Abstract
Aim
This study explored the effects of blood flow restriction (BFR) on mRNA responses of PGC‐1α (total, 1α1, and 1α4) and Na+,K+‐ATPase isoforms (NKA; α1‐3, β1‐3, and FXYD1) to an interval running session and determined whether these effects were related to increased oxidative stress, hypoxia, and fibre type‐specific AMPK and CaMKII signalling, in human skeletal muscle.
Methods
In a randomized, crossover fashion, 8 healthy men (26 ± 5 year and 57.4 ± 6.3 mL kg−1 min−1) completed 3 exercise sessions: without (CON) or with blood flow restriction (BFR), or in systemic hypoxia (HYP, ~3250 m). A muscle sample was collected before (Pre) and after exercise (+0 hour, +3 hours) to quantify mRNA, indicators of oxidative stress (HSP27 protein in type I and II fibres, and catalase and HSP70 mRNA), metabolites, and α‐AMPK Thr172/α‐AMPK, ACC Ser221/ACC, CaMKII Thr287/CaMKII, and PLBSer16/PLB ratios in type I and II fibres.
Results
Muscle hypoxia (assessed by near‐infrared spectroscopy) was matched between BFR and HYP, which was higher than CON (~90% vs ~70%; P < .05). The mRNA levels of FXYD1 and PGC‐1α isoforms (1α1 and 1α4) increased in BFR only (P < .05) and were associated with increases in indicators of oxidative stress and type I fibre ACC Ser221/ACC ratio, but dissociated from muscle hypoxia, lactate, and CaMKII signalling.
Conclusion
Blood flow restriction augmented exercise‐induced increases in muscle FXYD1 and PGC‐1α mRNA in men. This effect was related to increased oxidative stress and fibre type‐dependent AMPK signalling, but unrelated to the severity of muscle hypoxia, lactate accumulation, and modulation of fibre type‐specific CaMKII signalling.
Keywords: AMP‐activated protein kinase, blood flow restriction, Na+‐K+‐ATPase, oxidative stress, PGC‐1α, reactive oxygen species
1. INTRODUCTION
A decline in the ability to perform high‐intensity exercise coincides with a critical threshold of locomotor muscle fatigue,1 suggesting factors within or around skeletal muscle partly limit human exercise performance. One of these factors is the capacity for maintenance of resting transmembrane sodium (Na+) and potassium (K+) ion gradients,2, 3 which is determined primarily by the activity of the Na+,K+‐ATPase (NKA).4, 5, 6 The functional NKA complex is composed of several subunits, including a catalytic α, regulatory β, and an ancillary protein, phospholemman (FXYD), and these subunits are expressed as multiple isoforms (α1‐3, β1‐3, and FXYD1) in human skeletal muscle.7 Most of the isoforms have been shown to be regulated at the mRNA level by exercise,8, 9, 10, 11, 12, 13 and their relative distribution and assembly are critical for maximal NKA activity.14 However, little is known about the effects of different ergogenic interventions (eg hypoxia and cold‐water immersion) on exercise‐induced modulation of the expression of these isoforms in human skeletal muscle.7, 11, 15
Another limiting factor for human exercise performance is the muscle capability to generate ATP via oxidative phosphorylation.16 Accordingly, increases in both mitochondrial respiratory function and content (as assessed by citrate synthase activity) have been temporally related to enhanced exercise performance.17 A key determinant of these endurance‐type adaptations is the transcriptional co‐activator, the peroxisome proliferator‐activated receptor‐γ co‐activator 1α (PGC‐1α). It has recently been shown that human muscle contains different PGC‐1α isoforms,18 and these isoforms may regulate different aspects of the muscle response to exercise.19 Furthermore, there is evidence for18, 20 and against21, 22 some of these isoforms (PGC‐1α1 and PGC‐1α4) responding to different types of exercise. But like the NKA isoforms, there is limited evidence about the effects of different ergogenic strategies on the regulation of the level of these isoforms in human muscle.
One potential strategy to augment increases in the expression of these isoforms could be to exercise with reduced muscle blood flow (blood flow restriction, BFR). To our knowledge, no study has explored the effects of BFR on exercise‐induced mRNA responses of NKA isoforms and FXYD1 in skeletal muscle. Given these isoforms likely exert different functions in skeletal muscle23, 24 and are regulated at the mRNA level by muscle activity,7, 11 this would seem of great physiological relevance. Furthermore, although 3 studies have assessed the effects of BFR on exercise‐induced changes in PGC‐1α mRNA content in human muscle, only one study measured different PGC‐1α transcripts.25 In these studies, BFR either attenuated,25 augmented,26 or had no effect27 on changes in PGC‐1α mRNA content after exercise. These contradictory findings are likely related to the different experimental approaches used (eg type and intensity of exercise and the timing of BFR). Thus, to maximize the effectiveness of BFR training, there is need to improve our understanding of the physiological stressors involved in the regulation of the expression of NKA and PGC‐1α isoforms and how these stressors can be influenced by BFR in humans.
