; ertugliflozin 15 mg, 5.3%). The incidence of severe hypoglycaemia was 0.7%, 0.0% and 1.3% in the placebo/metformin, ertugliflozin 5 mg and ertugliflozin 15 mg groups, respectively.
3.3.3. Adjudicated adverse events
Two participants, both in the ertugliflozin 15 mg group, experienced fracture‐related AEs confirmed via adjudication. Both were non‐serious AEs of hand fracture. The first (Day 94, Phase A) occurred as a result of hitting the steering wheel of a car, and was confirmed by the CAC as a low‐trauma fracture; the second (Day 182, Phase A) was sustained from a pinch injury with a chain at work, and was confirmed by the CAC as a high‐trauma fracture. One event of mild acute pancreatitis occurred in the placebo/metformin group in Phase B. No participant experienced a renal event that met criteria for adjudication (any event or last recorded laboratory value of doubling of serum creatinine from baseline value [or ≥50% decrease in eGFR from baseline] or events of end‐stage renal disease or renal replacement therapy). Two participants, one in the placebo/metformin group and one in the ertugliflozin 5 mg group, met ≥1 criteria for hepatic adjudication. The event in the placebo/metformin group of elevated alanine aminotransferase (ALT), aspartate aminotransferase (AST) and bilirubin was adjudicated as a doubtful relationship to study medication (potential etiology, herbal supplements), while the event of elevated ALT and AST in the ertugliflozin group was adjudicated as possibly related to study medication. There were no confirmed cases of ketoacidosis.
3.3.4. Laboratory parameters
Changes from baseline at Week 52 in LDL‐C, HDL‐C and LDL‐C:HDL‐C ratio are shown in Table S3. At Week 52, eGFR was similar to baseline levels in all treatment groups (Figure 4). Mean (± SD) eGFR values at baseline were 86.2 (± 19.4), 88.5 (± 18.4) and 88.3 (± 18.0) mL/min/1.73 m2 in the placebo/metformin, ertugliflozin 5 mg and ertugliflozin 15 mg groups, respectively. Mean (SE) change from baseline to Week 52 in eGFR was 1.7 (1.2), 0.6 (1.0) and −0.8 (1.0) mL/min/1.73 m2, in the placebo/metformin, ertugliflozin 5 mg and ertugliflozin 15 mg groups, respectively. In the Phase A + B treatment period, 4 (2.8%), 5 (3.4%) and 5 (3.4%) participants in the placebo/metformin, ertugliflozin 5 mg and ertugliflozin 15 mg groups, respectively, experienced ≥1 occurrence of a decrease from baseline >30% in eGFR.
Figure 4.
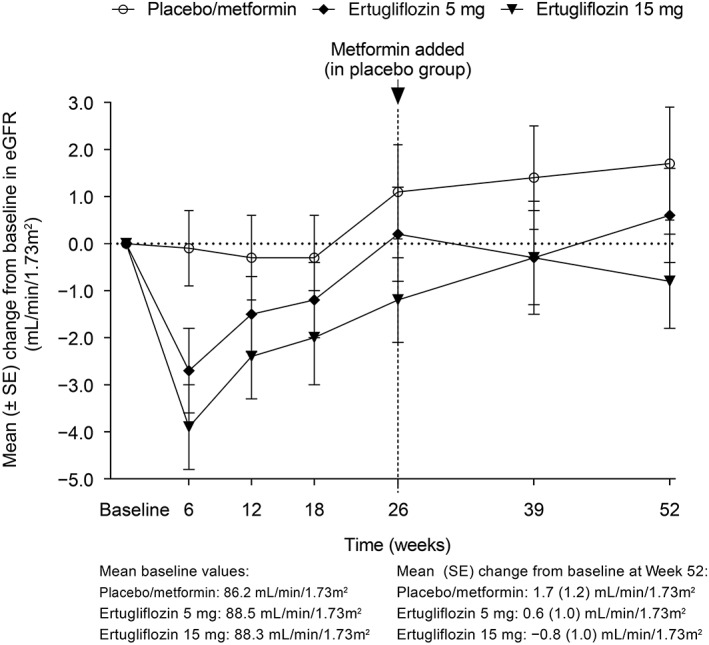
Change from baseline over time in eGFR (mL/min/1.73 m2). Abbreviations: eGFR, estimated glomerular filtration rate; SE, standard error
A summary of key laboratory data is provided in Table S4. Small mean increases from baseline in both magnesium and phosphate were observed in the ertugliflozin groups at Week 26; however, there was no further increase through Week 52. Small mean decreases in uric acid from baseline at Week 52 were observed in both ertugliflozin groups. Mean decreases in ALT and AST were observed in all treatment groups at Week 52. Mean increases from baseline in haemoglobin and haematocrit levels were observed through Week 52 in both ertugliflozin groups.
