Summary
We performed a systematic review on the neurological complications of chikungunya virus. Such complications are being reported increasingly, owing primarily to the scale of recent epidemics but also to a growing understanding of the virus' neurovirulence. We performed a thorough literature search using PubMed and Scopus databases, summating the data on all published reports of neurological disease associated with chikungunya virus. We appraised the data for each major condition in adults, children, and neonates, as well as evaluating the latest evidence on disease pathogenesis and management strategies. The review provides a comprehensive summary for clinicians, public health officials, and researchers tackling the challenges associated with this important emerging pathogen.
Keywords: acute disseminated encephalomyelitis, chikungunya, complications, congenital infections, encephalitis, Guillain‐Barré syndrome, myelitis, neonatal infection, neurological, optic neuritis, retinitis, uveitis
Abbreviations: Used in main text
- AIDP
acute inflammatory demyelinating polyneuropathy
- AMAN
acute motor axonal neuropathy
- AMSAN
acute motor and sensory axonal neuropathy
- CNS
central nervous system
- CSF
cerebrospinal fluid
- ECSA
East/Central/South African lineage
- GBS
Guillain‐Barré syndrome
- MRI
magnetic resonance imaging
- PCR
polymerase chain reaction
- RNA
ribonucleic acid
Used in tables only
- ADEM
acute disseminated encephalomyelitis
- BL
bullous lesions
- CIDP
chronic inflammatory demyelinating polyneuropathy
- CN
cranial nerve
- CRAO
central retinal artery occlusion
- DIC
disseminated intravascular coagulation
- EEG
electroencephalogram
- HI
haemagglutination inhibition
- Isol
viral isolation
- MFS
Miller Fisher syndrome
- MP
methylprednisolone
- PL
prodrome length
- PP
days postpartum
- RAPD
relative afferent papillary defect
- UMN/LMN
upper/lower motor neuron
- UR/I
urinary retention/incontinence
- VEP
visual evoked potential
- WCC
white cell count
1. INTRODUCTION
Chikungunya virus is an alphavirus (genus Alphavirus, family Togaviridae) that is primarily transmitted to humans by Aedes mosquitoes, and occasionally from mother to child. The word “chikungunya” originates from the Makonde language, spoken in Tanzania and Mozambique, meaning “that which bends up”1; this refers to the debilitating arthralgia often occurring in the acute phase of infection, along with fever, myalgia, headache, and rash. Although the first outbreaks were described in the 1960s, the virus was not considered a major public health problem until 2004, when it caused explosive outbreaks in the tropics. Severe complications of chikungunya infection, including neurological disease, are being recognised increasingly.
Classically, alphaviruses are described in 2 groups—the “old world” viruses, including Sindbis, O'nyong'nyong, and Ross River viruses, which cause a predominantly arthritic syndrome, and the “new world” viruses, including Eastern, Western, and Venezuelan equine encephalitis viruses, which are responsible for outbreaks of encephalitis.2 Chikungunya virus is now recognised as a cause of both arthritic and neurological diseases throughout the tropics.
Dengue and Zika are also arthropod‐borne viruses (arboviruses) that, like chikungunya, are transmitted by Aedes mosquitoes but are flaviviruses (genus Flavivirus, family Flaviviridae). All 3 arboviruses cause an initial fever‐arthralgia‐rash syndrome and are associated with neurological complications.3, 4, 5 Given increasing reports of cocirculation and coinfection of the 3 arboviruses in the Americas,6 the underlying viral aetiology in patients presenting with arbovirus‐associated neurological disease is not always clear. Therefore, understanding the similarities and differences between chikungunya‐, Zika‐, and dengue‐associated neurological diseases is of importance and will be addressed in this review.
We performed a systematic review for evidence of neurological disease associated with chikungunya virus. A well‐designed review was recently published on this topical subject7; our review approaches the disease spectrum in a different manner, accounting for the differences. Here, we use a broader search strategy, leading us to consider general features of the virus and disease before individually discussing the different neurological manifestations, the challenges in their diagnosis, sequelae of perinatal infection, and knowledge of the underlying disease mechanisms; we include comparison with Zika and dengue viruses throughout. Where provided, we present all data detailing the clinical information, investigations, management, and outcome for all cases described in the literature.
2. METHODS
We searched PubMed and Scopus for articles published up to 29 October 2017 using the following criteria: “chikungunya” AND (“neurolog*” OR “encephal*” OR “meningoencephalitis” OR “guillain‐barré syndrome” OR “myelitis” OR “myelopathy” OR “stroke” OR “ocular” OR “optic neuritis” OR “severe” OR “unusual manifestations” OR “neonatal” OR “congenital” OR “perinatal” OR “fatal”) (Figure 1). There were no language restrictions. All published studies describing patients with neurological complications of chikungunya were considered eligible for inclusion, comprising case reports and series, and case‐control, cohort, cross‐sectional, and pathogenesis studies. Articles that did not mention neurological complications of chikungunya were excluded. Data extracted included the numbers of patients with neurological complications, and where available, the time between systemic symptoms of infection and neurological disease, cerebrospinal fluid (CSF) findings, a summary of the clinical presentation, the author's diagnosis, treatment given, and outcome. The number of patients described per distinct neurological syndrome and aggregate data on diagnosis, treatment, and outcome were summarised.
Figure 1.
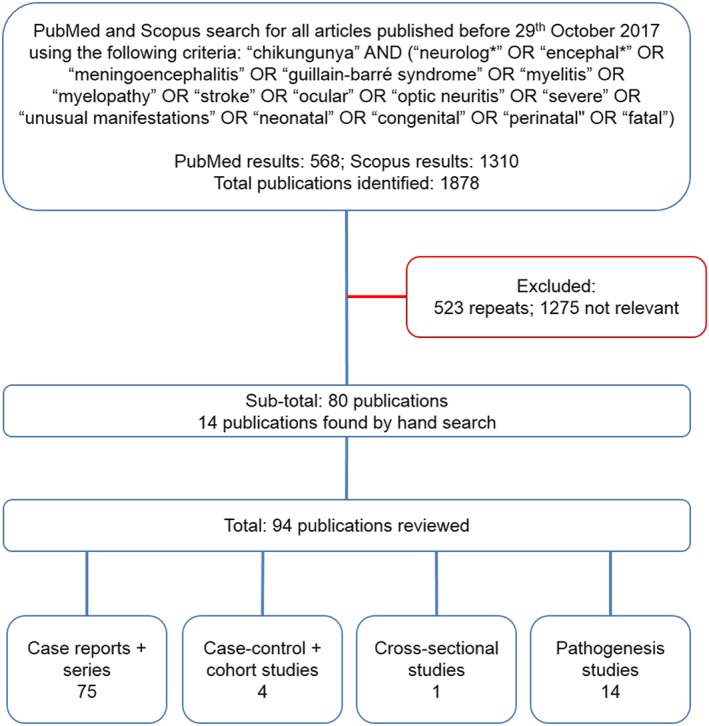
Search strategy to identify publications on neurological complications of chikungunya
3. ROLE OF THE FUNDING SOURCE
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
4. EPIDEMIOLOGY AND LINEAGE
Historical accounts suggest that chikungunya may have caused outbreaks as early as the 18th century,8 although the virus and its disease were first isolated and documented respectively in 1952 to 1953, in Tanzania.9, 10 Since then, 2 lineages, namely West African and East/Central/South African (ECSA), have been shown to circulate in sub‐Saharan Africa in a sylvatic cycle between mosquitoes and non‐human primates.11 The first documented human outbreaks were in southern Asia during the 1960s to 1970s12, 13 and were caused by the Asian strain, a descendent of the ECSA strain.14 After decades of low transmission, an ECSA divergent strain re‐emerged in 2004, having undergone 2 successive mutations of its envelope E1 glycoprotein.15 This new lineage, renamed the Indian Ocean Lineage spread from Kenya to cause explosive outbreaks throughout the islands of the Indian Ocean, India, and Southeast Asia, affecting millions.16 In late 2013, the emergence of the Asian strain was reported in the Caribbean,17 marking its first documented appearance in the Americas. It has since rapidly spread throughout 48 American countries and territories, causing over 2 million suspected cases to date.18 Of note, local circulation of the ECSA strain has also recently been reported in Bahia state, Brazil.19
5. DIAGNOSIS
As for other arboviruses, proving that chikungunya has caused neurological disease can be challenging.2, 5 Traditionally, a causal relationship between microbe and disease was based on Koch's postulates, as follows20:
The agent must be demonstrable in every case of the disease.
The agent is not present in other diseases.
After isolation in culture, the agent must be able to produce the disease in experimental animals.
The agent can be recovered from the experimental animal.
There are clear limitations to these in modern microbiology. For example, we know now that certain pathogens can cause multiple diseases, and indeed certain diseases can be caused by more than 1 pathogen (postulates 1 and 2). Furthermore, modern technologies such as polymerase chain reaction (PCR) assays have increased our detection rate of certain pathogens over isolation in culture (postulate 3). We therefore adapt these postulates today in studies regarding causality. Neurological disorders associated with arbovirus infection have an added layer of complexity, owing to the different samples used for testing for the presence of the viruses. The strongest evidence of causality comes from demonstrating that the virus is in the central nervous system (CNS), which is most often shown by detecting viral RNA in the CSF by PCR; alternatively, the virus may be cultured. In fatal cases, autopsy material may be positive by PCR. For many patients, the virus has cleared from the CSF by the time they present; in which case, the detection of CSF IgM antibody by enzyme‐linked immunosorbent assay is considered diagnostic. Interpretation is complicated for flavivirus infections because a positive Zika‐IgM test can result from cross‐reactivity of serum containing antibodies against dengue virus.21 Because chikungunya is an alphavirus, there is no serological cross‐reactivity with the flaviviruses, making diagnosis more straightforward (in areas where other alphaviruses are not circulating). It is not known for how long virus, RNA, or IgM remains detectable in chikungunya‐associated neurological disease, and whether this differs for the different neurological disorders. By analogy with similar arboviruses, we might expect the virus to be detectable for the first few days of illness, at which point it is replaced by antibody, which remains for weeks to months. Chikungunya virus is more readily detected by PCR or culture in the blood, because of its long and high viraemia; IgM antibody can also be detected in the blood. However, a positive blood test in a patient with neurological disease does not necessarily mean the virus caused the disease; infection may be coincidental, and care must be taken to exclude other possible causes. The virus can also be detected in urine, saliva, semen, and milk,22, 23, 24 but the same caveats apply.
6. CLINICAL FINDINGS
Seroprevalence studies have reported a range of asymptomatic rates of chikungunya infection, from 3% to 47%.25 In acute symptomatic infection, following an incubation period of approximately 3 days,26 there is an abrupt onset of fever, headache, rash, arthralgia, and myalgia, which typically last for 1 to 2 weeks.27 After this, seroconversion likely confers lifelong immunity.28 As well as neurological manifestations, chikungunya virus is associated with complications of the cardiovascular, renal, respiratory, hepatic, gastrointestinal, and adrenal systems, sometimes collectively referred to as “atypical features.”29, 30, 31 However, a disorder of the nervous system appears to be the most common severe complication of chikungunya infection (Table 1). In 2 studies investigating manifestations of chikungunya in patients requiring intensive care, a neurological disorder was the primary issue in 61%32 and 79%33 of chikungunya‐infected patients.
Table 1.
List of neurological syndromes and syndromes and diseases associated with chikungunya virus
| Described More Frequently | Described Less Frequently |
|---|---|
| Encephalopathy and encephalitis | Seizures with or without fever |
| Myelopathy and myelitis | Behavioural changes |
| Encephalomyelopathy | Sensorineural hearing loss |
| Myeloneuropathy | Stroke |
| Encephalomyeloneuropathy | Cerebellitis |
| Guillain‐Barré syndrome | Meningism |
| Acute disseminated encephalomyelitis | Third nerve palsy |
| Neonatal hypotonia | Encephaloneuropathy |
| Neuro‐ocular disease (uveitis, retinitis, optic neuritis) | Carpal tunnel syndrome |
| Bilateral total ophthalmoplegia | |
| Mild encephalitis with a reversible lesion of the splenium | |
| Bickerstaff brainstem encephalitis–Miller Fisher syndrome–Guillain‐Barré syndrome overlap |
In chikungunya‐associated neurological disease, the clinician must be vigilant for other complications in the same patient, a pattern also described in dengue but rarely in Zika infection. Amongst 99 cases of chikungunya‐associated neurological disease described in a study in India, 69 also had other complications involving, for example, the renal, hepatic, and respiratory systems.30 These patients should be managed using a multidisciplinary approach.
7. NEUROLOGICAL MANIFESTATIONS
The literature on neurological manifestations of chikungunya reflects that of the disease activity itself, with 5 publications between 1964 and 1971 and 89 since 2005, comprising 27 case reports, 48 case series, 1 case‐control study, 3 cohort studies, 1 cross‐sectional study, and 14 pathogenesis studies (Figure 1); autopsy data were included in one report found.34 Table 2 provides a summary of all cases of reported chikungunya‐associated neurological disease. In total, we found 856 cases of chikungunya‐associated neurological disease in the literature; 796 (93.0%) were in adults and children infected directly via mosquito, 60 (7.0%) were in neonates infected vertically from mother to child (Figure 2). Because patients were investigated to variable extents, we have categorised them according to the presenting clinical syndromes of encephalopathy, myelopathy, neuropathy, combinations of these, and neuro‐ocular disease; we then provided diagnoses, treatment, and outcome where available. A limitation of our study was that we could not incorporate diagnostic criteria, given the range of information available amongst all the cases published. The most common clinical presentation of neurological disease associated with adult and child chikungunya infection was encephalopathy; it accounts for 322 (40.5%) of the 781 patients described. Excluding ocular disease, 474 (77.3%) of all adult and child cases had a pure CNS disorder, 82 (13.4%) had a pure peripheral nervous system disorder, and 57 (9.3%) had a disorder of both the CNS and peripheral nervous system. Cases in which coinfection of chikungunya with another neurovirulent arbovirus was detected are not included in Table 2 but are further discussed below.
Table 2.