Blood flow restriction has typically been achieved by inflation of an occlusion cuff fixed around the limb(s) proximal to locomotor muscles and has been applied during various exercise modes, including walking, cycling, and resistance training.28, 29, 30 Inflation of the cuff compromises both the arterial and venous flow,31, 32 resulting in a hypoxic and more acidic intramuscular environment.31 Successive deflation of the cuff promotes local reactive hyperaemia.33 In combination, these mechanisms seem a powerful stimulus for amplifying the transient bursts in reactive oxygen species (ROS) levels and the resultant oxidative stress that accompany consecutive bouts of exercise.34, 35, 36 In rat and human skeletal muscles, ROS appear required for exercise‐induced increases in the mRNA content of the catalytic NKA isoforms (α1, α2, α3),10 and those of PGC‐1α in skeletal muscle cells in vitro.37 In the latter study, the effect of ROS was mediated by activation of the AMP‐activated protein kinase (AMPK). Several human studies have reported simultaneous increases in muscle AMPK activation (as assessed by protein phosphorylation) and PGC‐1α mRNA,38, 39, 40 supporting AMPK may be involved in the regulation of these mRNA transcripts. However, it remains unknown in humans whether muscle oxidative stress may be related to the effect of BFR on NKA‐ and PGC‐1α‐isoform mRNA content in skeletal muscle, and whether this effect is associated with increased AMPK signalling.
Disturbances in blood flow during exercise invoked by BFR may also affect muscle ion (K+ and Ca2+) homeostasis by modulating the function of ion channels and transport systems, including NKA.41, 42 Furthermore, substantial increases in muscle release of, and interstitial and venous blood, lactate have been reported in response to BFR exercise.31, 43 Both modulation of intracellular ion concentrations13, 44 and lactate45 have been implicated in transient, excitation‐induced increases in NKA and/or PGC‐1α mRNA levels. However, no study has explored if lactate is associated with exercise‐induced increases in the mRNA content of NKA and PGC‐1α isoforms in humans. In 2 independent cell culture studies, the Ca2+/calmodulin‐dependent protein kinase (CaMK) was shown to mitigate increases in NKA and PGC‐1α mRNA invoked by ionic perturbations in vitro.13, 46 Thus, activation of CaMKII due to ionic perturbations, or indirectly through stimulation of ROS production,47 could be another mechanism by which BFR could augment increases in NKA and PGC‐1α mRNA content in human muscle. However, this hypothesis remains to be evaluated.
The first aim of this study was to explore the effect of BFR on changes in the mRNA content of PGC‐1α (total and isoform 1α1 and 1α4) and NKA isoforms (α1‐3, β1‐3, and FXYD1) in response to a single, moderate‐intensity, interval exercise session in human skeletal muscle. The second aim was to elucidate some of the potential cellular stressors and molecular signalling proteins involved. Our working hypotheses were as follows: (1) BFR would augment the effect of exercise on the expression of NKA and PGC‐1α isoforms, and (2) higher expression of these isoforms would coincide with increases in markers representative of responses to oxidative stress (HSP27 protein content in type I and II muscle fibres and whole‐muscle catalase and heat‐shock protein 70, HSP70 mRNA), AMPK signalling (as assessed by the ACC Ser221/ACC ratio), and CaMKII activation (as determined by CaMKII Thr287/CaMKII). Evidence from astrocytes in vitro suggests the reperfusion phase and resulting tissue re‐oxygenation, rather than hypoxia, may be a primary stimulus underlying increases in the expression of NKA isoforms in response to hypoxia‐reperfusion.48 Thus, we designed our BFR protocol to induce multiple bursts in hypoxia‐reperfusion by incorporating repeated exercise bouts with BFR interspersed by periods with cuff deflation. We also included a hypoxic condition (ie exercising in normobaric, systemic hypoxia) to assess the hypothesis that (3) exercise‐induced increases in isoform expression in the BFR condition would not be attributed to the concomitant muscle hypoxia.
2. RESULTS
2.1. Na+,K+‐ATPase and FXYD1 mRNA transcripts (Figures 1 and 2)
Figure 1.
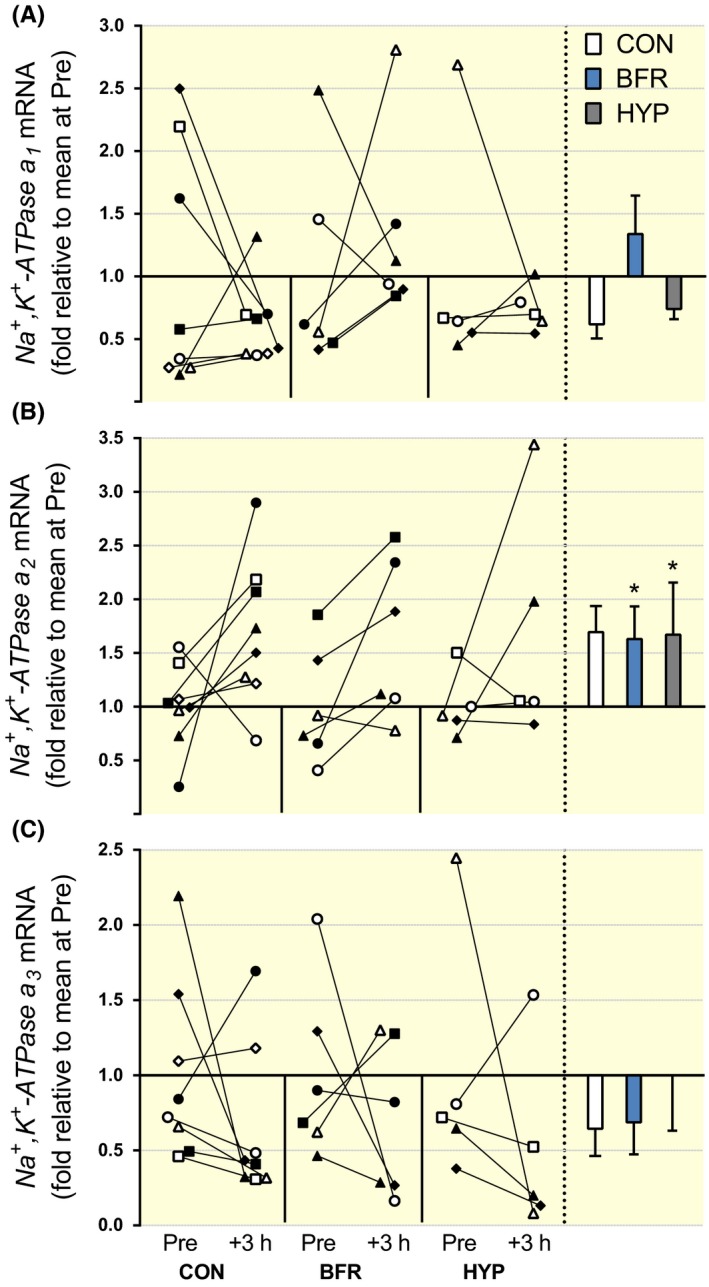
NKA‐α‐isoform mRNA responses to moderate‐intensity interval running performed without or with blood flow restriction or in systemic hypoxia. (A) α1, (B) α2, and (C) α3, mRNA content. Individual changes from before (Pre) to 3 hours after exercise (+3 hours) are displayed on the left with each symbol representing one participant across trials and figures. On the right are bars representing mean (±SEM) changes relative to Pre for exercise alone (CON, white; n = 8), with blood flow restriction (BFR, blue; n = 6) or in systemic hypoxia (HYP, grey; n = 5). *P ≤ .05, different from Pre
Figure 2.
