Abstract
The combination of oligonucleotides and synthetic supramolecular systems allows for novel and long‐needed modes of regulation of the self‐assembly of both molecular elements. Discotic molecules were conjugated with short oligonucleotides and their assembly into responsive supramolecular wires studied. The self‐assembly of the discotic molecules provides additional stability for DNA‐duplex formation owing to a cooperative effect. The appended oligonucleotides allow for positional control of the discotic elements within the supramolecular wire. The programmed assembly of these hybrid architectures can be modulated through the DNA, for example, by changing the number of base pairs or salt concentration, and through the discotic platform by the addition of discotic elements without oligonucleotide handles. These hybrid supramolecular‐DNA structures allow for advanced levels of control over 1D dynamic platforms with responsive regulatory elements at the interface with biological systems.
Keywords: DNA, hybrid systems, polymers, self-assembly, supramolecular chemistry
The combination of synthetic supramolecular systems with biomolecules such as proteins and DNA creates new opportunities for modulation of the function of the biomolecules through the supramolecular elements and, vice versa, for control over the assembly of the synthetic building blocks through the well‐structured biomolecular platform.1, 2, 3, 4 Programmable assemblies based on sequence‐designed DNA biomolecules have shown enormous potential in nanotechnology.5, 6, 7 Simultaneously, challenges for the optimal usage and implementation of DNA nanotechnology still exist, such as the need for specific salt concentrations for DNA‐duplex formation and the limited breath of chemical diversity achievable by structures composed exclusively of DNA.8 The research field of supramolecular DNA assembly2, 9 bridges a link between synthetic supramolecular structures and biological systems by expanding the diversity, complexity, and functionality available to DNA structures and by providing directionality and a higher level of assembly control to supramolecular systems. For instance, different groups have reported the introduction of synthetic vertices into oligonucleotides to control the geometry and stability of DNA hybrid structures.10, 11, 12, 13 Foldamers consisting of oligonucleotides with extended aromatic molecules integrated into their backbone have resulted in temperature‐responsive structures,14 and decoration of DNA structures with hydrophobic polymers of diverse length and composition was shown to control the self‐assembly properties of DNA prismatic cages, adding a higher level of complexity.15 The addition of lipids into DNA scaffolds has led to the development of supramolecular systems with novel functions, as highlighted in recent reviews.16, 17, 18 DNA hybrid‐grafted supramolecular polymers based on the self‐assembly properties of oligopyrenes have been reported, with the structure of the oligopyrenes dictating the morphology of the final assembly into helical 1D supramolecular polymers19, 20 and requiring a high temperature to incorporate additional monomers.21
Synthetic supramolecular systems have been designed to assemble in both small discrete as well as polymeric assemblies.22, 23 While great control over the interaction strengths between the supramolecular building blocks has been shown possible, control over the precise positioning of these supramolecular elements within the final assemblies has remained challenging. Concepts such as charge alternation or charge‐mediated clustering have shown promise for more general levels of control.24, 25 Combining DNA elements with synthetic supramolecular systems has the potential to address the fundamental challenge of exact positional control in polymeric supramolecular self‐assemblies. Hybrid structures of supramolecular polymeric systems and DNA thus promise to provide novel levels of control in both ways.
Bis‐pyridine‐based C 3‐symmetrical amphiphilic discotic molecules (BiPy‐Discs) have been reported to spontaneously assemble into fluorescent supramolecular wires in water,26, 27 which can operate as quasi‐1D scaffolds for the assembly and reversible exchange of proteins.28, 29 Like for other supramolecular polymers, positional control over individual monomers within the final polymeric assembly has remained elusive. Combining oligonucleotides (ODNs) with the BiPy‐Disc would, on the one hand, modulate the hybridization of the ODNs through the discotic molecules and, on the other hand, control the positioning of the discotic molecules in the supramolecular wires through the hybridization of appended ODNs (Figure 1). We therefore synthesized novel, hybrid supramolecular wires composed of short ODNs attached to BiPy‐Disc molecules and report on their properties, multi‐input regulation, and positional control.