Reports of neurological disease associated with chikungunya virus in adults and children
| Year of Case(s), Location | No. | Laboratory Evidence for Chikungunya | PL | CSF | Neurological Features | Diagnosis | Treatmenta and outcome |
|---|---|---|---|---|---|---|---|
| Encephalopathy (n = 322) | |||||||
| 1963‐1964, India35 | 2 | HI | … | … | Unknown | Encephalitis | 1 died, 1 unknown |
| 1962‐1964, Thailand,13 | 1 | HI/isol ser | … | … | Unknown | Meningoencephalitis | Unknown |
| 2005‐2006, Mayotte36 | 2 | PCR/IgM CSF/ser | … | … | Unknown | Meningoencephalitis | Unknown |
| 2005‐2006, Réunion33 | 14 | PCR CSF (2/12), PCR serum (4/10), IgM CSF (11/13), IgM serum (12/13) | … | … | Headaches, seizures, focal neurology, altered GCS | Encephalopathy | 4 died |
| 2005‐2006, Réunion29 | 84 | PCR/IgM CSF/ser/BL | … | … | Unknown | Encephalitis (69), meningoencephalitis (15) | 6 died |
| 2005‐2006, Réunion37 | 57b | PCR CSF (40/52), PCR serum (31/37), IgM CSF (21/52), IgM serum (32/37) | … | WCC↑ (21/57), prot↑ (37/55) | International Encephalitis Consortium criteria used to classify patients | Encephalitis (24), nonencephalitic CHIKV‐associated CNS disease (33) | 7 died, 12 disabled, 16 recovered, 22 unknown |
| 2006, India38 | 11 | IgM CSF/ser | … | … | Headaches, altered sensorium, ataxia, rigidity, opsoclonus; abnormal brain MRI (?no.) | Encephalopathy | 3 died |
| 2006, India30 | 37 | Isol/PCR/IgM CSF/serc | … | … | Unknown | Encephalitis | 7 died |
| 2006, India39 | 11 | Isol/PCR/IgM/HI CSF/ser (6) | … | … | Unknown | Encephalitis | 2 died |
| 2006, Réunion40 | 16 | PCR/IgM CSF/ser | … | … | Drowsiness, seizures, focal neurological signs; abnormal MRI brain (5) and EEG (?no.) | Encephalitis (12), encephalopathy (4) | 2 died, 5 disabled, 9 no neurological sequelae |
| 2006, India41 | 27 | PCR CSF (4) | ‖ | WCC↑ (6/20), prot↑ (14/20) | 59% abnormal behaviour; 22% drowsiness, extrapyramidal; 11% seizures; abnormal MRI brain (1/4) | Encephalitis | 21 improved, 4 no improvement, 2 died |
| 2007, Italy42 | 1 | PCR CSF and ser, HI | 5d | WCC↑, prot↑ | 83 y M; confusion, drowsiness | Encephalitis | Died after 3 d |
| 2008, Singapore43 | 1 | IgM ser | 3d | … | 45 y M; drowsiness, headache; abnormal MRI brain | Encephalitis | Antimicrobials. Full recovery |
| 2009, Thailand44 | 2 | IgM CSF | 3 d | WCC↑, prot↑ | 27 y F; drowsiness; abnormal MRI brain | Meningoencephalitis | Aciclovir. Full recovery at 6 mo |
| HI | 0 d | 85 y M; drowsiness, jerky movements; abnormal MRI brain | No improvement | ||||
| 2010‐2011, India45 | 4 | PCR and IgM CSF, IgM ser | 4 d | WCC↑, prot↑ | 32 y F; seizure, disorientation, neck stiffness | Encephalitis | Improved over 10 d |
| PCR CSF and ser | … | WCC↑, prot↑ | 50 y M; headache, disorientation, drowsiness, meningism | Encephalitis | Improved over 7 d | ||
| PCR CSF and IgM ser | 3 d | WCC↑, prot↑ | 23 y M; seizure, dysarthria, hiccups, quadriparesis, CN involvement; abnormal MRI brain | Meningoencephalopathy | MP. Improved over 3 wk, mild weakness | ||
| IgM CSF & ser | 7 d | Prot↑ | 29 y F; headache, neck stiffness, quadriparesis, drowsiness; abnormal MRI brain and spine | ADEM | MP. Recovered at 1 mo, some residual weakness | ||
| 2011,d India46 | 1 | IgM CSF and ser | 10 d | NAD | 55 y M; weakness, vertigo, ↓GCS, nystagmus, bulbar weakness; abnormal MRI brain and spine | Brainstem encephalitis (ADEM) | MP. Near‐complete recovery |
| 2011‐2012, Cambodia47 | 11 | PCR/isol CSF | … | … | <16 y; unknown | Meningoencephalitis | Unknown |
| 2012,d India48 | 1 | IgM ser | 7 d | WCC↑, prot N | 32 y M; seizures, stimulus sensitive myoclonus | Meningoencephalitis | Anticonvulsants. Fully recovered at 3 mo |
| 2014,d India49 | 1 | PCR, IgM CSF, and ser | 2 d | … | 12 y M; seizure, vomiting, altered sensorium, weakness, ↑UL tone and tremors, unequal pupils; abnormal CT | Encephalitis | Died after 6 d |
| 2014, Tonga50 | 1 | PCR ser, IgM CSF, and ser | 7 d | WCC N, prot↑ | 57 y M; altered mental status, seizure; abnormal MRI brain and EEG | Encephalitis | IVIG, anticonvulsants. Improved |
| 2014, L Antilles32 | 3 | PCR/IgM CSF/ser | … | … | Met Venkatesan51 criteria for encephalitis | Encephalitis | Unknown |
| 2015, Honduras52 | 18 | PCR ser (11)e | … | WCC↑, prot↑ | <12 mo (11); seizures/lethargy/bulging fontanelle/irritability/hyperalgesia; abnormal MRI brain (5/5), abnormal EEG (7/14) | Meningoencephalitis | 1 died, remaining unknown |
| 2015,d India53 | 3 | IgM CSF/ser | … | NAD | 19 d F; tonic seizures, poor feeding | Encephalopathy | Unknown |
| WCC↑, prot N | 23 d M; multifocal clonic seizures | Encephalopathy | |||||
| NAD | 25 d F; multifocal clonic seizures, poor feeding | ||||||
| 2015,d Colombia54 | 1 | IgM CSF/ser | 4 d | WCC↑,f prot↑ | 23 d M; seizures, stupor, severe thrombocytopenia; abnormal MRI brain | Encephalitis | ↓hearing, ↓tone, motor delays at 13 mo |
| 2016,d India55 | 1 | PCR CSF and ser | … | NAD | 55 y M; altered sensorium, GCS 12; abnormal MRI brain | Mild encephalitis with a reversible lesion of the splenium | Full recovery at 5 d |
| 2016, Brazilg | 2 | PCR CSF and ser, IgM ser | 6 d | WCC↑, prot N | 51y F; confusion, seizure, drowsiness, dysarthria | Encephalitis | Aciclovir. Full recovery |
| PCR CSF | 4 d | WCC↑, prot↑ | 84y M; confusion, dysarthria, dysphagia, quadriparesis; NP: myositis | IVIG, aciclovir, antibiotics, antifungals. No improvement | |||
| 2016, India56 | 3 | PCR ser | … | … | Children; seizures/altered sensorium | Meningoencephalitis | Recovered at 3‐4 d (2), died after 6 h (1) |
| 2016,d Brazil57 | 2 | IgM CSF/ser | 4d | WCC↑, prot↑ | 74 y M; confusion, drowsiness, paraparesis; abnormal MRI brain; NP: AMSAN | Encephalitis | IVIG, plasmapheresis. Improved at 3 mo |
| 6d | 83 y M; confusion, lethargy, required ventilation | Aciclovir, IVIG. Complete recovery at 8 d | |||||
| 2016, Brazil58 | 3 | IgM ser | 1 d | … | <5 y M; headache, seizures, GCS 3, areflexia; abnormal CT brain | Not given | Died 23 d after admission |
| 10 d | WCC N, prot↑ | 65 y M; seizures, GCS 9, required ventilation; abnormal CT brain | Aciclovir. Died 1 d after admission | ||||
| 9 d | … | 92 y F; ↓GCS, LL involuntary movements, required ventilation | Died 10 d after admission | ||||
| 2017,d Brazil59 | 1 | PCR CSF and ser and urine and saliva | 13 d | WCC↑, prot↑ | 57 y M; confusion | Meningoencephalitis, anterior uveitish | Aciclovir, steroids po and top, tropicamide top. Improved |
| Myelopathy (n = 19) | |||||||
| 2006, India38 | 4 | IgM CSF/ser | … | WCC↑, prot↑ | UR followed by paraparesis | Myelopathy | Improvement (unclear extent) |
| 2006, India39 | 5 | Isol/PCR/IgM/HI CSF/ser (2) | … | … | Unknown | Myelitis | Unknown |
| 2006, India41 | 7 | PCR CSF (2), isol (1) | —i | WCC↑ (2/6), prot↑ (2/6) | Para/quadriparesis (7), UR (6); abnormal MRI spine (1) | Myelopathy | 5 improved, 2 no improvement |
| 2015,d India60 | 1 | IgM CSF/ser | 2 wk | WCC N, prot↑ | 18 y M; quadriparesis, ↓sensation, UR, areflexia, myositis (↑creatine kinase); abnormal MRI C1‐C6 | Myelitis | MP. Slow, partial improvement |
| 2016, Brazil61 | 1 | IgM ser | 8 d | … | Paraparesis, T10 sensory level | Myelitis | Steroids po. Fully recovered at 2 mo |
| 2016, Brazilg | 1 | PCR CSF | 0 d | NAD | 20 y M; paraesthesia, triparesis, hyperreflexia, C6 sensory level, UR; abnormal MRI spine; NP NAD | Myelitis | MP. Partially improved |
| Encephalomyelopathy (n = 23) | |||||||
| 2005‐2006, Réunion29 | 1 | PCR/IgM CSF/ser/BL | … | … | Unknown | Myelomeningoencephalitis | Unknown |
| 2006, India38 | 7 | IgM CSF/ser | … | … | Unknown | Encephalomyelopathy | Unknown |
| 2006, India30 | 14 | Isol/PCR/IgM CSF/serc | … | … | Unknown | Encephalomyelitis (11), encephalomyelopathy (3) | 5 died |
| 2016, Brazilg | 1 | PCR ser and urine, IgM CSF and ser | 0 d | WCC↑, prot↑ | 76 y M; seizures, confusion, dysarthria, headache, neck stiffness, spastic paraparesis, T2‐T3 sensory level, UI | Encephalomyelitis | Antivirals, antibiotics. Unknown |
| Myeloneuropathy (n = 24) | |||||||
| 2006, India38 | 13 | IgM CSF/ser | Unknown | Myeloneuropathy | Unknown | ||
| 2006, India41 | 7 | PCR CSF (1) | <5 to 10‐20 d | WCC N (6/6), prot↑ (5/6) | Quadriparesis (6), UR (1); abnormal MRI spine (3); NP AIDP (7) | Myeloneuropathy | 4 improved, 2 no improvement, 1 died |
| 2009, Thailand44 | 1 | IgM CSF | 2 wk | WCC N, prot↑ | 44 y F; quadriparesis, dysphonia/phagia, facial diplegia, areflexia; abnormal MRI C4‐C5; NP AMSAN | Myeloneuropathy | IVIG. Rapid improvement, fully recovered at 6 mo |
| 2012,d India62 | 1 | IgM ser | 20 d | WCC↑, prot↑ | 56 y M; weakness and sensory loss; abnormal MRI spine C2‐C3 T5‐T7 | Myeloradiculopathy | Improved |
| 2014, Dom Rep63 | 1 | IgM and IgG ser | 10 d | WCC↑, prot↑ | 47 y F; L LL weakness, R LL pain, T12 sensory level; abnormal MRI T12‐L1 + cauda equina | Myeloradiculopathy | MP. Recovered at 6 mo, residual pain |
| 2016, Brazilg | 1 | PCR and IgM CSF | 2 d | WCC N, prot↑ | 63 y M; paraesthesia; flaccid areflexic paraparesis; T4 sensory level; UR; NP AMSAN | Myeloradiculitis | IVIG. No improvement |
| Encephalomyeloneuropathy (n = 24) | |||||||
| 2006, India38 | 9 | IgM CSF/ser | … | … | Unknown | Encephalomyeloneuropathy | Unknown |
| 2006, India30 | 12 | Isol/PCR/IgM CSF/ser | … | … | Unknown | Encephalomyeloneuritis (9), encephalomyeloneuropathy (3) | 1 died |
| 2008,d India34 | 2 | IgM CSF and ser | … | WCC↑,j prot↑ | 65 y M; drowsiness, neck stiffness, weakness; abnormal MRI brain and nerve root; NP AMAN | Encephalomyeloradiculitis | MP. No improvement |
| WCC↑, prot↑ | 74 y M; drowsiness, weakness; abnormal MRI brain and nerve root; NP “generalised sensorimotor peripheral neuropathy” | Dexamethasone. Died; brain autopsy gross oedema, cerebellar haemorrhages, small foci demyelination | |||||
| 2009, Singapore64 | 1 | PCR CSF, ser, urine, skin | 2 d | WCC N, prot↑ | 54 y M; weakness, confusion, vomiting, sensory level, shock, rhabdomyolysis, UR; NP AIDP; EEG encephalopathy | AIDP | IVIG. Full recovery |
| Neuropathy (n = 72) | |||||||
| 1963‐1964, India65 | 1 | HI ser | … | WCC↑,k prot↑ | Quadriparesis, facial diplegia, ↓R visual acuity | GBS | Complete slow recovery over months |
| 2005‐2006, Réunion29 | 4 | PCR/IgM CSF/ser/BL | … | … | Unknown | GBS | Unknown |
| 2005‐2006, Réunion33 | 1 | IgM CSF and ser | … | WCC N, prot↑ | 55 y M; weakness, hyporeflexia, facial palsy; NP “suggestive” of GBS | GBS | Moderately disabled at 6 mo |
| 2005‐2006, Réunion66 | 2 | IgM CSF | … | WCC N, prot↑ | NP sensory motor deficit (2) | GBS | IVIG. Rapid improvement |
| 2006, India41 | 7 | PCR CSF (1) | —i | Prot↑(5/6), WCC N (6/6) | Quadriparesis with AIDP (7) | Peripheral neuropathy | 6 improved; 1 no improvement |
| 2006, Réunion67 | 3 | IgM ser | 2 wk | WCC N, prot↑ | 51y F; quadriparesis, areflexia, facial diplegia; NP AIDP | GBS | IVIG. Partial recovery at 1 mo |
| PCR ser | 3 d | NAD | 60 y M; quadriparesis, L facial palsy, hypoaesthesia, areflexia; NP AIDP | IVIG. Good recovery at 1 mo, residual palsy | |||
| IgM & IgG ser | 1 wk | WCC N, prot↑ | 49 y F; paraparesis, proprioceptive ataxia, areflexia, facial diplegia, dyspnoea; NP AIDP | IVIG. Good recovery at 1 mo, residual palsy | |||
| 2006, India38 | 13 | IgM CSF/ser | … | … | Unknown | Neuropathy | Unknown |
| 2006, Réunion68 | 2 | IgM CSF & ser | 1 wk | WCC N, prot↑ | 51 y F; areflexia, facial diplegia, dyspnoea requiring ventilation; NP AIDP | GBS | IVIG. Good recovery at 2 mo |