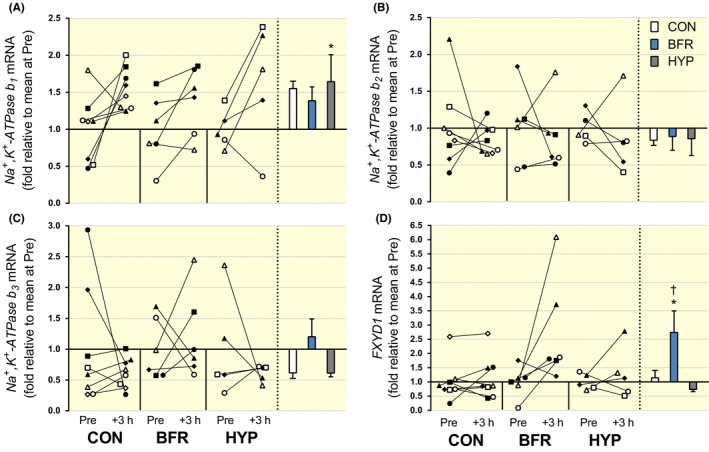
NKA‐β‐isoform and FXYD1 mRNA responses to moderate‐intensity interval running performed without or with blood flow restriction or in systemic hypoxia. (A) β1, (B) β2, (C) β3 and (D) FXYD1, mRNA content. Individual changes from before (Pre) to 3 hours after exercise (+3 hours) are displayed on the left with each symbol representing one participant across trials and figures. On the right are bars representing mean (±SEM) changes relative to Pre for exercise alone (CON, white; n = 8), with blood flow restriction (BFR, blue; n = 6) or in systemic hypoxia (HYP, grey; n = 5). *P < .05, different from Pre
NKAα1 mRNA was not changed in BFR (P = .90, d = 0.44), in CON (P = .39, d = 0.54), or in HYP (P = .43, d = 0.47; Figure 1A). There were no significant differences among conditions for the change in NKAα1 mRNA from Pre to +3 hours (P ≥ .54, d = 0.19‐0.62). NKAα2 mRNA increased from Pre to +3 hours in BFR (P = .050, d = 0.90), but there was no change in CON (P = .089, d = 1.1) or in HYP (P = .18, d = 1.0; Figure 1B). There were no differences among conditions for the change in NKAα2 mRNA from Pre to +3 hours (P ≥ .31, d = 0.26 to 0.45). NKAα3 mRNA was not changed in BFR (P = .47, d = 0.57), in CON (P = .26, d = 0.63), or in HYP (P = .071, d = 1.1; Figure 1C), and there were no differences among conditions for the change in NKAα3 mRNA from Pre to +3 hours (P ≥ .31, d = 0.11‐0.96).
NKAβ1 mRNA increased from Pre to +3 hours in CON (P = .049, d = 1.2), but there was no change in BFR (P = .064, d = 0.79) or in HYP (P = .077, d = 1.1; Figure 2A). There were no differences among conditions for the change in NKAβ1 mRNA from Pre to +3 hours (P ≥ .47, d = 0.04‐0.53). NKAβ2 mRNA was not changed in BFR (P = .69, d = 0.24), in CON (P = .51, d = 0.40), or in HYP (P = .55, d = 0.45; Figure 2B), and there were no differences among conditions for the change in NKAβ2 mRNA from Pre to +3 hours (P ≥ .76, d = 0.01‐0.10). NKAβ3 mRNA was also not changed in BFR (P = .63, d = 0.34), in CON (P = .58, d = 0.55), or in HYP (P = .40, d = 0.74; Figure 2C), with no differences among conditions for the change in NKAβ3 mRNA from Pre to +3 hours (P ≥ .34, d = 0.03‐0.73).
FXYD1 mRNA increased from Pre to +3 hours in BFR (P = .058, d = 1.1), but there was no change in CON (P = .51, d = 0.20) or in HYP (P = .42, d = 51; Figure 2D). There were no differences among conditions for the change in FXYD1 mRNA from Pre to +3 hours (P ≥ .19), although the effect size was large (0.95) for the comparison BFR vs CON. The effect size was lower for the remaining comparisons: CON vs HYP (d = 0.33) and BFR vs HYP (d = 0.66).
2.2. PGC‐1α mRNA transcripts (Figure 3)
Figure 3.
