Abstract
Aim
To evaluate the safety and efficacy of once‐weekly subcutaneous semaglutide as monotherapy or combined with an oral antidiabetic drug (OAD) vs an additional OAD added to background therapy in Japanese people with type 2 diabetes (T2D) inadequately controlled on diet/exercise or OAD monotherapy.
Methods
In this phase III, open‐label trial, adults with T2D were randomized 2:2:1 to semaglutide 0.5 mg or 1.0 mg, or one additional OAD (a dipeptidyl peptidase‐4 inhibitor, biguanide, sulphonylurea, glinide, α‐glucosidase inhibitor or thiazolidinedione) with a different mode of action from that of background therapy. The primary endpoint was number of adverse events (AEs) after 56 weeks.
Results
Baseline characteristics were balanced between treatment arms (601 randomized). More AEs were reported in the semaglutide 0.5 mg (86.2%) and 1.0 mg (88.0%) groups than in the additional OAD group (71.7%). These were typically mild/moderate. Gastrointestinal AEs were most frequent with semaglutide, which diminished over time. The mean glycated haemoglobin (HbA1c) concentration (baseline 8.1%) was significantly reduced with semaglutide 0.5 mg and 1.0 mg vs additional OAD (1.7% and 2.0% vs 0.7%, respectively; estimated treatment difference [ETD] vs additional OAD −1.08% and −1.37%, both P < .0001). Body weight (baseline 71.5 kg) was reduced by 1.4 kg and 3.2 kg with semaglutide 0.5 mg and 1.0 mg, vs a 0.4‐kg increase with additional OAD (ETD −1.84 kg and −3.59 kg; both P < .0001). For semaglutide‐treated participants, >80% achieved an HbA1c concentration <7.0% (Japanese Diabetes Society target).
Conclusions
Semaglutide was well tolerated, with no new safety issues identified. Semaglutide treatment significantly reduced HbA1c and body weight vs additional OAD treatment in Japanese people with T2D.
Keywords: GLP‐1 analogue, glycaemic control, incretin therapy, phase III study, randomized trial, type 2 diabetes
1. INTRODUCTION
The prevalence of type 2 diabetes (T2D) is rising in many countries, including Japan, influenced by increasing obesity and decreasing physical activity.1 If diet and exercise fail to achieve glycaemic control, the Japanese Diabetes Society guidelines recommend the addition of glucose‐lowering agents.2, 3 However, despite the availability of numerous such agents, achieving recommended glycaemic targets (eg, glycated haemoglobin [HbA1c] <7.0% [53.0 mmol/mol] [Japanese Diabetes Society, American Diabetes Association])3, 4 remains a challenge for many patients.5, 6 There is therefore a clinical need for individualized treatment,7 particularly to avoid weight gain and hypoglycaemia.8, 9
Unlike many other T2D therapies, glucagon‐like peptide‐1 receptor agonists (GLP‐1RAs) achieve glycaemic control and reduce body weight.10 Semaglutide (Novo Nordisk, Denmark) is a GLP‐1 analogue currently in development for the treatment of T2D. It has 94% homology to native GLP‐1,11 and is structurally similar to liraglutide,12 but has modifications resulting in a half‐life of ~1 week, making semaglutide appropriate for once‐weekly administration.11, 12 Semaglutide has been evaluated in the Semaglutide Unabated Sustainability in Treatment of Type 2 Diabetes (SUSTAIN) phase IIIa programme, which evaluated the efficacy and safety of semaglutide at all stages of the T2D disease continuum13, 14, 15, 16, 17, 18; however, Japanese regulatory guidelines require that the safety and efficacy of all investigational drugs are evaluated in combination with sulphonylureas in at least 100 Japanese patients and in combination with other approved oral antidiabetic drugs (OADs) in at least 50 Japanese patients in order to obtain approval.19 Hence, additional SUSTAIN phase III clinical trials were designed to investigate semaglutide treatment in Japanese populations.20, 21
The present trial evaluated the safety and efficacy of once‐weekly semaglutide (0.5 and 1.0 mg) as monotherapy or in combination with an OAD, vs an additional OAD, during 56 weeks of treatment in Japanese people with T2D insufficiently controlled on diet/exercise or OAD monotherapy.
2. MATERIALS AND METHODS
2.1. Trial design
This was a phase III randomized, open‐label, active‐controlled, parallel‐group (three groups), multicentre, single‐country trial to evaluate the safety and efficacy of once‐weekly treatment with semaglutide as monotherapy or in combination with one OAD in Japanese people with inadequately controlled T2D. Participants were selected and screened by the investigator and then randomized across 41 sites in a 2:2:1 ratio to receive semaglutide 0.5 mg or 1.0 mg once weekly, or one additional OAD (a dipeptidyl peptidase‐4 inhibitor, biguanide, sulphonylurea, glinide, α‐glucosidase inhibitor or thiazolidinedione) with a different mode of action from that of background therapy, as selected by the investigator.
The trial comprised a screening period (weeks −2 to 0), a treatment period (weeks 0–56) including the dose‐escalation period, and a follow‐up period (weeks 56‐61); with a total trial duration of 63 weeks (weeks −2 to 61; Figure S1 in File S1). This trial was conducted in compliance with the International Conference on Harmonisation Good Clinical Practice guidelines,22 and the Declaration of Helsinki.23 Brief details of the study design are available at http://clinicaltrials.gov (NCT02207374).
2.2. Study population
Japanese adults were eligible for participation. Key inclusion criteria were: T2D; stable treatment with diet/exercise therapy only for at least 30 days before screening or OAD monotherapy (sulphonylureas, glinides, α‐glucosidase inhibitors or thiazolidinediones) with approved Japanese labelling, in addition to diet/exercise therapy, for at least 60 days before screening; age ≥20 years at the time of signing informed consent; and HbA1c between 53 and 91 mmol/mol (7.0‐10.5%) at screening. Key exclusion criteria were: treatment with glucose‐lowering agent(s) other than those stated in the inclusion criteria within 60 days before screening, and treatment with once‐weekly GLP‐1RAs <90 days before screening (except for short‐term treatment [≤7 days in total] with insulin in connection with intercurrent illness); history of chronic or acute pancreatitis; impaired renal function (eGFR <30 mL/min/1.73 m 2 ); and acute coronary or cerebrovascular events in the 90 days before randomization. Further details on inclusion/exclusion criteria are in Table S1 in File S1.
2.3. Randomization and masking
Participants were randomly assigned in a 2:2:1 ratio to receive once‐weekly subcutaneous semaglutide (0.5 or 1.0 mg) or one additional OAD using an automated voice/web recognition system without human involvement at the randomization visit. Randomization was stratified according to pre‐trial treatment at screening (diet and exercise therapy, sulphonylureas, glinides, α‐glucosidase inhibitors or thiazolidinediones). The trial had an open‐label design because of the different dosing regimen between semaglutide and comparator treatment.
2.4. Drug administration
After a 2‐week screening period (diet and exercise therapy, sulphonylureas, glinides, α‐glucosidase inhibitors or thiazolidinediones), the participants received semaglutide 0.5 or 1.0 mg once weekly or an additional OAD for 56 weeks, followed by a 5‐week follow‐up period (Figure S1 in File S1). Semaglutide was administered by once‐weekly subcutaneous injection in the thigh, abdomen or upper arm and was to be taken on the same day of the week. Participants in the semaglutide arms followed a fixed‐dose escalation regimen of semaglutide 0.5 mg (maintenance dose reached after 4 weeks of 0.25 mg semaglutide once weekly) or semaglutide 1.0 mg (maintenance dose reached after 4 weeks of 0.25 mg semaglutide, followed by 4 weeks of 0.5 mg semaglutide). The type and dosage of the additional OAD was to be selected by the investigator according to the approved Japanese labelling, taking into account complementary modes of action and contraindications against different drug combinations. Participants who discontinued randomized treatment prematurely were asked to stay in the trial and continue all planned visit procedures in all treatment arms.