Figure 1.
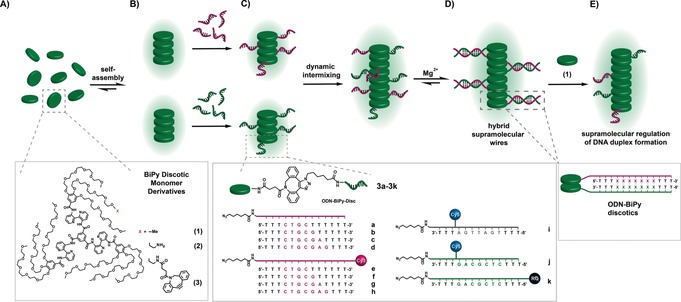
A) Schematic and chemical structure of BiPy‐Disc (1), NH2‐BiPy‐Disc (2) and DBCO‐BiPy‐Disc (3). B) Self‐assembly of discs in water and C) of the ODN‐BiPy‐Discs (3 a–3 k) ODN sequences used in this study (a–k). Sequences (a–d) and their Cy3‐labelled variants (e–h) and their complementary Cy5‐labelled (j) and quencher‐labelled (k) sequence, as well as non‐complementary reference sequence (i) D) Controlled positioning of the discs in the supramolecular wires owing to DNA‐duplex formation. E) Modulation of the DNA‐duplex by the addition of 1.
The novel supramolecular‐DNA assembly system is centered around the water‐soluble BiPy‐Disc molecule 1 (Figure 1). The mono‐functionalized NH2‐BiPy‐Disc (2) was used as starting material for subsequent ODN attachment by first reacting with an excess of dibenzocyclooctyne‐N‐hydroxysuccinimidyl ester (NHS‐DBCO) to yield the DBCO‐BiPy‐Disc (3). 3 was used as a platform for the monovalent attachment of a diverse array of oligonucleotides (a–k) by strain‐promoted azide–alkyne cycloaddition.30 Short ODNs thirteen bases in length, unlabeled (a–d) and dye‐labeled (e–h), were designed with varying numbers of complementary bases (four to seven bases) matching ODNs j and k, and aimed to avoid background DNA‐duplex formation between the ODNs at room temperature. The ODNs contained spacers of three thymine bases at both ends of the sequences to limit any repulsive interaction by the ethylene glycol chains.31 ODNs a–d were functionalized at the 5′ end with an azide group and conjugated with 3, to yield ODN‐BiPy‐Discs 3 a–3 d. ODNs e–h were additionally functionalized with a Cy3 dye at the 3′ to generate 3 e–3 h. By contrast, the complementary ODNs (j and k) were functionalized with an azide group at the 3′ position and a Cy5 dye (j) in the back‐bone or Iowa Black RQ (k) at the 5′ position to give 3 j and 3 k. Likewise, an ODN (i) bearing a Cy5 dye but fully non‐complementary to sequences a–h, was used to generate ODN‐BiPy‐Disc (3 i). All the ODN‐BiPy‐Discs were purified by precipitation with isopropanol to remove unreacted 3. Native polyacrylamide gel electrophoresis showed 1 and 3 a–3 k to have no electrophoretic mobility and to be pure and free from unconjugated ODNs (a–k) (Supporting Information, Figure S3 A). Thin layer chromatography also revealed full conversion and purity of the final ODN‐BiPy‐Discs (Figure S3 B). ES‐MS confirmed the identity of compounds 3 a–3 k (Supporting Information, Figure S4–14).
