Abstract
Aims
To estimate the incidence of Type 2 diabetes in children aged <17 years, compare this with similar data 10 years ago, and characterize clinical features at diagnosis in the UK and Republic of Ireland.
Methods
Using the British Paediatric Surveillance Unit reporting framework, cases of Type 2 diabetes diagnosed in children aged <17 years between 1 April 2015 and 30 April 2016 were reported each month.
Results
A total of 106 cases were reported, giving a UK incidence of 0.72/100 000 (95% CI 0.58–0.88). Children from ethnic minorities had significantly higher incidence compared with white children (0.44/100 000) with rates of 2.92/100 000 and 1.67/100 000, in Asian and BACBB (black/African/Caribbean/black British) children respectively. Sixty‐seven percent were girls and 81% had a family history of Type 2 diabetes. The mean BMI sd score at diagnosis was 2.89 (2.88, girls; 2.92, boys); 81% were obese. Children of Asian ethnicity had a significantly lower BMI sd score compared with white children (P<0.001). There was a trend in increased incidence from 2005 to 2015, with a rate ratio of 1.35 (95% CI 0.99–1.84), although this was not statistically significant (P=0.062). There was statistical evidence of increased incidence among girls (P=0.03) and children of South‐Asian ethnicity (P=0.01) when comparing the 2005 and 2015 surveys.
Conclusions
Type 2 diabetes remains far less common than Type 1 diabetes in childhood in the UK, but the number of cases continues to rise, with significantly increased incidence among girls and South‐Asian children over a decade. Female gender, family history, non‐white ethnicity and obesity were found to be strongly associated with the condition.
What's new?
The 2015/2016 UK incidence of Type 2 diabetes in children aged <17 years was 0.72 per 100 000 per year
The incidence of Type 2 diabetes amongst girls and South‐Asian children has risen significantly over the last decade.
Female gender, family history, non‐white ethnicity and obesity were strongly associated with Type 2 diabetes in childhood.
Comorbidities are commonly identified at diagnosis, including 37% with non‐alcoholic fatty liver disease and 21% with hypertension
The most common presenting complaint at diagnosis after osmotic symptoms (polyuria, polydipsia and weight loss) was recurrent, mainly genital, infections, although over a third of cases were asymptomatic and detected on obesity screening investigations
What's new?
The 2015/2016 UK incidence of Type 2 diabetes in children aged <17 years was 0.72 per 100 000 per year
The incidence of Type 2 diabetes amongst girls and South‐Asian children has risen significantly over the last decade.
Female gender, family history, non‐white ethnicity and obesity were strongly associated with Type 2 diabetes in childhood.
Comorbidities are commonly identified at diagnosis, including 37% with non‐alcoholic fatty liver disease and 21% with hypertension
The most common presenting complaint at diagnosis after osmotic symptoms (polyuria, polydipsia and weight loss) was recurrent, mainly genital, infections, although over a third of cases were asymptomatic and detected on obesity screening investigations
Introduction
Type 2 diabetes accounts for 90% of diabetes globally 1 and prevalence of Type 2 diabetes in adults is increasing in the UK 2. The highest rates of Type 2 diabetes in children and adolescents have been reported in studies from the USA 3, with the incidence rising from nine to 12.5 cases per 100 000 between 2002 and 2012 4. European countries report a lower incidence, with an Austrian study reporting 0.29 per 100 000 per year between 1999 and 2007 3. Recent results from a multinational European database of >27 000 children found that Type 2 diabetes accounted for 1.3% of all cases of diabetes. In Europe, the UK has the highest reported prevalence of childhood Type 2 diabetes 5. In 2005, the UK annual incidence of Type 2 diabetes in children aged <17 years was 0.53 per 100 000 per year 6. Among children in the UK, Type 2 diabetes is less common than Type 1 diabetes. The National Paediatric Diabetes Audit 2015–2016 reported that Type 2 diabetes accounted for 2.2% of the total 7.
Risk factors for developing childhood Type 2 diabetes are similar to those for adulthood, and include obesity, family history and ethnicity 8. A previous UK study reported 95% of children were overweight or obese at diagnosis 6, with similar findings from other countries 9. In the UK, incidence was substantially higher in ethnic minority groups: 3.9 per 100 000 in BACBB children, and 1.25 per 100 000 in children of South‐Asian ethnicity, compared with 0.35 per 100 000 in white children 6, with similar findings among non‐white ethnicities in other studies 3. Family history is an important risk factor, with 84% reporting a first‐ or second‐degree relative with Type 2 diabetes 6.
Type 2 diabetes in young people represents an extreme phenotype of the disease. Young people may present with complications of diabetes and are more likely to develop microvascular complications compared with young people with Type 1 diabetes 8. The long‐term risk of cardiovascular disease in young people diagnosed with Type 2 diabetes is worse than in those diagnosed later in life 11.