4. DISCUSSION
The results of this study demonstrate that 1 year of treatment with ertugliflozin improved glycaemic control and reduced body weight and SBP in adults with inadequately controlled T2DM despite diet and exercise. SGLT2 inhibitors lower glucose independently of insulin and β‐cell function.11, 12 Consequently, this mechanism may lend itself to durable HbA1c lowering in patients with T2DM. Ertugliflozin provided effective lowering of HbA1c, with mean reductions from baseline of approximately 1% at 1 year. The durable HbA1c lowering from Week 26 through Week 52 is consistent with the β‐cell–independent mechanism of action of SGLT2 inhibitors. Approximately one‐quarter of participants in the ertugliflozin groups had HbA1c <7% at Week 52 on ertugliflozin monotherapy. The proportion of participants in the ertugliflozin groups with HbA1c <6.5% at Week 52 was approximately half that observed in the placebo/metformin group. This may be explained, in part, by a lower mean HbA1c at baseline in the placebo/metformin group compared with the ertugliflozin groups, and the fact that participants in the placebo group who were not rescued through Week 26 probably had lower HbA1c values prior to initiating metformin in Phase B. Longer‐term glycaemic data will be available from other clinical studies in the programme (NCT01999218, NCT02033889, NCT01986881).
To minimize the length of time that participants with T2DM received no background therapy, the design of this study included a placebo‐switch paradigm. Participants initially randomized to placebo who had not required glycaemic rescue therapy were switched to blinded metformin at Week 26. The protocol used a rapid dose‐titration scheme and the median achieved dose of metformin in the Phase B portion of the study was 1790 mg/d. Approximately two‐thirds of participants were receiving a final metformin dose of 2000 mg/d. At the end of the study, participants who received ertugliflozin monotherapy achieved a change from baseline in HbA1c similar to that of those who received metformin, with means of −1.0%, −0.9% and −1.0% in the placebo/metformin, ertugliflozin 5 mg and ertugliflozin 15 mg groups, respectively. The reduction with metformin is in line with prior studies in which the same dose was used.13 Although this study was not powered for a formal comparison of the efficacy of the 2 ertugliflozin doses, the results for the proportion of participants with HbA1c <7.0% at Week 26, along with the reductions from baseline in HbA1c and FPG, are suggestive of incremental glycaemic efficacy with the 15‐mg dose relative to the 5‐mg dose. The reduction in body weight at Week 26 in the ertugliflozin groups was maintained through Week 52, with no evidence of weight regain. Participants in the ertugliflozin groups had reductions from baseline in SBP of approximately 2–3 mm Hg. The reduction in body weight observed after initiation of metformin in the placebo group is similar to that reported in 2 recent active‐controlled studies of dapagliflozin vs metformin, in which reductions of 1.3 to 1.4 kg were observed with metformin at 24 weeks.14 Similarly, in a head‐to‐head study of metformin vs dulaglutide, a 2.2 kg weight loss was observed at 26 weeks in patients who received metformin.15
Ertugliflozin was generally well tolerated throughout the 52‐week treatment period in this study population. The overall incidences of AEs, SAEs and AEs leading to discontinuation of study medication were similar across treatment groups. The incidence of GMIs was higher in the ertugliflozin groups compared with the placebo/metformin group, but there was no increased incidence of UTI, symptomatic hypoglycaemia or hypovolaemia AEs. Similar to reports for other SGLT2 inhibitors, the majority of GMI events occurred early in the study.16, 17 There was no increase in AEs associated with osmotic diuresis.
Initial mean reductions in eGFR in the ertugliflozin groups at Week 6 were followed by mean increases, returning approximately to baseline values by Week 52. In the ertugliflozin groups, changes seen in magnesium, phosphate and haemoglobin at Week 26 remained stable through Week 52. The clinical relevance (if any) of such changes observed with SGLT2 inhibitors is presently unknown. Analysis of lipid parameters showed a greater increase in LDL‐C and HDL‐C levels in the ertugliflozin groups compared with the placebo/metformin group, although there was no increase in the LDL‐C:HDL‐C ratio. Data from the ongoing ertugliflozin cardiovascular outcome trial (VERTIS CV, NCT01986881) will provide information on the effect of ertugliflozin on cardiovascular outcomes.