The effect of the ODNs on the general self‐assembly properties of the discs was monitored by using the fluorescence of the discs in the stacks. Solutions containing ODN‐BiPy‐Discs (3 a–3 d; 3 μm) showed fluorescence signal intensities comparable to that of 1 26 (Supporting Information, Figure S15 A). Charge screening by MgCl2 (1–5 mm, required for subsequent tuning of the DNA hybridization) did not perturb the stacking of the discs either (Supporting Information, Figure S16). The assembly of the ODN‐BiPy‐Discs was further analyzed by FRET studies, using the discotic core as the fluorescence donor and the Cy3 or Cy5 fluorophores on the ODNs as acceptors. Irradiation of a solution of ODN‐BiPy‐Disc 3 e, at 345 nm resulted in only a weak residual signal of the disc fluorescence at 520 nm and a strong new signal at 565 nm of the Cy3 dye (Figure S15 B). Reference experiments with free BiPy‐Disc and Cy3‐ODN intermixed, did not show any FRET. Analogous results were performed for a supramolecular wire composed of 3 j monomers, bearing a Cy5 dye. In this case, irradiation at 345 nm resulted in a fully quenched disc fluorescence signal and the appearance of only the Cy5 fluorescence at 665 nm (Figure S15 C). Mixtures of ODN‐BiPy‐Disc 3 j and BiPy‐Disc 1 were prepared to study the formation of mixed supramolecular wires and its dynamics. Directly after mixing 3 j and 1, the fluorescence spectra represented the simple addition of both individual components, with a concentration‐dependent increase of the disc fluorescence at 520 nm (Figure 2 A). Over time, mixing of the two supramolecular building blocks occurred to form supramolecular co‐polymers as judged by enhanced energy transfer, reaching equilibrium after approximately three hours (Figure 2 B).
Figure 2.
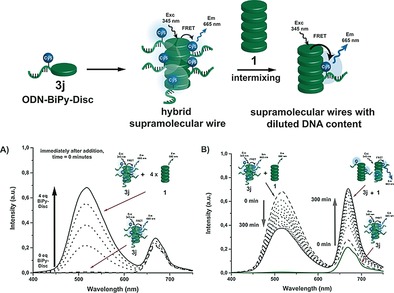
A) Fluorescence of the hybrid supramolecular wire 3 j (1 μm) directly after addition of 1–4 equiv BiPy‐Disc 1, when excited at 345 nm. B) Fluorescence spectra of mixture of 1 and 3 j, irradiated at 345 nm and monitored over time.
A set of Cy3/Cy5 conjugated ODN‐BiPy‐Discs, potentially capable of displaying FRET signals between their respective complementary sequences, was used as reporter to study ODN‐BiPy‐Disc heterodimerization in the supramolecular wires. The system with four complementary bases (3 e–3 j) strongly exemplifies the influence of the supramolecular wire on DNA hybridization (Figure 3). A 1:1 mixture of unmodified ODNs with four complementary base pairs e (0.5 μm) and j (0.5 μm), did not show any FRET signal under different salt concentrations (0–5 mm MgCl2; Figure 3 A). In contrast, when the same experiment was performed using a mixture of ODN‐BiPy‐Discs 3 e (0.5 μm) and 3 j (0.5 μm), a strong, MgCl2‐dependent FRET signal was observed (Figure 3 B). In the absence of MgCl2, no DNA‐duplex formation was observed, but a strong Cy3/Cy5‐FRET signal was obtained immediately upon addition of MgCl2 (1–5 mm). The systems reached equilibrium 20 to 30 min after addition of MgCl2. Similar results were obtained for the supramolecular wires featuring five (3 f–3 j), six (3 g–3 j), and seven (3 h–3 j) complementary base pairs (Supporting Information, Figure S17). A control set of ODN‐BiPy‐Discs (3 e and 3 i), with zero complementary bases, exhibited only a very weak FRET signal (Figure 3 C) presumably because the negatively charged oligonucleotides repel and thus orientate away from each other along the supramolecular stack. A weak FRET signal has also been observed previously for the same discotic molecules appended with fluorescent proteins.29 These sets of experiments thus confirmed the following: 1) two different discotic molecules are co‐positioned through the ODNs, 2) the DNA‐duplex formation is specific to the ODN sequences, and 3) hybridization is strengthened by the supramolecular BiPy‐Disc assembly. Remarkably, the extra stability engendered by the Disc–Disc interaction allowed DNA duplex formation under low salt concentrations and at very low numbers of complementary base pairs.