The health, economic and societal impact of early‐onset Type 2 diabetes is concerning. We aimed to estimate the incidence and to characterize the demographics and presentation of Type 2 diabetes in children aged <17 years in the UK and Republic of Ireland and to compare these with our previous study conducted 10 years ago.
Methods
A prospective monthly surveillance of >3400 consultant paediatricians in the UK and Republic of Ireland using the British Paediatric Surveillance Unit (BPSU), based at the Royal College of Paediatrics and Child Health, UK was undertaken to identify new cases of Type 2 diabetes in children aged <17 years. The BPSU is an active reporting system in which an orange report card containing a list of conditions is sent monthly, by post or electronically, to all consultant paediatricians. Respondents report cases they have seen in the previous month for conditions named on the card or tick ‘Nothing to report’. The BPSU forward the reporting clinician's details to the research team, who send a proforma requesting the child's clinical details. The study ran for 13 months (as per previous BPSU studies allowing a lead‐in month) from April 2015 to April 2016.
Diabetes mellitus was defined according to American Diabetic Association 12 definition with fasting glucose level >7 mmol/l, random glucose level >11.1 mmol/l or stimulated glucose level >11.1 mmol/l after a standard oral glucose tolerance test or HbA1c >48 mmol/mol (6.5%). Type 2 diabetes was distinguished from other types of diabetes using the following criteria: (1) presence of raised insulin level (>132 pmol/l) or raised C peptide level (>0.6 nmol/l) 13, 14 or (2) the child was managed off insulin therapy for >9 months in the absence of typical Type 1 diabetes auto‐antibodies. The latter definition is likely to be based on a clinical re‐evaluation after diagnosis when a clinical course suggests a diagnosis other than Type 1 diabetes. Exclusion criteria included: Type 1 diabetes (positive auto‐antibodies and/or persisting insulin requirement from diagnosis); maturity‐onset diabetes of the young; diabetes developing in a person with a known diabetes‐associated syndrome, such as Prader–Willi or Bardet–Biedl syndromes; diagnosis of diabetes while on medical therapy with a known diabetogenic medication and pancreatic failure.
The initial study proforma collected information about the child, clinical presentation, diagnostic details and management. Clinicians were asked to provide evidence of comorbidities including hypertension (defined as systolic blood pressure above the 95th centile for sex, age and height centile 15), renal disease (presence of macro/microalbuminuria), polycystic ovarian syndrome (evidenced by oligo or secondary amenorrhoea, ultrasonography or a biochemical picture of luteinizing hormone:follicle‐stimulating hormone ratio >3, low sex hormone binding globulin) and non‐alcholic fatty liver disease (alanine aminotransferase >50 mg/dl for boys and >44 mg/dl for girls in obese children or ultrasonography indicating hepatic steatosis with other aetiologies excluded 16). A section allowed clinicians to report other relevant clinical features. A follow‐up proforma was sent 1 year after diagnosis. Case eligibility was determined on review of the questionnaire by the investigators (T.C., J.H.S and T.B.).
The methodology was the same used with the BPSU study in 2004–2005 6 to allow direct comparison.
Ethics approval
This study was approved by the National Research Ethics Service Committee South West, Central Bristol, UK [14/SW/1143] and by the Health Research Authority [15/CAG/0102].
Statistical analysis
The incidence, defined as number of new cases per population over a given period (usually 1 year), was calculated using valid cases reported between May 2015 and April 2016 (12 consecutive months). The population denominator was obtained from the ‘Population Estimates Summary for the UK, Mid‐2015’ table, published by the Office for National Statistics (http://www.ons.gov.uk). Ethnicity‐specific incidence rates were calculated for English and Welsh children only using the ‘Ethnic Group by Age in England and Wales 2011’ data (http://www.ons.gov.uk). Data for ethnicity by age were only available for these two countries. The 95% CIs for incidence rates were calculated using the exact Poisson method 17. Poisson regression models were used to compare the incidences rates in the different ethnic groups. Each ethnic group was compared with the white ethnicity, baseline group, and the P values from the Wald test are reported in Table 1. BMI z‐scores (sd scores) at diagnosis were calculated from weight and height, age and gender using the 1990 UK growth standard curves 18. For each ethnic group, we reported the mean difference in BMI sd score from the white ethnic group, as the baseline group, 95% CI, and the Wald test P value (Table 2). Overweight and obesity were defined as described in Cole et al. 19 as follows: sd scores ≥1.30 and ≥2.37, for boys and ≥1.19 and ≥2.25 for girls, respectively. Simple linear regression models were used to compare the BMI sd scores according to ethnic group. Because the BMI sd scores are adjusted, by definition, for age and gender, the resulting estimates consider any potential confounding effects of these variables when comparing the BMI across the ethnic groups.
Table 1.