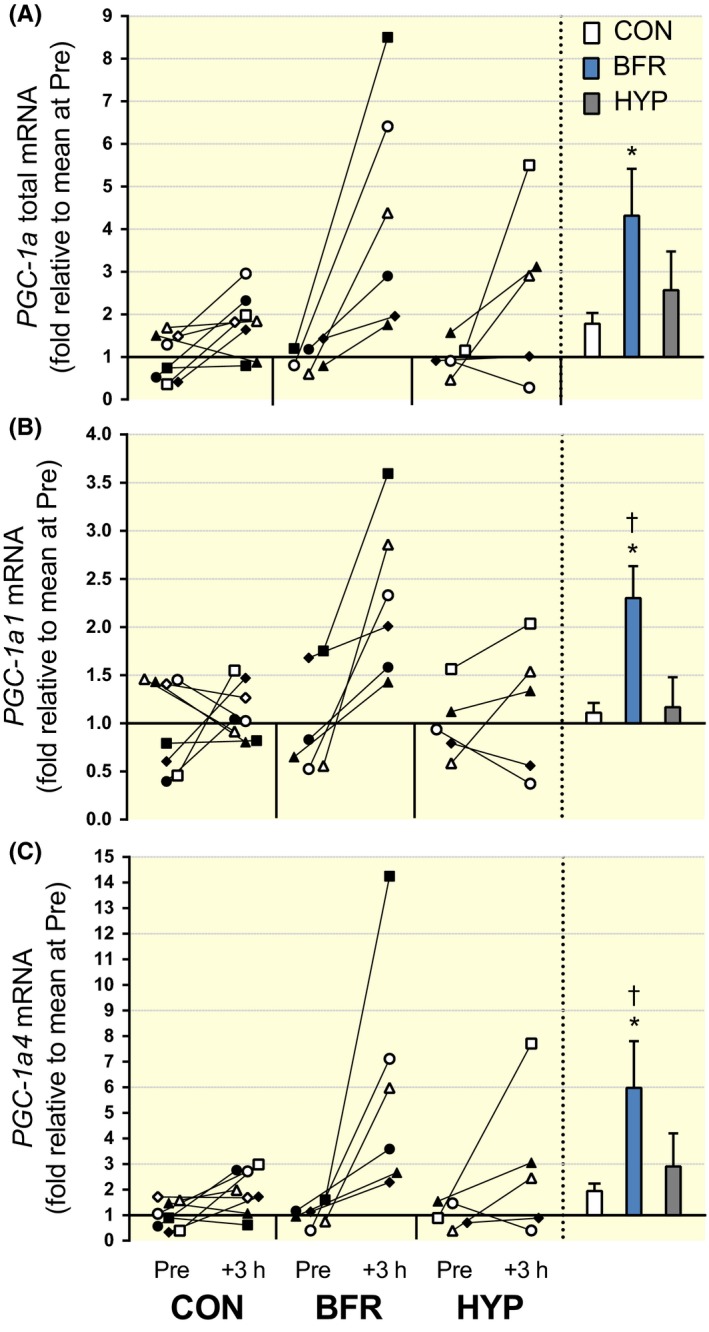
PGC‐1α total and PGC‐1α‐isoform mRNA responses to moderate‐intensity interval running performed without or with blood flow restriction or in systemic hypoxia. (A) PGC‐1α total, (B) PGC‐1α1 and (C) PGC‐1α4, mRNA content. Individual changes from before (Pre) to 3 hours after exercise (+3 hours) are displayed on the left with each symbol representing one participant across trials and figures. On the right are bars representing mean (±SEM) changes relative to Pre for exercise alone (CON, white; n = 8), with blood flow restriction (BFR, blue; n = 6) or in systemic hypoxia (HYP, grey; n = 5).*P ≤ .05, different from Pre; †P ≤ .05, different from CON and HYP
PGC‐1α total mRNA increased from Pre to +3 hours in BFR (P = .031, d = 1.3) and in CON (P = .047, d = 1.1), but was not altered in HYP (P = .12, d = 1.1; Figure 3A). There were no differences among conditions for the change in PGC‐1α total mRNA from Pre to +3 hours, although there was a low P‐value (.088) and large effect size (1.11) for the comparison BFR vs CON. The P‐value was higher and the effect size lower for the remaining comparisons: CON vs HYP (P = .36, d = 0.74) and BFR vs HYP (P = .38, d = 0.52). PGC‐1α1 mRNA increased from Pre to +3 hours in BFR (P = .010, d = 1.36), but was not altered in CON (P = .65, d = 0.29) or in HYP (P = .52, d = 0.35; Figure 3B). The increase in BFR was greater compared to that in CON (P = .047, d = 1.34), but not compared to that in HYP, although the P‐value was low (.075) and the effect size large (1.19) for this comparison. There was no difference for the change from Pre to +3 hours between CON and HYP (P = .75, d = 21). PGC‐1α4 mRNA increased from Pre to +3 hours in BFR (P = .037, d = 1.3), but was not increased in CON (P = .10, d = 1.1) or in HYP (P = .18, d = 0.96). There were no differences among conditions for the change in PGC‐1α4 mRNA from Pre to +3 hours (P ≥ .11), although the effect size was large (1.1) for the comparison BFR vs CON. The effect size was moderate for the remaining comparisons: CON vs HYP (0.58) and BFR vs HYP (0.61).
2.3. Muscle hypoxia and indicators of responses to oxidative stress (Figure 4)
Figure 4.
