Abstract
It was examined how ventral striatum responses to rewards develop across adolescence and early adulthood and how individual differences in state‐ and trait‐level reward sensitivity are related to these changes. Participants (aged 8–29 years) were tested across three waves separated by 2 years (693 functional MRI scans) in an accelerated longitudinal design. The results confirmed an adolescent peak in reward‐related ventral striatum, specifically nucleus accumbens, activity. In early to mid‐adolescence, increases in reward activation were related to trait‐level reward drive. In mid‐adolescence to early adulthood decreases in reward activation were related to decreases in state‐level hedonic reward pleasure. This study demonstrates that state‐ and trait‐level reward sensitivity account for reward‐related ventral striatum activity in different phases of adolescence and early adulthood.
Short abstract
The title for this Special Section is The Developing Brain: Evidence for Plasticity during Childhood and Adolescence, edited by Amanda E. Guyer, Koraly Pérez‐Edgar, and Eveline A. Crone
Adolescence has often been described as a period of exploration and novelty seeking (Hauser, Iannaccone, Walitza, Brandeis, & Brem, 2015). On the one hand, novelty seeking can lead to increased risk‐taking behavior, which might have potentially damaging health consequences (Dahl, 2004). On the other hand, novelty seeking is an important aspect of normal explorative behavior with positive outcomes, such as seeking out new friendships (Telzer, 2016), and contributes to behavioral flexibility and greater learning (Crone & Dahl, 2012). An important factor that drives novelty seeking and explorative behavior in adolescence is reward sensitivity (Abler, Walter, Erk, Kammerer, & Spitzer, 2006; Demaree, DeDonno, Burns, & Erik Everhart, 2008; Hawes et al., 2017; Telzer, 2016; Van Duijvenvoorde, Peters, Braams, & Crone, 2016). Increases in reward sensitivity in adolescence have been explained in terms of asynchronous development of subcortical brain regions, including the ventral striatum and amygdala, relative to cortical brain regions (Casey, Galván, & Somerville, 2016; Ernst & Fudge, 2009). Prior studies have demonstrated that reward sensitivity is linked to ventral striatum activity in adolescence, but how reward sensitivity relates to neural activity patterns across adolescent development is not yet well understood (e.g., Braams, Van Duijvenvoorde, Peper, & Crone, 2015; Urošević, Collins, Muetzel, Lim, & Luciana, 2012). This three‐wave longitudinal study set out to examine the relation between state‐ and trait‐level reward sensitivity and neural activity in response to reward outcomes in the ventral striatum across adolescence.
Several recent studies have examined ventral striatum activity to rewards across developmental periods. In particular, the nucleus accumbens (NAcc) of the ventral striatum has been shown to be involved in reward processing across a variety of domains, such as gaining money, social status, or positive social feedback (Bhanji & Delgado, 2014; Izuma, Saito, & Sadato, 2008; Liu, Hairston, Schrier, & Fan, 2011; Sescousse, Caldú, Segura, & Dreher, 2013). Several empirical studies have demonstrated that the ventral striatum is more active in adolescents than in children and adults when receiving rewards in gambling tasks (Galvan et al., 2006; Van Leijenhorst, Zanolie, et al., 2010), with a peak in reward‐related activity around age 16–17 years (Braams et al., 2015; Silverman, Jedd, & Luciana, 2015), although inconsistent findings have been reported as well (see review by Galvan, 2010). We aimed to confirm the adolescent peak in NAcc reward activation in a follow‐up study of Braams et al. (2015), which included two data waves of this study. We extended these analyses using three data waves and thereby examined the transition into young adulthood using a within‐person design. We also sought to determine how state‐ and trait‐level reward sensitivity levels related to increases in reward‐related NAcc activity across early and mid‐adolescence and declines in NAcc activity across late adolescence and early adulthood.
Several prior studies suggested that the NAcc plays an important role in adolescents’ tendency to seek out rewarding and exciting experiences (Telzer, 2016; Van Duijvenvoorde et al., 2016). In previous studies it was shown that dopamine release from the ventral striatum, especially from the NAcc, is involved in the hedonic impact or the pleasure experienced in rewarding situations (Telzer, 2016; Wahlstrom, White, & Luciana, 2010). Hence, one type of behavioral reward sensitivity that may be involved in age‐related changes in reward‐related ventral striatum activation is the pleasure people experience when receiving rewards. This type of reward sensitivity was previously related to the actual rewards obtained (Telzer, 2016; Wahlstrom et al., 2010) and is therefore henceforth referred to as state‐level reward sensitivity. Another type of reward sensitivity that may be associated with age‐related changes in reward‐related ventral striatum activation is individuals’ general motivation to approach rewards (Carver & White, 1994). Increased ventral striatum activation to rewards has been associated with higher reward drive, that is the drive to pursue rewards or to achieve a goal (Braams et al., 2015), and more fun‐seeking tendencies (Van Duijvenvoorde et al., 2014). In addition, a decline in NAcc volume in late adolescence, which is posed to be related to a lower density of synapses or less pruning, has been associated with a decrease in the tendency to approach rewards (Urošević et al., 2012). This type of reward sensitivity relates to someone's general tendency to seek out rewards and is henceforth referred to as trait‐level reward sensitivity. In this study, we examined how behavioral state‐ and trait‐level reward sensitivity (i.e., pleasure derived from obtaining task‐specific rewards and general desire to obtain rewards, respectively) contribute to fluctuations in NAcc reward sensitivity.
We tested these questions using functional MRI (fMRI) with an accelerated longitudinal design with three time points, each separated by 2 years. Results of the first and second time point of this study are reported in Braams, Peters, Peper, Güroğlu, and Crone (2014) and Braams et al. (2015). We acquired functional scans of NAcc responses to rewards versus losses when participants (8–29 years of age) played a gambling task that involved making a heads‐or‐tails guess with 50% chance of winning. State‐level reward sensitivity was measured using self‐reports of how much participants enjoyed winning and losing in the fMRI task, and trait‐level reward sensitivity was measured using the Behavioral Activation System (BAS) scale (Carver & White, 1994). There are currently no studies that have examined changes in ventral striatum reward sensitivity with a design including more than two time points (Braams et al., 2015; Lamm et al., 2014), and to our knowledge, no studies have focused on the decline in NAcc activity in early adulthood. On the basis of prior findings, we hypothesized that reward‐related NAcc activation peaks in mid‐adolescence (Braams et al., 2015; Silverman et al., 2015). We further expected a positive relation between NAcc activity and state‐level reward sensitivity (i.e., pleasure from winning; Dohmen, Falk, Fliessbach, Sunde, & Weber, 2011) and trait‐level reward sensitivity (i.e., general motivation to approach rewards; Simon et al., 2010). On the basis of prior studies, we specifically expected positive relations between the trait‐level drive to pursue rewards and personal goals (measured with the BAS Drive scale), and fun‐seeking tendencies (measured with the BAS Fun Seeking scale; Braams et al., 2015; Van Duijvenvoorde et al., 2014). Specifically, we tested whether these two types of behavioral reward sensitivity measures accounted for the increase in NAcc response from early to mid‐adolescence and the decrease in NAcc response from mid‐ to late adolescence and adulthood. As such, the findings will provide insights in the underlying mechanisms involved in age‐related differences in explorative behaviors across adolescence and early adulthood.