Incidence rates for Type 2 diabetes in children aged 0–16 years in England and Wales, according to ethnic group
| Group | Ethnicity | Population | No. cases | Incidence ratea, b | 95% CI | P value (Wald test) |
|---|---|---|---|---|---|---|
| 1 | All Ethnicities | 11 274 750 | 88 | 0.78 | 0.63–0.96 | |
| White: total | 8 921 552 | 39 | 0.44 | 0.31–0.60 | Baseline group | |
| 2 | Mixed: total | 577 065 | <5 | 0.52 | 0.11–1.52 | 0.772 |
| White and black | 84 471 | <5 | 2.37 | 0.29–8.55 | 0.020 | |
| Other mixed | 124 917 | <5 | 0.80 | 0.02–4.46 | 0.550 | |
| 3 | Asian: total | 1 096 304 | 32 | 2.92 | 2.00–4.12 | 2*10‐15 |
| Indian | 287 027 | <5 | 1.05 | 0.22–3.05 | 0.146 | |
| Pakistani | 388 889 | 15 | 3.86 | 2.16–6.36 | 8*10‐13 | |
| Bangladeshi | 163 308 | 8 | 4.90 | 2.11–9.65 | 5*10‐10 | |
| Other Asian | 201 002 | 6 | 2.99 | 1.10–6.50 | 1*10‐5 | |
| 4 | BACBB: total | 537 938 | 9 | 1.67 | 0.77–3.18 | 3*10‐4 |
| Caribbean | 111 569 | <5 | 1.79 | 0.22–6.48 | 0.052 | |
| African | 314 374 | 5 | 1.59 | 0.52–3.71 | 0.007 | |
| Other Black | 111 996 | <5 | 1.79 | 0.22–6.45 | 0.052 | |
| 5 | Other: total | 141 891 | <5 | 0.70 | 0.02–3.93 | 0.637 |
| Unknown ethnicity | <5 |
Rates are presented per 100 000/year.
Poisson regression model was used to compare the incidence rates of the ethnicity groups (labeled 1–5 in the first column). Each ethnic group and subgroup was compared with group 1, as the baseline group. There was strong evidence that groups 3 (and all its subgroups except those of Indian origin) and 4 had a higher rate than group 1. For black/African/Caribbean/black British there was evidence of a higher rate than group 1. Whereas there was no evidence that groups 2 and 5 were differed from 1.
Table 2.
Comparisons of average BMI z‐scores for Type 2 diabetes in children aged 0–16 years from different ethnic groups in the UK
| Group | Ethnicity | Mean BMI z‐scorea, b | Difference from baseline (95%CI)c | P value (Wald test) |
|---|---|---|---|---|
| 1 | White | 3.07 | 0 | Baseline group |
| 2 | Mixed | 3.50 | 0.43 (–0.23, 1.10) | 0.199 |
| 3 | Asiand | 2.54 | –0.53 (–0.81, –0.24) | 4*10‐4 |
| 4 | BACBB | 3.08 | 0.00 (–0.38, 0.39) | 0.968 |
| 5 | Other | 2.76 | –0.31 (–0.91, 0.30) | 0.315 |
Linear regression model was used to compare the BMI z‐score of the ethnic groups, labelled 1–5 in the first column, with group 1 (those whose ethnicity was defined as white), as the baseline group.
The estimated mean of BMI z‐score for each ethnic group.
The estimated difference (and 95% confidence interval of difference) in means between the corresponding group, reported in the first column, and the baseline group.
There were a strong evidence that the Asian group had a lower average of BMI z‐scores than the baseline group. It was estimated that a child with Type 2 diabetes whose ethnic group defined as ‘Asian’ had, on average, 0.53 (95% CI 0.24–0.81) lower BMI z‐score than a white child with Type 2 diabetes. There was no evidence that other groups were differed from the baseline group.
Overall UK incidence, gender‐specific and ethnic‐specific incidence rates for 2015 were compared with the corresponding rates for 2005 using incidence rate ratio, defined as the ratio of incidence in 2015 to 2005 (with null value equal to 1). P values computed from the z‐test for log‐ratio of incidence rates are reported in Table 3. For comparisons of ethnic group‐specific rates, findings were based on the England figures only, to be comparable with 2005 results. R Statistical software (version 3.3.2) were used for the analysis.
Table 3.