| IgM ser | 2 wk | 48 y F; weakness, paraesthesia, areflexia, dyspnoea; NP “peripheral neuropathy, conduction block” | IVIG. Good recovery | ||||
| 2006, India39 | 2 | Nil | … | … | Unknown | GBS | Unknown |
| 2006, Indial,69 | 4 | IgM CSF/ser | … | … | Progressive, symmetrical, ascending quadriparesis with areflexia (4), required ventilation (1) | Acute flaccid paralysis | MP. Improved: rapidly (3), by 1 mo (1) |
| 2014, L Antilles32 | 6 | PCR/IgM CSF/ser | … | … | Unknown | GBS | Unknown |
| 2014‐2015, Fr Poly70 | 9 | PCR/IgM CSF/ser | … | WCC N, prot↑ | Sensorimotor deficit (8), facial diplegia (1); NP mixed axonal and demyelinating (9) | GBS | IVIG (9). NP returned to approximately normal at 3 mo |
| 2014‐2015, L Antilles71 | 13 | PCR CSF (3)/ser (3), IgM ser (13) | 1‐22 d | WCC N, prot↑ | Mean age 61; severe (6), autonomic dysfunction (5), required ventilation (5) | AIDP (7), AMSAN (2), MFS (2), pharyngeal‐cervical‐brachial weakness (1), Bickerstaff brainstem encephalitis (1) | IVIG (12), plasma exchange (2). 2 died, 8 improved, 1 severe residual symptoms, 1 CIDP, 1 unknown |
| 2016, Brazilg | 1 | PCR urine, IgM ser | 7 d | WCC↑, prot↑ | 67 y F; flaccid areflexic quadriparesis, dysphagia, impaired sensation; NP AMSAN | GBS | IVIG. No improvement |
| 2016,d Colombia72 | 1 | PCR and IgM ser | … | WCC N, prot↑ | 77 y F; paraesthesia, bilateral hemiparesis, impaired sensation, hyporeflexia; NP AIDP | GBS | IVIG. Fully recovered at 8 wk |
| 2016, Brazil58 | 1 | IgM ser | 15 d | WCC↑, prot↑ | 51 y F; quadriparesis, neck stiffness | Not given | Antibiotics. Died after 38 h |
| 2016,d India73 | 2 | IgM CSF/ser | 17 d | … | 18 y M; areflexic quadriparesis, dysphagia, dyspnoea; NP AMAN | GBS | Plasmapheresis. Partial improvement |
| 12 d | … | 20 y M; flaccid quadriparesis, facial and bulbar weakness; NP AIDP | |||||
| Ocular disease (n = 78) h | |||||||
| 2006, India74 | 14 | IgM ser | 11.0 dm | … | Visual field defect (14), ↓visual acuity (13), pain (1), floaters (1), diplopia (1), RAPD (9), disc oedema (9), VII CN palsy (2), delayed VEP | Papillitis (6), neuroretinitis (3), retrobulbar neuritis (3), demyelination optic tract (2) | MP 3 d, steroids po 2 wk. 10 improved, 4 poor outcome |
| 2006, India75 | 37 | IgM ser | 33.2 dm | … | Primary presenting complaint ↓visual acuity, unilateral (30), bilateral (7) | Anterior uveitis (11), panuveitis (5), optic neuritis (4), lagophthalmos and sixth nerve palsy (3), retrobulbar neuritis (3), retinitis and vitritis (2), bilateral neuroretinitis (1), keratitis (3), CRAO (1), choroiditis (2), retinal detachment (2) | Visual acuity of 26 followed up: 11 improved, 12 remained stable, 3 worsened |
| 2006, India38 | 2 | IgM CSF/ser | … | … | ↓visual acuity | Bilateral retinal haemorrhage (1), branch retinal artery occlusion (1) | Steroids intravitreal. Minimal (1)/partial (1) improvement |
| 2006, India76 | 9 | IgM ser | 4‐12 wk | … | ↓visual acuity/pain/red eye | Episcleritis (1), anterior uveitis (5), retinitis (3) | Indomethacin po; steroids, homatropine, diclofenac, timolol top; aciclovir iv/po, steroids po. Recovered well (9) |
| 2007, India77 | 10 | IgM ser | 1‐6 wk | … | ↓visual acuity (10), pain (10), bilateral (3), visual field defects (10), disc oedema (10), RAPD (7), delayed VEP | Papillitis (7), retrobulbar neuritis (1), perineuritis (1), neuroretinitis (1) | MP 3 d, steroids po 2 wk. Rapid improvement (9), persistent RAPD/visual field/colour vision defect (4/6/2) |
| 2007,d India78 | 1 | PCR and IgM ser | 2 wk | … | 48 y F; bilateral ↓visual acuity, bilateral centrocaecal scotoma, retinal haemorrhage | Bilateral neuroretinitis | Steroids po. Visual acuity 20/30 R, 20/20 L at 2 mo |
| 2009,d India79 | 1 | PCR aqueous humour | 1 wk | … | 20 y F; L ↓visual acuity, tripod dendritic pattern of keratic precipitates | Fuchs heterochromic iridocyclitis and cataract | Cataract surgery. Recovered well |
| 2010, India80 | 1 | IgM ser | 4 wk | … | 27 y F; bilateral ↓visual acuity, R RAPD, bilateral retinitis posterior pole, macular oedema, serous detachment | Anterior uveitis and retinitis | Steroids po. Gradual recovery |
| 2011,d India81 | 1 | PCR ser | 1 wk | … | 65 y M; bilateral ↓visual acuity, neuroretinitis, cotton wool spots, retinal haemorrhages | Bilateral neuroretinitis | Steroids, aciclovir po. Partial improvement |
| 2015,d Dom Rep82 | 1 | IgM and IgG ser | 4 d | NAD | 47 y F; bilateral ↓visual acuity, photophobia, optic nerve head oedema, hyperpigmented scars, serous detachment temporal macula, represented with floaters; abnormal MRI orbits | Panuveitis, retinal detachment | Steroids po and top, cyclopentolate top, mycophenolate. Improved |
| 2016,d Dom Rep83 | 1 | IgM and IgG ser | 20 d | … | 44 y F; ↓visual acuity, floaters, keratic precipitates, anterior chamber cells, Koeppe nodules | Intermediate uveitis | Steroids po and top. Rapid improvement |
| Other focal neurology (n = 233) | |||||||
| 1962‐1964, Thailand13 | 1 | HI/isol CSF/ser | … | … | Febrile convulsions | Febrile convulsions | Unknown |
| 1963‐1964, India65 | 2 | HI | 14 d | … | Limb paresis and slurring of speech | Not given | Recovered |
| 7 d | Vocal hoarseness and nasal regurgitation | ||||||
| 1964, India65 | 1 | HI/isol CSF/ser | 4 d | … | 12 y M; bilateral total ophthalmoplegia, loss of accommodation reflex | CN palsy | Complete recovery at 1 wk |
| 3 | HI/isol CSF/ser | … | NAD | Delirium, coma, meningism, sluggish pupils, dysarthria | Not given | Unknown | |
| 1964, India84, 85 | 12 | PCR/IgM CSF/ser | … | … | Seizures in infants (3), children (9); associated with fever (12), focal (2), ↓GCS (4) | Seizures | Died (1), residual neurological deficit (2) |
| 2005‐2006, Réunion66 | 21 | PCR/IgM CSF/ser | … | WCC N, prot↑ (12) | Confusion (20), headache (7), epilepsy (6), meningism (1), motor deficit (1), sensory deficit (2); EEG diffuse slowing (13), epileptic activity (3), NAD (2) | Not given | Died (5), generally good outcome (16) |
| 2005‐2006, Réunion29 | 12 | PCR/IgM CSF/ser/BL | … | … | Seizures | Seizures | Unknown |
| 2005‐206, Réunion29 | 5 | PCR/IgM CSF/ser/BL | … | … | Unknown | Stroke (2), cerebellitis (3) | Unknown |
| 2006, Réunion40 | 14 | PCR/IgM CSF/ser | … | … | Nuchal rigidity, Kernig/Brudzinski sign, photophobia, tense fontanelle (4) | Meningeal syndrome | Mild/no neurological sequelae (1)/(3) |
| Febrile seizures (10) | Asthenia (1), no neurological sequelae (9) | ||||||
| 2006, India86 | 20 | IgM ser | … | WCC↑ (9), prot↑ (20) | Altered mental status (20), psychosis (6), seizures (15), CN deficit (20), hemiparesis (1), LMN paraparesis (3), involuntary movements (4), optic neuritis (2) | Not clear | 13 gradual full improvement, 1 blind, 6 died |
| 2006, India87 | 8 | PCR CSF/ser | … | WCC↑ (2/5) | Altered mental status, meningism, seizures, status epilepticus, aphasia | Not given | At discharge, normal GCS (6), ↓GCS (2) |
| 2006, Réunion88 | 25 | PCR CSF (8), IgM/PCR ser | … | WCC↑ (1/17), prot↑ (3/17) | Paediatric cohort: convulsion, confusion, behavioural disorders, meningism; abnormal MRI (2/8), abnormal EEG (8/10) | Not given | Unknown |
| 2006, India38 | 18 | IgM CSF/ser | … | … | Unknown | Encephaloneuropathy (8), carpal tunnel syndrome (10) | Unknown |
| 2007,d India89 | 1 | IgM CSF/ser | 13 d | WCC N, prot↑ | 45 y M; asymmetric quadriparesis, dysphagia, clonus, dystonia; abnormal MRI brain | ADEM | MP. Walking independently at 12 d |
| 2007,d India90 | 1 | IgM ser | 2‐3 d | … | 15 y F; sudden‐onset profound L‐sided hearing loss, tinnitus | Sensorineural hearing loss | No improvement at 1 mo |
| 2011,d India91 | 1 | IgM ser | 5 d | WCC↑, prot↑ | 26 y F; spastic quadriplegia, impaired sensation, UR; abnormal MRI brain and spine | ADEM | MP. Good clinical and radiological recovery |
| 2013,d India92 | 1 | IgM CSF/ser | 9 d | WCC↑, prot↑ | 8 y M; flaccid quadriparesis, R UMN facial palsy, seizure, UR; abnormal MRI brain and spine | ADEM | MP. Minimal improvement at 6 mo |
| 2014, Martinique93 | 1 | IgM and IgG ser | 2 d | … | 62 y M; isolated unilateral third nerve palsy | CN palsy | Improved at 6 mo |
| 2014, L Antilles32 | 2 | PCR/IgM CSF/ser | … | … | Diffuse brain ischaemia leading to brain death | Not given | Died |
| 2014, Fr Poly7 | 1 | IgM ser | 6 d | WCC↑, prot↑ | 74 y M; hypoesthesia, flaccid quadriplegia, dyspnoea, GCS 3, CN palsies, required ventilation; abnormal MRI brain, abnormal EEG, NP axonal polyneuropathy | Bickerstaff brainstem encephalitis‐MFS‐GBS overlap | IVIG, MP. Normal mental function and residual paresis at 1.5 y |
| 2015, Honduras52 | 59 | PCR ser | … | … | Seizures | Seizures | Unknown |
| 2016, India56 | 2 | PCR ser (1), IgM ser (1) | <7 d | … | Children; hyperactivity, insomnia, aggressive behaviour, hallucinations, behaviour changes | Not given | Recovered at 4 d (1), persistent behavioural problems at 4 wk (1) |
| 2016, Brazil61 | 21 | IgM ser | … | … | Seizures, altered consciousness, weakness, impaired sensation, sphincter dysfunction, persecutory delusions, suicidal/aggressive behaviour, insomnia, headache | Not given | Unknown |
| 2016, Brazilg | 1 | PCR ser and urine, IgM ser | 16d | Prot N | 17 y M; L hemiparesis and numbness, facial palsy, impaired sensation; abnormal MRI brain | ADEM | MP. Improved |
| 2017,d India94 | 1 | IgM ser | 10d | … | 5 y F; bilateral ophthalmoplegia, blurring of vision | Bilateral ophthalmoplegia | Steroids po. Unknown |
Abbreviations: /, or (eg, IgM CSF/ser = not specified whether IgM detected in CSF or serum); …, data unavailable; ADEM, acute disseminated encephalomyelitis; AIDP, acute inflammatory demyelinating polyneuropathy; AMAN, acute motor axonal neuropathy; AMSAN, acute motor and sensory axonal neuropathy; BL, bullous lesions; CHIKV, chikungunya virus; CIDP, chronic inflammatory demyelinating polyneuropathy; CN, cranial nerve; CRAO, central retinal artery occlusion; CSF, cerebrospinal fluid; CT, computed tomography; Dom Rep, Dominican Republic; EEG, electroencephalogram; F, female; Fr Poly, French Polynesia; GBS, Guillain‐Barré syndrome; GCS, Glasgow coma score; HI, haemagglutination inhibition; IgM, immunoglobulin M; IVIG, intravenous immunoglobulin; isol = viral isolation; L, left; L Antilles, Lesser Antilles; LL, lower limb; LMN, lower motor neuron; M, male; MFS, Miller Fisher syndrome; MP, methylprednisolone; MRI, magnetic resonance imaging; N, normal; NAD, no abnormality detected; PCR, polymerase chain reaction; PL, prodrome length (time between initial infection and onset of neurology); prot, protein (↑, >0.4 g/L for adults, >1.5 g/L for neonates); R, right; RAPD, relative afferent papillary defect; ser, serum; UI, urinary incontinence; UL, upper limb; UMN, upper motor neuron; UR, urinary retention; VEP, visual evoked potential; WCC, white cell count (↑, >5 cells/μL).
Treatments are in italics.
Five vertically transmitted cases.
Neurological sequelae in cases with positive dengue virus IgM as well as CHIKV were attributed to CHIKV.
Date of article submission, date of case unclear.
Seven of these patients did not have laboratory evidence of chikungunya infection and were not described further; their age and whether they were vertically transmitted cases were not reported.
WCC reported as “>5”; normal neonatal WCC ranges from 0 to 30 cells/μL.
Unpublished findings from Mehta R, Soares C, Medialdea‐Carrera R, et?al. (2017) “The spectrum of neurological disease associated with Zika and chikungunya viruses in adults in Rio de Janeiro, Brazil: a case series.”