Method
Participants
This study is part of the Braintime longitudinal study, which has been conducted at Leiden University in 2011, 2013, and 2015. Data from the first and the second time points have been previously published (e.g., Braams, Güroğlu, et al., 2014; Braams, Peters, et al., 2014; Braams et al., 2015). At the first time point (T1) we collected data of 299 participants (M age = 13.98 years, SD age = 3.68 years, rangeage = 8.01–25.95 years; 153 female), at the second time point (T2) of 287 participants (M age = 15.84 years, SD age = 3.57 years, rangeage = 9.92–26.61 years; 149 females), and at the third time point (T3) of 275 participants (M age = 17.91 years, SD age = 3.68 years, rangeage = 11.94–28.72 years; 143 female). At T2 and T3 all participants who indicated to be willing to participate again were invited for participation. This meant that participants who did not participate at T2 could participate again at T3. At T2 and T3, 32 participants could not participate in the MRI session due to dental braces. From these participants, we obtained questionnaire measures (self‐report BAS and Pleasure from winning versus losing, described below). Participants’ estimated intelligence scores were obtained at T1 and T2 and these scores did not correlate with age (Braams et al., 2015). From all participants in our sample (N = 287), there were 235 (81.9%) participants with European parents and with at least three (out of four) European grandparents, and nine participants (3.1%) with European parents and with fewer than three European grandparents. The remaining participants (N = 27; 9.4%) were from diverse ethnic backgrounds, and from 16 participants (5.6%) data was missing.
There were 248 valid scans obtained for the analyses at T1, 226 valid scans at T2, and 219 scans at T3. Scans obtained at T2 and T3 of participants who had developed a neurological or psychiatric disorder at T2 and scans obtained at T3 of participants who had developed a disorder at T3 were excluded from the analyses. Table S1 provides a detailed overview of reasons for exclusion of the brain scans. We also excluded the self‐report data from participants with neuropsychological disorders.
Across the three waves of the study, there were in total 12 participants who did not participate at T2 (4 female, 8 male) and 19 participants who did not participate at T3 (6 female, 13 male). Those who participated at T2 were significantly younger at T1 than those who did not participate at T2 (M age = 13.8 and 15.6, respectively, p < .01), but there was no such effect when comparing those who participated at T3 and those who did not participate at T3 on age at T1 (p = .08). These two groups did not differ significantly on our outcome measures (described below): BAS Drive (T2: p = .50, T3: p = 1.00), BAS Fun Seeking (T2: p = .32, T3: p = .10), BAS Reward Responsiveness (T2: p = .40, T3: p = .88), and Pleasure from winning versus losing (T2: p = .46, T3: p = .16).
Procedure
Participants were scanned three times with a 2‐year interval (∆ in years T1–T2: M = 1.99, SD = 0.10; ∆ in years T2–T3: M = 2.02, SD = 0.09). All participants aged 18 years and older gave written consent for participation. Parents of participants under the age of 18 also provided their written consent and the under aged participants gave written assent. Before scanning, the participants were familiarized with the scanner environment using a mock scanner and practiced the fMRI task. Adult participants, participants 12–17 years of age, and participants under the age of 12 years received 60, 30, and 20€, respectively, for their participation. Participants could win a small additional endowment of 3–6€ when playing the fMRI task. Participants younger than 18 years received 10€ for filling out the questionnaires, and adult participants received 15€.
fMRI Task
Participants played a heads‐or‐tails gambling game in which they guessed heads or tails on each trial (Figure S1; also see Braams, Güroğlu, et al., 2014; Braams, Peters, et al., 2014; Braams et al., 2015). If they guessed correctly, they won coins, and if they guessed incorrectly they lost coins. Chances of winning were 50%. Participants were explained that the coins won in the task would translate to real money. See Supporting Information for a more detailed description of the task.
Pleasure From Winning Versus Losing
After the MRI session participants indicated how much pleasure they experienced when winning and losing coins during the task on an 11‐point scale ranging from 0 (I did not like winning/losing at all) to 10 (I really liked winning/losing). For the analyses, we used difference scores (Pleasure from winning versus losing) to keep this measure consistent with the fMRI‐contrast (NAcc activation during winning > losing). At T1, these two questions were administered to all adolescents, but not adults. At T2 and T3 all participants filled out these questions.
Participants indicated pleasure from winning and losing on an 11‐point scale ranging from 0 (I did not like winning/losing at all) to 10 (I really liked winning/losing). At T3, a sample of 28 participants received the same questions measuring pleasure with an 11‐point scale (M age = 24.22, SD = 0.59, 17 females), but the majority of the participants received the questions on a 10‐point scale (ranging from 1 to 10; 209 participants, 105 females‐, M age = 17.26, SD = 2.07) due to a program change. The results were similar with and without the group of 28 participants who received the questions with an 11‐point scale at T3. Therefore only the results with the complete sample are reported.
Behavioral Inhibition System/Behavioral Activation System
From the Behavioral Inhibition System/BAS scales, we used the BAS scales as a measure of reward sensitivity. The BAS scales contain 13 items and was administered to asses three different types of underlying motivations of behavior: Positive responsiveness to rewards (i.e., the affective response to rewards; BAS Reward Responsiveness), a desire for new rewards and the tendency to seek out for rewards (BAS Fun Seeking), and the drive to obtain rewards or to achieve a goal (BAS Drive; Carver & White, 1994). Participants indicated how well a statement described them on a 4‐point scale ranging from 1 (strongly agree) to 4 (strongly disagree). The scores are recoded such that a higher score indicated a higher sensitivity to rewards. In this study, we were specifically interested in the BAS Drive and BAS Fun Seeking subscales given prior evidence for their association with ventral striatum activation during adolescence (Braams et al., 2015; Van Duijvenvoorde et al., 2014). However, for completeness, we also included the BAS subscale Reward Responsiveness.
We also examined how BAS Drive, BAS Fun Seeking, and BAS Reward Responsiveness correlated with Pleasure from winning versus losing within T1, T2, and T3 using partial correlation analyses controlling for age. These analyses show that at T1, Pleasure from winning versus losing correlated positively with BAS Drive (r = .16, p = .01) and BAS Reward Responsiveness (r = .20, p < .01). At T2, Pleasure from winning versus losing correlated positively with BAS Drive (r = .16, p = .01) and BAS Fun Seeking (r = .18, p < .01). At T3, Pleasure from winning versus losing correlated positively with BAS Reward Responsiveness (r = .16, p = .02). There were no significant correlations between Pleasure from winning versus losing with BAS Fun Seeking at T1, BAS Reward Responsiveness at T2, and BAS Drive and BAS Fun Seeking at T3 (ps > .43).
MRI Data Acquisition
Scans were acquired with a 3T Philips Achieva MRI scanner (Leiden University Medical Center, Leiden, The Netherlands). The scanning procedure included a (a) localizer scan, (b) Blood oxygenation level dependent (BOLD) T2*‐weighted gradient echo planar images (repetition time [TR] = 2.2 s, echo time [TE] = 30 ms, sequential acquisition, 38 slices of 2.75 mm, field of view [FOV] = 220 × 220 × 114.7 mm), and a (c) anatomical 3D T1‐weighted image (TR = 9.754 ms, TE = 4.59 ms, 8° flip angle, 140 slices, 0.875 × 0.875 × 1.2 mm, and FOV = 224.000 × 168.000 × 177.333 mm). Two functional runs were obtained at T1 and T2. At T3, one functional run was obtained in which all trials were presented in the same run. The first two volumes of the functional scans were discarded to allow for equilibration of T1 saturation effects.
fMRI Data Analysis
The data were analyzed using SPM8 software (http://www.fil.ion.ucl.ac.uk/spm/). Preprocessing steps of functional images included realignment, slice‐time correction, and smoothing with a Gaussian filter of 6 mm full‐width at half maximum. Functional and structural images were spatially normalized to T1 templates. Templates were based on the Montreal Neurological Institute 305 stereotactic space. Statistical analyses were performed using the general linear model in SPM8 (Wellcome Trust Centre for Neuroimaging, University College London, Londen, The United Kingdom). Regressors were modeled as zero‐duration events at feedback onset and convolved with a canonical hemodynamic response function.
In this study, we investigated NAcc activation in the win > lose contrast when playing for self. We used an anatomical mask of the left and right NAcc thresholded at 40% from the Harvard‐Oxford subcortical atlas, which included 28 (left NAcc) and 26 voxels (right NAcc). The MarsBar toolbox (Brett, Anton, Valabregue, & Poline, 2002) was used to extract the parameter estimates of the left and right Nacc for our analyses (also see Braams et al., 2015). We focused on the NAcc, because this region has been highlighted as a core region in the ventral striatum involved in reward processing (Braams et al., 2015; Telzer, 2016), and because we aimed to explain age‐related changes in NAcc activity related to rewards reported in Braams et al. (2015).