Comparisons between 2005 and 2015 UK incidence rates for Type 2 diabetes in children aged 0–16 years for whole population and for populations classified by gender and ethnicity
| 2005 | 2015 | Ratio of 2015/2005 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Population | No. of cases | IR* | 95% CI | Population | No. of cases | IR | 95% CI | IRR | 95% CI | P value (Z‐test)b | |
| Total | 12 495 153 | 67 | 0.53 | 0.41–0.68 | 13 008 432 | 94 | 0.72 | 0.58–0.88 | 1.35 | 0.99–1.84 | 0.062 |
| Gender | |||||||||||
| Females | 6 098 516 | 38 | 0.62 | 0.44–0.86 | 6 345 915 | 62 | 0.98 | 0.75–1.25 | 1.57 | 1.05–2.35 | 0.029 |
| Males | 6 396 637 | 29 | 0.45 | 0.30–0.65 | 6 662 517 | 32 | 0.48 | 0.33–0.68 | 1.06 | 0.64–1.75 | 0.822 |
| Ethnicity† | |||||||||||
| White | 10 857 143 | 38 | 0.35 | 0.20–0.50 | 8 366 173 | 36 | 0.43 | 0.30–0.60 | 1.23 | 0.78–1.94 | 0.374 |
| BACBB | 333 333 | 13 | 3.90 | 2.10–6.70 | 533 457 | 9 | 1.69 | 0.77–3.20 | 0.43 | 0.18–1.01 | 0.053 |
| South Asiansa | 800 000 | 10 | 1.25 | 0.60–2.40 | 827 786 | 26 | 3.14 | 2.05–4.60 | 2.51 | 1.21–5.21 | 0.013 |
IR, incidence rate; IRR, incidence rate ratio (2015 rate/2005 rate).
*Rates are presented per 100 000/year. †Ethnicity comparisons were based on England figures only to be comparable with 2005 findings. Rates are presented per 100 000/year.
South‐Asian ethnicity includes only the Indian, Pakistani and Bangladeshi ethnic groups to follow those of the 2005 study methodology.
P values were computed by using the Z‐test with the log‐ratio of the incidence rates.
The descriptive statistics included all 106 cases (UK and Republic of Ireland).
Results
A total of 155 case notifications were received during the 13‐month study period. The mean monthly BPSU card return compliance rate was 92%. For the 155 notifications, 148 questionnaires (95%) were received (Fig. 1), 29 cases were excluded (eight were diagnosed outside the study period, in seven cases another type of diabetes was diagnosed, in five cases the individual was aged >17 years at diagnosis, and nine cases did not meet the criteria for diabetes or the diabetes was associated with known associated syndrome/medical treatment, or was made outside the UK/Republic of Ireland). After case review, 119 cases met the diagnostic criteria for Type 2 diabetes. Thirteen of these were excluded, as duplicate cases (two clinicians reporting cases with the same, unique National Health Service identification number). A total of 106 cases were identified as newly confirmed (104 from the UK, two from the Republic of Ireland).
Figure 1.
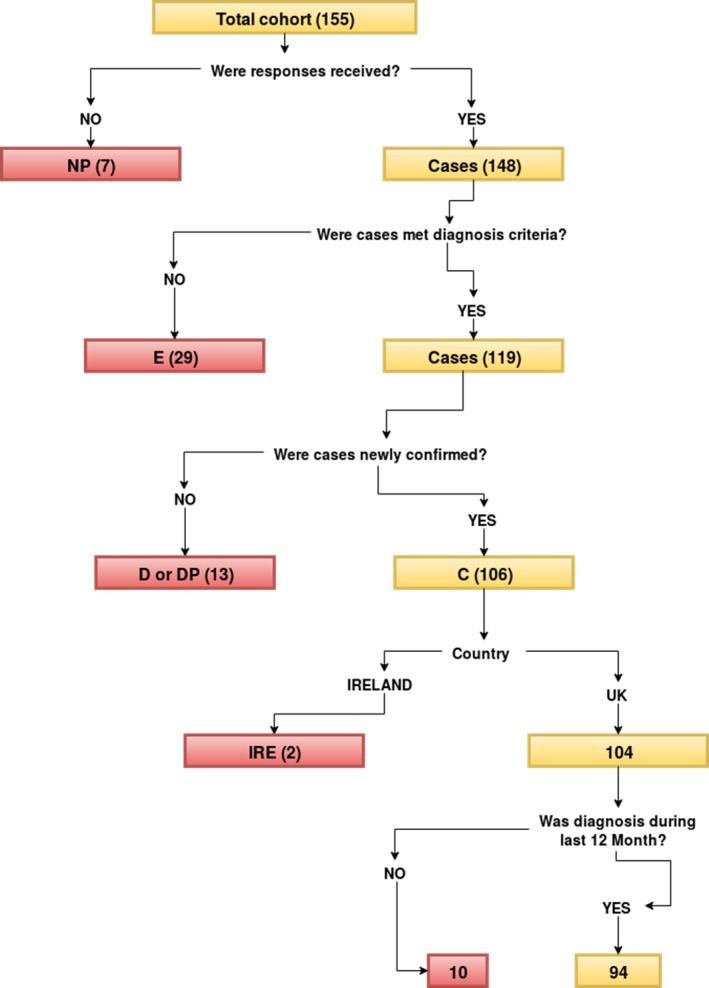
Flowchart of cases’ classification. D, Duplicate report; DP, Duplicate positive (i.e. duplicate of confirmed case); IRE, Republic of Ireland; NP, No response received
In all, 67% of cases were in girls (71/106). The median (range) age at diagnosis was 14.3 (7.9–16.9) years [girls: 14.2 (7.9–16.9) years; boys 14.5 (9.9–16.7) years]. 44% of the cases the children were white, in 4% of cases the ethnic group was uncertain and 52% of the cases were in ethnic minority groups, predominantly Asian/Asian‐British (65%) and BACBB (25%). Of the total cohort, 34% were Asian or Asian‐British (36 cases) and 13% were BACBB (14 cases).