One patient with both encephalitis and anterior uveitis has not been included in the ocular disease section.
Range of prodrome lengths: <5 to 10‐20 d (24) and >30 d (3) for encephalitis; <5 to 20‐30 d for myelopathy; <5 to 10‐20 d for GBS.
WCC reported as “few cells”; unclear actual number per microlitre.
WCC mildly elevated, 8 lymphocytes/field.
Andaman and Nicobar Islands.
Mean.
Figure 2.
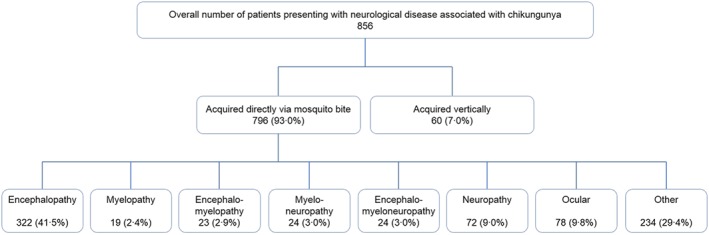
Presentations of nervous system disease associated with chikungunya infection
Neurological disease following chikungunya virus infection was first reported during an outbreak in 1964 in Madras, India.65 Four cases with chikungunya confirmed serologically or by viral isolation were described. Two presented with a meningoencephalitic picture (“delirium or coma, and signs of meningeal irritation with nuchal rigidity and Kernig's sign, sluggish pupillary reaction etc”), one with acute flaccid paralysis and elevated CSF protein, suggestive of Guillain‐Barré syndrome (GBS), and one with transient dysarthria. Since then, neurological manifestations have been reported throughout the Indian Ocean, South Asia, the Pacific islands, Southern Europe, the Caribbean, and South America, ranging from mild behavioural disorders to severe acute syndromes of both CNS and peripheral nervous system (Figure 3).
Figure 3.
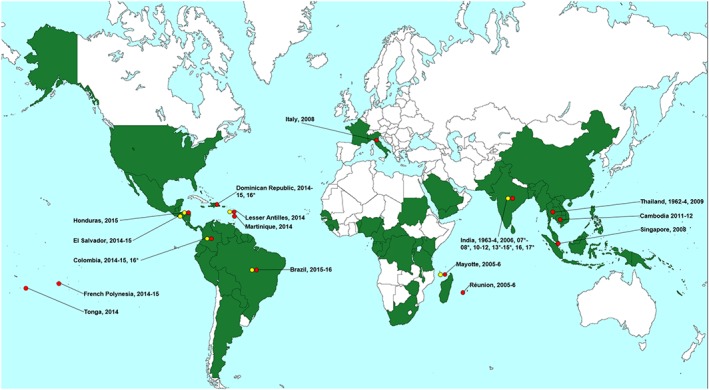
Global distribution of chikungunya virus and countries/territories with reported associated neurology. Key:  adult and child neurological disease associated with chikungunya infection;
adult and child neurological disease associated with chikungunya infection;  vertically acquired neurological disease in the neonate associated with chikungunya infection. *unclear date of case(s), year refers to year of publication. Data regarding global distribution of chikungunya virus acquired from the Centers for Disease Control and Prevention website95
vertically acquired neurological disease in the neonate associated with chikungunya infection. *unclear date of case(s), year refers to year of publication. Data regarding global distribution of chikungunya virus acquired from the Centers for Disease Control and Prevention website95
Given the large spectrum of neurological disease and scarce epidemiological data, estimating the incidence of neurological disease amongst all systemically symptomatic chikungunya infections is difficult. In one study from the 2006 Indian outbreak,39 18 (4.4%) of 405 suspected chikungunya cases attending the recruiting hospital over 3 months developed neurological complications; this study did not, of course, include the many people with uncomplicated chikungunya infection who did not seek hospital attention. An epidemiological study of the 2005 to 2006 Réunion Island outbreak found approximately 0.3% of all chikungunya infections resulted in “atypical” cases,29 of which 24.1% of the adults presented with abnormal neurology. Thus, approximately 0.1% (1 case per 1000) of all chikungunya infections developed neurological disease.
It has been suggested that severe complications of chikungunya infection typically arise in those with co‐morbidities.96 The above epidemiological study describing 610 atypical cases of chikungunya infection showed that underlying respiratory disease, cardiac disease, and hypertension were all independently associated with severe complications, including neurological disease.29 However, a study from India of 124 atypical chikungunya cases did not identify co‐morbidity as a significant risk factor for systemic complications or fatality30; similarly, in a case series of chikungunya‐associated GBS, 6 (67%) of 9 cases did not have any co‐morbidities.70 Thus, although underlying co‐morbidities may play a role in neurological and other complications of chikungunya, they are not an indispensable requisite. Interestingly, age has been consistently reported as a significant risk factor for severe manifestations of chikungunya infection, both in the elderly (>60‐65)29, 30, 37 and in infants.37
Future studies will need to determine whether initially asymptomatic chikungunya infections, ie, without a primary fever, arthralgia, or rash syndrome, can cause neurological disease. Although none have been reported to date, the possibility has not yet been investigated in adults. In vertical transmission, a retrospective study from the Réunion Island outbreak found that of 38 symptomatic neonates (with combinations of fever, rash, and oedema), 2 mothers had been asymptomatic (with a positive chikungunya PCR or IgM).97 However, mild symptoms may have been missed by the mothers owing to poor recall, and whether their neonates developed neurological manifestations was unclear.
Cocirculation of chikungunya, Zika, and dengue viruses has been reported in much of South America6 and is a potential problem in all areas of the world where Aedes mosquitoes are endemic. Aedes albopictus mosquitoes have the ability to deliver more than 1 arbovirus in their saliva, raising the possibility of simultaneous transmission of the viruses.98 Furthermore, coinfection of arboviruses has been detected in patients presenting with neurological disease.99, 100 Given that all 3 arboviruses are known to be neurovirulent, it is unclear whether in these patients, their neurological disease is associated with one or more of their infections. Coinfection has also been reported in pregnant women,101, 102 the significance of which for the neonate is unclear. The most common coinfection to be reported in all patients is with chikungunya and dengue viruses, although this may be due to the greater number of epidemics of these viruses so far compared with Zika. Albeit rarely, coinfection with all 3 viruses has been reported, including in patients with neurological disease.99, 103 The differences in disease pathogenesis, presentation, and severity between monoinfections and coinfections are currently unknown. It is clear, however, that in endemic areas, pregnant patients with a fever‐arthralgia‐rash syndrome and all patients presenting with acute neurological disease (regardless of previous viral symptoms) should be investigated for all 3 of chikungunya, Zika, and dengue viruses.
The following sections evaluate the evidence for each of the neurological syndromes that has been described in association with chikungunya virus infection.
7.1. Encephalopathy and encephalitis
Encephalopathy, defined by the International Encephalitis Consortium as “a clinical state of altered mental status, manifesting as confusion, disorientation, behavioural changes or other cognitive impairment,”51 is one of the most common neurological presentations for arboviral infections. Whilst in some patients this may be due to encephalitis—ie, brain inflammation associated with direct viral infection—in others, it may be a non‐specific manifestation of a severe systemic disease, for example, due to hypoperfusion of the brain.3, 5, 104 Strictly speaking, encephalitis is a pathological diagnosis, but for practical purposes, it can be diagnosed in an encephalopathic patient if there is surrogate evidence of brain inflammation, for example, from a CSF pleocytosis, brain imaging, or focal changes on electroencephalogram.2, 5
In one study from Réunion Island, where the authors were careful to accurately define encephalitis using the international criteria, the estimated cumulative incidence rate for chikungunya‐associated encephalitis was 8.6 per 100 000 people during the 2005 to 2006 outbreak, which led to a 2‐fold increased incidence of all encephalitis in the region.37 Twenty‐four patients with encephalitis were reported (5 of whom were neonates infected perinatally), presenting with altered mental status, as well as seizures and focal neurological signs in some. When compared with encephalopathic patients who did not meet criteria for encephalitis, the encephalitic patients had more severe CNS disease and required more intensive care support; this was despite there being no significant difference between chikungunya viral loads and IgM titres in the serum or CSF, which may indicate a role for predisposing host factors in chikungunya virus neurovirulence. Overall, our literature review revealed that 251 of the 322 (78.0%) patients who presented with an isolated encephalopathy syndrome had a diagnosis of encephalitis, whereas 66 (20.5%) and 2 (0.6%) had a diagnosis of encephalopathy and acute disseminated encephalomyelitis, respectively. Additionally, involvement of the meninges was also reported in 55 (17.1%) cases.
Symptoms of encephalitis begin between 0 and 13 days following the onset of systemic features of infection (see Table 2). As is the case in other arboviral encephalitides, a CSF pleocytosis is not always seen.40, 41 Unlike encephalitis caused by other CNS pathogens such as herpes simplex virus and cytomegalovirus, which have characteristic imaging abnormalities, chikungunya encephalitis in adults and children does not appear to show a distinct pattern. Described abnormalities include oedema or non‐specific haemorrhage on computed tomography, and increased T2+/− fluid‐attenuated inversion recovery signal (Figure 4) or restricted diffusion signal in several areas of the cerebrum on magnetic resonance imaging (MRI).34, 40, 44 Many cases do not show any imaging abnormalities at all.37, 40 Although there are non‐specific slowing of brain waves in some patients, there is no specific electroencephalogram pattern.40, 50
Figure 4.
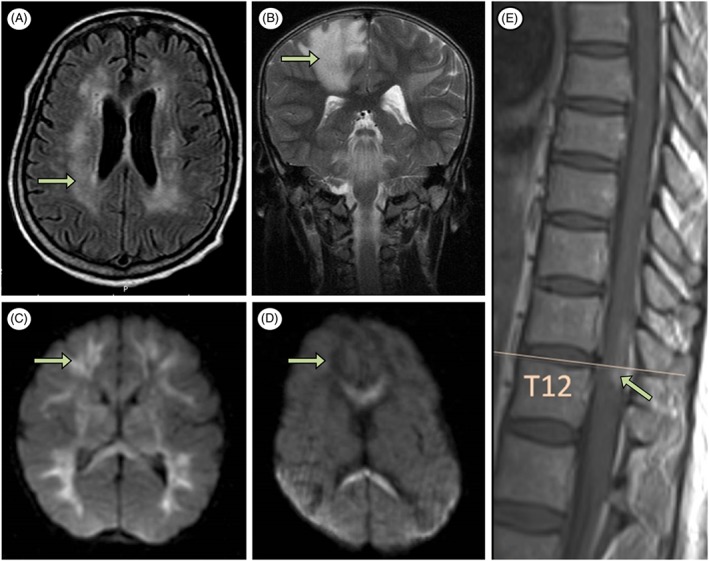
Central nervous system imaging abnormalities in patients with chikungunya infection. A, Signal abnormality involving the periventricular white matter in an 85‐year‐old patient with encephalitis (axial fluid‐attenuated inversion recovery).44 B, Confluent areas of signal abnormality consistent with demyelination in an 8‐year‐old patient with acute disseminated encephalomyelitis (coronal T2).92 C, Signal abnormality (hyperintense) involving the corpus callosum and the frontal and parietal lobes in neonate A with vertically acquired encephalopathy (day 6, axial diffusion weighted imaging).105 D, Signal abnormality (hypointense) involving the frontal and parietal lobes in neonate B with vertically acquired encephalopathy (day 21, axial diffusion‐weighted imaging).105 E, Signal abnormality at T12 in a 47‐year‐old patient with myeloradiculopathy (sagittal T1)63
In the Réunion Island study, 7 of the 57 patients (aged 4 d‐88 y) with either encephalopathy or encephalitis died in hospital or shortly after discharge; of the 10 adults followed up after 3 years, 3 had persistent neurological sequelae in the form of epilepsy, postinfectious dementia, and cognitive disorder.37 Taking into account the attrition in the follow‐up cohort, a range from 18% to 43% of patients were estimated to have neurological sequelae. Similarly, a paediatric series from the same outbreak reported 5 (31%) of 16 children with chikungunya‐associated encephalopathy or encephalitis had residual neurological deficit, whilst 2 (13%) died.40 From our literature review, of the 127 patients cases for whom follow‐up data were reported, 62 (48.8%) had complete or near‐complete recovery, 25 (19.7%) had residual neurological deficit, and 40 (31.5%) died.
7.2. Myelopathy and myelitis
Chikungunya virus can cause myelopathy, symptoms of spinal cord disease, which may present with limb weakness, sensory changes, hyperreflexia, and bowel and bladder disturbances, depending on the level of the lesion and extent to which the cord is involved. If cord inflammation is confirmed by MRI, a CSF pleocytosis, or elevated CSF IgG index, showing local immunoglobulin production, then it is classified as myelitis.106
The incidence of spinal cord disease after chikungunya infection is not known, but it is likely to be less than that of encephalopathy, given that the 90 cases described in the literature comprise less than a third of the 322 cases of encephalopathy. Myelopathy and myelitis usually occur as part of more widespread neurological disease. Of the 90 patients identified, 47 had myelopathy as part of more widespread CNS disease, with encephalopathy or encephalitis; for 48, there was also peripheral nervous system disease in the form or radiculopathy or neuropathy; just 19 patients had a pure myelopathy syndrome. Spinal cord disease typically presents 0 day (Mehta et al, unpublished findings) to 3 weeks after the first clinical feature of infection (fever, arthralgia, or rash).41 Patients present with weakness in 2, 3, or 4 limbs, sometimes accompanied by one or more of paraesthesia in the limbs, a sensory level, or urinary retention. In our literature review, of the 12 patients with a pure myelopathy where CSF data were provided, 6 (50%) had a CSF pleocytosis. Similarly, MRI abnormalities are variable. They may range from changes “suggestive of demyelinating pathology” to extensive T2/fluid‐attenuated inversion recovery hyperintensity from the cervicomedullary junction to the C6 level.60
No deaths have been reported for chikungunya‐infected patients with a pure myelopathy syndrome; follow‐up data were reported for 13 patients, 11 of whom improved, although the extent of this was often unclear.