Figure 3.
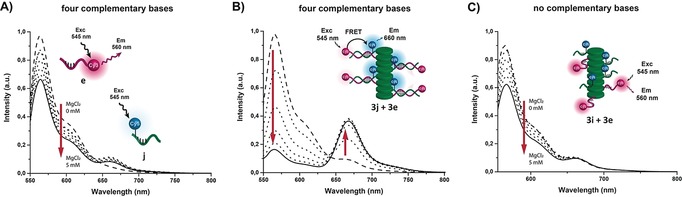
Extent of DNA‐duplex formation under different MgCl2 concentrations (0–5 mm) using ODNs with four complementary bases. A) Change in Cy3/Cy5 FRET signals upon duplex formation with complementary unmodified ODNs (0.5 μm). B) Significant change in Cy3/Cy5 FRET signals upon duplex formation with complementary ODN‐BiPy‐Disc 3 e and 3 j (0.5 μm). C) Cy3/Cy5 FRET signals with ODN‐BiPy‐Disc (3 i) lacking of complementary bases.
The stabilizing effect of the Disc elements on DNA‐duplex formation was quantified by titration experiments. ODN‐BiPy‐Disc 3 k, functionalized with a quencher (RQ) at the 5′ end, was titrated into solutions containing ODN‐BiPy‐Discs with the corresponding complementary sequence (3 e–3 h) and Cy3 dye (Supporting Information, Figure S19). Low MgCl2 concentrations were used to determine the dissociation constant for the supramolecular wires with four, five, and six complementary bases (Table 1). The dissociation constant of the system with seven complementary bases (3 h–3 k) was, even at these low MgCl2 concentrations, already too strong to be determined. The dissociation constants of ODNs alone, with 9, 10, and 11 complementary bases, were also determined, as these showed similar overall K D values as the hybrid systems.
Table 1.
Dissociation constants (K D) for the series of ODN‐BiPy‐Disc duplexes 3 e–3 h+3 k, experimental and theoretical ΔG°.
| Discs/ODNs | K D [nm] | ΔG
o[a]
[kcal mol−1] |
ΔG
o[b]
[kcal mol−1] |
ΔΔG
o
[kcal mol−1] |
|---|---|---|---|---|
| 3 e+3 k | 1.4×103 | −8.0 | −1.9 | −6.1 |
| 3 f+3 k | 39 | −10 | −4.1 | −6.0 |
| 3 g+3 k | 7.6 | −11 | −5.1 | −6.0 |
| 3 h+3 k | <5 | <−11 | −7.5 | – |
| l+k | 8.7×103 | −6.9 | −7.9 | – |
| m+k | 3.5×102 | −8.8 | −10 | – |
| n+k | <5 | <−11 | −12 | – |
[a] Experimentally determined ΔG o at 0.5 mm Mg2+. [b] Calculated ΔG o of the DNA component alone at 0.5 mm Mg2+ and 10 mm Na+, using software package UNAFold.
The binding energy for the final DNA‐duplex complex (ΔG o) was calculated from the measured dissociation constant values and serves to analyze the contribution of the BiPy‐Disc to the DNA‐duplex formation. The theoretical ΔG o of the DNA hybridization only was calculated using the software package UNAFold (Unified Nucleic Acid Folding).32 From the ΔΔG o value between the ΔG o of the experimental result and the computed ΔG o for DNA hybridization only, the contribution of the BiPy‐Discs to the overall assembly could be calculated. A value of at least −6.0 kcal mol−1 was obtained for the ΔΔG o. This extra ΔG o imposed by the discotic molecules is approximately equivalent to the binding energy of an additional four base pairs. Additionally, it should be noted that the results were calculated under equal MgCl2 conditions (0.5 mm) as used for the measurement but with an additional NaCl concentration (10 mm) since the program does not permit calculations with lower NaCl concentrations. The data on the DNA‐only measurements (Figure S19 B and Table 1) show that the addition of NaCl in the calculations leads to a larger calculated ΔG o for the DNA component as compared to the actual measured values. The calculated ΔΔG o as incurred by the discotic elements thus represent a minimal contribution of the discotic component to the overall affinity.