The mean BMI sd score at diagnosis was 2.89 (girls, 2.88; boys, 2.92). Of the 106 cases, 102 (96%) were at least overweight and 81% (86/106) were obese. A total of 2% had a normal BMI and 2% had no BMI measured. Comparisons of BMI at diagnosis for each ethnic group are shown in Fig. 2, and Table 2 shows comparisons of the mean BMI by ethnicity, compared with white children. Asian children had a lower BMI at presentation than white children (P <0.001; Table 2).
Figure 2.
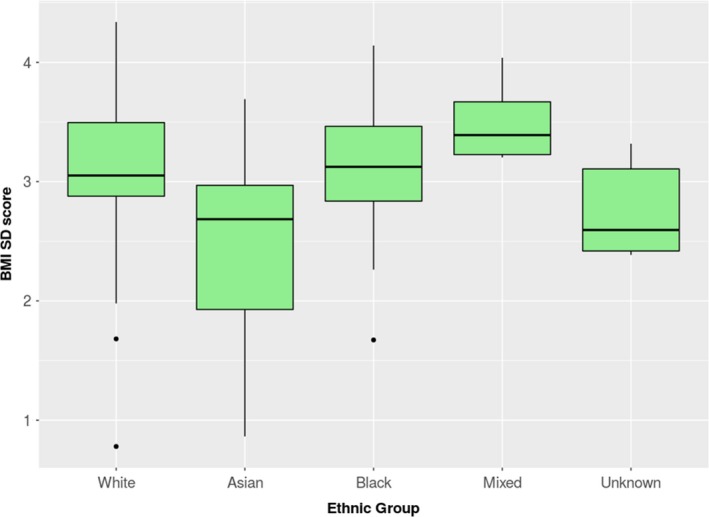
Box‐plots for the BMI z‐scores (sd scores) of children aged 0–16 years in UK, grouped by their ethnicity
A family history of Type 2 diabetes was present in 81% of cases, with a first‐degree relative affected in 70% of cases (17% both parents affected, 50% mother alone affected, 23% father alone affected, 10% sibling alone affected) and a second‐degree relative in 11%.
In 35% of cases (37/106) the child was asymptomatic at diagnosis, with diagnosis being made after an incidental glucose finding or during a clinical assessment for obesity‐related comorbidities. In 53% of cases (56/106), osmotic symptoms suggestive of diabetes were present (either polyuria, polydipsia, nocturia or weight loss). Recurrent infections were reported in 18% of cases (19/106), with genital/perineal infections being the most common (47%). Other infections included cellulitis/skin abscesses (26%) and urinary tract infections (11%). Lethargy was reported in 15% of the children (16/106). Half of all cases had evidence of ketonuria at diagnosis. Fewer than five cases presented with diabetic ketoacidosis or a hyperosmolar hyperglycaemic state.
At diagnosis, non‐alcoholic fatty liver disease was reported in 37%, hypertension in 21%, dyslipidaemia in 9%, renal disease in 3% and psychological comorbidities in 2% of cases.
A total of 104 newly confirmed cases were from the UK (excluding two from Republic of Ireland), of which 94 were diagnosed in the last consecutive 12 months of the study (May 2015 to April 2016) and were used for calculations of incidence. The estimated UK population aged <17 years in mid‐2015 was 13 008 432 (http://www.ons.gov.uk), resulting in a national UK incidence of Type 2 diabetes in children aged 0–16 years of 0.72 per 100 000 (95% CI 0.58–0.88). For the ethnic breakdown of incidence, data from England and Wales were available. When compared with white children [0.44 per 100 000 (95% CI 0.31–0.60)], there was strong evidence that Asian [2.92 per 100 000 (95% CI 2.00–4.12); P <0.001] and BACBB children [1.67 per 100 000 (95% CI 0.77–3.18); P <0.001] children had a higher incidence of Type 2 diabetes (Table 1).
Comparing 2005 with 2015 data, there was a trend towards increased incidence of Type 2 diabetes (incidence rate ratio 1.35, 95% CI 0.99–1.84), although this was not statistically significant (P=0.062; Table 3). There was statistical evidence that the incidence had increased among girls (P=0.03) and South‐Asian children (P=0.01) over the decade (Table 3).