Mixed‐Model Building Procedure
We used a mixed models approach in R for our analyses (R Core Team, 2014) using the nlme package (Pinheiro, Bates, DebRoy, Sarkar, & R Core Team, 2013). The first aim was to determine age‐related patterns (linear, quadratic, or cubic) of NAcc activation, Pleasure from winning versus losing, and BAS subscale scores (BAS Drive, BAS Fun Seeking, and BAS Reward Responsiveness). A linear relation between age and the outcome variable indicates an age‐related increase or decrease. A quadratic relation between age and the outcome variables indicates a nonlinear adolescent‐specific U or inverted U‐pattern. A cubic relation between age and the outcome variable indicates a nonlinear adolescent emerging or declining pattern. We used the variables of interest as dependent variables in the models and added age as a polynomial predictor, and since the data were nested within subjects, we used a random intercept for subjects (also see Braams et al., 2015). All models were fitted following a formal model‐fitting procedure (see also Braams et al., 2015), and we compared models with one degree of freedom difference. That is, we compared the null model (with a fixed and random intercept) with the linear model, the linear model with the quadratic model, and the cubic model with the quadratic model. We also investigated whether a main effect of sex or a Sex × Age interaction effect explained additional variance. Sex was dummy coded such that male participants were labeled as 1 and female participants as 0.
To test for the effects of individual differences in self‐reported state‐ and trait‐level reward sensitivity on NAcc activity, we investigated whether individual differences in BAS‐scores and Pleasure from winning versus losing were linearly associated with NAcc activity in separate multilevel models. We were specifically interested in testing whether these indices contributed differentially to the increase and decrease in NAcc activity across age. Therefore, the participants were separated in two age groups: adolescents younger than 16.0 years, and 16.0 years and older. The cut‐off of 16 years of age is based on an estimation of the age where NAcc activation peaks in our data (at 15.3 and 15.1 years of age for the left and right NAcc respectively). For these analyses, we again started with a null model, and then added the variable of interest as a linear predictor. In the next step, we compared this model with a model including both the variable of interest and age. We also tested whether a main effect of sex and an interaction effect between sex and the variable of interest explained additional variance. We used the Akaike information criterion (AIC; Akaike, 1974) to compare the model fits, and the log likelihood ratio to assess significance, but we also report the Bayesian information criterion (BIC; Schwarz, 1978). We reported the results with a significance threshold of p < .05. We also indicated which results survived a threshold corrected for multiple comparisons. We assessed these corrected thresholds using a method that accounts for dependency between different variables, for example, when variables are components of the same psychological construct (http://www.quantitativeskills.com/sisa/calculations/bonfer.htm; Perneger, 1998; Sankoh, Huque, & Dubey, 1997). We used a total of three constructs as independent variables: (a) NAcc activation, (b) the three BAS scales (Drive, Fun Seeking, and Reward Responsiveness), and (c) Pleasure from winning versus losing. To correct for multiple comparisons, we adjusted the most commonly used significance threshold of .05. We first calculated an adjusted significance threshold for the first two constructs accounting for the mean correlation of the variables within constructs (i.e., mean correlation of left and right NAcc activity within T1, T2, and T3 of .79, and of the three BAS scales within T1, T2, and T3 of .35). The adjusted significance threshold for analyses with NAcc activity as the dependent variable was .043, and with one of the BAS scales as the dependent variable was .024. The threshold for analyses in which Pleasure from winning versus losing was used as a dependent variable was set to .05. Next we divided these adjusted significance thresholds by three (i.e., the number of constructs). The resulting adjusted significance thresholds corrected for multiple testing were (a) .014 when left or right NAcc activity was the dependent variable, (b) .008 when BAS Drive, BAS Fun Seeking, or BAS Reward Responsiveness was the dependent variable, and (c) .017 when Pleasure from winning versus losing was the dependent variable.
Results
Age‐Related Patterns
For each measure (i.e., NAcc activation for winning > losing for the self, Pleasure from winning versus losing as state‐level reward sensitivity, and BAS scores as trait‐level reward sensitivity), we tested whether they showed a linear, quadratic, or cubic relation with age. We also tested whether sex explained additional variance. The intraclass correlations of these measures ranged from .21 to .61 (see Table 1). Information regarding the number of observations and participants’ ages in the analyses is listed in Table 1. Furthermore, information regarding the model‐fitting procedure (AIC and BIC values) is listed in Table 2, significance levels of the model comparisons are listed in Table S2, and the statistical parameters of the best fitting models are listed in Table 3. A visual representation of the raw data can be found in Figure S2.
Table 1.
For Each Measure, Number of Observations, Age Range, and Intraclass Correlations (ICC) With 95% Confidence Interval (95% CI) at Time 1, Time 2, and Time 3
| Dependent variable | N (females) | Age range (years) | ICC T1, T2, T3 | ||||
|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T1 | T2 | T3 | ICC (95% CI) | |
| Left NAcc win > lose | 248 (131) | 226 (112) | 219 (116) | 8.41–25.96 | 9.92–26.36 | 11.94–28.46 | .30 (.10, .46) |
| Right NAcc win > lose | 248 (131) | 226 (112) | 219 (116) | 8.41–25.96 | 9.92–26.36 | 11.94–28.46 | .21 (−.01, .39) |
| Pleasure from winning versus losing | 260 (133) | 241 (124) | 224 (116) | 8.01–17.91 | 9.92–26.36 | 11.94–28.46 | .65 (.55, .74) |
| BAS Drive | 277 (145) | 273 (141) | 241 (130) | 8.01–25.96 | 9.92–26.36 | 11.94–28.46 | .62 (.53, .70) |
| BAS Fun Seeking | 277 (145) | 273 (141) | 241 (130) | 8.01–25.96 | 9.92–26.36 | 11.94–28.46 | .60 (.50, .69) |
| BAS Reward Responsiveness | 277 (145) | 273 (141) | 241 (130) | 8.01–25.96 | 9.92–26.36 | 11.94–28.46 | .61 (.51, .69) |
BAS = Behavioral Activation System; NAcc = nucleus accumbens.
Table 2.
AIC and BIC Values for Null, Linear, Quadratic, and Cubic Models to Describe the Relation With Age and Each of the Measures Reported
| Model | Null | Linear | Quadratic | Cubic | If best fitting model has an effect of sex | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dependent variable | AIC | BIC | AIC | BIC | AIC | BIC | AIC | BIC | Effect | Model | AIC | BIC |
| Left NAcc win > lose | 3,045 | 3,059 | 3,043 | 3,062 | 3,035 | 3,057 | 3,037 | 3,064 | — | — | — | — |
| Right NAcc win > lose | 3,098 | 3,112 | 3,096 | 3,114 | 3,086 | 3,109 | 3,088 | 3,115 | — | — | — | — |
| Pleasure from winning versus losing | 3,519 | 3,533 | 3,500 | 3,519 | 3,500 | 3,523 | 3,502 | 3,530 | Main effect | Linear | 3,491 | 3,514 |
| Interaction with age | Linear | 3,493 | 3,514 | |||||||||
| BAS Drive | 3,440 | 3,454 | 3,436 | 3,454 | 3,437 | 3,461 | 3,435 | 3,463 | Main effect | Cubic | 3,436 | 3,469 |
| Interaction with age | Cubic | 3,434 | 3,480 | |||||||||
| BAS Fun Seeking | 3,174 | 3,188 | 3,176 | 3,194 | 3,177 | 3,201 | 3,169 | 3,197 | — | — | — | — |
| BAS Reward Responsiveness | 3,180 | 3,194 | 3,181 | 3,200 | 3,183 | 3,206 | 3,178 | 3,206 | Main effect | Cubic | 3,174 | 3,207 |
| Interaction with age | Cubic | 3,177 | 3,224 | |||||||||
Preferred models are in bold type. AIC = Akaike information criterion; BAS = Behavioral Activation System; BIC = Bayesian information criterion; NAcc = nucleus accumbens.