Ertugliflozin, administered to adults with inadequately controlled T2DM despite diet and exercise, improved glycaemic control and reduced body weight and SBP. Both doses provided a clinically meaningful reduction from baseline in HbA1c that was maintained over 52 weeks. There was no increase in the incidence of symptomatic hypoglycaemia, UTI or hypovolaemia AEs with ertugliflozin, but the incidence of GMIs was higher compared with placebo/metformin.
Supporting information
Table S1. Participants’ baseline demographics and clinical characteristics (all participants treated).
Table S2. Longitudinal data analysis model results (excluding rescue approach, FAS).
Table S3. Percent change from baseline at week 52 in LDL‐C, HDL‐C and ratio of LDL‐C/HDL‐C.
Table S4. Summary of key laboratory data.
Figure S1. Trial scheme showing the design of the study.
Figure S2. Participant disposition Phase A and Phase B. Abbreviation: eGFR, estimated glomerular filtration rate.
Figure S3. Participant disposition Phase A + B. The “Study terminated by Sponsor” category included any participant who was discontinued (from study drug and/or from the trial) because the site was closed by Pfizer. Abbreviation: eGFR, estimated glomerular filtration rate.
ACKNOWLEDGMENTS
Medical writing support was provided by Helen Jones, PhD of Engage Scientific Solutions (Horsham, UK) and was funded by Pfizer, Inc. and Merck & Co., Inc.
Parts of this study were previously presented at the Scientific Sessions of the American Diabetes Association 77th Annual Scientific Sessions, 2017 (San Diego, California) and at the 53rd Annual European Association for the Study of Diabetes (EASD) Congress 2017 (Lisbon, Portugal).
Conflict of interest
R. A. has received research support or consultancy fees from Amgen, AstraZeneca, Becton Dickinson, Boehringer Ingelheim, Bristol‐Myers Squibb, Eli Lilly, Janssen, Merck & Co., Novo Nordisk, Pfizer, Sanofi and Takeda. J. F. has received research support or consultancy fees from AbbVie, AstraZeneca, Boehringer Ingelheim, Bristol‐Myers Squibb, CeQur, Eli Lilly, Ionis Pharmaceuticals, Janssen, Johnson & Johnson, Ligand Pharmaceuticals, Merck & Co., Novo Nordisk, Pfizer, Sanofi, Theracos and vTv Therapeutics. B. L. is an employee of and owns stock in Merck & Co., Inc. A. G. owns stock in Merck & Co., Inc. A. G., A. D. and S. G. T. are employees of and own stock in Pfizer, Inc.
Author contributions
Author contributions to this study were as follows: Design: A. D., S. G. T. Analysis: A. G., A. D., B. L., S. G. T. Writing the manuscript: R. A., J. F., A. G., A. D., B. L., S. G. T.
Aronson R, Frias J, Goldman A, Darekar A, Lauring B, Terra SG. Long‐term efficacy and safety of ertugliflozin monotherapy in patients with inadequately controlled T2DM despite diet and exercise: VERTIS MONO extension study. Diabetes Obes Metab. 2018;20:1453–1460. https://doi.org/10.1111/dom.13251
Funding information This study was sponsored by Pfizer, Inc., Groton, Connecticut and Merck & Co., Inc., Kenilworth, New Jersey.