The ΔΔG o values can be used to calculate the effective molarity (EM)33 for the appended ODNs on the supramolecular wire. In this case, the EM is a quantitative parameter for the evaluation of the chelate cooperative effect in DNA‐duplex formation. The resulting EM for our supramolecular system ranges between 0.2 and 20 mm (see the Supporting Information for the calculation). The different self‐assembly properties of charged discotic molecules24, 28 and the underestimation of the ΔΔG o (see above) imply that the EM is most probably somewhat larger. These EM values are comparable to those obtained for other supramolecular systems. For instance, for the formation of highly optimal intramolecular H‐bonds, using coordination complexes between zinc porphyrins and pyridine ligands (EM from 3 to 240 mm).34
The DNA‐duplex formation in the context of the ODN‐BiPy‐Disc is thus in part driven by the self‐assembly of the supramolecular wire. Therefore, modulation of the degree of duplex formation should be possible through copolymerization with 1. The system with the weakest DNA hybridization based on four complementary base pairs (3 e–3 j) should be the most sensitive in this regard. Irradiation of a solution containing 3 e and 3 j (both at 0.5 μm; 1 mm of MgCl2) at 545 nm resulted in the Cy3/Cy5 FRET signal consistent with the DNA‐duplex formation (Figure 4 A). Upon addition of 1 (10 μm) as supramolecular monomer the Cy3/Cy5 FRET signal decreased over a period of 2 h. Simultaneously, the FRET from the discotic core to the Cy3 and Cy5 dyes increased upon intermixing of 1 (Supporting Information, Figure S18). Both FRET events are strong evidence of the disruption of DNA‐duplex formation, through the intercalation of 1 between the hybridized 3 e–3 j pairs.
Figure 4.
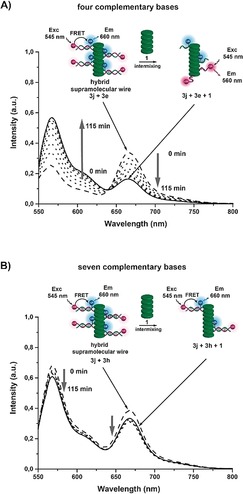
A) Change in fluorescence spectra over time of a 1:1 mixture of complementary (4 bases) ODN‐BiPy Discs 3 j+3 e (0.5 μm) with 1 mm of MgCl2 when irradiated at 545 nm upon addition of 10 equivalents of 1. The decrease in FRET signal shows disruption of DNA duplex. B) Fluorescence spectra of a 1:1 mixture of complementary (7 bases) ODN‐BiPy Discs 3 j+3 h under the same experimental conditions. No significant changes are observed because the DNA duplex is too stable to be dissociated by the addition of 1.
The copolymerization with 1 was also performed on the supramolecular wire featuring ODNs with seven complementary base pairs (3 h–3 j; Figure 4 B). In this case the addition of 1 to the 3 h–3 j mixture did not result in a change in the Cy3/Cy5 FRET signal. The energy transfer from the discotic core to the Cy3 and Cy5 dyes did increase but to a lesser extent than for the 3 e–3 j mixture (Figure S18). While 1 thus does insert into the wire, the DNA hybridization yields supramolecular copolymers in which the joined positioning of one 3 h and one 3 j is maintained. In this case the DNA hybridization is sufficiently strong to maintain dimer formation in supramolecular wires constructed from three different monomers.