Table 3.
Statistical Parameters (Regression Coefficients [b], Significance Level (p), and 95%‐Confidence Interval for the bs) for the Best Fitting Models Testing the Relation Between Age and Each of the Measures Reported in the Table
| Dependent variable | Fixed effects | b | p | 95% Confidence interval β | |
|---|---|---|---|---|---|
| Min | Max | ||||
| Left NAcc win > lose | Intercept | 1.65 | < .001 | 1.43 | 1.87 |
| Age, 1 | −0.01 | .62 | −0.06 | 0.04 | |
| Age, 2 | −0.01 | < .001 | −0.02 | −0.01 | |
| Right NAcc win > lose | Intercept | 1.78 | < .001 | 1.56 | 2.00 |
| Age, 1 | −0.02 | .50 | −0.06 | 0.03 | |
| Age, 2 | −0.02 | < .001 | −0.02 | −0.01 | |
| Pleasure from winning versus losing | Intercept | 4.31 | < .001 | 3.96 | 4.62 |
| Age, 1 | −0.14 | < .001 | 0.08 | 0.20 | |
| Sex | 0.87 | < .001 | 0.36 | 1.36 | |
| BAS Drive | Intercept | 10.99 | < .001 | 10.66 | 11.31 |
| Age, 1 | 0.13 | .01 | 0.03 | 0.23 | |
| Age, 2 | 0.00 | .61 | −0.02 | 0.01 | |
| Age, 3 | 0.00 | .72 | 0.00 | 0.00 | |
| Sex | −0.31 | .20 | −0.77 | 0.16 | |
| Age, 1 × Sex | −0.04 | .54 | −0.19 | 0.10 | |
| Age, 2 × Sex | 0.02 | .05 | −0.04 | 0.00 | |
| Age, 3 × Sex | 0.00 | .13 | 0.00 | 0.00 | |
| BAS Fun Seeking | Intercept | 11.56 | < .001 | 11.37 | 11.76 |
| Age, 1 | 0.07 | .02 | 0.01 | 0.13 | |
| Age, 2 | 0.01 | .01 | 0.00 | 0.02 | |
| Age, 3 | 0.00 | < .01 | 0.00 | 0.00 | |
| BAS Reward Responsiveness | Intercept | 17.25 | < .001 | 17.00 | 17.50 |
| Age, 1 | 0.07 | .02 | 0.01 | 0.13 | |
| Age, 2 | 0.01 | .07 | 0.00 | 0.02 | |
| Age, 3 | 0.00 | .02 | 0.00 | 0.00 | |
| Sex | −0.41 | .01 | −0.74 | −0.09 | |
Linear age terms are represented by “Age, 1,” quadratic terms by “Age, 2,” and cubic terms by “Age, 3.” BAS = Behavioral Activation System; NAcc = nucleus accumbens.
Reward‐Related NAcc Activation
The developmental pattern of left and right NAcc response to winning versus losing was best described by a quadratic relation (p = .001 [left], and p < .001 [right], remains significant after correction for multiple comparisons). As can be seen in Figure 1A, this relation indicates that reward‐related NAcc activation peaks in mid‐adolescence (at 15.3 and 15.1 years of age for the left and right NAcc respectively). There was no main effect of sex or an Age × Sex interaction effect.
Figure 1.
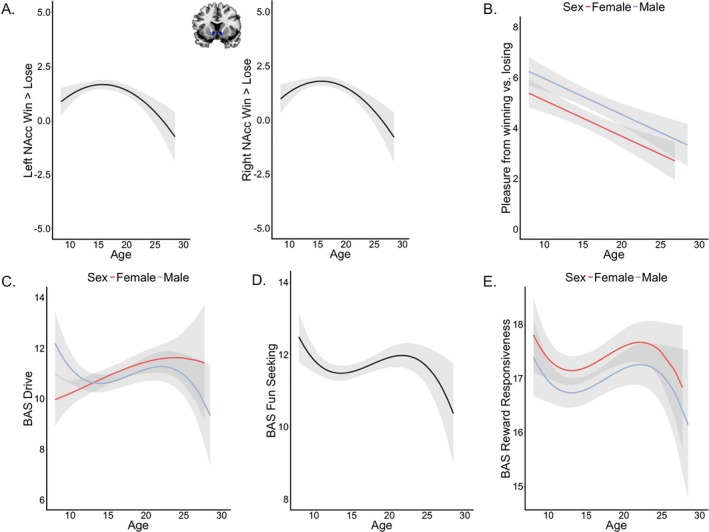
Development of (A) left and right nucleus accumbens (NAcc) activation during winning versus losing, (B) self‐reported Pleasure from winning versus losing, (C) Behavioral Activation System (BAS) drive, (D) BAS Fun Seeking, and (E) BAS Reward Responsiveness across development. The smooth lines represent the predicted values and the light ribbon their 95%‐confidence interval according to the best fitting model. Red and blue fitted lines indicate different age effects for males and females. A black fitted line indicates general age effects (no interaction with sex).
State‐Level Reward Sensitivity: Pleasure From Winning Versus Losing
Self‐reported pleasure from winning versus losing coins showed a negative linear relation with age and there was a main effect of sex (p < .001, significant after correction for multiple comparisons). These results indicate that Pleasure from winning versus losing decreases across adolescence and boys liked winning relatively more than losing compared to girls (Figure 1B).
Trait‐Level Reward Sensitivity: BAS
The relation between BAS Drive and age was best described by a cubic model with a main effect of sex and an Age × Sex interaction (p = .02, uncorrected for multiple comparisons; Figure 1C). Follow‐up analyses of the interaction effect showed a significant linear increase in BAS Drive scores with age for girls (linear age term: b = .12, SE = .05, p < .01, quadratic age term: p = .62, cubic age term: p = .72), and a cubic age effect on BAS Drive for males (linear age term: p = .10, quadratic age term: b = .02, SE = .01, p = .02, cubic age term: b = .00, SE = .00, p < .01).
A cubic model best described the relation between age and BAS Fun Seeking (p < .01, uncorrected for multiple comparisons; Figure 1D). There was no effect of sex in this model. Finally, the cubic model with a main effect of sex best explained the relation between age and BAS Reward Responsiveness. Girls scored higher on BAS Reward Responsiveness than boys (Figure 1E).
Brain‐Behavior Relations in Reward Sensitivity
Next, we tested the role of developmental differences in self‐reported pleasure from winning versus losing, and BAS subscales on NAcc activation in early to mid‐adolescents (< 16 years of age) and mid‐adolescents to young adults (≥ 16 years of age) separately. We used a model fitting procedure in which the linear term of the variable of interest was added before the linear term of age. Tables S3, 4, and 5 give a detailed overview of the significance levels of the model comparisons, model fits (AIC and BIC values), and the statistical parameters of the best fitting models, respectively. Plots of the raw data can be found in Figure S3.
Table 4.