Rescue medication was offered if fasting plasma glucose (FPG) exceeded predefined criteria (Supporting Information).
2.5. Trial objectives and endpoints
The primary objective of the study was to compare the safety of once‐weekly semaglutide (0.5 mg and 1.0 mg) vs OAD therapy during 56 weeks of treatment in Japanese people with T2D. The secondary objective was to compare the efficacy of once‐weekly semaglutide (0.5 mg and 1.0 mg) vs OAD therapy after 56 weeks of treatment.
All endpoints were prespecified in the clinical trial protocol, including the primary and all supportive secondary efficacy/safety endpoints. The primary endpoint was the number of treatment‐emergent adverse events (TEAEs) during 56 weeks of treatment. Supportive secondary safety endpoints included the number of severe or blood glucose (BG)‐confirmed symptomatic hypoglycaemic episodes, change from baseline in safety variables (including haematology, biochemistry, calcitonin and pulse), and the occurrence of anti‐semaglutide antibodies. An external event adjudication committee performed validation of selected adverse events (AEs) or other events (Table S2 in File S1).
Supportive secondary efficacy endpoints included change from baseline in: HbA1c, FPG, self‐measured plasma glucose (SMPG, mean of 7‐point profile and mean postprandial increment, over all meals [measurements performed with capillary blood were automatically calibrated to plasma‐equivalent glucose values, shown on the display of the BG meter and documented by the trial participant]), body weight, body mass index (BMI), waist circumference, blood pressure, lipids and other markers of glucose metabolism and β‐cell function. FPG and SMPG are reported in mg/dL (1 mmol/L = 18.02 mg/dL). Secondary responder endpoints were the proportion of participants achieving HbA1c targets of <7% or ≤6.5%, the proportion of participants achieving HbA1c targets of <7% with no severe or BG‐confirmed symptomatic hypoglycaemia (defined as severe according to the American Diabetes Association classification or BG‐confirmed by plasma glucose <3.1 mmol/L, with symptoms consistent with hypoglycaemia) and no weight gain, and the proportion of participants achieving weight loss of ≥5% and ≥10%.
2.6. Sample size calculation
A total of 595 participants were planned to be randomized for treatment, stratified by pre‐trial treatment: 170 participants on diet and exercise therapy; 170 participants on sulphonylurea monotherapy; and 85 participants on each of the other three OAD monotherapies (glinides, α‐glucosidase inhibitors and thiazolidinediones). The sample size and stratification was determined based on Japanese regulatory guidelines,19 which require the following minimum numbers of semaglutide‐treated completers in each group: semaglutide only (100); semaglutide with a sulphonylurea (100); semaglutide with a glinide (50); semaglutide with an α‐glucosidase inhibitor (50); and semaglutide with a thiazolidinedione (50). The sample size assumed a premature discontinuation rate from randomized treatment of 25%, accounting for the 2:2:1 randomization.
2.7. Statistical analysis
Safety and efficacy were assessed in the modified intention‐to‐treat population, which consisted of all randomized participants who were exposed to at least one dose of randomized treatment, as specified in the trial protocol. For safety, we used only data obtained before premature treatment discontinuation, with an ascertainment window of 42 days to define TEAEs. For efficacy, the assessment used data obtained before the initiation of any rescue medication or before premature treatment discontinuation. We also carried out supportive sensitivity analyses using all data obtained during the trial, regardless of whether participants were on or off treatment or had received rescue medication, for both efficacy and safety.
Overall, safety endpoints were summarized descriptively in line with regulatory requirements. AEs, hypoglycaemic episodes and changes from baseline in HbA1c and body weight were also assessed by pre‐trial treatment.
The main analysis model for numerical efficacy endpoints was the mixed model for repeated measurements (MMRM), with treatment and pre‐trial treatment as fixed factors and baseline value as a covariate, all nested within visit. From the model, the estimated differences between each semaglutide dose level and the comparator at week 56 and corresponding two‐sided P values and 95% confidence intervals (CIs) are presented. With the exception of HbA1c, body weight, SMPG, FPG, BMI, waist circumference and blood pressure, values were log‐transformed, subject to analysis, and the estimated treatment effects of each semaglutide dose level vs comparator are presented as treatment ratios. For HbA1c and weight loss targets, missing data at week 56 were imputed from the MMRM used for the corresponding continuous endpoint and subsequently classified. Treatment comparisons were based on a logistic regression model including the same fixed factors and associated baseline value as covariate.
The MMRM analysis relied on the ‘missing at random’ assumption. To investigate whether the results from the MMRM approach are robust towards deviations from the assumption of ‘missing at random’, prespecified complementary and separate analyses for change in HbA1c and change in body weight at 56 weeks were performed by varying the method for handling missing data. Finally, as a sensitivity analysis, the primary MMRM analysis was repeated based on all data collected post‐randomization, regardless of whether participants were on or off treatment or received rescue medication.
3. RESULTS
3.1. Participant disposition and baseline characteristics
The trial began on August 4, 2014 and ended on February 27, 2016, per protocol. In total, 601 people were randomized 2:2:1 to semaglutide 0.5 mg (n = 239), semaglutide 1.0 mg (n = 241) or additional OAD (n = 121; Figure 1). Of the 121 participants assigned to additional OAD treatment, 120 were treated with one of the following OADs: dipeptidyl peptidase‐4 inhibitors (n = 74), biguanides (n = 31), sulphonylureas (n = 7), α‐glucosidase inhibitors (n = 3), thiazolidinediones (n = 4), or glinides (n = 1).
Figure 1.
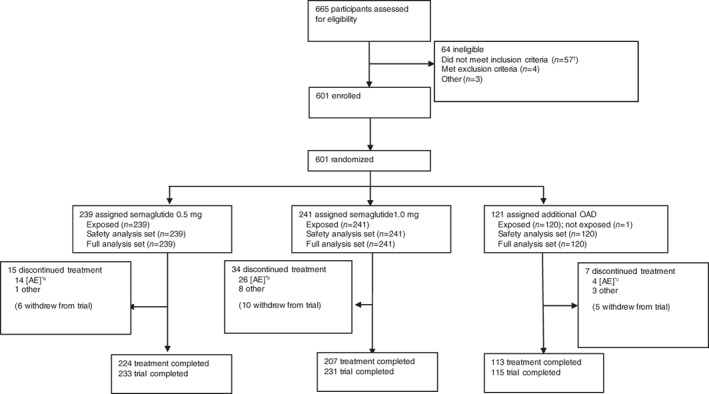
Flow of participants through the trial. *Reflects primary reason for treatment discontinuation, as judged by the investigator. †55 participants did not have a glycated haemoglobin (HbA1c) value within the specified range and 2 participants were not on stable treatment with diet/exercise therapy only for at least 30 days before screening, or on oral antidiabetic drug (OAD) monotherapy, in addition to diet/exercise therapy for at least 60 days before screening. aSeven participants discontinued because of gastrointestinal (GI) adverse events (AEs) and seven discontinued because of other AEs. bEighteen participants discontinued because of GI AEs and 8 discontinued because of other AEs. cNo participants discontinued because of GI AEs and 4 discontinued because of other AEs. Numbers in brackets within treatment discontinuation denote participants who also withdrew from trial, as those who discontinued treatment had the option to continue follow‐up. Trial completers were participants who were exposed, did not discontinue treatment prematurely, did not withdraw from trial and who attended a follow‐up visit
The numbers of participants who received rescue medication were: none in the semaglutide 0.5 mg group, 1 in the semaglutide 1.0 mg group, and 8 in the additional OAD group. Premature treatment discontinuation occurred in 15 participants on semaglutide 0.5 mg, 34 on semaglutide 1.0 mg and 7 on additional OAD. The main reason for premature treatment discontinuation was AEs (Figure 1). Baseline characteristics were generally well balanced among the three groups (Table 1 and Table S3 in File S1).