In summary, combining ODNs with discotic‐based supramolecular wires resulted in a dual‐responsive supramolecular‐DNA hybrid system. The programmed assembly of the hybrid architectures and positional control of the monomers can be modulated through the DNA, by changing the number of base pairs or the salt concentration. The dynamic nature of the supramolecular wire also allows for a DNA‐orthogonal control over the extent of the DNA‐duplex formation, by the content of non‐functionalized discotic monomers.
The hybrid oligonucleotide‐discotic supramolecular wires provide for an increased stability for DNA‐duplex formation. This enabled the formation of duplexes between complementary sequences as short as four base pairs. The additional stability imposed by the discotic system is comparable to approximately four to five extra base pairs. The ability of these hybrid DNA–supramolecular assemblies to induce short oligonucleotide duplex formation at low salt concentrations suggests applications in biologically relevant media. Moreover, the appended ODNs enable a specific positioning of the discotic molecules within the final supramolecular assemblies as paired dimers. This allows entry into the long desired positional control over monomeric units in supramolecular polymers, for example, for the programmable recruitment of proteins within functional molecular systems.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
Acknowledgements
We thank Katja Petkau‐Milroy for providing 1 and Jurriaan Huskens, University of Twente, for discussions on the effective molarity. This work was funded by The Netherlands Organization for Scientific Research (NWO) through Gravity Program 024.001.035, VICI Grant 016.150.366 and through Marie Curie Independent Grant MSCA‐IF‐EF‐ST and Marie Curie Innovative Training Network MULTI‐APP (2015–2019).
M. Á. Alemán García, E. Magdalena Estirado, L.-G. Milroy, L. Brunsveld, Angew. Chem. Int. Ed. 2018, 57, 4976.
References
- 1. Uhlenheuer D. A., Petkau K., Brunsveld L., Chem. Soc. Rev. 2010, 39, 2817. [DOI] [PubMed] [Google Scholar]
- 2. McLaughlin C. K., Hamblin G. D., Sleiman H. F., Chem. Soc. Rev. 2011, 40, 5647. [DOI] [PubMed] [Google Scholar]
- 3. Luo Q., Hou C., Bai Y., Wang R., Liu J., Chem. Rev. 2016, 116, 13571–13632. [DOI] [PubMed] [Google Scholar]
- 4. van Dun S., Ottmann C., Milroy L.-G., Brunsveld L., J. Am. Chem. Soc. 2017, 139, 13960–13968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Seeman N. C., Nature 2003, 421, 427–431. [DOI] [PubMed] [Google Scholar]
- 6. Chen Y.-J., Groves B., Muscat R. A., Seelig G., Nat. Nanotechnol. 2015, 10, 748–760. [DOI] [PubMed] [Google Scholar]
- 7. Seeman N. C., Sleiman H. F., Nat. Rev. Mater. 2017, 3, 17068. [Google Scholar]
- 8. Pinheiro A. V., Han D., Shih W. M., Yan H., Nat. Nanotechnol. 2011, 6, 763–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aldaye F. A., Palmer A. L., Sleiman H. F., Science 2008, 321, 1795–1799. [DOI] [PubMed] [Google Scholar]
- 10. Scheffler M., Dorenbeck A., Jordan S., Wüstefeld M., von Kiedrowski G., Angew. Chem. Int. Ed. 1999, 38, 3311–3315; [PubMed] [Google Scholar]; Angew. Chem. 1999, 111, 3513–3518. [Google Scholar]
- 11. Aldaye F. A., Sleiman H. F., Angew. Chem. Int. Ed. 2006, 45, 2204–2209; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2006, 118, 2262–2267. [Google Scholar]