7.3. Acute disseminated encephalomyelitis
Like other acute viral infections, chikungunya can trigger an acute inflammatory syndrome involving the brain parenchyma and spinal cord, which is thought to be an immune‐mediated response to infection, rather than due to direct viral invasion. The diagnosis of this monophasic illness is usually based on finding focal or multifocal, poorly demarcated white matter demyelinating lesions on MRI.107, 108
Six cases of acute disseminated encephalomyelitis (5 adults and 1 child) have been described, with the disease starting 5 to 16 days after the initial fever‐arthralgia‐rash symptoms of chikungunya infection. Patients presented with a variety of neurological features, including headache; drowsiness; cranial nerve involvement such as facial nerve palsy, vertigo, nystagmus, and bulbar weakness; limb weakness; sensory disturbance; and urinary retention. MRI of the brain and/or spine suggested demyelinating pathology. All 6 were treated with intravenous methylprednisolone; the outcome varied between good clinical and radiological recovery91 and permanent neurological disability with confinement to a wheelchair and long‐term urinary catheterisation.92
7.4. Guillain‐Barré syndrome
Chikungunya‐associated peripheral neuropathy without CNS disease has been described for 72 patients in case reports or series, the majority of whom were described as having GBS. In one series of 4 patients with acute flaccid paralysis, no CSF or neurophysiology results were reported, making diagnosis difficult.69 Other causes of acute flaccid paralysis, such as anterior myelitis, have not yet been reported in association with chikungunya virus.
Two studies from Réunion Island showed an increased incidence of GBS following a large outbreak of chikungunya. One from the 2014 to 2015 outbreak reported 9 patients with chikungunya‐associated GBS, representing a 4‐ to 9‐fold increase in the island's annual GBS incidence.70 The increase in the incidence of GBS following the 2006 chikungunya virus outbreak on Réunion Island was estimated to be around 22% compared with the year before.68 A study from Martinique and Guadeloupe also showed an increase in incidence of GBS following the 2014 chikungunya virus outbreak, but to a lesser extent (2‐fold).71
Clinically, chikungunya‐associated GBS resembles GBS associated with other infections such as Campylobacter jejuni, presenting with symmetrical, bilateral flaccid weakness, often with paraesthesia and/or cranial nerve palsy.109 The 4 reports in the literature detailing the time interval between chikungunya infection and onset of neurological features describe a prodrome of 3 to 17 days, compatible with a parainfectious or postinfectious syndrome (Table 2).67, 68, 73
Unlike infections such as C. jejuni, which is associated with a more severe pure motor variant of GBS,110 chikungunya appears to be associated with the full range of GBS variants, as determined by neurophysiological studies, including disorders of motor and sensory axons, and myelin sheaths, sometimes in combination (see Table 2). Further investigation is required to determine risk factors and markers for the different GBS variants, including antiganglioside antibodies.
For the 36 cases where treatment was described, 28 (78%) received intravenous immunoglobulin, 2 (6%) intravenous immunoglobulin and plasma exchange, 4 (11%) intravenous methylprednisolone, and 2 (6%) plasmapheresis. Forty (87%) of the 46 cases for whom follow‐up data were available improved. In some cases, this was rapid; in most, it had occurred by 3 months.
7.5. Ocular complications
Although photophobia and conjunctivitis are associated with the acute phase of chikungunya infection,111 many later ocular complications have been described up to 12 weeks after infection, which may require emergency management. These include disease of the uvea, retina, and optic nerve. As well as inflammation, other pathologies have been described, including retinal detachment, intraretinal haemorrhage, and branch retinal artery occlusion (Table 2).38, 75 Our literature review found 78 cases of ocular complications of chikungunya infection.
In a retrospective study from India of 37 Indian patients with acute ocular manifestations and IgM‐confirmed chikungunya infection, uveitis was the most common diagnosis, occurring in 16 (43%) patients;75 48 controls from the chikungunya‐endemic area, selected from patients attending the hospital for nonacute problems including cataract and refractive error, were all negative for chikungunya IgM. Recovery was variable—of the 26 patients followed up, the visual acuity improved in 11 (42%), remained the same in 12 (46%), and worsened in 3 (12%). Overall, of the 67 patients with ocular disease we identified for whom follow‐up data were reported, 46 (69%) recovered well, whereas 21 (31%) showed minimal or no improvement.
Although most of the serious ocular complications occur days to weeks after the acute chikungunya infection, in one report, 5 of 14 optic neuritis cases occurred simultaneously with the systemic disease onset.74 This suggests a more direct viral effect may be important, as well a postviral immune response.
Disease relapse has been reported in one report, which described bilateral uveitis and retinal detachment, with loss of visual acuity starting 4 days after symptoms of chikungunya infection.82 Having received a week's course of oral and topical steroids and recovered within 6 weeks, the patient re‐presented 3 months later with floaters and keratic precipitates; clinicians should be vigilant for relapse in such cases.
7.6. Disease affecting multiple components of the nervous system
As well as the distinct syndromes described above, chikungunya infection is associated with complex diseases involving multiple parts of the nervous system causing, for example, encephalomyelopathy (23 patients identified in our literature review), myeloneuropathy (24), and encephalomyeloneuropathy (24). Where follow‐up data were available, unlike in pure myelopathy, more of these patients had an unfavourable outcome (8 deaths, 4 no improvement) compared with those who improved (8).
7.7. Other
A handful of other neurological disorders have been associated with chikungunya, albeit in smaller numbers. A study from the Réunion outbreak described 32 patients presenting with behavioural changes including attention disorders, irritability, and memory issues.37 Other reports have described febrile seizures, isolated cranial nerve palsies, stroke, and hearing loss (Table 1), and one report (in press) describes the possibility of an association with chronic fatigue syndrome. Although it is difficult to definitively associate these isolated disorders with chikungunya infection, the full spectrum of chikungunya‐associated neurological diseases appears to be broad.
7.7.1. Perinatally acquired neurological disease
Most of the evidence on chikungunya causing neonatal disease relates to transmission in the intrapartum period, rather than earlier in pregnancy. A wide range of severe manifestations has been described affecting neonates whose mothers had acute chikungunya infection near the time of delivery (Table 3). A case series from Colombia from 2014 to 2015 described 8 infants who required admission to an intensive care unit after contracting chikungunya infection perinatally.31 All mothers and neonates were PCR and IgM positive for chikungunya in serum; the neonates presented with severe diseases including meningoencephalitis, respiratory distress, sepsis, necrotising enterocolitis, myocarditis, and pericarditis.
Table 3.
Reports of perinatally acquired neurological disease associated with chikungunya virus
| Year of case(s), Location | No. | Evidence for Chikungunya | PP | CSF | Neurological Features | Treatmenta and Outcome |
|---|---|---|---|---|---|---|
| Perinatal encephalopathy (n = 35) | ||||||
| 2005‐2006, Réunion37 | 5 | Ne: PCR CSF | … | … | Fulfilled International Encephalitis Consortium criteria for encephalitis | Cerebral palsy and blindness (1), poor neurodevelopmental performance (1) |
| 2005‐206, Mayotte36 | 3 | Ne&M: PCR/IgM CSF/ser | … | … | Unknown | Unknown |
| 2005‐2006, Réunion112 | 4 | Ne&M: PCR/IgM ser | 3‐7 d | … | Seizures; EEG consistent with encephalitis | Survived |
| 2010, India113 | 2 | Ne&M: PCR ser | 5 d | NAD | Altered sensorium, apnoeic seizures | Spastic diplegia, epilepsy, mental retardation |
| 3 d | NAD | Apnoeic seizures, lethargy | ↓tone, cerebral palsy, ↓vision, mental retardation | |||
| 2014‐2015, Colombia31 | 2 | Ne&M: PCR/IgM ser | … | … | Unknown | Unknown |
| 2014‐2015, El Salvador, Colombia, and Dom Rep114 | 12 | Ne: PCR/IgM ser/CSF (10) | … | … | Unknown | Unknown |
| 2015, Brazil115 | 1 | Ne: PCR CSF, ser, urine, saliva | 6 d | WCC N, prot↑ | Seizures; abnormal MRI brain | Anticonvulsants. Improved at 17 d |
| 2015, Honduras52 | 3 | PCR ser | … | … | Unknown | Unknown |
| 2016, India116 | 2 | Ne&M: IgM ser | 5 d | … | Dizygotic twins; both had seizures, required ventilation, thrombocytopenia; abnormal MRI brain | Both improved and discharged at 24 d |
| 2016c, Brazil117 | 1 | Ne: PCR CSF; M: IgM ser | 4 | WCC ↑, prot ↑ | Prostration, lethargy, seizures, required ventilation, thrombocytopenia; abnormal MRI brain and EEG | Cerebral palsy, microcephaly, epilepsy at 1 y |
| Perinatal brain haemorrhage (n = 7) | ||||||
| 2005‐206, Réunion105 | 2 | Ne&M: PCR/IgM CSF/ser | … | … | DIC, transient scattered parenchymal petechiae (1), cerebellar haematoma (1) | Unknown |
| 2005‐2006, Réunion97 | 2 | Ne&M: PCR/IgM CSF/ser | … | … | Unknown | Unknown |
| 2005‐206, Réunion112 | 1 | Ne&M: PCR and IgM ser | 3‐7 d | … | Severe thrombocytopenia, cerebral haemorrhage | Survived |
| 2015, Brazil118 | 1 | Nil | 4 d | NAD | Intraventricular bleed (cranial US), lethargy | Improved, discharged after 17 d |
| 2012, India119 | 1 | M: IgM ser; Ne: NAD | 3 | NAD | Lethargic, severe thrombocytopenia, focal bleeds basal ganglia, and subcortical areas | Fully recovered |
| Perinatal other (n = 18) | ||||||
| 2005‐2006, Réunion97 | 17b | Ne&M: PCR/IgM CSF/ser | … | … | Seizures (6); hypotonia (17) | Unknown |
| 2005‐2006, Mayotte36 | 1 | Ne&M: PCR/IgM CSF/ser | … | … | Hypotonia | Unknown |
Abbreviations: …, data unavailable; CSF, cerebrospinal fluid; DIC, disseminated intravascular coagulation; Dom Rep, Dominican Republic; EEG, electroencephalogram; IgM, immunoglobulin M; M, mother; MRI, magnetic resonance imaging; N, normal; NAD, no abnormality detected; Ne, neonate; PCR, polymerase chain reaction; PP, onset of neurological disease days postpartum; prot, protein (↑, >0.4 g for adults, >1.5 g for neonates); ser, serum; WCC, white cell count (↑, >5 cells/μL); VEP, visual evoked potential.
Treatments are in italics.
At least 17 patients; unclear whether seizures and hypotonia were seen in the same or different patients.
Patient had a subarachnoid haemorrhage and optic atrophy in addition to encephalitis.
One study from Réunion Island described 739 mothers who experienced symptoms of chikungunya infection during pregnancy, 39 of whom were symptomatic in the intrapartum period (between 2 d before and 2 d after delivery).105 Of these 39 mothers, all infants were asymptomatic at birth, but 19 developed acute disease a median 4 (range 3‐7) days after delivery, giving a vertical transmission rate of approximately 50% for mothers symptomatic in the intrapartum period. The initial clinical features in the affected neonates included fever, distress, poor feeding, petechiae, and a maculopapular rash. Nine cases (47%) were reported to have developed encephalopathy. Cerebrospinal fluid for all 9 showed normal biochemistry and white cell counts, and chikungunya PCR was detected in 5. Magnetic resonance imaging data from this study, combined with a later follow‐up study,120 show that amongst neonates developing encephalopathy or encephalitis after perinatally acquired chikungunya, severe white matter injury is well characterised in a 3‐stage pattern: cytotoxic brain oedema (ischaemia), vasogenic oedema (reperfusion), and mass reduction (demyelination).105, 120
In addition to these severe features, hypotonia has been described in 17 neonates with chikungunya infection from Réunion Island,97 as well as intracerebral haemorrhage secondary to clotting abnormalities.105, 112 Interestingly, neonatal spinal cord and peripheral nervous system diseases have not been reported.
With regard to prevention of transmission, no studies have found a protective effect of caesarean section.105, 114 The risk factors for vertical transmission are not understood, although in one study, the viral load of chikungunya in the placentas of the 19 transmitters was found to be significantly higher than in 13 nontransmitters.105 Interestingly, one of the transmitters gave birth to dizygous twins, of whom one acquired infection but the other did not.
In addition to overt diseases in some perinatally infected neonates soon after delivery, there is also evidence for impacts on longer‐term development. In one study comparing the neurocognitive function at approximately 2 years of age for 33 children with and 135 without perinatal chikungunya infection, significant differences in development quotients were found, including movement, coordination, language, and sociability.120 Importantly, even those infected at birth without obvious clinical features of neurological disease, such as encephalopathy, had significantly worsened neurocognitive function than uninfected children. Thus, the neurological effects of vertically transmitted chikungunya may not be obvious at birth, emphasising the importance of follow‐up of this cohort. The 12 cases with encephalopathy at birth showed still more severe developmental deficit, including cerebral palsy and microcephaly.
Maternal chikungunya virus infection earlier in pregnancy does not appear to affect the fetus; in the above study investigating 739 mothers with chikungunya infection, 700 were symptomatic outside of the intrapartum period, and none of these infants developed symptoms of chikungunya.105
Of note, in 3 of 7 miscarriages occurring before 22 weeks, chikungunya RNA was detected in amniotic fluid for all 3 and placenta and fetal brain for 2. However, no significant increase in antepartum fetal deaths was seen during the chikungunya outbreak as compared with previous years. Another study of 1400 mothers from Réunion Island121 found no effect of antepartum chikungunya infection on pregnancy outcomes. Together, these data would suggest that although antepartum congenital infection has been detected in miscarried fetuses, given the lack of epidemiological evidence for a causal association between infection and miscarriage, this may have been an incidental finding.
Zika virus is also now known to cause devastating neurological disease in neonates, which is of global concern.122 Whereas chikungunya appears to be most damaging around the time of birth, Zika has been associated with neurological sequelae in infections at all stages of pregnancy.101 Another important difference is in the initial presentation of neonatal disease—in perinatal chikungunya infections, fever‐rash and neurological symptoms are only seen at approximately 4 days postpartum. In the Zika congenital syndrome, the damage is done in utero; thus, neonates can be born with clear evidence of infection, such as microcephaly. Despite the magnitude of outbreaks throughout the tropics, there is a paucity of data on the effects of dengue virus in pregnancy. A recent large retrospective study from Brazil reported an increased risk of preterm birth associated with dengue infection, with no difference in the rate of congenital malformations.123 Adverse outcomes in neonates following perinatal dengue infection, such as thrombocytopenia, dengue haemorrhagic fever, and dengue shock syndrome, have been described in a handful of case reports and series in neonates124, 125, 126; but unlike chikungunya and Zika, apart from one case of anoxic encephalopathy,127 neurological complications have not been reported.
7.8. Pathophysiology
The mechanisms by which chikungunya virus affects the nervous system have not been fully elucidated. The following section discusses progress on some of the important unanswered questions, including how certain patients develop neurological disease after chikungunya infection and others do not, whether the virus acts directly or indirectly towards neurons and if the process differs in the CNS and peripheral nervous system, and the significance of the phylogenetic strain and factors driving placental transmission.