Table 1.
Baseline characteristics of trial populations
| Semaglutide 0.5 mg | Semaglutide 1.0 mg | Additional OAD | Total | |||||
|---|---|---|---|---|---|---|---|---|
| Meana | (SD) | Meana | (SD) | Meana | (SD) | Meana | (SD) | |
| Age, years | 58.0 | (10.6) | 58.7 | (10.2) | 59.2 | (10.1) | 58.5 | (10.3) |
| Male/female, % | 69.5/30.5 | – | 72.2/27.8 | – | 74.2/25.8 | – | 71.5/28.5 | – |
| HbA1c, mmol/mol | 64.4 | (9.8) | 65.5 | (10.5) | 65.1 | (9.8) | 65.0 | (10.1) |
| HbA1c < % | 8.0 | (0.9) | 8.1 | (1.0) | 8.1 | (0.9) | 8.1 | (0.9) |
| FPG, mmol/L | 8.9 | (1.9) | 8.9 | (2.1) | 9.0 | (1.9) | 8.9 | (2.0) |
| FPG, mg/dL | 159.8 | (34.5) | 160.4 | (38.0) | 161.7 | (34.8) | 160.4 | (36.0) |
| Diabetes duration, years | 8.1 | (6.0) | 9.4 | (6.5) | 9.3 | (7.0) | 8.8 | (6.4) |
| Body weight, kg | 71.0 | (15.4) | 71.7 | (15.9) | 72.2 | (14.9) | 71.5 | (15.5) |
| BMI, kg/m 2 | 26.2 | (4.8) | 26.4 | (4.7) | 26.7 | (4.6) | 26.4 | (4.7) |
| eGFR (MDRD), mL/min/1.73 m 2 | 101.4 | (21.7) | 101.6 | (24.0) | 102.0 | (22.5) | 101.6 | (22.8) |
| Pre‐trial treatment, n (%) | ||||||||
| Diet and exercise therapy | 68 | (28.5) | 68 | (28.2) | 35 | (29.2) | 171 | (28.5) |
| Sulphonylureas | 68 | (28.5) | 69 | (28.6) | 33 | (27.5) | 170 | (28.3) |
| Glinides | 34 | (14.2) | 36 | (14.9) | 17 | (14.2) | 87 | (14.5) |
| α‐ glucosidase inhibitors | 35 | (14.6) | 34 | (14.1) | 18 | (15.0) | 87 | (14.5) |
| Thiazolidinedione | 34 | (14.2) | 34 | (14.1) | 17 | (14.2) | 85 | (14.2) |
Abbreviations: BMI, body mass index; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; HbA1c, glycated haemoglobin; MDRD, modification of diet in renal disease; OAD, oral antidiabetic drug.
Values are arithmetic means.
3.2. Safety endpoints
3.2.1. Primary safety endpoint
Overall, more participants reported TEAEs in the semaglutide‐treatment groups (86.2% and 88.0% with semaglutide 0.5 mg and 1.0 mg, respectively) than in the additional OAD treatment group (71.7%; Table 2).
Table 2.
Treatment‐emergent adverse events summary by system organ class and incidence of hypoglycaemia
| Semaglutide 0.5 mg | Semaglutide 1.0 mg | Additional OAD | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | (%) | E | R | N | (%) | E | R | N | (%) | E | R | |
| Overview of treatment‐emergent AEs | ||||||||||||
| Number of participants | 239 | 241 | 120 | |||||||||
| AEs (total) | 206 | (86.2) | 909 | 335.5 | 212 | (88.0) | 954 | 371.5 | 86 | (71.7) | 269 | 197.9 |
| Fatal | 1 | (0.4) | 1 | 0.4 | 0 | – | – | 1 | 0.8 | 1 | 0.7 | |
| Serious | 19 | (7.9) | 25 | 9.2 | 12 | (5.0) | 13 | 5.1 | 8 | (6.7) | 10 | 7.4 |
| Severity | ||||||||||||
| Severe | 10 | (4.2) | 12 | 4.4 | 3 | (1.2) | 3 | 1.2 | 2 | (1.7) | 3 | 2.2 |
| Moderate | 29 | (12.1) | 46 | 17.0 | 29 | (12.0) | 37 | 14.4 | 15 | (12.5) | 20 | 14.7 |
| Mild | 202 | (84.5) | 851 | 314.1 | 209 | (86.7) | 914 | 356.0 | 80 | (66.7) | 246 | 181.0 |
| Leading to premature treatment discontinuation | 14 | (5.9) | 16 | 5.9 | 26 | (10.8) | 40 | 15.6 | 4b | (3.3)b | N/A | N/A |
| AEs reported by ≥ 5% of participants, by system organ class and preferred term | ||||||||||||
| Infections and infestations | 129 | (54.0) | 231 | 85.3 | 111 | (46.1) | 184 | 71.7 | 59 | (49.2) | 105 | 77.2 |
| Nasopharyngitisa | 81 | (33.9) | 132 | 48.7 | 75 | (31.1) | 105 | 40.9 | 41 | (34.2) | 60 | 44.1 |
| Pharyngitisa | 12 | (5.0) | 15 | 5.5 | 9 | (3.7) | 9 | 3.5 | 2 | (1.7) | 3 | 2.2 |
| Gastroenteritisa | 15 | (6.3) | 16 | 5.9 | 5 | (2.1) | 6 | 2.3 | 2 | (1.7) | 2 | 1.5 |
| Gastrointestinal disorders | 129 | (54.0) | 262 | 96.7 | 130 | (53.9) | 300 | 116.8 | 24 | (20.0) | 40 | 29.4 |
| Constipationa | 45 | (18.8) | 50 | 18.5 | 36 | (14.9) | 40 | 15.6 | 5 | (4.2) | 5 | 3.7 |
| Nauseaa | 29 | (12.1) | 38 | 14.0 | 46 | (19.1) | 70 | 27.3 | 1 | (0.8) | 1 | 0.7 |
| Diarrhoeaa | 24 | (10.0) | 33 | 12.2 | 38 | (15.8) | 49 | 19.1 | 8 | (6.7) | 9 | 6.6 |
| Abdominal discomforta | 15 | (6.3) | 15 | 5.5 | 15 | (6.2) | 16 | 6.2 | 0 | – | – | – |
| Vomitinga | 13 | (5.4) | 17 | 6.3 | 14 | (5.8) | 21 | 8.2 | 2 | (1.7) | 2 | 1.5 |
| Investigations | 40 | (16.7) | 66 | 24.4 | 58 | (24.1) | 81 | 31.5 | 9 | (7.5) | 11 | 8.1 |
| Lipase increaseda | 21 | (8.8) | 25 | 9.2 | 33 | (13.7) | 36 | 14.0 | 2 | (1.7) | 2 | 1.5 |
| Amylase increaseda | 7 | (2.9) | 8 | 3.0 | 13 | (5.4) | 15 | 5.8 | 1 | (0.8) | 1 | 0.7 |
| Metabolism and nutrition disorders | 24 | (10.0) | 28 | 10.3 | 34 | (14.1) | 40 | 15.6 | 4 | (3.3) | 4 | 2.9 |
| Decreased appetitea | 18 | (7.5) | 22 | 8.1 | 32 | (13.3) | 36 | 14.0 | 0 | – | – | – |
| Eye disorders | 35 | (14.6) | 49 | 18.1 | 36 | (14.9) | 39 | 15.2 | 9 | (7.5) | 9 | 6.6 |
| Diabetic retinopathya | 11 | (4.6) | 11 | 4.1 | 16 | (6.6) | 16 | 6.2 | 6 | (5.0) | 6 | 4.4 |
| Musculoskeletal and connective tissue disorders | 35 | (14.6) | 43 | 15.9 | 46 | (19.1) | 55 | 21.4 | 22 | (18.3) | 26 | 19.1 |
| Back paina | 9 | (3.8) | 10 | 3.7 | 13 | (5.4) | 13 | 5.1 | 10 | (8.3) | 10 | 7.4 |