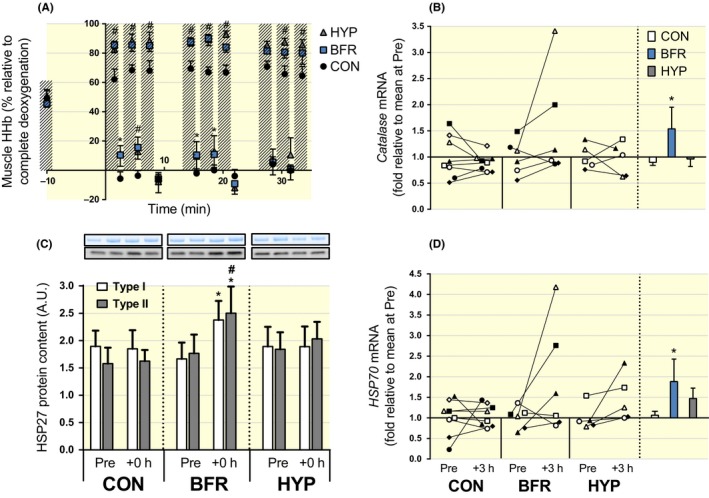
Changes in muscle hypoxia and indicators of responses to oxidative stress in response to moderate‐intensity interval running performed without or with blood flow restriction or in systemic hypoxia. (A) Muscle hypoxia (ie deoxygenated haemoglobin, Muscle HHb) as assessed by near‐infrared spectroscopy during moderate‐intensity running without (CON, black symbols; n = 8) or with blood flow restriction (BFR, blue symbols; n = 8), or in systemic hypoxia (HYP, grey symbols; n = 8). Hashed bars represent exercise bouts. #P ≤ .05, BFR and HYP different from CON. *P ≤ .05, BFR different from CON. (B) Catalase, and (D) heat‐shock protein 70 (HSP70), mRNA expression. Individual changes from before (Pre) to 3 hours after exercise (+3 hours) are displayed on the left with each symbol representing one participant across trials and figures. On the right are bars representing mean (±SEM) changes relative to Pre for moderate‐intensity running without (CON, white) or with blood flow restriction (BFR, blue), or in systemic hypoxia (HYP, grey). *P ≤ .05, different from Pre. (C) Heat‐shock protein 27 protein content in type I (white bars) and type II fibres (grey bars) at rest before (Pre) and immediately after (+0 hour) exercise. Representative Western blots are indicated above the corresponding bars. *P ≤ .05, increase vs Pre within BFR. #P ≤ .05, BFR different from CON within fibre type. Data are means ± SEM
Muscle hypoxia, as assessed by deoxygenated haemoglobin concentration (muscle HHb), was higher during exercise in BFR (P = .007) and HYP (P = .007), relative to CON, except for the 7th bout of exercise (P ≥ .45). During the recovery from the 1st (P = .002), 4th (P = .005), and 5th bout (P = .016), muscle HHb was higher in BFR, relative to CON. During the recovery from the 2nd bout, muscle HHb was higher in BFR (P < .001) and HYP (P = .037) compared to CON (Figure 4A). No differences were detected between BFR and HYP at any time point (P = .1). Catalase mRNA content increased in BFR (P = .024, d = 0.70), but there was no change in CON (P = .881, d = 0.34) or in HYP (P = .505, d = 0.15; Figure 4B). HSP27 protein content increased from Pre to +0 hour in both type I (P = .003) and type II fibres (P = .004) in BFR, with the increase in type II fibres being greater relative to CON (P = .030). No changes occurred in CON (P ≥ .80) or in HYP (P ≥ .32; Figure 4C). HSP70 mRNA increased in BFR (P = .057, d = 0.86), but there was no significant change in CON (P = .669, d = 0.18) or in HYP (P = .176, d = 0.95; Figure 4D).
2.4. Muscle metabolites (Figure 5)
Figure 5.
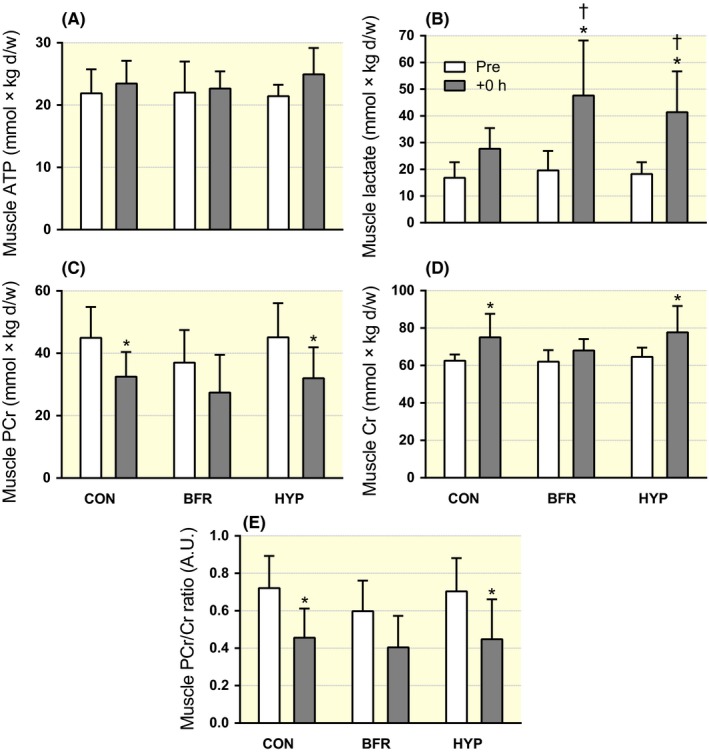
Changes in muscle ATP and lactate concentration in response to moderate‐intensity interval running performed without (CON) or with blood flow restriction (BFR) or in systemic hypoxia (HYP). (A) ATP, (B) lactate, (C) phosphocreatine (PCr), (D) creatine (Cr) and (E) PCr/Cr ratio before (Pre, white) and immediately after exercise (+0 hour, grey). n = 8 for all conditions. Data are means ± SEM. *P < .05, different from Pre. †P < .05, different from CON
ATP remained unchanged in all conditions (P = .904; Figure 5A). Lactate increased in BFR (P < .001) and in HYP (P < .001), but was not significantly changed in CON (P = .075). The increases in BFR and HYP were greater than CON (P = .017 and .015, respectively; Figure 5B). PCr decreased in CON (P = .027) and in HYP (P = .011), but was not altered in BFR (P = .335; Figure 5C). Similarly, Cr increased in CON (P = .027) and in HYP (P = .011), but was not changed in BFR (P = .335; Figure 5D). The PCr/Cr ratio decreased in CON (P = .026) and in HYP (P = .018), but was not altered in BFR (P = .261; Figure 5E). PCr and Cr content, and PCr/Cr ratio, were not different between conditions at Pre or +0 hour (P > .05).