A study from India compared the cytokine profile for patients with and without neurological complications following chikungunya infection.128 Of those with neurological disease, 4 had encephalitis and 1 had “neuropathy.” Concentrations of 4 cytokines (TNF‐α, IFN‐α, IL‐6, and monokine induced by IFN‐γ) were found to be significantly higher in patients with neurological disease secondary to chikungunya, as opposed to uncomplicated chikungunya infection. However, the role of these cytokines in disease pathogenesis is still unclear.
Whether the virus affects the nervous system directly or indirectly via a triggered immune‐mediated effect is also unknown; scarce evidence argues for both. For the former, both RNA and virus have been isolated from CSF in severe CNS disease,30 consistent with direct neuroinvasion. Neurons, astrocytes, and oligodendrocytes (but not microglia) are susceptible to chikungunya infection in vitro; the former 2 cell types were shown to undergo apoptosis postinfection.129, 130 In vivo, subcutaneous inoculation in macaques resulted in morphological changes in astrocytes, including cell body hypertrophy and alteration in the pattern of branching of their primary processes.131 In relation to the latter, immune‐mediated hypothesis, this study also showed upregulation of TLR2 in grey matter astrocytes, a gene associated with the innate immune response. The clinical neurological state of the macaques was not reported, which adds to the uncertainty of whether the immune response was protective or pathogenic in these cases. Another study that subcutaneously inoculated mice with chikungunya virus detected upregulation of TLR3 in the brain, a gene that is also associated with the innate immune response.132 Amongst other clinical signs, these mice developed hind‐limb paralysis, dehydration, and weight loss, and 25% of them died after 1 week. Interestingly, pretreatment with polyinosinic:polycytidylic acid (a TLR3 agonist and interferon inducer), which caused upregulation of proinflammatory cytokines, chemokines, antiviral genes, and IFN‐β, was protective clinically and promoted viral clearance from the brain, arguing for a protective innate and adaptive immune response, at least in CNS disease. In concordance, faster viral clearance after chikungunya infection was seen in wild‐type mice compared with a TLR3‐knockout model; this was thought to be secondary to increased antibody‐neutralising activity in the wild‐type mice.133 Chikungunya‐infected TLR3‐knockout mice had increased viral dissemination throughout the viscera, including the brain. Along with a U‐shaped pattern of age‐specific incidence, this critical role of TLR3 is reminiscent of susceptibility to HSV encephalitis.
Patients diagnosed with myeloneuropathy or encephalomyeloneuropathy exhibit disease of both the CNS and peripheral nervous system. Given the association between chikungunya and GBS, it is not clear whether in these cases, there is one underlying pathological process involving both the CNS and peripheral nerves, or dual pathology, with a myelopathy ± encephalopathy centrally and GBS peripherally. For example, a case report from India described a 73‐year‐old man who, a week after chikungunya infection, was admitted with drowsiness, weakness, and absent reflexes and eventually died.34 His CSF and MRI showed evidence of CNS involvement, and an electromyogram showed a sensorimotor neuropathy. A brain autopsy showed subarachnoid haemorrhage, ischaemic changes, and small foci of demyelination without identification of viral inclusion bodies. Clearly, this patient had involvement of both CNS and peripheral nervous system at the same time, but it is not clear whether the same pathological process was responsible for both. Elucidation of these mechanisms may help to better guide management strategies.
Neurological disease secondary to chikungunya has been reported in areas with both ECSA (or ECSA‐diverged Indian Ocean lineage) and Asian strains, but whether these strains have differing neurovirulence is unknown. One study compared the effect of intracerebral inoculation of Asian and ECSA‐diverged strains in mice.134 Both spread within the brain to a similar extent, but the Asian strain was associated with higher mortality than the ECSA‐diverged strain. Upregulation of a gene associated with apoptosis was seen in the former, whilst antiapoptosis, antiviral, and CNS protective gene upregulation were seen in the latter. This potentially suggests a higher neurovirulence of the Asian strain, and comparative clinical data from countries such as Brazil, where both strains are circulating, will be useful.
On neonatal neurological disease, given that caesarean section is not protective, vertical transmission is unlikely to occur via the birth canal, as is the case in other neonatal infections such as herpes simplex.135 Furthermore, the placenta seems to act as a barrier to transmission, as one study reported (as unpublished data) that placental cells from infected neonates were negative when labelled with antichikungunya antibody.105 One hypothesis raised by the authors is that uterine contractions result in breaches of this placental barrier, allowing passive passage of the virus.
7.9. Management
There are currently no specific antiviral agents or vaccines for chikungunya virus.136 Various in vitro compounds active against chikungunya virus have been reported, including both direct‐acting and host‐targeting antivirals; however, most of these compounds have yet to find their way into in vivo models and clinical trials.137 Two of the few that have been tested clinically, chloroquine and ribavirin, are already widely used in the treatment of other diseases and have a known safety profile. However, chloroquine was found not to have any benefit for arthritic chikungunya when compared with a nonsteroidal anti‐inflammatory drug in a randomised clinical trial.138 Ribavirin, on the other hand, had promising results in a small case series of 10 patients with severe arthritis post–chikungunya infection.139 To the best of our knowledge, no antiviral has been evaluated in the management of chikungunya‐associated neurological disease, which therefore remains the same as that of neurological disease without associated chikungunya infection: for patients with encephalitis, those with a reduced Glasgow coma score (GCS) require assessment by intensive care specialists and may need intubation, ventilatory support, correction of electrolyte abnormalities, management of raised intracranial pressure, and enhancement of cerebral perfusion pressure.140 In patients with myelitis, corticosteroids are the standard first‐line treatment, despite the lack of trial evidence for their use in this scenario.141 The management of GBS focuses on immunotherapy with intravenous immunoglobulin or plasma exchange, and ventilatory support if the innervation of respiratory muscles is affected.109
Although not yet commercially available, it is hoped that a vaccine for chikungunya is on the horizon. Two phase 1 clinical trials have shown a good safety and immunogenicity profile to date.142, 143 A recent study that tested an insect‐specific alphavirus as the vaccine platform found promising results in mice and macaques, including immunogenicity after a single dose.144
8. CONCLUSION
Neurological disease associated with chikungunya virus is being reported increasingly, in part because of the recent introduction of the virus to the South American population and associated large outbreaks. Clinicians and public health officials globally face challenges from the wide range of associated neurological disease and the complicating factor that dengue and Zika viruses are transmitted by the same mosquito vectors and have broadly similar epidemiology. In endemic areas, chikungunya virus should be tested for in all patients presenting with acute neurological disease and all mothers presenting with fever, arthralgia, or rash; neonates with suspected infection should be followed up for at least 2 years for evidence of neurodevelopmental delay, regardless of the initial presentation (Table 4). Future challenges include understanding the full scope of chikungunya neurological disease, in both neonatal and adult infection, and their underlying pathophysiological mechanisms. It is hoped that new direct therapeutic and vaccine candidates, some of which have shown promise in early studies, will augment the current supportive management strategies.
Table 4.
For the clinician—summary
| Adults and Children (Transmission Directly Via Mosquito Bite) | Neonates (Vertical Transmission) |
|---|---|
| Patients in areas endemic for chikungunya, Zika, or dengue presenting with an acute neurological disorder should be investigated for all 3 arboviruses | Neonates born to mothers experiencing symptoms of chikungunya infection near the time of delivery require admission and observation for signs of vertical transmission for at least 7 d postpartum, as they may be asymptomatic for the first few days of life |
| Encephalitis is the most commonly reported neurological complication associated with chikungunya; encephalitis has a worse prognosis than encephalopathy alone; a CSF pleocytosis is not always seen | Neonates born to mothers infected outside of the peripartum period are usually unaffected by chikungunya virus |
| In myelitis associated with chikungunya, CSF pleocytosis and magnetic resonance imaging changes are not always seen | Caesarean section does not appear to be protective in vertical transmission of chikungunya |
| Guillain‐Barré syndrome associated with chikungunya follows a similar course compared with other infections such as Campylobacter jejuni; most patients recover after immunomodulatory treatment | Neonates infected with chikungunya should be followed up for at least 2 y, regardless of symptoms in the first week of life; the neurodevelopment of those without clinical encephalopathy at birth can still be affected |
| Disease of both the central and peripheral nervous systems in the same patient can be seen in association with chikungunya infection | |
| Ophthalmological complications associated with chikungunya have been reported both at the time of infection and up to 12 wk after; some reports describe treating with steroids, recovery is variable | |
| Following chikungunya infection, complications of other organs can also occur at the same time as disease of the nervous system; such cases should be managed using a multidisciplinary approach | |
| There is currently no available antiviral treatment or vaccine for chikungunya |
AUTHOR CONTRIBUTION
All authors performed the literature search and reviewed the included articles. R.M. wrote the initial draft, and all authors commented on and edited the manuscript.
CONFLICT OF INTEREST
We declare no competing interests.
ACKNOWLEDGEMENTS
We are very grateful to Dr Nicholas Beale for critically reviewing the ocular complications section of the article. T.S. receives funding from the United Kingdom Medical Research Council; the National Institute for Health Research Health Protection Research Unit (NIHR HPRU) in Emerging and Zoonotic Infections at the University of Liverpool, in partnership with Public Health England (PHE) and Liverpool School of Tropical Medicine (LSTM); and the European Union's Horizon 2020 research and innovation programme under grant agreement no. 734584. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, the Department of Health or Public Health England. No sponsorship was received to write this review.
Mehta R, Gerardin P, de Brito CAA, Soares CN, Ferreira MLB, Solomon T. The neurological complications of chikungunya virus: A systematic review. Rev Med Virol. 2018;28:e1978 https://doi.org/10.1002/rmv.1978
Contributor Information
Ravi Mehta, Email: ravi.mehta@liverpool.ac.uk.
Tom Solomon, Email: tsolomon@liv.ac.uk.
REFERENCES
- 1. Robinson MC. An epidemic of virus disease in Southern Province, Tanganyika Territory, in 1952‐53. I. Clinical features. Trans R Soc Trop Med Hyg. 1955;49(1):28‐32. [DOI] [PubMed] [Google Scholar]
- 2. Solomon T, Whitley RJ, Scheld MW, Whitley RJ, Marra CM. Arthropod‐borne viral encephalitides In: Infections of the Central Nervous System. Philadelphia, Pennsylvania: Lippincott Williams & Wilkins; 2004. [Google Scholar]
- 3. Carod‐Artal FJ, Wichmann O, Farrar J, Gascon J. Neurological complications of dengue virus infection. Lancet Neurol. 2013;12(9):906‐919. [DOI] [PubMed] [Google Scholar]
- 4. Beckham JD, Pastula DM, Massey A, Tyler KL. Zika virus as an emerging global pathogen: neurological complications of Zika virus. JAMA Neurol. 2016;73(7):875‐879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Solomon T, Dung NM, Vaughn DW, et al. Neurological manifestations of dengue infection. Lancet. 2000;355(9209):1053‐1059. [DOI] [PubMed] [Google Scholar]
- 6. Rodriguez‐Morales AJ, Villamil‐Gomez WE, Franco‐Paredes C. The arboviral burden of disease caused by co‐circulation and co‐infection of dengue, chikungunya and Zika in the Americas. Travel Med Infect Dis. 2016;14(3):177‐179. [DOI] [PubMed] [Google Scholar]
- 7. Cerny T, Schwarz M, Schwarz U, Lemant J, Gerardin P, Keller E. The range of neurological complications in chikungunya fever. Neurocrit Care. 2017. [DOI] [PubMed] [Google Scholar]
- 8. Carey DE. Chikungunya and dengue: a case of mistaken identity? J Hist Med Allied Sci. 1971;26(3):243‐262. [DOI] [PubMed] [Google Scholar]
- 9. Lumsden WH. An epidemic of virus disease in Southern Province, Tanganyika Territory, in 1952‐53. II. General description and epidemiology. Trans R Soc Trop Med Hyg. 1955;49(1):33‐57. [DOI] [PubMed] [Google Scholar]
- 10. Ross RW. The Newala epidemic. III. The virus: isolation, pathogenic properties and relationship to the epidemic. J Hyg (Lond). 1956;54(2):177‐191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Weaver SC, Forrester NL. Chikungunya: evolutionary history and recent epidemic spread. Antiviral Res. 2015;120:32‐39. [DOI] [PubMed] [Google Scholar]
- 12. Shah KV, Gibbs CJ Jr, Banerjee G. Virological investigation of the epidemic of haemorrhagic fever in Calcutta: isolation of three strains of chikungunya virus. Indian J Med Res. 1964;52:676‐683. [PubMed] [Google Scholar]
- 13. Nimmannitya S, Halstead SB, Cohen SN, Margiotta MR. Dengue and chikungunya virus infection in man in Thailand, 1962‐1964 I. Observations on hospitalized patients with hemorrhagic fever. Am J Trop Med Hyg. 1969;18(6):954‐971. [DOI] [PubMed] [Google Scholar]
- 14. Powers AM, Brault AC, Tesh RB, Weaver SC. Re‐emergence of chikungunya and o'nyong‐nyong viruses: evidence for distinct geographical lineages and distant evolutionary relationships. J Gen Virol. 2000;81(Pt 2):471‐479. [DOI] [PubMed] [Google Scholar]
- 15. Tsetsarkin KA, Vanlandingham DL, McGee CE, Higgs S. A single mutation in chikungunya virus affects vector specificity and epidemic potential. PLoS Pathog. 2007;3(12):e201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Staples JE, Breiman RF, Powers AM. Chikungunya fever: an epidemiological review of a re‐emerging infectious disease. Clin Infect Dis. 2009;49(6):942‐948. [DOI] [PubMed] [Google Scholar]
- 17. Leparc‐Goffart I, Nougairede A, Cassadou S, Prat C, de Lamballerie X. Chikungunya in the Americas. Lancet. 2014;383(9916):514. [DOI] [PubMed] [Google Scholar]
- 18. Pan American Health Organisation . New cases of chikungunya in the Americas 2013‐2017. 2017; http://ais.paho.org/phip/viz/ed_chikungunya_amro.asp. Accessed 28/06/2017.
- 19. Nunes MR, Faria NR, de Vasconcelos JM, et al. Emergence and potential for spread of chikungunya virus in Brazil. BMC Med. 2015;13:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Oxford Reference . http://www.oxfordreference.com/view/10.1093/oi/authority.20110803095930707. Accessed 10/12/17.