| EAC‐confirmed AEs | ||||||||||||
| Cardiovascular events | 1 | (0.4) | 1 | 0.4 | 1 | (0.4) | 1 | 0.4 | 0 | – | – | – |
| Coronary revascularization | 0 | – | – | – | 1 | (0.4) | 1 | 0.4 | 0 | – | – | – |
| Heart failure | 1 | (0.4) | 1 | 0.4 | 0 | – | – | – | 0 | – | – | – |
| Neoplasms | 15 | (6.3) | 17 | 6.3 | 21 | (8.7) | 27 | 10.5 | 6 | (5.0) | 6 | 4.4 |
| Benign | 11 | (4.6) | 13 | 4.8 | 19 | (7.9) | 23 | 9.0 | 3 | (2.5) | 3 | 2.2 |
| Colorectal | 7 | (2.9) | 8 | 3.0 | 8 | (3.3) | 10 | 3.9 | 0 | |||
| Malignant | 4 | (1.7) | 4 | 1.5 | 3 | (1.2) | 3 | 1.2 | 3 | (2.5) | 3 | 2.2 |
| Hypoglycaemia | ||||||||||||
| Severe or BG‐confirmed symptomatic | 3 | (1.3) | 4 | 1.5 | 6 | (2.5) | 8 | 3.1 | 2 | (1.7) | 2 | 1.5 |
Abbreviations: ADA, American Diabetes Association; AE, adverse event; BG, blood glucose; E, number of events; EAC, event adjudication committee; N, number of participants experiencing at least 1 event; OAD, oral antidiabetic drug; N/A, not applicable: the data are not available as the relationship between adverse event and action taken was not collected for comparator treatment (please see (b)); R, event rate per 100 years of treatment exposure.
Treatment‐emergent AEs include events that are collected from first exposure to the follow‐up visit scheduled 5 weeks (+1 week visit window) after the last trial product dose. Severe or BG‐confirmed symptomatic hypoglycaemia: an episode that is severe according to the ADA classification or BG‐confirmed by a plasma glucose value <3.1 mmol/L (56 mg/dL), with symptoms consistent with hypoglycaemia.
Most frequent AEs by system organ class and preferred term in ≥5%.
These participants cited AEs as the primary reason for discontinuing treatment when completing their end‐of‐trial form. However, the relationship between reporting of an adverse event during the trial and the action taken were not collected on the AE form for comparator treatments as, in this trial, the additional OAD was not considered as trial product. Number of episodes and event rate are therefore not available.
3.2.2. Secondary safety endpoints
Serious AEs (SAEs) were reported by 7.9%, 5.0% and 6.7% of participants treated with, respectively, semaglutide 0.5 mg, 1.0 mg and additional OAD, with no differences seen across groups according to system organ class (Table S4 in File S1). Two deaths occurred during the trial, one in the semaglutide 0.5 mg group (assessed as unlikely to be related to trial product by the investigator), and the other in the additional OAD group. Both were confirmed by the event adjudication committee as non‐cardiovascular deaths (Table 2).
Typically, AEs were mild to moderate in severity. The proportion of participants discontinuing treatment as a result of AEs was 5.9% with semaglutide 0.5 mg and 10.8% with semaglutide 1.0 mg (Table 2). The most frequent AEs, and most common reason for discontinuation in semaglutide‐treated participants, were gastrointestinal events. These events diminished over time (Table 2; Figure S2A–D in File S1). A similar proportion of participants in all arms reported diabetic retinopathy (Table 2).
Blood glucose‐confirmed symptomatic hypoglycaemia was infrequently reported (Table 2) and occurred almost exclusively in combination with sulphonlyureas (10 of 11 episodes in total). There were no reported episodes of severe hypoglycaemia.
Event adjudication committee‐confirmed neoplasms were reported in 6.3%, 8.7% and 5.0% of participants treated with, respectively, semaglutide 0.5 mg, semaglutide 1.0 mg and additional OAD. In the semaglutide treatment groups, these were mostly benign and colorectal in nature, while in the additional OAD group there were a similar number of benign and malignant neoplasms. No cases of medullary thyroid carcinoma were reported, and no differences in malignant neoplasms were observed across different tissues/organ systems (Table 2).
Cholelithiasis was reported in 4 participants (1.7%) treated with semaglutide 0.5 mg, 2 participants (0.8%) treated with semaglutide 1.0 mg, and none treated with additional OAD. There were no events of pancreatitis. Levels of pancreatic enzymes increased similarly with semaglutide 0.5 mg and 1.0 mg, and were significantly higher than with additional OAD (lipase: semaglutide 0.5 mg, estimated treatment ratio [ETR] 1.27 [95% CI 1.18 to 1.35]; semaglutide 1.0 mg, ETR 1.33 [95% CI 1.25 to 1.43]; amylase: semaglutide 0.5 mg, ETR 1.12 [95% CI 1.07 to 1.17]; semaglutide 1.0 mg, ETR 1.14 [95% CI 1.09 to 1.20], all P < .0001 vs additional OAD).
The event adjudication committee confirmed heart failure in a participant treated with semaglutide 0.5 mg and coronary revascularization in a participant treated with semaglutide 1.0 mg (Table 2). Pulse rate significantly increased from baseline with semaglutide 0.5 mg and 1.0 mg, compared with additional OAD (Table 3).
Table 3.