2.5. Venous blood lactate, pH, and K+ concentration (Figure 6)
Figure 6.
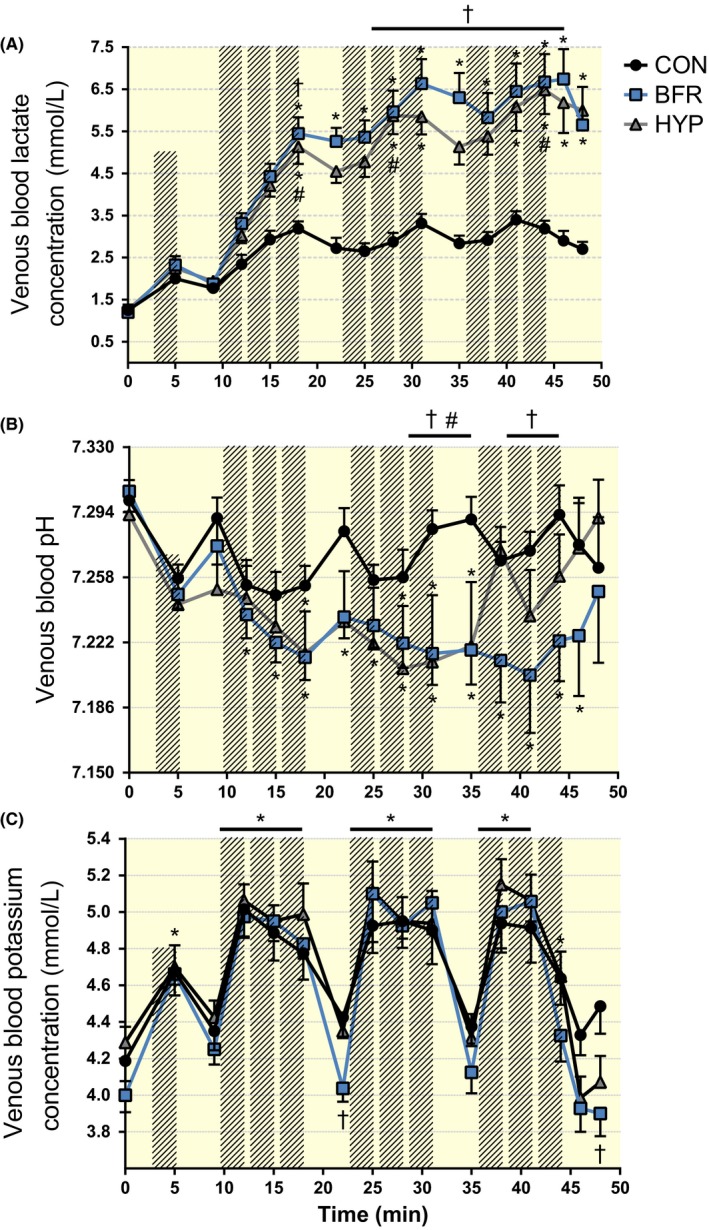
Changes in venous blood lactate, pH and potassium ion (K+) concentration in response to moderate‐intensity interval running performed without or with blood flow restriction or in systemic hypoxia. (A) Lactate, (B) pH and (C) K+ concentration during moderate‐intensity interval running without (CON, black symbols) or with blood flow restriction (BFR, blue symbols), or in systemic hypoxia (HYP, grey symbols). Hashed bars represent running bouts. n = 8 for all conditions. Data are means ± SEM. *P < .05, different from rest; †P < .05, BFR different from CON; #P < .05, HYP different from CON
In BFR, blood lactate concentration ([lac−]) increased (P < .05) after the 3rd exercise bout and remained elevated throughout the trial compared to rest. Blood [lac−] was higher (P < .05) in BFR than in CON after the 3rd bout, the 5th to 9th bout, and after 3 minutes of recovery. In HYP, blood [lac−] increased (P < .05) after the 3rd, 5th, 6th, 8th and 9th bout and in recovery, compared to rest. Blood [lac−] was higher (P < .05) in HYP than in CON after the 3rd, 5th and 9th exercise bout. In CON, blood [lac−] remained unchanged throughout the trial, compared to rest (P > .05; Figure 6A).
In BFR, blood pH dropped (P < .05) following the 1st exercise bout and remained lower (P < .05) compared to rest throughout the trial and 3 minutes into recovery, but returned to resting level after 6 minutes of recovery (P > .05). The drop in pH in BFR was lower, relative to CON, after the 6th, before the 7th, and after the 8th and 9th, bout relative to CON (P < .05). In HYP, blood pH was lower, compared to rest, following the 3rd, 5th, and 6th bout, and before the 7th bout, but returned to resting level after the 7th bout, from where it remained unchanged. The drop in pH in HYP was lower following the 6th and before the 7th bout, relative to CON (P < .05). In CON, blood pH remained unchanged throughout the trial (P > .05; Figure 6B).