AIC and BIC Values for the Models Testing the Relation Between NAcc Activation and Each of the Predictors
| Dependent variable | Left NAcc win > lose | Right NAcc win > lose | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model | Null | Predictor | Predictor + age | Null | Predictor | Predictor + age | ||||||
| Predictor | AIC | BIC | AIC | BIC | AIC | BIC | AIC | BIC | AIC | BIC | AIC | BIC |
| Early‐mid adolescents | ||||||||||||
| Pleasure from winning versus losing | 1,631 | 1,643 | 1,631 | 1,646 | 1,633 | 1,652 | 1,662 | 1,674 | 1,662 | 1,677 | 1,663 | 1,683 |
| BAS Drive | 1,581 | 1,593 | 1,578 | 1,594 | 1,580 | 1,599 | 1,629 | 1,641 | 1,626 | 1,641 | 1,627 | 1,647 |
| BAS Fun Seeking | 1,581 | 1,593 | 1,580 | 1,595 | 1,581 | 1,601 | 1,629 | 1,641 | 1,630 | 1,646 | 1,632 | 1,651 |
| BAS Reward Responsiveness | 1,581 | 1,593 | 1,583 | 1,598 | 1,584 | 1,603 | 1,629 | 1,641 | 1,630 | 1,646 | 1,632 | 1,651 |
| Mid‐late adolescents and young adults | ||||||||||||
| Pleasure from winning versus losing | 1,201 | 1,212 | 1,195 | 1,211 | 1,174 | 1,193 | 1,223 | 1,234 | 1,211 | 1,226 | 1,187 | 1,205 |
| BAS Drive | 1,369 | 1,380 | 1,371 | 1,386 | 1,352 | 1,371 | 1,380 | 1,391 | 1,382 | 1,397 | 1,360 | 1,379 |
| BAS Fun Seeking | 1,369 | 1,380 | 1,370 | 1,386 | 1,352 | 1,371 | 1,380 | 1,391 | 1,382 | 1,397 | 1,360 | 1,379 |
| BAS Reward Responsiveness | 1,369 | 1,380 | 1,370 | 1,385 | 1,352 | 1,370 | 1,380 | 1,391 | 1,381 | 1,396 | 1,360 | 1,379 |
Preferred models are in bold type. AIC = Akaike information criterion; BAS = Behavioral Activation System; BIC = Bayesian information criterion; NAcc = nucleus accumbens.
Table 5.
Statistical Parameters (Regression Coefficients [b], Significance Level (p), and 95%‐Confidence Interval for the bs for the Best Fitting Models Testing the Relation Between NAcc Activation and Each of the Measures Reported in the Table
| Fixed effects | b | p | 95% Confidence interval β | ||
|---|---|---|---|---|---|
| Min | Max | ||||
| Early to mid‐adolescents | |||||
| Pleasure from winning versus losing | |||||
| Left NAcc | Intercept | 1.46 | < .001 | 1.19 | 1.73 |
| Right NAcc | Intercept | 1.58 | < .001 | 1.31 | 1.84 |
| BAS Drive | |||||
| Left NAcc | Intercept | 0.05 | .94 | −1.14 | 1.24 |
| BAS Drive | 0.13 | .02 | 0.02 | 0.24 | |
| Right NAcc | Intercept | 0.10 | .87 | −1.18 | 1.27 |
| BAS Drive | 0.14 | .02 | 0.03 | 0.25 | |
| BAS Fun Seeking | |||||
| Left NAcc | Intercept | 1.39 | < .001 | 1.12 | 1.67 |
| Right NAcc | Intercept | 1.53 | < .001 | 1.26 | 1.81 |
| BAS Reward Responsiveness | |||||
| Left NAcc | Intercept | 1.39 | < .001 | 1.12 | 1.67 |
| Right NAcc | Intercept | 1.53 | < .001 | 1.26 | 1.81 |
| Mid‐adolescents to young adults | |||||
| Pleasure from winning versus losing | |||||
| Left NAcc | Intercept | 4.72 | < .001 | 3.20 | 6.24 |
| Pleasure from winning versus losing | 0.08 | .05 | 0.00 | 0.15 | |
| Age | −0.19 | < .001 | −0.26 | −0.11 | |
| Right NAcc | Intercept | 4.85 | < .001 | 3.32 | 6.38 |
| Pleasure from winning versus losing | 0.12 | < .01 | 0.04 | 0.20 | |
| Age | −0.20 | < .001 | −0.27 | −0.12 | |
BAS = Behavioral Activation System; NAcc = nucleus accumbens.
Trait‐Level Reward Sensitivity (BAS Scales) as Predictors for NAcc Activation
For the younger age group (early to mid‐adolescents, < 16.0 years of age), the relation between left and right NAcc and BAS Drive was best explained by a positive linear relation (p = .023 [left] and .020 [right], corrected significance threshold .014). There was no interaction with sex. These results show that participants who reported stronger BAS Drive showed higher activity in NAcc for winning versus losing (Figure 2A). There was no such relation in the older age groups (> 16 years of age, mid‐adolescence to adulthood). Furthermore, there were no relations between NAcc activation and the BAS Fun Seeking and BAS Reward Responsiveness subscale in either age group.
Figure 2.
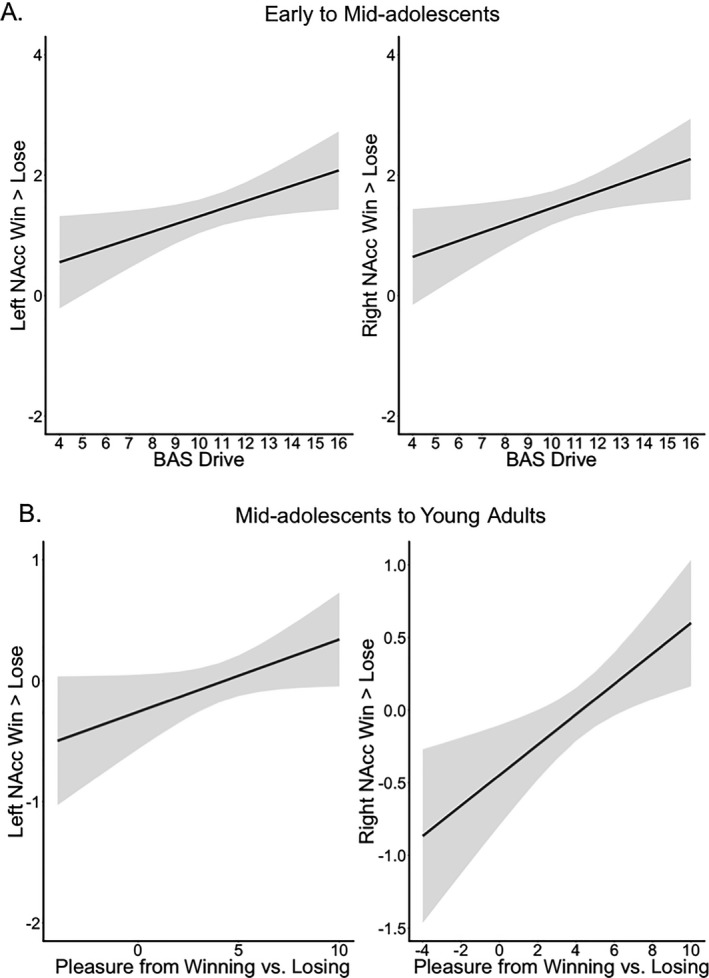
Relation between left and right nucleus accumbens (NAcc) activation during winning versus losing and (A) Behavioral Activation System (BAS) drive scores from early to mid‐adolescents, and (B) Pleasure from winning versus losing corrected for the main effect of age from mid‐ to late adolescents and young adults. The smooth lines represent the predicted values and the light gray ribbon their 95% confidence interval according to the best fitting model. A black fitted line indicates general age effects (no interaction with sex).
State‐Level Reward Sensitivity (Pleasure from Winning Versus Losing) as a Predictor for NAcc Activation
There was no relation between NAcc activation and Pleasure of winning versus losing in the younger age group (early to mid‐adolescence). For mid‐ to late adolescents and young adults (≥ 16.0 years of age), the relation between left and right NAcc activation and Pleasure from winning versus losing was best explained by a positive linear relation (model: ps < .001, remain significant after correction for multiple comparisons; bs: p = .047 for left NAcc, uncorrected for multiple comparisons, and p = .0025 for right NAcc, significant after correction for multiple comparisons). Sex did not explain additional variance. Thus, in mid to late adolescence and early adulthood, participants who reported less pleasure for winning money showed less NAcc activation for winning versus losing (Figure 2B).
NAcc Activation as a Function of Predictor × Age Group Interaction
We also tested whether the strength of the relation between NAcc activation and individual differences in BAS Drive, and Pleasure from winning versus losing was significantly different for the younger age group (< 16.0 years) and the older age group (≥ 16.0 years). We built separate models containing a main effect of the predictor of interest (BAS Drive or Pleasure from winning versus losing) and a Predictor of Interest × Age Group interaction term. The analyses revealed no significant interaction between age group and BAS Drive, and age groups and Pleasure from winning versus losing (ps > .06). Possibly, the interaction was under powered to detect changing contributions over age. Therefore, effects per age group should not be interpreted as specific age effects.