REFERENCES
- 1. Scheen AJ. SGLT2 inhibitors: benefit/risk balance. Curr Diab Rep. 2016;16:92. [DOI] [PubMed] [Google Scholar]
- 2. Ferrannini E, Solini A. SGLT2 inhibition in diabetes mellitus: rationale and clinical prospects. Nat Rev Endocrinol. 2012;8:495‐502. [DOI] [PubMed] [Google Scholar]
- 3. Amin NB, Wang X, Jain SM, Lee DS, Nucci G, Rusnak JM. Dose‐ranging efficacy and safety study of ertugliflozin, a sodium‐glucose co‐transporter 2 inhibitor, in patients with type 2 diabetes on a background of metformin. Diabetes Obes Metab. 2015;17:591‐598. [DOI] [PubMed] [Google Scholar]
- 4. Amin NB, Wang X, Mitchell JR, Lee DS, Nucci G, Rusnak JM. Blood pressure‐lowering effect of the sodium glucose co‐transporter‐2 inhibitor ertugliflozin, assessed via ambulatory blood pressure monitoring in patients with type 2 diabetes and hypertension. Diabetes Obes Metab. 2015;17:805‐808. [DOI] [PubMed] [Google Scholar]
- 5. Rosenstock J, Frias J, Páll D, et al. Effect of ertugliflozin on glucose control, body weight, blood pressure and bone density in type 2 diabetes mellitus inadequately controlled on metformin monotherapy (VERTIS MET). Diabetes Obes Metab. 2018;20:520‐529. [DOI] [PubMed] [Google Scholar]
- 6. Dagogo‐Jack S, Liu J, Eldor R, et al. Efficacy and safety of the addition of ertugliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin and sitagliptin: the VERTIS SITA2 placebo‐controlled randomized study. Diabetes Obes Metab. 2018;20:530‐540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pratley RE, Eldor R, Raji A, et al.. Ertugliflozin plus sitagliptin versus either individual agent over 52 weeks in patients with type 2 diabetes mellitus inadequately controlled with metformin: the VERTIS FACTORIAL randomized trial. Diabetes Obes Metab. 2018;20:1111–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Terra SG, Focht K, Davies M, et al. Phase III efficacy and safety study of ertugliflozin monotherapy in people with type 2 diabetes mellitus inadequately controlled with diet and exercise alone. Diabetes Obes Metab. 2017;19:721‐728. [DOI] [PubMed] [Google Scholar]
- 9. Liang K, Zeger S. Longitudinal data analysis of continuous and discrete responses for pre‐post design. Sankhya: Indian J Stat B. 2000;62:134‐148. [Google Scholar]
- 10. Miettinen O, Nurminen M. Comparative analysis of two rates. Stat Med. 1985;4:213‐226. [DOI] [PubMed] [Google Scholar]
- 11. Chao EC, Henry RR. SGLT2 inhibition—a novel strategy for diabetes treatment. Nat Rev Drug Discov. 2010;9:551‐559. [DOI] [PubMed] [Google Scholar]
- 12. Jurczak MJ, Lee HY, Birkenfeld AL, et al. SGLT2 deletion improves glucose homeostasis and preserves pancreatic beta‐cell function. Diabetes. 2011;60:890‐898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hirst JA, Farmer AJ, Ali R, Roberts NW, Stevens RJ. Quantifying the effect of metformin treatment and dose on glycemic control. Diabetes Care. 2012;35:446‐454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Henry RR, Murray AV, Marmolejo MH, Hennicken D, Ptaszynska A, List JF. Dapagliflozin, metformin XR, or both: initial pharmacotherapy for type 2 diabetes, a randomised controlled trial. Int J Clin Pract. 2012;66:446‐456. [DOI] [PubMed] [Google Scholar]
- 15. Umpierrez G, Tofé Povedano S, Pérez Manghi F, Shurzinske L, Pechtner V. Efficacy and safety of dulaglutide monotherapy versus metformin in type 2 diabetes in a randomized controlled trial (AWARD‐3). Diabetes Care. 2014;37:2168‐2176. [DOI] [PubMed] [Google Scholar]
- 16. Nyirjesy P, Sobel JD, Fung A, et al. Genital mycotic infections with canagliflozin, a sodium glucose co‐transporter 2 inhibitor, in patients with type 2 diabetes mellitus: a pooled analysis of clinical studies. Curr Med Res Opin. 2014;30:1109‐1119. [DOI] [PubMed] [Google Scholar]
- 17. Geerlings S, Fonseca V, Castro‐Diaz D, List J, Parikh S. Genital and urinary tract infections in diabetes: impact of phar‐macologically‐induced glucosuria. Diabetes Res Clin Pract. 2014;103:373‐381. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Participants’ baseline demographics and clinical characteristics (all participants treated).
Table S2. Longitudinal data analysis model results (excluding rescue approach, FAS).
Table S3. Percent change from baseline at week 52 in LDL‐C, HDL‐C and ratio of LDL‐C/HDL‐C.
Table S4. Summary of key laboratory data.
Figure S1. Trial scheme showing the design of the study.
Figure S2. Participant disposition Phase A and Phase B. Abbreviation: eGFR, estimated glomerular filtration rate.
Figure S3. Participant disposition Phase A + B. The “Study terminated by Sponsor” category included any participant who was discontinued (from study drug and/or from the trial) because the site was closed by Pfizer. Abbreviation: eGFR, estimated glomerular filtration rate.