In all trials, blood K+ concentration ([K+]) increased after warm‐up, and after the 1st to 8th exercise bout, compared to rest. In CON, blood [K+] was also elevated (P < .05) after the 9th bout, relative to rest. Compared to CON, blood [K+] was lower (P < .05) in BFR 4 minutes into recovery from the 3rd bout, and 6 minutes into recovery from the 9th bout, with no differences at other time points (P > .05), nor between HYP and CON at all time points (P > .05; Figure 6C).
2.6. AMPK and ACC total and phosphorylated protein content (Figures 7 and 8)
Figure 7.
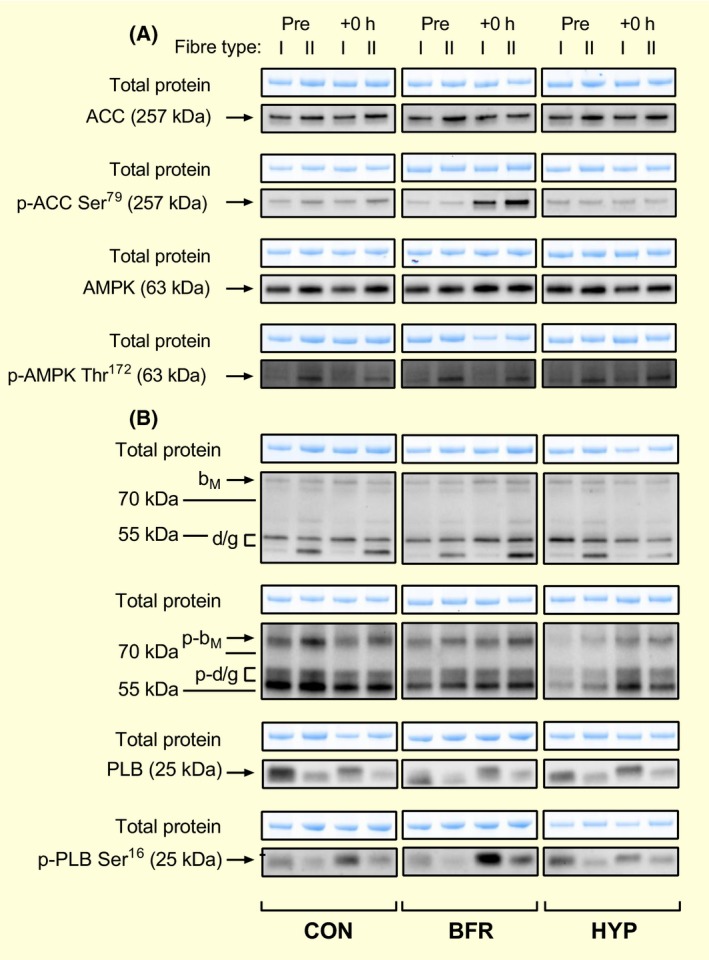
Representative blots for AMPKα, ACC, CaMKII and phospholamban (PLB) protein abundance and phosphorylation in type I and II human skeletal muscle fibres. Protein abundance and phosphorylation of (A) AMPK and ACC, and (B) CaMKII and PLB in human skeletal muscle in response to moderate‐intensity interval running without (CON) or with blood flow restriction (BFR), or in systemic hypoxia (HYP) before (Pre) and immediately after (+0 hour) exercise. Total protein was determined in each lane from the stain‐free gel images obtained after electrophoresis. CaMKII isoforms (βM and σ/γ) are indicated in (B)
Figure 8.
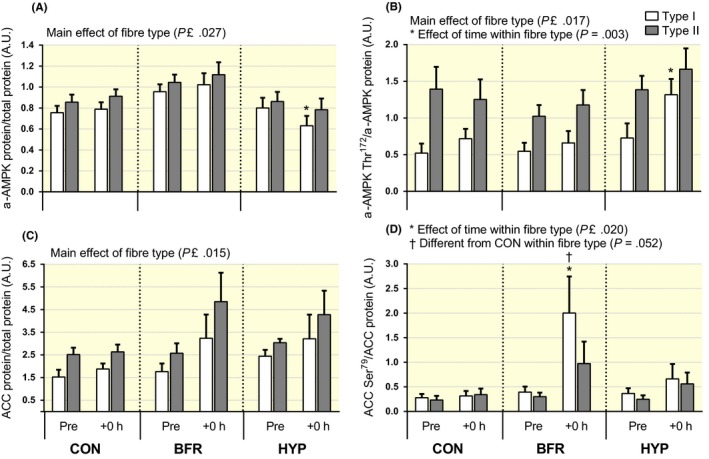
Changes in AMPKα and ACC protein abundance and phosphorylation in type I and II human skeletal muscle fibres in response to moderate‐intensity interval running performed without or with blood flow restriction or in systemic hypoxia. (A) AMPKα protein, (B) AMPKα phosphorylation at Thr172 normalized to AMPKα protein, (C) ACC protein and (D) ACC phosphorylation at Ser79 normalized to ACC protein in type I (white bars) and type II (grey bars) fibres before (Pre) and immediately after (+0 hour) exercise. n = 8 for all conditions. Data are means ± SEM. *P < .05, different from rest within condition and fibre type; †P ≤ .05, BFR different from HYP in (A), and from CON in (D)
Representative blots for AMPK and ACC are shown in Figure 7A.