Discussion
The goal of this three‐wave accelerated longitudinal study was to test the developmental trajectory of reward‐related NAcc activation across ages 8–29 years and how behavioral state‐ and trait‐level reward sensitivity related to these changes. The results confirmed that NAcc activity to rewards peaks in mid‐adolescence consistent with our previous findings based on data from the first two waves of the study reported by Braams et al. (2015). In addition, it was found that developmental differences in self‐reported motivation to approach rewards (trait‐level reward sensitivity) and the immediate pleasure from winning (state‐level reward sensitivity) contributed to these changes. Below, we set out how these two different types of reward sensitivity explained NAcc activation in early to mid‐adolescence and in mid‐adolescence to early adulthood.
Consistent with previous studies, we found that NAcc activation during the receipt of a reward peaks in mid‐adolescence (Braams et al., 2015; Galvan et al., 2006; Silverman et al., 2015; Telzer, 2016; Van Leijenhorst, Gunther Moor, et al., 2010). Our results demonstrate that mid‐adolescents respond to a greater extent to rewards than children, early adolescents, late adolescents, and young adults, and extend previous findings by showing that this developmental trajectory continues until at least into the late twenties. It has previously been argued that adolescence is a time of stronger dopamine release, which may also contribute to the greater reward sensitivity in the NAcc in mid‐adolescence (Wahlstrom et al., 2010). This study is the first to show results of NAcc activation during receipt of rewards measured at three time points, and the accelerated longitudinal design of the study precludes influence of cohort effects (Crone & Elzinga, 2015; Ordaz, Foran, Velanova, & Luna, 2013).
Given that the peak of reward activation was predicted around the age of 16 years, we separately tested whether variance in NAcc activity could be explained between ages 8–16 years, and between ages 16–29 years by trait‐level reward sensitivity as measured with the BAS scales (Urošević et al., 2012) and state‐level reward sensitivity as measured with a scale assessing immediate pleasure from rewards (Salimpoor, Benovoy, Larcher, Dagher, & Zatorre, 2011). In younger adolescents (8–16 years of age), higher levels of a self‐reported drive to pursue and achieve personal goals, that is, trait‐level reward sensitivity, were associated with stronger NAcc activity to rewards. This finding suggests that the rise in NAcc activity is stronger for adolescents with a higher motivation to obtain rewards (Simon et al., 2010), such as the drive to obtain rewards or the desire for rewards (Braams et al., 2015; Van Duijvenvoorde et al., 2014). Our finding suggests that higher NAcc responses to rewards may relate to the drive to seek out novel experiences. It should be noted that in this study the relation between reward drive and NAcc activation was not significant after Bonferroni correction for multiple comparisons and should therefore be replicated in future studies. In addition, the longitudinal design allows for a better estimation of brain‐behavior relations than cross‐sectional studies but does not allow for causal inferences, because patterns may coincide over time in relation to a third factor, such as changes in pubertal hormones (Braams et al., 2015; Forbes et al., 2010; Op de Macks et al., 2011). Nonetheless, the findings are consistent with prior studies (Braams et al., 2015; Urošević et al., 2012; Van Duijvenvoorde et al., 2014) and show that individual differences in reward drive are an important factor to investigate in future research.
Another important question for future research is to test why effects were specific for reward drive. No significant relation was found between NAcc activity and other forms of trait‐level reward sensitivity measured in our study, such as fun‐seeking tendencies (cf. Van Duijvenvoorde et al., 2014) and affective responses to rewards. Possibly, these forms of reward sensitivity are distinctly related to NAcc responses to rewards and, by extension, to novelty seeking behaviors. In addition, this implies that they are distinct constructs within trait‐level reward sensitivity. However, to test this question of specificity of reward drive in more detail, it will be important to test relations with multiple reward types in future research.
A final question concerns the relation between neural responses to rewards and measures of state‐ and trait‐level reward sensitivity between mid‐adolescence and adulthood. In older adolescents and young adults (16–29 years of age), reducing levels of NAcc activity were associated with less reward pleasure experiences when receiving rewards in the task (i.e., state‐level reward sensitivity). This suggests that the age‐related decrease in state‐level reward sensitivity can possibly be explained by a decrease in NAcc activation. This finding fits with previous findings showing that ventral striatum activation and dopamine release from the striatum were related to pleasure experienced during listening to music and during winning money in a simple estimation task (Dohmen et al., 2011; Salimpoor et al., 2011). The incentive in these types of simple reward tasks may be lower for late adolescents and young adults than early adolescents. Possibly, NAcc activity scales with the reduction in pleasure obtained when gaining rewards in a simple gambling task in adulthood.
This study also had several limitations that deserve attention. First, although we have often linked ventral striatum activation to explorative behaviors, we did not assess these behaviors in our study. Prior studies have found that increased self‐reported risk propensity (Galvan, Hare, Voss, Glover, & Casey, 2007) and risky decision making (Van Duijvenvoorde et al., 2014) are associated with increased reward‐related ventral striatum activity. In future research, it will be important to include measures that represent real‐life explorative behaviors. Second, we could not identify an interaction on NAcc activation between the self‐report measures of state‐ and trait‐level reward sensitivity measures and the two age groups. Therefore, we cannot conclude that the relations between NAcc activation and state‐ and trait‐level reward sensitivity are significantly different between the two age groups. Third, in this study we contrasted NAcc activity for winning and losing. This manner of presenting the results does not allow for distinguishing whether NAcc activity was driven by wins or losses (Braams et al., 2015). Hence, the results should be interpreted as a relative difference, and future studies should include an appropriate baseline condition, for example, in which participants do not win or lose coins.
To conclude, in this study we demonstrated that reward‐related NAcc activation peaks in mid‐adolescence and declines again in late adolescence and early adulthood. We show that the increase in NAcc activation to rewards in early to mid‐adolescence is driven by developmental differences in a general (trait‐level) drive to pursue personal goals. The decrease in NAcc activation in late adolescence and adulthood was related to a decrease in state‐level hedonic reward ratings. A strength of this study was the use of longitudinal measurements, which are pivotal for understanding trajectories of change, given that these reduce cohort effects and provide more power for detecting change (Crone & Elzinga, 2015; Ordaz et al., 2013). Furthermore, longitudinal measurements are essential for testing how changes in neural activity co‐vary with individual differences (Telzer, Fuligni, Lieberman, & Galván, 2013). Most studies on ventral striatum activity to date are based on cross‐sectional studies, but there are some exceptions that are based on assessments from two time points (Braams et al., 2015; Lamm et al., 2014; Van Duijvenvoorde et al., 2014). Importantly, with the third time point included in this study, we were not only able to study adolescence but also to capture the transition from late adolescence to early adulthood. Future longitudinal studies should further examine (a) how individual differences in NAcc sensitivity to rewards in adolescence relate to real‐life explorative behaviors and future achievements and (b) what motivates older adolescents and adults to obtain rewards and how this relates to NAcc reward responses. Importantly, future longitudinal studies should examine how rewards in different contexts, for example, when participants gain rewards for others or play a more complex reward task, affect neural reward mechanisms and behavior across adolescence and early adulthood (Rosenbaum, Venkatraman, Steinberg, & Chein, 2017). Together, our findings set the stage for future research into unique contributions of motivational factors for the neural underpinnings of explorative behaviors, which might ultimately help adolescents and young adults to become successful adults.