Discussion
In the UK, the incidence of Type 2 diabetes among girls and South‐Asian children is increasing. The first study to quantify accurately the incidence in the UK was in 2005 6, using a similar methodology to that used in the present study and thereby allowing comparison a decade later. Sixty‐seven cases were reported in 2004–2005, while the present study identified 106 cases, with incidence rising from 0.53 per 100 000 (95% CI 0.41–0.68) in 2004–2005 to 0.72 per 100 000 (95% CI 0.58–0.88). The incidence in white children in 2004–2005 was 0.35 per 100 000 (95% CI 0.2–0.5) and in 2015–2016 it was 0.43 per 100 000 (95% CI 0.30–0.58), demonstrating a modest increase. Generally, the higher national incidence is a result of significantly higher rates among ethnic minorities, despite Asian and BACBB children accounting for 9.7% and 4.8% of the population in England and Wales respectively. Compared with white children, Asian children had an incidence nearly seven times and BACBB children nearly four times greater. The rise in cases among Asian children means that they represent a higher proportion of cases compared with BACBB children, while previously the reverse was described 6. Previous estimates of the prevalence of Type 2 diabetes among young people of Asian ethnicity may be underestimates because a proportion were initially misdiagnosed as having Type 1 diabetes 20. The UK incidence is still lower than in the USA 4, Japan and Taiwan 3 but higher than in previous studies from Europe 3. The higher incidence in ethnic minority groups has been described in many previous studies 4. It is notable that only one case in 2005 and two cases from 2015 were reported from the Republic of Ireland. It could be speculated that this relates to the less diverse ethnic background of the citizens in that country.
A major driver of the development of Type 2 diabetes is obesity. Most children in our cohort were overweight or obese. With 53% of the cases presenting with osmotic symptoms including weight loss, the BMI recorded at diagnosis may be lower than earlier in the disease process. Asian children present with Type 2 diabetes at a significantly lower BMI than do white children, which has been described previously 21 and may be related to ethnic differences in visceral fat distribution which corresponds to increased metabolic risk. Obesity prevalence in the UK has increased dramatically over the last 20 years. Childhood obesity increased between 1994 and 2003, although the overall trend stabilized between 2004 and 2013. In the 11–14‐year age group, however, there was a continued trend in increased obesity albeit at a slower rate than the previous decade 22. The increased incidence of Type 2 diabetes may not be solely explained by rising obesity rates but may also be attributable to greater awareness of the disease in the paediatric population, changing demographics in the UK and better screening for comorbidities related to obesity.
Family history of Type 2 diabetes clearly plays a role in developing the condition in childhood. The interplay between genetic predisposition and obesogenic environment (which families often share) can be difficult to characterize. Impaired glucose handling is seen more frequently in non‐obese children with a family history of Type 2 diabetes than in those with no family history 23, suggesting the importance of genetic predisposition. Two‐thirds of the cases were female, significantly higher than in 2005 6. This female preponderance has been described previously 10 and the UK National Paediatric Diabetes Audit reported a female: male ratio of ~2:1 7. Girls tend to reach puberty earlier and puberty is associated with fat accumulation. This may contribute to greater insulin resistance in girls than in boys of a similar age. Physical activity levels also tend to be lower among adolescent girls than boys 24 and this may be detrimental to metabolic health.
The present study suggests the importance of screening for Type 2 diabetes in obese individuals, as 35% of our cases were asymptomatic at diagnosis. UK national guidelines recommend assessment (including consideration of measurement of fasting glucose and insulin, HbA1c and an oral glucose tolerance test) of comorbidities related to obesity, including diabetes in those above the 98th centile for BMI 25. Recurrent infections, especially those affecting the genitalia, were the most common presentation after osmotic symptoms. We advocate screening for Type 2 diabetes in overweight children presenting with recurrent perineal or cutaneous infections. Ketoacidosis is a rare presentation in Type 2 diabetes but does occur, as evidenced by cases in our cohort.
Non‐alcoholic fatty liver disease was the most commonly reported comorbidity (37%) and has previously been reported in approximately half of adolescents with Type 2 diabetes 26. A recent study in children and adolescents with non‐alcoholic fatty liver disease reported a prevalence of impaired glucose tolerance of 23.4% and of Type 2 diabetes of 6.5% 27. Our finding of hypertension in 21% of children at diagnosis is consistent with other studies, which have reported rates of hypertension in adolescents of 10–55% at diagnosis 28, 29, although it was lower than the 34% rate reported in the 2005 cohort 30. In 11 cases no blood pressure data were available, therefore, cases of hypertension may have been missed. Our observation of renal disease in 3% of cases is low compared with previous studies 29, which have reported rates of 7–22% of microalbuminuria at diagnosis in adolescents. This relatively small number of cases with renal disease is similar to the number observed in the 2005 cohort, in which early nephropathy was rare (4%) 30. This may reflect either a lack of screening for renal disease by clinicians, as 32% of cases did not report data on albuminuria, earlier diagnosis of diabetes, or a lower incidence of microalbuminuria. Certainly, as the rate of progression of microalbuminuria and renal disease is rapid in adolescents with Type 2 diabetes, with 28% prevalence of microalbuminuria reported at 1.3 years after diagnosis 29, careful screening and monitoring is crucial. Follow‐up data on this cohort will give valuable insights into any progression and change in prevalence of nephropathy.