- 21. Smith DW, Mackenzie J. Zika virus and Guillain‐Barre syndrome: another viral cause to add to the list. Lancet. 2016;387(10027):1486‐1488. [DOI] [PubMed] [Google Scholar]
- 22. Campos GS, Bandeira ACA, Rocha VFD, Dias JP, Carvalho RH, Sardi SI. First detection of chikungunya virus in breast milk. Pediatr Infect Dis J. 2017. [DOI] [PubMed] [Google Scholar]
- 23. Bandeira AC, Campos GS, Rocha VF, et al. Prolonged shedding of chikungunya virus in semen and urine: a new perspective for diagnosis and implications for transmission. IDCases. 2016;6:100‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Musso D, Teissier A, Rouault E, Teururai S, de Pina JJ, Nhan TX. Detection of chikungunya virus in saliva and urine. Virol J. 2016;13:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gay N, Rousset D, Huc P, et al. Seroprevalence of Asian lineage chikungunya virus infection on Saint Martin Island, 7 months after the 2013 emergence. Am J Trop Med Hyg. 2016;94(2):393‐396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rudolph KE, Lessler J, Moloney RM, Kmush B, Cummings DA. Incubation periods of mosquito‐borne viral infections: a systematic review. Am J Trop Med Hyg. 2014;90(5):882‐891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Burt FJ, Rolph MS, Rulli NE, Mahalingam S, Heise MT. Chikungunya: a re‐emerging virus. Lancet. 2012;379(9816):662‐671. [DOI] [PubMed] [Google Scholar]
- 28. Galatas B, Ly S, Duong V, et al. Long‐lasting immune protection and other epidemiological findings after chikungunya emergence in a Cambodian rural community, April 2012. PLoS Negl Trop Dis. 2016;10(1):e0004281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Economopoulou A, Dominguez M, Helynck B, et al. Atypical chikungunya virus infections: clinical manifestations, mortality and risk factors for severe disease during the 2005‐2006 outbreak on Reunion. Epidemiol Infect. 2009;137(4):534‐541. [DOI] [PubMed] [Google Scholar]
- 30. Tandale BV, Sathe PS, Arankalle VA, et al. Systemic involvements and fatalities during chikungunya epidemic in India, 2006. J Clin Virol. 2009;46(2):145‐149. [DOI] [PubMed] [Google Scholar]
- 31. Villamil‐Gomez W, Alba‐Silvera L, Menco‐Ramos A, et al. Congenital chikungunya virus infection in Sincelejo, Colombia: a case series. J Trop Pediatr. 2015;61(5):386‐392. [DOI] [PubMed] [Google Scholar]
- 32. Crosby L, Perreau C, Madeux B, et al. Severe manifestations of chikungunya virus in critically ill patients during the 2013‐2014 Caribbean outbreak. Int J Infect Dis. 2016;48:78‐80. [DOI] [PubMed] [Google Scholar]
- 33. Lemant J, Boisson V, Winer A, et al. Serious acute chikungunya virus infection requiring intensive care during the Reunion Island outbreak in 2005‐2006. Crit Care Med. 2008;36(9):2536‐2541. [DOI] [PubMed] [Google Scholar]
- 34. Ganesan K, Diwan A, Shankar SK, Desai SB, Sainani GS, Katrak SM. Chikungunya encephalomyeloradiculitis: report of 2 cases with neuroimaging and 1 case with autopsy findings. AJNR Am J Neuroradiol. 2008;29(9):1636‐1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chatterjee SN, Chakravarti SK, Mitra AC, Sarkar JK. Virological investigation of cases with neurological complications during the outbreak of haemorrhagic fever in Calcutta. J Indian Med Assoc. 1965;45(6):314‐316. [PubMed] [Google Scholar]
- 36. Sissoko D, Malvy D, Giry C, et al. Outbreak of chikungunya fever in Mayotte, Comoros archipelago, 2005‐2006. Trans R Soc Trop Med Hyg. 2008;102(8):780‐786. [DOI] [PubMed] [Google Scholar]
- 37. Gerardin P, Couderc T, Bintner M, et al. Chikungunya virus‐associated encephalitis: a cohort study on la Reunion Island, 2005‐2009. Neurology. 2016;86(1):94‐102. [DOI] [PubMed] [Google Scholar]
- 38. Wadia RA. Neurotropic virus (chikungunya) and a neuropathic aminoacid (homocysteine). Ann Indian Acad Neurol. 2007;10(4):198‐213. [Google Scholar]
- 39. Suryawanshi SD, Dube AH, Khadse RK, et al. Clinical profile of chikungunya fever in patients in a tertiary care centre in Maharashtra, India. Indian J Med Res. 2009;129(4):438‐441. [PubMed] [Google Scholar]
- 40. Robin S, Ramful D, Le Seach F, Jaffar‐Bandjee MC, Rigou G, Alessandri JL. Neurologic manifestations of pediatric chikungunya infection. J Child Neurol. 2008;23(9):1028‐1035. [DOI] [PubMed] [Google Scholar]
- 41. Chandak NH, Kashyap RS, Kabra D, et al. Neurological complications of chikungunya virus infection. Neurol India. 2009;57(2):177‐180. [DOI] [PubMed] [Google Scholar]
- 42. Casolari S, Briganti E, Zanotti M, et al. A fatal case of encephalitis associated with chikungunya virus infection. Scand J Infect Dis. 2008;40(11‐12):995‐996. [DOI] [PubMed] [Google Scholar]
- 43. Kee AC, Yang S, Tambyah P. Atypical chikungunya virus infections in immunocompromised patients. Emerg Infect Dis. 2010;16(6):1038‐1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chusri S, Siripaitoon P, Hirunpat S, Silpapojakul K. Case reports of neuro‐chikungunya in southern Thailand. Am J Trop Med Hyg. 2011;85(2):386‐389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Taraphdar D, Roy B, Chatterjee S. Chikungunya virus infection amongst the acute encephalitis syndrome cases in West Bengal, India. Indian J Med Microbiol. 2015;33(5):153‐156. [DOI] [PubMed] [Google Scholar]
- 46. Gauri LA, Ranwa BL, Nagar K, Vyas A, Fatima Q. Post chikungunya brain stem encephalitis. J Assoc Physicians India. 2012;60:68‐70. [PubMed] [Google Scholar]
- 47. Horwood PF, Duong V, Laurent D, et al. Aetiology of acute meningoencephalitis in Cambodian children, 2010‐2013. Emerg Microbes Infect. 2017;6(5):e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kalita J, Kumar P, Misra UK. Stimulus‐sensitive myoclonus and cerebellar ataxia following chikungunya meningoencephalitis. Infection. 2013;41(3):727‐729. [DOI] [PubMed] [Google Scholar]
- 49. Shaikh NJ, Raut CG, Sinha DP, Manjunath MJ. Detection of chikungunya virus from a case of encephalitis, Bangalore, Karnataka state. Indian J Med Microbiol. 2015;33(3):454‐455. [DOI] [PubMed] [Google Scholar]
- 50. Nelson J, Waggoner JJ, Sahoo MK, Grant PM, Pinsky BA. Encephalitis caused by chikungunya virus in a traveler from the Kingdom of Tonga. J Clin Microbiol. 2014;52(9):3459‐3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Venkatesan A, Tunkel AR, Bloch KC, et al. Case definitions, diagnostic algorithms, and priorities in encephalitis: consensus statement of the international encephalitis consortium. Clin Infect Dis. 2013;57(8):1114‐1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Samra JA, Hagood NL, Summer A, Medina MT, Holden KR. Clinical features and neurologic complications of children hospitalized with chikungunya virus in Honduras. J Child Neurol. 2017;32(8):712‐716. [DOI] [PubMed] [Google Scholar]
- 53. Gupta D, Bose A, Rose W. Acquired neonatal chikungunya encephalopathy. Indian J Pediatr. 2015;82(11):1065‐1066. [DOI] [PubMed] [Google Scholar]
- 54. Alvarado‐Socarras JL, Ocampo‐Gonzalez M, Vargas‐Soler JA, Rodriguez‐Morales AJ, Franco‐Paredes C. Congenital and neonatal chikungunya in Colombia. J Pediatr Infect Dis Soc. 2016;5(3):e17‐e20. [DOI] [PubMed] [Google Scholar]
- 55. Nagpal K, Agarwal P, Kumar A, Reddi R. Chikungunya infection presenting as mild encephalitis with a reversible lesion in the splenium: a case report. J Neurovirol. 2017. [DOI] [PubMed] [Google Scholar]
- 56. Singh A, Jain R. Neurological manifestations of chikungunya in children. Indian Pediatr. 2017;54(3):249. [DOI] [PubMed] [Google Scholar]
- 57. Scott SSO, Braga‐Neto P, Pereira LP, et al. Immunoglobulin‐responsive chikungunya encephalitis: two case reports. J Neurovirol. 2017. [DOI] [PubMed] [Google Scholar]
- 58. Sa PKO, Nunes MM, Leite IR, et al. Chikungunya virus infection with severe neurologic manifestations: report of four fatal cases. Rev Soc Bras Med Trop. 2017;50(2):265‐268. [DOI] [PubMed] [Google Scholar]
- 59. Rocha VFD, de Oliveira AHP, Bandeira AC, et al. Chikungunya virus infection associated with encephalitis and anterior uveitis. Ocul Immunol Inflamm. 2017;1‐3. [DOI] [PubMed] [Google Scholar]
- 60. Choudhary N, Makhija P, Puri V, Khwaja GA, Duggal A. An unusual case of myelitis with myositis. J Clin Diagn Res. 2016;10(5):OD19‐OD20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Martins HAL, Bernardino SN, Ribas KH, Santos CC, Antunes T, et al. Outbreak of neuro‐chikungunya in Northeastern Brazil. J Neuroinfect Dis. 2016;7(2). [Google Scholar]
- 62. Krishnan M, Rahul K. Chikungunya myeloradiculopathy: a rare complication. J Glob Infect. 2012;4(4):207‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Banilad AM, Batra A, Colorado RA, Lyons JL. Myeloradiculopathy associated with chikungunya virus infection. J Neurovirol. 2016;22(1):125‐128. [DOI] [PubMed] [Google Scholar]
- 64. Chung SJ, Chlebicki MP. A case of Addisonian crisis, acute renal failure, vesiculobullous rash, rhabdomyolysis, neurological disturbances and prolonged viraemia in a patient on long term steroids. J Clin Virol. 2013;57(3):187‐190. [DOI] [PubMed] [Google Scholar]
- 65. Thiruvengadam KV, Kalyanasundaram V, Rajgopal J. Clinical and pathological studies on chikungunya fever in Madras city. Indian J Med Res. 1965;53(8):729‐744. [PubMed] [Google Scholar]
- 66. Tournebize P, Charlin C, Lagrange M. Neurological manifestations in chikungunya: about 23 cases collected in Reunion Island. Rev Neurol (Paris). 2009;165(1):48‐51. [DOI] [PubMed] [Google Scholar]
- 67. Wielanek AC, Monredon JD, Amrani ME, Roger JC, Serveaux JP. Guillain‐Barre syndrome complicating a chikungunya virus infection. Neurology. 2007;69(22):2105‐2107. [DOI] [PubMed] [Google Scholar]
- 68. Lebrun G, Chadda K, Reboux AH, Martinet O, Gauzere BA. Guillain‐Barre syndrome after chikungunya infection. Emerg Infect Dis. 2009;15(3):495‐496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Singh SS, Manimunda SP, Sugunan AP, Sahina, Vijayachari P. Four cases of acute flaccid paralysis associated with chikungunya virus infection. Epidemiol Infect. 2008;136(9):1277‐1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Oehler E, Fournier E, Leparc‐Goffart I, et al. Increase in cases of Guillain‐Barre syndrome during a chikungunya outbreak, French Polynesia, 2014 to 2015. Euro Surveill. 2015;20(48):30079. [DOI] [PubMed] [Google Scholar]
- 71. Balavoine S, Pircher M, Hoen B, et al. Guillain‐Barre syndrome and chikungunya: description of all cases diagnosed during the 2014 outbreak in the French West Indies. Am J Trop Med Hyg. 2017;97(2):356‐360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Villamil‐Gomez W, Silvera LA, Paez‐Castellanos J, Rodriguez‐Morales AJ. Guillain‐Barre syndrome after chikungunya infection: a case in Colombia. Enferm Infecc Microbiol Clin. 2016;34(2):140‐141. [DOI] [PubMed] [Google Scholar]
- 73. Agarwal A, Vibha D, Srivastava AK, Shukla G, Prasad K. Guillain‐Barre syndrome complicating chikungunya virus infection. J Neurovirol. 2017. [DOI] [PubMed] [Google Scholar]
- 74. Mittal A, Mittal S, Bharati MJ, Ramakrishnan R, Saravanan S, Sathe PS. Optic neuritis associated with chikungunya virus infection in South India. Arch Ophthalmol. 2007;125(10):1381‐1386. [DOI] [PubMed] [Google Scholar]
- 75. Lalitha P, Rathinam S, Banushree K, Maheshkumar S, Vijayakumar R, Sathe P. Ocular involvement associated with an epidemic outbreak of chikungunya virus infection. Am J Ophthalmol. 2007;144(4):552‐556. [DOI] [PubMed] [Google Scholar]
- 76. Mahendradas P, Ranganna SK, Shetty R, et al. Ocular manifestations associated with chikungunya. Ophthalmology. 2008;115(2):287‐291. [DOI] [PubMed] [Google Scholar]
- 77. Rose N, Anoop TM, John AP, Jabbar PK, George KC. Acute optic neuritis following infection with chikungunya virus in southern rural India. Int J Infect Dis. 2011;15(2):e147‐e150. [DOI] [PubMed] [Google Scholar]
- 78. Mahesh G, Giridhar A, Shedbele A, Kumar R, Saikumar SJ. A case of bilateral presumed chikungunya neuroretinitis. Indian J Ophthalmol. 2009;57(2):148‐150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mahendradas P, Shetty R, Malathi J, Madhavan HN. Chikungunya virus iridocyclitis in Fuchs' heterochromic iridocyclitis. Indian J Ophthalmol. 2010;58(6):545‐547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Vishwanath S, Badami K, Sriprakash KS, Sujatha BL, Shashidhar SD, Shilpa YD. Post‐fever retinitis: a single center experience from south India. Int Ophthalmol. 2014;34(4):851‐857. [DOI] [PubMed] [Google Scholar]
- 81. Nair AG, Biswas J, Bhende MP. A case of bilateral chikungunya neuroretinitis. J Ophthalmic Inflamm Infect. 2012;2(1):39‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Scripsema NK, Sharifi E, Samson CM, Kedhar S, Rosen RB. Chikungunya‐associated uveitis and exudative retinal detachment: a case report. Retin Cases Brief Rep. 2015;9(4):352‐356. [DOI] [PubMed] [Google Scholar]
- 83. Lin J, Chen RW, Hazan A, Weiss M. Chikungunya virus infection manifesting as intermediate uveitis. Ocul Immunol Inflamm. 2016;1‐3. [DOI] [PubMed] [Google Scholar]
- 84. Jadhav M, Namboodripad M, Carman RH, Carey DE, Myers RM. Chikungunya disease in infants and children in Vellore: a report of clinical and haematological features of virologically proved cases. Indian J Med Res. 1965;53(8):764‐776. [PubMed] [Google Scholar]
- 85. Carey DE, Myers RM, DeRanitz CM, Jadhav M, Reuben R. The 1964 chikungunya epidemic at Vellore, South India, including observations on concurrent dengue. Trans R Soc Trop Med Hyg. 1969;63(4):434‐445. [DOI] [PubMed] [Google Scholar]
- 86. Rampal SM, Meena H. Neurological complications in chikungunya fever. J Assoc Physicians India. 2007;55:765‐769. [PubMed] [Google Scholar]
- 87. Lewthwaite P, Vasanthapuram R, Osborne JC, et al. Chikungunya virus and central nervous system infections in children, India. Emerg Infect Dis. 2009;15(2):329‐331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ernould S, Walters H, Alessandri JL, et al. Chikungunya in paediatrics: epidemic of 2005‐2006 in Saint‐Denis, Reunion Island. Arch Pediatr. 2008;15(3):253‐262. [DOI] [PubMed] [Google Scholar]
- 89. Musthafa AK, Abdurahiman P, Jose J. Case of ADEM following chikungunya fever. J Assoc Physicians India. 2008;56:473. [PubMed] [Google Scholar]
- 90. Bhavana K, Tyagi I, Kapila RK. Chikungunya virus induced sudden sensorineural hearing loss. Int J Pediatr Otorhinolaryngol. 2008;72(2):257‐259. [DOI] [PubMed] [Google Scholar]
- 91. Maity P, Roy P, Basu A, Das B, Ghosh USA. Case of ADEM following chikungunya fever. J Assoc Physicians India. 2014;62(5):441‐442. [PubMed] [Google Scholar]
- 92. Das S, Sarkar N, Majumder J, Chatterjee K, Aich B. Acute disseminated encephalomyelitis in a child with chikungunya virus infection. J Pediatr Infect Dis. 2013;9:37‐41. [Google Scholar]
- 93. Benzekri R, Hage R, Merle H. Third cranial nerve palsy in the setting of chikungunya virus infection. Am J Trop Med Hyg. 2016;95(1):180‐181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Dutta P, Sharma A. A case of atypical ophthalmoplegia after chikungunya fever. Int Ophthalmol. 2017. [DOI] [PubMed] [Google Scholar]
- 95. Centers for Disease Control and Prevention . Countries and territories where chikungunya cases have been reported (as of April 22, 2016). 2016; https://www.cdc.gov/chikungunya/geo/. Accessed 02/01/2017.