Supporting information
Figure S1. Example of One Trial of the Functional MRI (fMRI) Task
Figure S2. Raw Data of (A) Left and Right Nucleus Accumbens (NAcc) Activation During Winning Versus Losing, (B) Self‐Reported Pleasure from Winning Versus Losing, (C) Behavioral Activation System (BAS) Drive, (D) BAS Fun Seeking, and (E) BAS Reward Responsiveness Across Development
Figure S3. Raw Data of the Relation Between Left and Right Nucleus Accumbens (NAcc) Activation During Winning Versus Losing and (A) Behavioral Activation System (BAS) Drive Scores From Early to Mid‐Adolescent Males and Females, and (B) Pleasure From Winning Versus Losing Corrected for the Main Effect of Age From Mid‐ to Late Adolescents and Young Adult Males and Females
Table S1. Number of Scans Obtained at T1, T2, and T3
Table S2. Significance Levels Model Comparisons Testing the Relation With Age
Table S3. Significance Levels Model Comparisons Testing the Relation With Nucleus Accumbens (NAcc) Activation
Appendix S1. Functional MRI (fMRI) Task
The authors thank Anna van Duijvenvoorde, Babette Langeveld, Batsheva Mannheim, Bianca Westhoff, Cédric Koolschijn, Dianne van der Heide, Erik de Water, Jochem Spaans, Jorien van Hoorn, Kiki Zanolie, Kyra Lubbers, Laura van der Aar, Mara van der Meulen, Marije Stolte, Rosa Meuwese, Sabine Peters, Sandy Overgaauw, and Suzanne van der Groep for their support during data collection. This work was supported by a European Research Council (ERC) starting grant awarded to Eveline A. Crone (ERC‐2010‐StG‐263234), and two VENI grants from the Netherlands Science Foundation (NWO) awarded to Jiska S. Peper (NWO‐VENI 451‐10‐007) and Berna Guroğlu (NWO‐VENI 451‐10‐021).
References
- Abler, B. , Walter, H. , Erk, S. , Kammerer, H. , & Spitzer, M. (2006). Prediction error as a linear function of reward probability is coded in human nucleus accumbens. NeuroImage, 31, 790–795. https://doi.org/10.1016/j.neuroimage.2006.01.001 [DOI] [PubMed] [Google Scholar]
- Akaike, H. (1974). A new look at the statistical model identification. IEEE Transactions on Automatic Control, 19, 716–723. https://doi.org/10.1109/TAC.1974.1100705 [Google Scholar]
- Bhanji, J. P. , & Delgado, M. R. (2014). The social brain and reward: Social information processing in the human striatum. Wiley Interdisciplinary Reviews. Cognitive Science, 5(1), 61–73. https://doi.org/10.1002/wcs.1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braams, B. R. , Güroğlu, B. , De Water, E. , Meuwese, R. , Koolschijn, P. C. , Peper, J. S. , & Crone, E. A. (2014). Reward‐related neural responses are dependent on the beneficiary. Social Cognitive and Affective Neuroscience, 9, 1030–1037. https://doi.org/10.1093/scan/nst077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braams, B. R. , Peters, S. , Peper, J. S. , Güroğlu, B. , & Crone, E. A. (2014). Gambling for self, friends, and antagonists: Differential contributions of affective and social brain regions on adolescent reward processing. NeuroImage, 100, 281–289. https://doi.org/10.1016/j.neuroimage.2014.06.020 [DOI] [PubMed] [Google Scholar]
- Braams, B. R. , Van Duijvenvoorde, A. C. K. , Peper, J. S. , & Crone, E. A. (2015). Longitudinal changes in adolescent risk‐taking: A comprehensive study of neural responses to rewards, pubertal development, and risk‐taking behavior. Journal of Neuroscience, 35, 7226–7238. https://doi.org/10.1523/jneurosci.4764-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett, M. , Anton, J. , Valabregue, R. , Poline, J. (2002). Region of interest analysis using an SMP toolbox. Paper Presented at: 8th International Conference on Functional mapping of the Human Brain.
- Carver, C. S. , & White, T. L. (1994). Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS scales. Journal of Personality and Social Psychology, 67, 319 https://doi.org/10.1037/0022-3514.67.2.319 [Google Scholar]
- Casey, B. J. , Galván, A. , & Somerville, L. H. (2016). Beyond simple models of adolescence to an integrated circuit‐based account: A commentary. Developmental Cognitive Neuroscience, 17(Suppl. 1), 128–130. https://doi.org/10.1016/j.dcn.2015.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone, E. A. , & Dahl, R. E. (2012). Understanding adolescence as a period of social‐affective engagement and goal flexibility. Nature Reviews Neuroscience, 13, 636–650. https://doi.org/10.1038/nrn3313 [DOI] [PubMed] [Google Scholar]
- Crone, E. A. , & Elzinga, B. M. (2015). Changing brains: How longitudinal functional magnetic resonance imaging studies can inform us about cognitive and social‐affective growth trajectories. Wiley Interdisciplinary Reviews: Cognitive Science, 6(1), 53–63. https://doi.org/10.1002/wcs.1327 [DOI] [PubMed] [Google Scholar]
- Dahl, R. E. (2004). Adolescent brain development: A period of vulnerabilities and opportunities. Keynote address. Annals of the New York Academy of Sciences, 1021(1), 1–22. https://doi.org/10.1196/annals.1308.001 [DOI] [PubMed] [Google Scholar]
- Demaree, H. A. , DeDonno, M. A. , Burns, K. J. , & Erik Everhart, D. (2008). You bet: How personality differences affect risk‐taking preferences. Personality and Individual Differences, 44, 1484–1494. https://doi.org/10.1016/j.paid.2008.01.005 [Google Scholar]
- Dohmen, T. , Falk, A. , Fliessbach, K. , Sunde, U. , & Weber, B. (2011). Relative versus absolute income, joy of winning, and gender: Brain imaging evidence. Journal of Public Economics, 95, 279–285. https://doi.org/10.1016/j.jpubeco.2010.11.025 [Google Scholar]
- Ernst, M. , & Fudge, J. L. (2009). A developmental neurobiological model of motivated behavior: Anatomy, connectivity and ontogeny of the triadic nodes. Neuroscience & Biobehavioral Reviews, 33, 367–382. https://doi.org/10.1016/j.neubiorev.2008.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes, E. E. , Ryan, N. D. , Phillips, M. L. , Manuck, S. B. , Worthman, C. M. , Moyles, D. L. , … Dahl, R. E. (2010). Healthy adolescents’ neural response to reward: Associations with puberty, positive affect, and depressive symptoms. Journal of the American Academy of Child & Adolescent Psychiatry, 49, 162–172. e165. https://doi.org/10.1016/j.jaac.2009.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan, A. (2010). Adolescent development of the reward system. Frontiers in Human Neuroscience, 4, 6 https://doi.org/10.3389/neuro.09.006.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan, A. , Hare, T. A. , Parra, C. E. , Penn, J. , Voss, H. , Glover, G. , & Casey, B. J. (2006). Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk‐taking behavior in adolescents. Journal of Neuroscience, 26, 6885 https://doi.org/10.1523/JNEUROSCI.1062-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan, A. , Hare, T. , Voss, H. , Glover, G. , & Casey, B. (2007). Risk‐taking and the adolescent brain: Who is at risk? Developmental Science, 10 https://doi.org/10.1111/j.1467-7687.2006.00579.x [DOI] [PubMed] [Google Scholar]
- Hauser, T. U. , Iannaccone, R. , Walitza, S. , Brandeis, D. , & Brem, S. (2015). Cognitive flexibility in adolescence: Neural and behavioral mechanisms of reward prediction error processing in adaptive decision making during development. NeuroImage, 104, 347–354. https://doi.org/10.1016/j.neuroimage.2014.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawes, S. W. , Chahal, R. , Hallquist, M. N. , Paulsen, D. J. , Geier, C. F. , & Luna, B. (2017). Modulation of reward‐related neural activation on sensation seeking across development. NeuroImage, 147, 763–771. https://doi.org/10.1016/j.neuroimage.2016.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izuma, K. , Saito, D. N. , & Sadato, N. (2008). Processing of social and monetary rewards in the human striatum. Neuron, 58, 284–294. https://doi.org/10.1016/j.neuron.2008.03.020 [DOI] [PubMed] [Google Scholar]