Surveys relying on clinicians reporting data have the potential to underestimate the true incidence of a disease. Clinical information was available from 95% of notifications, thus there was only a small proportion (5%) of missing data resulting from incomplete reporting. Over the study period, there was a mean monthly orange card return of 92%, leaving potential for cases not being reported. There was a mean monthly case notification card return of 93% in the study in 2005 6; therefore, the potential for error was similar in the two studies. It is recommended that children with diabetes are managed in secondary care, however, it is possible that some young people, especially those aged 16–17 years, with Type 2 diabetes are managed in primary care. These cases would not be detected by the BPSU reporting system.
Our data suggest the number of cases of childhood Type 2 diabetes is rising in the UK, although Type 2 diabetes in children is still unusual compared Type 1 diabetes 7. Female gender, non‐white ethnicity and a family history of Type 2 diabetes were strongly associated with the disease. Follow‐up data from the present cohort will characterize the management, clinical course and development of comorbidities in this population. Further work is needed to prevent the development of Type 2 diabetes and to understand the processes leading to Type 2 diabetes developing amongst children with obesity. The long‐term health and economic implications of the rising incidence in the UK will become evident in the coming decades, although this should be of great concern to clinicians and policy makers.
Funding sources
The study was funded by a grant from the National Institute for Health Research Translational Research Collaboration for Rare Diseases (NIHR RD‐TRC).
Competing interests
None declared.
Acknowledgements
Data on Birmingham children were collected with the support of the NIHR Wellcome Clinical Research Facility staff.
Disclaimer
This is an independent opinion from a Biomedical Research Unit (now Centre) in the NIHR Biomedical Research Centre and Unit Funding Scheme. The views expressed in this publication are those of the authors and not necessarily those of the National Health Service, the NIHR or the Department of Health.
Diabet. Med. 35, 737–744 (2018)
References
- 1. Zimmet P, Alberti KGMM, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001; 414: 782–787. [DOI] [PubMed] [Google Scholar]
- 2. Sharma M, Nazareth I, Petersen I. Trends in incidence, prevalence and prescribing in type 2 diabetes mellitus between 2000 and 2013 in primary care: a retrospective cohort study. BMJ Open 2016; 6: e010210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fazeli Farsani S, Van Der Aa MP, Van Der Vorst MMJ, Knibbe CAJ, De Boer A. Global trends in the incidence and prevalence of type 2 diabetes in children and adolescents: A systematic review and evaluation of methodological approaches. Diabetologia 2013; 56: 1471–1488. [DOI] [PubMed] [Google Scholar]
- 4. Mayer‐Davis EJ, Lawrence JM, Dabelea D, Divers J, Isom S, Dolan L et al Incidence Trends of Type 1 and Type 2 Diabetes among Youths, 2002–2012. N Engl J Med 2017; 376: 1419–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ehtisham S, Hattersley AT, Dunger DB, Barrett TG. First UK survey of paediatric type 2 diabetes and MODY. Arch Dis Child 2004; 89: 526–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Haines L, Wan KC, Lynn R, Barrett TG. Shield JPH. Rising incidence of type 2 diabetes in children in the U.K. Diabetes Care 2007; 30: 1097–1101. [DOI] [PubMed] [Google Scholar]
- 7. Royal College of Paediatrics and Child Health . National Paediatric Diabetes Audit 2015–2016 Report 1: Care Processes and Outcomes, National Paediatric Diabetes Audit Parent and carer report, February 2017. Available at https://www.rcpch.ac.uk/improving-child-health/quality-improvement-and-clinical-audit/national-paediatric-diabetes-audit-n-0#2015-16 Last accessed 28 February 2018.