- 96. Weaver SC, Lecuit M. Chikungunya virus and the global spread of a mosquito‐borne disease. N Engl J Med. 2015;372(13):1231‐1239. [DOI] [PubMed] [Google Scholar]
- 97. Ramful D, Carbonnier M, Pasquet M, et al. Mother‐to‐child transmission of chikungunya virus infection. Pediatr Infect Dis J. 2007;26(9):811‐815. [DOI] [PubMed] [Google Scholar]
- 98. Vazeille M, Mousson L, Martin E, Failloux AB. Orally co‐infected Aedes albopictus from La Reunion Island, Indian Ocean, can deliver both dengue and chikungunya infectious viral particles in their saliva. PLoS Negl Trop Dis. 2010;4(6):e706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Acevedo N, Waggoner J, Rodriguez M, et al. Zika virus, chikungunya virus, and dengue virus in cerebrospinal fluid from adults with neurological manifestations, Guayaquil, Ecuador. Front Microbiol. 2017;8:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Zambrano H, Waggoner JJ, Almeida C, Rivera L, Benjamin JQ, Pinsky BA. Zika virus and chikungunya virus coinfections: a series of three cases from a single center in Ecuador. Am J Trop Med Hyg. 2016;95(4):894‐896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Brasil P, Pereira JP Jr, Moreira ME, et al. Zika virus infection in pregnant women in Rio de Janeiro. N Engl J Med. 2016;375(24):2321‐2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Villamil‐Gomez WE, Rodriguez‐Morales AJ, Uribe‐Garcia AM, et al. Zika, dengue, and chikungunya co‐infection in a pregnant woman from Colombia. Int J Infect Dis. 2016;51:135‐138. [DOI] [PubMed] [Google Scholar]
- 103. Villamil‐Gomez WE, Gonzalez‐Camargo O, Rodriguez‐Ayubi J, Zapata‐Serpa D, Rodriguez‐Morales AJ. Dengue, chikungunya and Zika co‐infection in a patient from Colombia. J Infect Public Health. 2016. [DOI] [PubMed] [Google Scholar]
- 104. Rolle A, Schepers K, Cassadou S, et al. Severe sepsis and septic shock associated with chikungunya virus infection, Guadeloupe, 2014. Emerg Infect Dis. 2016;22(5):891‐894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Gerardin P, Barau G, Michault A, et al. Multidisciplinary prospective study of mother‐to‐child chikungunya virus infections on the island of La Reunion. PLoS Med. 2008;5(3):e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Transverse Myelitis Consortium Working Group . Proposed diagnostic criteria and nosology of acute transverse myelitis. Neurology. 2002;59(4):499‐505. [DOI] [PubMed] [Google Scholar]
- 107. Krupp LB, Tardieu M, Amato MP, et al. International pediatric multiple sclerosis study group criteria for pediatric multiple sclerosis and immune‐mediated central nervous system demyelinating disorders: revisions to the 2007 definitions. Mult Scler J. 2013;19(10):1261‐1267. [DOI] [PubMed] [Google Scholar]
- 108. Sejvar JJ, Kohl KS, Bilynsky R, et al. Encephalitis, myelitis, and acute disseminated encephalomyelitis (ADEM): case definitions and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25(31):5771‐5792. [DOI] [PubMed] [Google Scholar]
- 109. Willison HJ, Jacobs BC, van Doorn PA. Guillain‐Barre syndrome. Lancet. 2016;388(10045):717‐727. [DOI] [PubMed] [Google Scholar]
- 110. Jacobs BC, van Doorn PA, Schmitz PI, et al. Campylobacter jejuni infections and anti‐GM1 antibodies in Guillain‐Barre syndrome. Ann Neurol. 1996;40(2):181‐187. [DOI] [PubMed] [Google Scholar]
- 111. Mahendradas P, Avadhani K, Shetty R. Chikungunya and the eye: a review. J Ophthalmic Inflamm Infect. 2013;3(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Robillard P‐Y, Boumahni B, Gérardin P, et al. Transmission verticale materno‐fœtale du virus chikungunya. Presse Med. 2006;35(5:785‐788. [DOI] [PubMed] [Google Scholar]
- 113. Shenoy S, Pradeep GC. Neurodevelopmental outcome of neonates with vertically transmitted chikungunya fever with encephalopathy. Indian Pediatr. 2012;49(3):238‐240. [PubMed] [Google Scholar]
- 114. Torres JR, Falleiros‐Arlant LH, Duenas L, Pleitez‐Navarrete J, Salgado DM, Castillo JB. Congenital and perinatal complications of chikungunya fever: a Latin American experience. Int J Infect Dis. 2016;51:85‐88. [DOI] [PubMed] [Google Scholar]
- 115. Bandeira AC, Campos GS, Sardi SI, Rocha VF, Rocha GC. Neonatal encephalitis due to chikungunya vertical transmission: first report in Brazil. IDCases. 2016;5:57‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Karthiga V, Kommu PP, Krishnan L. Perinatal chikungunya in twins. J Pediatr Neurosci. 2016;11(3):223‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Ramos R, Viana R, Brainer‐Lima A, et al. Perinatal chikungunya virus‐associated encephalitis leading to postnatal‐onset microcephaly and optic atrophy. Pediatr Infect Dis J. 2017. [DOI] [PubMed] [Google Scholar]
- 118. Lyra PP, Campos GS, Bandeira ID, et al. Congenital chikungunya virus infection after an outbreak in Salvador, Bahia, Brazil. AJP Rep. 2016;6(3):e299‐e300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Gopakumar H, Ramachandran S. Congenital chikungunya. J Clin Neonatol. 2012;1(3):155‐156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Gerardin P, Samperiz S, Ramful D, et al. Neurocognitive outcome of children exposed to perinatal mother‐to‐child chikungunya virus infection: the CHIMERE cohort study on Reunion Island. PLoS Negl Trop Dis. 2014;8(7):e2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Fritel X, Rollot O, Gerardin P, et al. Chikungunya virus infection during pregnancy, Reunion, France, 2006. Emerg Infect Dis. 2010;16(3):418‐425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika virus and birth defects‐‐reviewing the evidence for causality. N Engl J Med. 2016;374(20):1981‐1987. [DOI] [PubMed] [Google Scholar]
- 123. Nascimento LB, Siqueira CM, Coelho GE, Siqueira JB Jr. Symptomatic dengue infection during pregnancy and livebirth outcomes in Brazil, 2007‐13: a retrospective observational cohort study. Lancet Infect Dis. 2017. [DOI] [PubMed] [Google Scholar]
- 124. Ribeiro CF, Lopes VG, Brasil P, Coelho J, Muniz AG, Nogueira RM. Perinatal transmission of dengue: a report of 7 cases. J Pediatr. 2013;163(5):1514‐1516. [DOI] [PubMed] [Google Scholar]
- 125. Sharma S, Jain S, Rajaram S. Spectrum of maternofetal outcomes during dengue infection in pregnancy: an insight. Infect Dis Obstet Gynecol. 2016;2016:5046091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Witayathawornwong P, Jirachanchai O, Kasemsut P, Mahawijit N, Srisakkwa R. Severe perinatal dengue hemorrhagic fever in a low birth weight infant. Southeast Asian J Trop Med Public Health. 2012;43(1):62‐67. [PubMed] [Google Scholar]
- 127. Arragain L, Dupont‐Rouzeyrol M, O'Connor O, et al. Vertical transmission of dengue virus in the peripartum period and viral kinetics in newborns and breast milk: new data. J Pediatr Infect Dis Soc. 2016. [DOI] [PubMed] [Google Scholar]
- 128. Kashyap RS, Morey S, Bhullar S, et al. Determination of toll‐like receptor‐induced cytokine profiles in the blood and cerebrospinal fluid of chikungunya patients. Neuroimmunomodulation. 2014;21(6):338‐346. [DOI] [PubMed] [Google Scholar]
- 129. Abraham R, Mudaliar P, Padmanabhan A, Sreekumar E. Induction of cytopathogenicity in human glioblastoma cells by chikungunya virus. PLoS One. 2013;8(9):e75854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Das T, Hoarau JJ, Jaffar Bandjee MC, Maquart M, Gasque P. Multifaceted innate immune responses engaged by astrocytes, microglia and resident dendritic cells against chikungunya neuroinfection. J Gen Virol. 2015;96(Pt 2):294‐310. [DOI] [PubMed] [Google Scholar]
- 131. Inglis FM, Lee KM, Chiu KB, et al. Neuropathogenesis of chikungunya infection: astrogliosis and innate immune activation. J Neurovirol. 2016;22(2):140‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Priya R, Patro IK, Parida MM. TLR3 mediated innate immune response in mice brain following infection with chikungunya virus. Virus Res. 2014;189:194‐205. [DOI] [PubMed] [Google Scholar]
- 133. Her Z, Teng TS, Tan JJ, et al. Loss of TLR3 aggravates CHIKV replication and pathology due to an altered virus‐specific neutralizing antibody response. EMBO Mol Med. 2015;7(1):24‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Chiam CW, Chan YF, Ong KC, Wong KT, Sam IC. Neurovirulence comparison of chikungunya virus isolates of the Asian and East/Central/South African genotypes from Malaysia. J Gen Virol. 2015;96(11):3243‐3254. [DOI] [PubMed] [Google Scholar]
- 135. Gnann JW Jr, Whitley RJ. Genital herpes. N Engl J Med. 2016;375(19):1906. [DOI] [PubMed] [Google Scholar]
- 136. Silva LA, Dermody TS. Chikungunya virus: epidemiology, replication, disease mechanisms, and prospective intervention strategies. J Clin Invest. 2017;127(3):737‐749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Abdelnabi R, Neyts J, Delang L. Antiviral strategies against chikungunya virus. Methods Mol Biol. 2016;1426:243‐253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Chopra A, Saluja M, Venugopalan A. Effectiveness of chloroquine and inflammatory cytokine response in patients with early persistent musculoskeletal pain and arthritis following chikungunya virus infection. Arthritis Rheumatol. 2014;66(2):319‐326. [DOI] [PubMed] [Google Scholar]
- 139. Ravichandran R, Manian M. Ribavirin therapy for chikungunya arthritis. J Infect Dev Ctries. 2008;2(2):140‐142. [PubMed] [Google Scholar]
- 140. Solomon T, Michael BD, Smith PE, et al. Management of suspected viral encephalitis in adults—Association of British Neurologists and British Infection Association National Guidelines. J Infect. 2012;64(4):347‐373. [DOI] [PubMed] [Google Scholar]
- 141. Frohman EM, Wingerchuk DM. Clinical practice. Transverse myelitis. N Engl J Med. 2010;363(6):564‐572. [DOI] [PubMed] [Google Scholar]
- 142. Ramsauer K, Schwameis M, Firbas C, et al. Immunogenicity, safety, and tolerability of a recombinant measles‐virus‐based chikungunya vaccine: a randomised, double‐blind, placebo‐controlled, active‐comparator, first‐in‐man trial. Lancet Infect Dis. 2015;15(5):519‐527. [DOI] [PubMed] [Google Scholar]
- 143. Chang LJ, Dowd KA, Mendoza FH, et al. Safety and tolerability of chikungunya virus‐like particle vaccine in healthy adults: a phase 1 dose‐escalation trial. Lancet. 2014;384(9959):2046‐2052. [DOI] [PubMed] [Google Scholar]
- 144. Erasmus JH, Auguste AJ, Kaelber JT, et al. A chikungunya fever vaccine utilizing an insect‐specific virus platform. Nat Med. 2017;23(2):192‐199. [DOI] [PMC free article] [PubMed] [Google Scholar]