- Lamm, C. , Benson, B. E. , Guyer, A. E. , Perez‐Edgar, K. , Fox, N. A. , Pine, D. S. , & Ernst, M. (2014). Longitudinal study of striatal activation to reward and loss anticipation from mid‐adolescence into late adolescence/early adulthood. Brain and Cognition, 89, 51–60. https://doi.org/10.1016/j.bandc.2013.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X. , Hairston, J. , Schrier, M. , & Fan, J. (2011). Common and distinct networks underlying reward valence and processing stages: A meta‐analysis of functional neuroimaging studies. Neuroscience and Biobehavioral Reviews, 35, 1219–1236. https://doi.org/10.1016/j.neubiorev.2010.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Op de Macks, Z. A. , Moor, B. G. , Overgaauw, S. , Güroğlu, B. , Dahl, R. E. , & Crone, E. A. (2011). Testosterone levels correspond with increased ventral striatum activation in response to monetary rewards in adolescents. Developmental Cognitive Neuroscience, 1, 506–516. https://doi.org/10.1016/j.dcn.2011.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordaz, S. J. , Foran, W. , Velanova, K. , & Luna, B. (2013). Longitudinal growth curves of brain function underlying inhibitory control through adolescence. Journal of Neuroscience, 33, 18109–18124. https://doi.org/10.1523/JNEUROSCI.1741-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perneger, T. V. (1998). What's wrong with Bonferroni adjustments. British Medical Journal, 316, 1236–1238. https://doi.org/10.1136/bmj.316.7139.1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro, J. , Bates, D. , DebRoy, S. , & Sarkar, D. ; R Core Team . (2013). nlme: Linear and nonlinear mixed effects models. R package version 3.1‐113. Retrieved from https://cran.r-project.org/package=nlme
- R Core Team . (2014). R: A language and environment for statistical computing. Vienna, Austria: Foundation for Statistical Computing. [Google Scholar]
- Rosenbaum, G. M. , Venkatraman, V. , Steinberg, L. , & Chein, J. M. (2017). The influences of described and experienced information on adolescent risky decision making. Developmental Review. https://doi.org/10.1016/j.dr.2017.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimpoor, V. N. , Benovoy, M. , Larcher, K. , Dagher, A. , & Zatorre, R. J. (2011). Anatomically distinct dopamine release during anticipation and experience of peak emotion to music. Nature Neuroscience, 14, 257–262. https://doi.org/10.1038/nn.2726 [DOI] [PubMed] [Google Scholar]
- Sankoh, A. J. , Huque, M. F. , & Dubey, S. D. (1997). Some comments on frequently used multiple endpoint adjustment methods in clinical trials. Statistics in Medicine, 16, 2529–2542. https://doi.org/10.1038/nn.2726 [DOI] [PubMed] [Google Scholar]
- Schwarz, G. (1978). Estimating the dimension of a model. Annals of Statistics, 6, 461–464. https://doi.org/10.1214/aos/1176344136 [Google Scholar]
- Sescousse, G. , Caldú, X. , Segura, B. , & Dreher, J.‐C. (2013). Processing of primary and secondary rewards: A quantitative meta‐analysis and review of human functional neuroimaging studies. Neuroscience & Biobehavioral Reviews, 37, 681–696. https://doi.org/10.1016/j.neubiorev.2013.02.002 [DOI] [PubMed] [Google Scholar]
- Silverman, M. H. , Jedd, K. , & Luciana, M. (2015). Neural networks involved in adolescent reward processing: An activation likelihood estimation meta‐analysis of functional neuroimaging studies. NeuroImage, 122, 427–439. https://doi.org/10.1016/j.neuroimage.2015.07.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon, J. J. , Walther, S. , Fiebach, C. J. , Friederich, H.‐C. , Stippich, C. , Weisbrod, M. , & Kaiser, S. (2010). Neural reward processing is modulated by approach‐and avoidance‐related personality traits. NeuroImage, 49, 1868–1874. https://doi.org/10.1016/j.neuroimage.2009.09.016 [DOI] [PubMed] [Google Scholar]
- Telzer, E. H. (2016). Dopaminergic reward sensitivity can promote adolescent health: A new perspective on the mechanism of ventral striatum activation. Developmental Cognitive Neuroscience, 17, 57–67. https://doi.org/10.1016/j.dcn.2015.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer, E. H. , Fuligni, A. J. , Lieberman, M. D. , & Galván, A. (2013). Ventral striatum activation to prosocial rewards predicts longitudinal declines in adolescent risk taking. Developmental Cognitive Neuroscience, 3, 45–52. https://doi.org/10.1016/j.dcn.2012.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urošević, S. , Collins, P. , Muetzel, R. , Lim, K. , & Luciana, M. (2012). Longitudinal changes in behavioral approach system sensitivity and brain structures involved in reward processing during adolescence. Developmental Psychology, 48, 1488–1500. https://doi.org/10.1037/a0027502. doi:10.1037/a0027502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Duijvenvoorde, A. C. K. , Op de Macks, Z. A. , Overgaauw, S. , Moor, B. G. , Dahl, R. E. , & Crone, E. A. (2014). A cross‐sectional and longitudinal analysis of reward‐related brain activation: Effects of age, pubertal stage, and reward sensitivity. Brain and Cognition, 89, 3–14. https://doi.org/10.1016/j.bandc.2013.10.005 [DOI] [PubMed] [Google Scholar]
- Van Duijvenvoorde, A. C. K. , Peters, S. , Braams, B. R. , & Crone, E. A. (2016). What motivates adolescents? Neural responses to rewards and their influence on adolescents’ risk taking, learning, and cognitive control. Neuroscience & Biobehavioral Reviews, 70, 135–147. https://doi.org/10.1016/j.neubiorev.2016.06.037 [DOI] [PubMed] [Google Scholar]
- Van Leijenhorst, L. , Gunther Moor, B. , Op de Macks, Z. A. , Rombouts, S. A. , Westenberg, P. M. , & Crone, E. A. (2010). Adolescent risky decision‐making: Neurocognitive development of reward and control regions. NeuroImage, 51, 345–355. https://doi.org/10.1016/j.neuroimage.2010.02.03 [DOI] [PubMed] [Google Scholar]
- Van Leijenhorst, L. , Zanolie, K. , Van Meel, C. S. , Westenberg, P. M. , Rombouts, S. A. R. B. , & Crone, E. A. (2010). What motivates the adolescent? Brain regions mediating reward sensitivity across adolescence. Cerebral Cortex, 20(1), 61–69. https://doi.org/10.1093/cercor/bhp078 [DOI] [PubMed] [Google Scholar]
- Wahlstrom, D. , White, T. , & Luciana, M. (2010). Neurobehavioral evidence for changes in dopamine system activity during adolescence. Neuroscience and Biobehavioral Reviews, 34, 631–648. https://doi.org/10.1016/j.neubiorev.2009.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Example of One Trial of the Functional MRI (fMRI) Task
Figure S2. Raw Data of (A) Left and Right Nucleus Accumbens (NAcc) Activation During Winning Versus Losing, (B) Self‐Reported Pleasure from Winning Versus Losing, (C) Behavioral Activation System (BAS) Drive, (D) BAS Fun Seeking, and (E) BAS Reward Responsiveness Across Development
Figure S3. Raw Data of the Relation Between Left and Right Nucleus Accumbens (NAcc) Activation During Winning Versus Losing and (A) Behavioral Activation System (BAS) Drive Scores From Early to Mid‐Adolescent Males and Females, and (B) Pleasure From Winning Versus Losing Corrected for the Main Effect of Age From Mid‐ to Late Adolescents and Young Adult Males and Females
Table S1. Number of Scans Obtained at T1, T2, and T3
Table S2. Significance Levels Model Comparisons Testing the Relation With Age
Table S3. Significance Levels Model Comparisons Testing the Relation With Nucleus Accumbens (NAcc) Activation
Appendix S1. Functional MRI (fMRI) Task