- 8. Wilmot EG, Davies MJ, Yates T, Benhalima K, Lawrence IG, Khunti K. Type 2 diabetes in younger adults: the emerging UK epidemic. Postgrad Med J 2010; 86: 711–718. [DOI] [PubMed] [Google Scholar]
- 9. Liu LL, Lawrence JM, Davis C, Liese AD, Pettitt DJ, Pihoker C et al Prevalence of overweight and obesity in youth with diabetes in USA: The SEARCH for Diabetes in Youth Study. Pediatr Diabetes 2010; 11: 4–11. [DOI] [PubMed] [Google Scholar]
- 10. Wilmot E, Idris I. Early onset type 2 diabetes: risk factors, clinical impact and management. Ther Adv Chronic Dis 2014; 5: 234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pinhas‐Hamiel O, Zeitler P. Acute and chronic complications of type 2 diabetes mellitus in children and adolescents. Lancet 2007; 369: 1823–1831. [DOI] [PubMed] [Google Scholar]
- 12. Association AD. Type 2 Diabetes in Children and Adolescents. Pediatrics 2000; 105: 671–680. [DOI] [PubMed] [Google Scholar]
- 13. Williams CL, Hayman LL, Daniels SR, Robinson TN, Steinberger J, Paridon S et al Cardiovascular health in childhood: A statement for health professionals from the Committee on Atherosclerosis, Hypertension, and Obesity in the Young (AHOY) of the Council on Cardiovascular Disease in the Young. American Heart Association. Circulation 2002; 106: 143–160. [DOI] [PubMed] [Google Scholar]
- 14. Jones AG, Hattersley AT. The clinical utility of C‐peptide measurement in the care of patients with diabetes. Diabet Med 2013; 30: 803–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. BeAssociation IPH . Blood Pressure Levels for Boys and Girls by Age and Height Percentile ‐ child_tbl.pdf. 2011; 4. Available at https://www.nhlbi.nih.gov/files/docs/guidelines/child_tbl.pdf Last accessed 28 February 2018.
- 16. Vos MB, Abrams SH, Barlow SE, Caprio S, Daniels SR, Kohli R et al NASPGHAN Clinical Practice Guideline for the Diagnosis and Treatment of Nonalcoholic Fatty Liver Disease in Children. J Pediatric Gastroenterol Nutr 2016; 64: 319–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ulm K. A simple method to calculate the confidence interval of a standardized mortality ratio (SMR). Am J Epidemiol 1990; 131: 373–375. [DOI] [PubMed] [Google Scholar]
- 18. Cole TJ, Freeman JV, Preece MA. Body mass index reference curves for the UK, 1990. Arch Dis Child 1995; 73: 25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 2000; 320: 1240–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Harron KL, Feltbower RG, McKinney PA, Bodansky HJ, Campbell FM, Parslow RC. Rising rates of all types of diabetes in south Asian and non‐south Asian children and young people aged 0‐29 years in West Yorkshire, U.K., 1991‐2006. Diabetes Care 2011; 34: 652–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nakagami T, Qiao Q, Carstensen B, Nhr‐Hansen C, Hu G, Tuomilehto J et al Age, body mass index and Type 2 diabetes‐associations modified by ethnicity. Diabetologia 2003; 46: 1063–1070. [DOI] [PubMed] [Google Scholar]
- 22. van Jaarsveld CHM, Gulliford MC. Childhood obesity trends from primary care electronic health records in England between 1994 and 2013: population‐based cohort study. Arch Dis Child 2015; 100: 214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rodríguez‐Moran M, Guerrero‐Romero F, Aradillas‐García C, Violante R, Simental‐Mendia LE et al Obesity and family history of diabetes as risk factors of impaired fasting glucose: implications for the early detection of prediabetes. Pediatr Diabetes 2010; 11: 331–336. [DOI] [PubMed] [Google Scholar]
- 24. Sallis JF, Prochaska JJ, Taylor WC. A review of correlates of physical activity of children and adolescents. Med Sci Sports Exerc 2000; 32: 963–975. [DOI] [PubMed] [Google Scholar]
- 25. Viner RM, White B, Barrett T, Candy DCA, Gibson P, Gregory JW et al Assessment of childhood obesity in secondary care: OSCA consensus statement. Arch Dis Child Educ Pract 2012; 97: 98–105. [DOI] [PubMed] [Google Scholar]
- 26. Bloomgarden ZT. Nonalcoholic Fatty Liver Disease and Insulin Resistance in Youth. Diabetes Care 2007; 30: 1663, LP–1669. [DOI] [PubMed] [Google Scholar]
- 27. Newton KP, Hou J, Crimmins NA, Lavine JE, Barlow SE, Xanthakos SA et al Prevalence of prediabetes and type 2 diabetes in children with nonalcoholic fatty liver disease. JAMA Pediatr 2016; 170: e161971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zdravkovic V, Daneman D, Hamilton J. Presentation and course of Type 2 diabetes in youth in a large multi‐ethnic city. Diabet Med 2004; 21: 1144–1148. [DOI] [PubMed] [Google Scholar]
- 29. Eppens MC, Craig ME, Cusumano J, Hing S, Chan AKF, Howard NJ et al Prevalence of diabetes complications in adolescents with type 2 compared with type 1 diabetes. Diabetes Care 2006; 29: 1300–1306. [DOI] [PubMed] [Google Scholar]
- 30. Shield JPH, Lynn R, Wan KC, Haines L, Barrett TG. Management and 1 year outcome for UK children with type 2 diabetes. Arch Dis Child 2009; 94: 206–209. [DOI] [PubMed] [Google Scholar]