Key outcomes by treatment group at week 56
| Baselinea | Semaglutide 0.5 mg | Semaglutide 1.0 mg | Additional OAD | |||||
|---|---|---|---|---|---|---|---|---|
| Mean [SD] | Change from baseline at week 56 [SE]b | ETD [95% CI]b | P b | Change from baseline at week 56 [SE]b | ETD [95% CI]b | P b | Change from baseline at week 56 [SE]b | |
| Glycaemia endpoints | ||||||||
| HbA1c, mmol/mol | 65.0 [10.1] | −19.1 [0.5] | −11.79 [−13.58; −10.00] | <.0001 | −22.2 [0.5] | −14.94 [−16.75; −13.13] | <.0001 | −7.3 [0.7] |
| HbA1c, % | 8.1 [0.9] | −1.7 [<0.1] | −1.08 [−1.24; −0.91] | <.0001 | −2.0 [<0.1] | −1.37 [−1.53; −1.20] | <.0001 | −0.7 [0.1] |
| FPG, mmol/L | 8.9 [2.0] | −2.3 [0.1] | −1.66 [−1.94; −1.38] | <.0001 | −2.7 [0.1] | −2.03 [−2.32; −1.75] | <.0001 | −0.7 [0.1] |
| FPG, mg/dL | 160.4 [36.0] | −42.3 [1.5] | −29.91 [−35.02; −24.79] | <.0001 | −49.0 [1.5] | −36.63 [−41.79; 31.46] | <.0001 | −12.4 [2.1] |
| 7‐point SMPG, mg/dL | ||||||||
| Mean | 222.1 [44.8] | −59.2 [2.1] | −28.81 [−36.15; −21.47] | <.0001 | −67.0 [2.2] | −36.63 [−44.00; −29.25] | <.0001 | −30.4 [3.1] |
| Postprandial increment | 83.1 [40.2] | −23.2 [2.3] | −8.00 [−15.81; −0.19] | .0448 | −27.5 [2.3] | −12.32 [−20.17; −4.46] | .0022 | −15.2 [3.3] |
| 7‐point SMPG, mmol/L | ||||||||
| Mean | 12.3 [2.5] | −3.3 [0.1] | −1.60 [−2.01; −1.19] | <.0001 | −3.7 [0.1] | −2.03 [−2.44; −1.62] | <.0001 | −1.7 [0.2] |
| Postprandial increment | 4.6 [2.2] | −1.3 [0.1] | −0.44 [−0.88;−0.01] | .0448 | −1.5 [0.1] | −0.68 [−1.12;−0.25] | .0022 | −0.8 [0.2] |
| Body weight endpoints | ||||||||
| Body weight, kg | 71.5 [15.5] | −1.4 [0.2] | −1.84 [−2.67; −1.01] | <.0001 | −3.2 [0.2] | −3.59 [−4.43; −2.75] | <.0001 | 0.4 [0.3] |
| BMI, kg/m 2 | 26.3 [4.8] | −0.6 [0.1] | −0.70 [−1.02; −0.39] | <.0001 | −1.2 [0.1] | −1.37 [−1.69; −1.05] | <.0001 | 0.1 [0.1] |
| Waist circumference, cm | 91.9 [11.9] | −2.2 [0.3] | −1.86 [−2.86; −0.87] | .0003 | −3.3 [0.3] | −2.94 [−3.95; −1.94] | <.0001 | −0.4 [0.4] |
| Blood pressure and pulse rate | ||||||||
| SBP, mm Hg | 129.2 [13.0] | −2.0 [0.8] | −2.12 [−4.81; 0.57] | .1224 | −3.7 [0.8] | −3.87 [−6.59; −1.15] | .0054 | 0.2 [1.1] |
| DBP, mm Hg | 77.0 [9.7] | −0.8 [0.5] | −0.20 [−1.88; 1.48] | .8150 | −1.0 [0.5] | −0.46 [−2.16; 1.23] | .5934 | −0.6 [0.7] |
| Pulse rate, bpm | 72.8 [10.5] | 4.2 [0.6] | 2.58 [0.70; 4.46] | .0072 | 4.9 [0.6] | 3.36 [1.46; 5.26] | .0005 | 1.6 [0.8] |
| Treatment targets and composite endpoints | ||||||||
|---|---|---|---|---|---|---|---|---|
| Semaglutide 0.5 mg | Semaglutide 1.0 mg | Additional OAD | ||||||
| Participants achieving target | OR [95% CI]c | P c | Participants achieving target | OR [95% CI]c | P c | Participants achieving target | ||
| Proportion achieving HbA1ctargets, n (%) | ||||||||
| <53 mmol/mol (<7.0%) | 200 (84) | 9.42 [5.39; 16.46] | <.0001 | 220 (91) | 23.06 [11.99; 44.36] | <.0001 | 50 (42) | |
| ≤48 mmol/mol (≤6.5%) | 169 (71) | 17.76 [9.64; 32.72] | <.0001 | 193 (80) | 35.76 [18.66; 68.50] | <.0001 | 18 (15) | |
| Proportion achieving HbA1c <53 mmol/mol without severe or BG‐confirmed symptomatic hypoglycaemia and without weight gain, n (%) | 148 (62) | 7.63 [4.40; 13.25] | <.0001 | 178 (74) | 15.79 [8.84; 28.21] | <.0001 | 24 (20) | |
| Proportion achieving body weight reduction, n (%) | ||||||||
| ≥5% | 63 (26) | 5.61 [2.51; 12.51] | <.0001 | 112 (46) | 14.83 [6.69; 32.85] | <.0001 | 7 (6) | |
| ≥10% | 20 (8) | 7.41 [1.42; 38.73] | .0176 | 42 (17) | 17.69 [3.48; 89.99] | .0005 | 1 (1) | |
Abbreviations: BG, blood glucose; BMI, body mass index; BP, blood pressure; bpm, beats per minute; CI, confidence interval; DBP, diastolic blood pressure; ETD, estimated treatment difference; FPG, fasting plasma glucose; HbA1c, glycated haemoglobin; OAD, oral antidiabetic drug; OR, odds ratio; SBP, systolic blood pressure; SMPG, self‐measured plasma glucose.
Baseline: observed mean for the entire trial population.
Estimated means and treatment differences (each semaglutide dose vs additional OAD) at week 56 are from a mixed model for repeated measurements (MMRM) using data obtained before the initiation of any rescue medication or before premature treatment discontinuation, with the exception of pulse rate values, which are based on the data before premature treatment discontinuation. P values are from 2‐sided testing for the null hypothesis of no treatment difference (each dose of semaglutide vs additional OAD).
ORs (each semaglutide dose vs additional OAD) and P values are based on a logistic regression model using data obtained before the initiation of any rescue medication or before premature treatment discontinuation. Missing HbA1c and body weight data at week 56 are imputed from an MMRM and subsequently classified. P values are two‐sided testing for the null hypothesis of no treatment difference (each dose of semaglutide vs additional OAD).
Calcitonin levels were consistently low and similar across all treatment groups (data not shown). Three semaglutide‐treated participants developed anti‐semaglutide antibodies (2 on semaglutide 0.5 mg and 1 on semaglutide 1.0 mg), and cross‐reaction with endogenous GLP‐1 occurred in 1 participant. No anti‐semaglutide antibodies had an in vitro neutralizing effect on semaglutide. Allergic reactions were reported in 17 participants (7.1%) on semaglutide 0.5 mg, 16 participants (6.6%) on semaglutide 1.0 mg, and 5 participants (4.2%) on additional OAD treatment. Injection‐site reactions occurred in 1 participant (0.4%) each in the semaglutide 0.5 mg and 1.0 mg groups.
There were no clinically relevant changes in other safety laboratory assessments, physical examination, electrocardiograms or fundoscopy/fundus photography.
3.3. Efficacy endpoints
3.3.1. Glycaemic control
At week 56, mean HbA1c (baseline 8.1%) was significantly reduced with semaglutide 0.5 mg and 1.0 mg vs the additional OAD group (Figure 2A,B, Table 3). HbA1c was consistently reduced from baseline to a greater extent with semaglutide 0.5 mg and 1.0 mg, compared with additional OAD, in all pre‐trial treatment groups (Figure S3 in File S1). Statistical sensitivity analyses for change in HbA1c at week 56 supported the main result (Figure S4A in File S1). Significantly higher proportions of participants achieved the American Diabetes Association and Japanese Diabetes Society HbA1c target of <7.0% and the American Association of Clinical Endocrinologists target24 of ≤6.5% (Figure 2C,D and Table 3).
Figure 2.