- 12. Zimmermann J., Cebulla M. P. J., Mönninghoff S., von Kiedrowski G., Angew. Chem. Int. Ed. 2008, 47, 3626–3630; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2008, 120, 3682–3686. [Google Scholar]
- 13. Yu H., Alexander D. T. L., Aschauer U., Häner R., Angew. Chem. Int. Ed. 2017, 56, 5040–5044; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2017, 129, 5122–5126. [Google Scholar]
- 14. Wang W., Wan W., Zhou H.-H., Niu S., Li A. D. Q., J. Am. Chem. Soc. 2003, 125, 5248–5249. [DOI] [PubMed] [Google Scholar]
- 15. Chidchob P., Edwardson T. G. W., Serpell C. J., Sleiman H. F., J. Am. Chem. Soc. 2016, 138, 4416–4425. [DOI] [PubMed] [Google Scholar]
- 16. Trinh T., Chidchob P., Bazzi H. S., Sleiman H. F., Chem. Commun. 2016, 52, 10914–10917. [DOI] [PubMed] [Google Scholar]
- 17. Liu K., Zheng L., Ma C., Göstl R., Herrmann A., Chem. Soc. Rev. 2017, 46, 5147–5172. [DOI] [PubMed] [Google Scholar]
- 18. Albert S. K., Golla M., Thelu H. V. P., Krishnan N., Varghese R., Chem. Eur. J. 2017, 23, 8348–8352. [DOI] [PubMed] [Google Scholar]
- 19. Vyborna Y., Vybornyi M., Häner R., J. Am. Chem. Soc. 2015, 137, 14051–14054. [DOI] [PubMed] [Google Scholar]
- 20. Vyborna Y., Vybornyi M., Häner R., Chem. Commun. 2017, 53, 5179–5181. [DOI] [PubMed] [Google Scholar]
- 21. Vyborna Y., Vybornyi M., Häner R., Bioconjugate Chem. 2016, 27, 2755–2761. [DOI] [PubMed] [Google Scholar]
- 22. Aida T., Meijer E. W., Stupp S. I., Science 2012, 335, 813–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mattia E., Otto S., Nat. Nanotechnol. 2015, 10, 111–119. [DOI] [PubMed] [Google Scholar]
- 24. Albertazzi L., Martinez-Veracoechea F. J., Leenders C. M. A., Voets I. K., Frenkel D., Meijer E. W., Proc. Natl. Acad. Sci. USA 2013, 110, 12203–12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Görl D., Zhang X., Stepanenko V., Würthner F., Nat. Commun. 2015, 6, 7009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brunsveld L., Zhang H., Glasbeek M., Vekemans J. A. J. M., Meijer E. W., J. Am. Chem. Soc. 2000, 122, 6175–6182. [Google Scholar]
- 27. Müller M. K., Brunsveld L., Angew. Chem. Int. Ed. 2009, 48, 2921–2924; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2009, 121, 2965–2968. [Google Scholar]
- 28. Petkau-Milroy K., Sonntag M. H., van Onzen A. H. A. M., Brunsveld L., J. Am. Chem. Soc. 2012, 134, 8086–8089. [DOI] [PubMed] [Google Scholar]
- 29. Petkau-Milroy K., Uhlenheuer D. A., Spiering A. J. H., Vekemans J. A. J. M., Brunsveld L., Chem. Sci. 2013, 4, 2886. [Google Scholar]
- 30. Debets M. F., van Berkel S. S., Schoffelen S., Rutjes F. P. J. T., van Hest J. C. M., van Delft F. L., Chem. Commun. 2010, 46, 97–99. [DOI] [PubMed] [Google Scholar]
- 31. Machado A. H. E., Lundberg D., Ribeiro A. J., Veiga F. J., Miguel M. G., Lindman B., Olsson U., Langmuir 2010, 26, 13102–13109. [DOI] [PubMed] [Google Scholar]
- 32. Markham N. R., Zuker M. in Bioinformatics (Ed.: J. M. Keith), Humana Press, Totowa, NJ, 2008, pp. 3–31. [Google Scholar]
- 33. Hobert E. M., Doerner A. E., Walker A. S., Schepartz A., Isr. J. Chem. 2013, 53, 567–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sun H., Hunter C. A., Navarro C., Turega S., J. Am. Chem. Soc. 2013, 135, 13129–13141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary