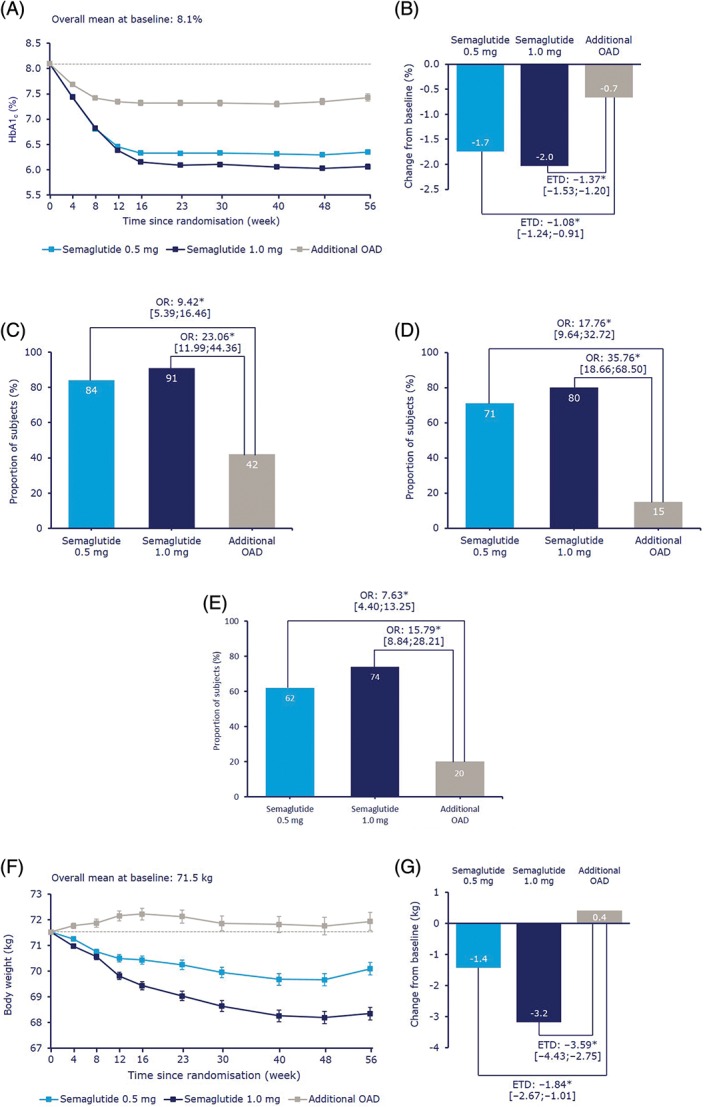
Semaglutide 0.5 mg and 1.0 mg once weekly, compared with an additional OAD, mean glycated haemoglobin (HbA1c) by week, A; change in mean HbA1c after 56 weeks, B; the proportion of participants achieving HbA1c <7.0%, C; ≤6.5%, D and HbA1c <7.0% with no severe or blood glucose, BG‐confirmed symptomatic hypoglycaemia and no weight gain at week 56, E; mean body weight by week, F and change in mean body weight after 56 weeks, G. Values in A, B, F and G are estimated means (±standard errors) from a mixed model for repeated measurements using data obtained before the initiation of any rescue medication or before premature treatment discontinuation. Dotted line is the overall mean value at baseline. Values in C, D, E, H and I are observed proportions using ‘on‐treatment without rescue medication’ data from subjects in the full analysis set. Missing data are imputed from a mixed model for repeated measurements and subsequently classified. BG‐confirmed, plasma glucose <3.1 mmol/L (56 mg/dL). BG, blood glucose; ETD, estimated treatment difference; HbA1c, glycosylated haemoglobin; OAD, oral antidiabetic drug; OR, odds ratio. *P < .0001. †P < .02
The proportion of participants achieving HbA1c <7.0% with no severe or BG‐confirmed symptomatic hypoglycaemia and no weight gain at week 56 was also greater with semaglutide vs additional OAD (Figure 2E, Table 3). Overall, more participants treated with semaglutide achieved any HbA1c reduction than participants treated with an additional OAD (Figure S6A in File S1).
At week 56, mean FPG, SMPG postprandial increment and mean 7‐point SMPG were all significantly reduced with semaglutide 0.5 mg and 1.0 mg vs additional OAD (Table 3).
3.3.2. Body weight
Mean body weight (baseline 71.5 kg) was significantly decreased in participants treated with semaglutide 0.5 mg and 1.0 mg, respectively, vs an increase in participants treated with additional OAD, and the treatment differences were statistically significant (Figure 2F,G and Table 3). Statistical sensitivity analyses for change in body weight at week 56 supported the main result (Figure S4B in File S1). Significantly higher proportions of semaglutide‐treated participants achieved a weight loss of ≥5% or ≥10% than in the additional OAD group (Figure S5 in File S1 and Table 3). Overall, more participants treated with semaglutide achieved a reduction in body weight than participants treated with an additional OAD (Figure S6B in File S1). BMI and waist circumference were significantly reduced in participants treated with semaglutide 0.5 mg and 1.0 mg vs additional OAD treatment (Table 3).
3.3.3. Glucose metabolism and β‐cell function
Pro‐insulin and pro‐insulin: insulin ratios were significantly reduced in participants treated with both semaglutide 0.5 mg and 1.0 mg vs participants receiving additional OAD. C‐peptide and homeostatic model assessment of β‐cell function were significantly increased with both semaglutide doses compared with additional OAD. Plasma glucagon and homeostatic model assessment of insulin resistance were significantly decreased with semaglutide 1.0 mg vs additional OAD, and insulin was significantly increased in participants treated with semaglutide 0.5 mg compared with participants treated with additional OAD (Figure S7 in File S1).
3.3.4. Other secondary efficacy endpoints
At week 56, there was a reduction from baseline in blood pressure in participants treated with semaglutide, compared with participants treated with additional OAD. This reduction was significant for systolic blood pressure with semaglutide 1.0 mg vs additional OAD (Table 3).
All lipids, except free fatty acids with semaglutide 0.5 mg and HDL cholesterol with both doses, showed significant reductions with semaglutide treatment, compared with additional OAD (Table S5 in File S1).
4. DISCUSSION
Overall, semaglutide was well tolerated and no new safety findings were identified in this trial. The primary endpoint of TEAEs occurred in more participants receiving semaglutide than those receiving one additional OAD. The proportion of participants reporting SAEs was similar across all treatment groups. The difference in AEs was primarily driven by gastrointestinal‐related AEs, although these were of mild or moderate severity and diminished over time, and are a well‐known side effect of GLP‐1RAs.25 Gastrointestinal‐related AEs were the most frequently reported events leading to premature treatment discontinuation. The AE profile was similar to that of other currently available GLP‐1RAs and generally similar to that seen in the global SUSTAIN trials.13, 14, 15, 16, 17, 18
The cardiovascular safety of semaglutide is of interest, in light of the recent global SUSTAIN 6 trial, which did not include any Japanese participants. SUSTAIN 6 was designed to assess the non‐inferiority of semaglutide, compared with placebo, for cardiovascular outcomes in participants with T2D. Results showed a significant cardioprotective effect with semaglutide treatment, with the first occurrence of cardiovascular death, non‐fatal myocardial infarction or non‐fatal stroke occurring in 6.6% of participants treated with semaglutide 0.5 mg or 1.0 mg, compared with 8.9% in participants treated with placebo (hazard ratio 0.74 [95% CI 0.58 to 0.95]; P < .001 for non‐inferiority; P = .02 for superiority).18
The efficacy results of this trial were favourable, showing that both semaglutide 0.5 mg and 1.0 mg produce significant and clinically relevant improvements in glycaemic control and body weight when used as monotherapy or in combination with one OAD (a sulphonylurea, glinide, α‐glucosidase inhibitor or thiazolidinedione), compared with one additional OAD, in Japanese participants with T2D. These improvements were sustained over 56 weeks of treatment, and results were supported by all sensitivity analyses. Furthermore, a very high and significantly greater proportion of participants receiving semaglutide vs additional OAD treatment achieved the American Diabetes Association and Japanese Diabetes Society target of HbA1c <7.0% (<53.0 mmol/mol) (84% and 91% of 0.5 mg and 1.0 mg semaglutide‐treated participants, respectively, vs 42% in the additional OAD group). Importantly, a higher proportion of participants treated with semaglutide vs additional OAD achieved this target with no severe or BG‐confirmed symptomatic hypoglycaemia and no weight gain at week 56. The efficacy of semaglutide on HbA1c and body weight was consistent across background treatments.
These findings generally correspond with those from the global SUSTAIN trials,13, 14, 15, 16, 17, 18 in which superior and clinically meaningful improvements in glycaemic control and body weight were achieved with semaglutide compared with placebo in SUSTAIN 1 and SUSTAIN 515, 17; with sitagliptin in SUSTAIN 213; with exenatide extended release in SUSTAIN 316; and with insulin glargine in SUSTAIN 4.14 In addition, the reduction in body weight was sustained for up to 2 years.18
A potential limitation of the present trial is the open‐label design, which was necessary because of the different appearances and administration methods of the medications used. The small sample size of the comparator group is also a limitation, although the sample size calculation is in line with Japanese guidelines regarding clinical trial design.21 The fact that the comparator group was subdivided between 6 different OADs compounds the sample size limitation further. The effects of using metformin and semaglutide exposure have been previously investigated in the phase IIIa programme, which included Japanese participants (SUSTAIN 2, SUSTAIN 5).13, 17 Similar efficacy and no safety issues were evident for the combination with metformin. Also, this study population may not accurately reflect that of the Japanese T2D population because more than two‐thirds of participants were male.
Overall, the combination of a predictable safety profile, coupled with glycaemic control and body weight reduction, indicates the potential of semaglutide for use in Japanese people for controlling T2D.5
In conclusion, in the present trial, semaglutide treatment was well tolerated in Japanese participants with T2D. AEs were more frequently reported with both doses of semaglutide than with 1 additional OAD, primarily driven by gastrointestinal AEs; the proportion of participants reporting SAEs was similar across treatment groups. No new safety issues were identified and the safety profile of semaglutide was similar to that of other GLP‐1RAs. In addition, semaglutide treatment significantly reduced HbA1c and body weight, and improved lipids and systolic blood pressure. Changes in glycaemic control and body weight were sustained for 56 weeks and were consistent across different background treatments.
Supporting information
File S1. Supporting information files.
Figure S1 Trial design.
Figure S2. Time course of constipation (A), nausea (B), diarrhoea (C) and vomiting (D).
Figure S3. HbA1c by pre‐trial treatment Semaglutide 0.5 mg and 1.0 mg once weekly, compared with an additional OAD, change in HbA1c after 56 weeks in patients on diet and exercise therapy; sulphonylureas (SU); glinide; α‐glucosidase inhibitor (α‐GI); and thiazolidinediones (TZD).
Figure S4. Statistical sensitivity analyses for HbA1c (A) and body weight (B)
Figure S5. Semaglutide 0.5 mg and 1.0 mg once weekly, compared with an additional OAD, proportion of subjects achieving ≥5% (A) and ≥10% body weight reduction (B)
Figure S6. Cumulative distribution of change from baseline in HbA1c (A) and (B) body weight at week 56
Figure S7. Glucose metabolism and beta‐cell function
Table S1. Full inclusion and exclusion criteria
Table S2. Events types predefined for event adjudication committee (EAC) review
Table S3. Baseline HbA1c and body weight by baseline treatment strata
Table S4. Treatment‐emergent serious adverse events (by system organ class)
Table S5. Additional secondary endpoints at week 56
ACKNOWLEDGMENTS
We thank all the participants, investigators and trial‐site staff who were involved in the conduct of the trial. We also thank Hrvoje Vrazic (Novo Nordisk) for review and input to the manuscript, and Jamil Bacha, PhD (AXON Communications) for medical writing and editorial assistance, who received compensation from Novo Nordisk.
Conflict of interest
K.K. has received honoraria or consulting fees from Astellas Pharma, AstraZeneca KK, MSD KK, Ono Pharmaceutical, Kissei Pharmaceutical, Kowa Pharmaceutical, Sanofi KK, Sanwakagaku Kenkyusyo, Sumitomo Dainippon Pharma, Mitsubishi Tanabe Pharma, Novartis Pharma KK, Novo Nordisk, Nippon Boehringer Ingelheim, Taisho Toyama Pharmaceutical and Takeda, and research grants from Taisho Pharmaceutical, Mitsubishi Tanabe Pharma and Nippon Boehringer Ingelheim. Y.Y. has received honoraria or consulting fees from Novo Nordisk. H.W. has received honoraria for consulting from Boehringer Ingelheim, Daiichi Sankyo, Dainippon Sumitomo Pharma, Eli Lilly, Kowa Pharmaceutical, Merck Sharp & Dohme, Novo Nordisk, Novartis, Ono Pharmaceutical, Sanofi, Sanwa Kagaku Kenkyusho, Takeda, Astellas Pharma, Mitsubishi Tanabe Pharma, AstraZeneca, Kyowa Hakko Kirin and Kissei Pharmaceutical, and grants from AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo, Dainippon Sumitomo Pharma, Eli Lilly, Kissei Pharma, Merck Sharp & Dohme, Mitsubishi Tanabe Pharma, Mochida Pharmaceutical, Novartis, Novo Nordisk, Pfizer, Sanofi, Sanwakagaku Kenkyusho, Takeda, Terumo Corp, Novartis, Astellas Pharma, Abbott Japan, Ono Pharmaceutical, Kyowa Hakko Kirin Co. Ltd, Kowa Pharmaceutical, Johnson & Johnson, Taisho Toyama Pharmaceutical, Nitto Boseki Company, Bayer, Bristol‐Myers Squibb and Benefit one Health care. A.A. has received consulting and/or speaker fees from Novo Nordisk, Eli Lilly Japan, Nippon Boehringer Ingelheim, Kowa Pharmaceutical, Novartis International, Astellas Pharma, Mitsubishi Tanabe Pharma, Taisho Toyama Pharmaceutical, Kyowa Hakko Kirin, Sumitomo Dainippon Pharma, Sanofi, MSD, Takeda, AstraZeneca, Ono Pharmaceutical, Sanwa Kagaku Kenkyusho and Daiichi Sankyo, and research grants from Novo Nordisk. T.N. is an employee of Novo Nordisk and holds shares in the company. J.Z. is an employee of Novo Nordisk and holds shares in the company. A.K. has received honoraria and consultation fees from AstraZeneca.
Author contributions
Author contributions were as follows: K.K.: study design, study conduct/data collection, data analysis, writing the manuscript; Y.Y.: writing the manuscript; H.W.: study conduct/data collection, data analysis; A.A.: writing the manuscript; T.N.: study design, data analysis, writing the manuscript; J.Z.: study design, study conduct/data collection, data analysis, writing the manuscript; A.K.: study conduct/data collection, writing the manuscript.
Kaku K, Yamada Y, Watada H, et al. Safety and efficacy of once‐weekly semaglutide vs additional oral antidiabetic drugs in Japanese people with inadequately controlled type 2 diabetes: A randomized trial. Diabetes Obes Metab. 2018;20:1202–1212. https://doi.org/10.1111/dom.13218
Funding information Novo Nordisk A/S, Funded by Novo Nordisk A/S; http://ClinicalTrials.gov identifier: NCT02207374.
REFERENCES
- 1. Mukai N, Doi Y, Ninomiya T, et al. Trends in the prevalence of type 2 diabetes and prediabetes in community‐dwelling Japanese subjects: The Hisayama Study. J Diabetes Investig. 2014;5(2):162‐169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Japanese Diabetes Society . Evidence‐based Practice Guideline for the Treatment of Diabetes in Japan 2013. Goals and Strategies for Diabetes Management. http://www.jds.or.jp/modules/en/index.php?content_id=44. Accessed May 23, 2017.
- 3. Japanese Diabetes Society . Treatment Guide for Diabetes 2014–2015 [in Japanese]. http://www.jds.or.jp/modules/en/index.php?content_id=34. Accessed May 23, 2017.
- 4. American Diabetes Association . Standards of medical care in diabetes. 5. Glycemic targets. Diabetes Care. 2016;39(suppl 1):S39‐S46. [DOI] [PubMed] [Google Scholar]
- 5. UK Prospective Diabetes Study Group . Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837‐853. [PubMed] [Google Scholar]
- 6. Diabetes Control Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. N Engl J Med. 1993;329(14):977‐986. [DOI] [PubMed] [Google Scholar]
- 7. Stark Casagrande S, Fradkin JE, Saydah SH, Rust KF, Cowie CC. The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988‐2010. Diabetes Care. 2013;36(8):2271‐2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Handelsman Y, Bloomgarden ZT, Grunberger G, et al. American Association of Clinical Endocrinologists and American College of Endocrinology ‐ clinical practice guidelines for developing a diabetes mellitus comprehensive care plan ‐ 2015. Endocr Pract. 2015;21(suppl 1):1‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient‐centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38(1):140‐149. [DOI] [PubMed] [Google Scholar]
- 10. Trujillo JM, Nuffer W, Ellis SL. GLP‐1 receptor agonists: a review of head‐to‐head clinical studies. Ther Adv Endocrinol Metab. 2015;6(1):19‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kapitza C, Nosek L, Jensen L, Hartvig H, Jensen CB, Flint A. Semaglutide, a once‐weekly human GLP‐1 analog, does not reduce the bioavailability of the combined oral contraceptive, ethinylestradiol/levonorgestrel. J Clin Pharmacol. 2015;55(5):497‐504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lau J, Bloch P, Schäffer L, et al. Discovery of the once‐weekly glucagon‐like peptide‐1 (GLP‐1) analogue semaglutide. J Med Chem. 2015;58(18):7370‐7380. [DOI] [PubMed] [Google Scholar]
- 13. Ahrén B, Masmiquel L, Kumar H, et al. Efficacy and safety of once‐weekly semaglutide versus once‐daily sitagliptin as an add‐on to metformin, thiazolidinediones, or both, in patients with type 2 diabetes (SUSTAIN 2): a 56‐week, double‐blind, phase 3a, randomised trial. Lancet Diabetes Endocrinol. 2017;5(5):341‐354. [DOI] [PubMed] [Google Scholar]
- 14. Aroda VR, Bain SC, Cariou B, et al. Efficacy and safety of once‐weekly semaglutide versus once‐daily insulin glargine as add‐on to metformin (with or without sulfonylureas) in insulin‐naive patients with type 2 diabetes (SUSTAIN 4): a randomised, open‐label, parallel‐group, multicentre, multinational, phase 3a trial. Lancet Diabetes Endocrinol. 2017;5(5):355‐366. [DOI] [PubMed] [Google Scholar]
- 15. Sorli C, Harashima SI, Tsoukas GM, et al. Efficacy and safety of once‐weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double‐blind, randomised, placebo‐controlled, parallel‐group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol. 2017;5(4):251‐260. [DOI] [PubMed] [Google Scholar]
- 16. Ahmann A, Capehorn M, Charpentier G, et al. Efficacy and safety of once‐weekly semaglutide vs exenatide ER after 56 weeks in subjects with type 2 diabetes (SUSTAIN 3). Diabetes. 2016;65(suppl 1):A49. [DOI] [PubMed] [Google Scholar]
- 17. Rodbard H, Lingvay I, Reed J, et al. Efficacy and safety of semaglutide once‐weekly vs placebo as add‐on to basal insulin alone or in combination with metformin in subjects with type 2 diabetes (SUSTAIN 5). Diabetologia. 2016;59(suppl 1):S364‐S365. [Google Scholar]
- 18. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834‐1844. [DOI] [PubMed] [Google Scholar]
- 19. Ministry of Health, Labour and Welfare . Guideline for Clinical Evaluation of Oral Hypoglycaemic Agents. 2010. https://www.pmda.go.jp/files/000208194.pdf. Accessed February 5, 2018.
- 20. http://ClinicialTrials.gov. A trial comparing the safety and efficacy of semaglutide once weekly versus sitagliptin once daily in Japanese subjects with type 2 diabetes (SUSTAIN). https://clinicaltrials.gov/ct2/show/NCT02254291. Latest update August 18, 2017. Accessed February 5, 2018.
- 21. http://ClinicialTrials.gov. A trial comparing the safety and efficacy of semaglutide once weekly in monotherapy or in combination with one OAD in Japanese subjects with type 2 diabetes (SUSTAIN). https://clinicaltrials.gov/ct2/show/NCT02207374. Accessed February 5, 2018.
- 22. International Conference on Harmonisation Working Group . International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Harmonised Tripartite Guideline: Guideline for Good Clinical Practice E6 (R1). 1996. https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf. Accessed February 5, 2018.
- 23. World Medical Association . Declaration of Helsinki Ethical Principles for Medical Research Involving Human Subjects 52nd WMA General Assembly, Edinburgh, Scotland, October 2000 Last amended with Note of Clarification on Paragraph 29 by the WMA General Assembly, Washington 2002; and Note of Clarification on Paragraph 30 by the WMA General Assembly, Tokyo. 2004. https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects. Accessed February 5, 2018.
- 24. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm – 2016 executive summary. Endocr Pract. 2016;22(1):84‐113. [DOI] [PubMed] [Google Scholar]
- 25. Sun F, Chai S, Yu K, et al. Gastrointestinal adverse events of glucagon‐like peptide‐1 receptor agonists in patients with type 2 diabetes: a systematic review and network meta‐analysis. Diabetes Technol Ther. 2015;17(1):35‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
File S1. Supporting information files.
Figure S1 Trial design.
Figure S2. Time course of constipation (A), nausea (B), diarrhoea (C) and vomiting (D).
Figure S3. HbA1c by pre‐trial treatment Semaglutide 0.5 mg and 1.0 mg once weekly, compared with an additional OAD, change in HbA1c after 56 weeks in patients on diet and exercise therapy; sulphonylureas (SU); glinide; α‐glucosidase inhibitor (α‐GI); and thiazolidinediones (TZD).
Figure S4. Statistical sensitivity analyses for HbA1c (A) and body weight (B)
Figure S5. Semaglutide 0.5 mg and 1.0 mg once weekly, compared with an additional OAD, proportion of subjects achieving ≥5% (A) and ≥10% body weight reduction (B)
Figure S6. Cumulative distribution of change from baseline in HbA1c (A) and (B) body weight at week 56
Figure S7. Glucose metabolism and beta‐cell function
Table S1. Full inclusion and exclusion criteria
Table S2. Events types predefined for event adjudication committee (EAC) review
Table S3. Baseline HbA1c and body weight by baseline treatment strata
Table S4. Treatment‐emergent serious adverse events (by system organ class)
Table S5. Additional secondary endpoints at week 56