In HYP, α‐AMPK protein abundance decreased (P = .023) from Pre to +0 hour in type I, but did not change in type II (P = .11) fibres. In BFR and CON, α‐AMPK abundance was not altered in either fibre type (P ≥ .42; Figure 8A). The α‐AMPK abundance was higher in type II vs type I fibres in all conditions (P ≤ .027). In HYP, the phosphorylation of α‐AMPK at Thr172 relative to total α‐AMPK abundance (α‐AMPK Thr172/α‐AMPK) increased in type I (P = .003), but not in type II (P = .558) fibres. In BFR and CON, there was no change in α‐AMPK Thr172/α‐AMPK in either fibre type (P ≥ .11). The α‐AMPK Thr172/α‐AMPK was higher in type II vs type I fibres in all conditions (P ≤ .017; Figure 8B).
ACC protein abundance was not altered in both fibre types in all conditions (P ≥ .17), and overall, it was higher in type II vs type I fibres (P ≤ .015; Figure 8C). The phosphorylation of ACC at Ser79 to total ACC abundance (ACC Ser79/ACC) increased from Pre to +0 hour in type I fibres in BFR (P ≤ .020), with the increase being higher relative to CON (P = .052). In the same condition, there was no change in ACC Ser79/ACC in type II fibres (P = .260). No changes in ACC Ser79/ACC occurred in CON and HYP (P ≥ .21; Figure 8D).
2.7. CaMKII and phospholamban total and phosphorylated protein content (Figures 7 and 9)
Figure 9.
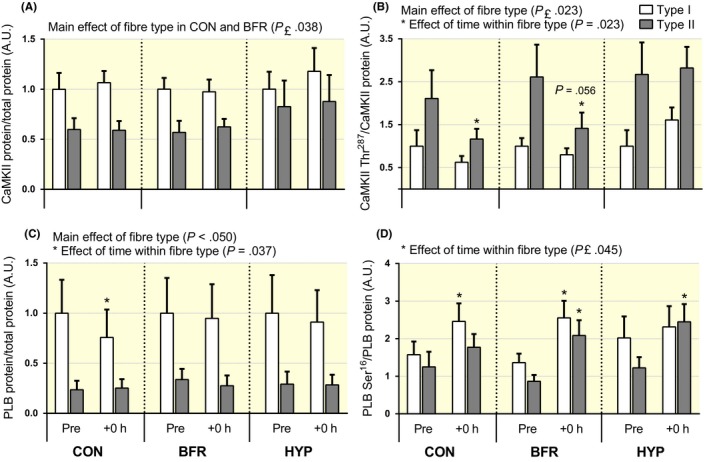
Changes in CaMKII and phospholamban (PLB) protein abundance and phosphorylation in type I and II human skeletal muscle fibres in response to moderate‐intensity interval running performed without or with blood flow restriction or in systemic hypoxia. (A) CaMKII protein, (B) CaMKII phosphorylation at Thr287 normalized to CaMKII protein, (C) PLB protein and (D) PLB phosphorylation at Ser16 normalized to PLB protein in type I (white bars) and type II (grey bars) fibres before (Pre) and immediately after (+0 hour) exercise. n = 8 for all conditions. Data are means ± SEM. *P < .05, different from rest within condition and fibre type
Representative blots for CaMKII and PLB are shown in Figure 7B.
CaMKII protein abundance did not change in either fibre type in all conditions (P ≥ .11). In BFR and CON, it was higher in type I vs type II fibres (main effect of fibre type, P ≤ .038), but not different between fibre types in HYP (P = .20; Figure 9A). Phosphorylation of CaMKII at Thr287 to total CaMKII abundance (CaMKII Thr287/CaMKII) decreased in type II fibres in CON (P = .023) and tended to decrease in BFR (P = .056), but did not change in HYP (P = .75). No changes in CaMKII Thr287/CaMKII occurred in type I fibres in any condition (P ≥ .21). CaMKII Thr287/CaMKII was significantly higher in type II vs type I fibres in all conditions (main effect of fibre type, P ≤ .023; Figure 9B).
In type I fibres, PLB protein abundance decreased in CON (P = .037), whereas it did not change in BFR (P = .79) or in HYP (P = .43) in the same fibre type. PLB abundance did not change in type II fibres in any condition (P ≥ .29). PLB abundance was lower in type II vs type I fibres (main effect of fibre type, P ≤ .050; Figure 9C). The phosphorylation of PLB at Ser16 relative to total PLB abundance (PLB Ser16/PLB) increased in type I fibres in CON (P = .023) and in BFR (P = .010), but it remained unchanged in HYP in the same fibre type (P = .41). In type II fibres, PLB Ser16/PLB increased in BFR (P ≤ .026) and in HYP (P = .025), but did not change in CON (P = .35; Figure 9D).
3. DISCUSSION
The main novel findings of the present study, which are summarized in Figure 10, were that moderate‐intensity interval running performed with blood flow restriction (BFR) increased the mRNA content of the NKA regulatory subunit, FXYD1 (~2.7‐fold), and of PGC‐1α (total, 4.3‐fold), 1α1 (2.3‐fold), and 1α4 (6‐fold), in human skeletal muscle. These responses to BFR were associated with increases in indicators of responses to oxidative stress (HSP27 protein in both fibre types, 70%; catalase and HSP70 mRNA, 1.5‐ to 1.9‐fold) and fibre type‐dependent AMPK downstream signalling, reflected by elevated (2‐fold) ACC Ser79/ACC ratio in type I, but not in type II, fibres. Furthermore, the effect of BFR on changes in FXYD1 and PGC‐1α mRNA levels was unrelated to the severity of muscle hypoxia, lactate accumulation, and fibre type‐specific modulation of the CaMKII Thr287/CaMKII ratio.
Figure 10.
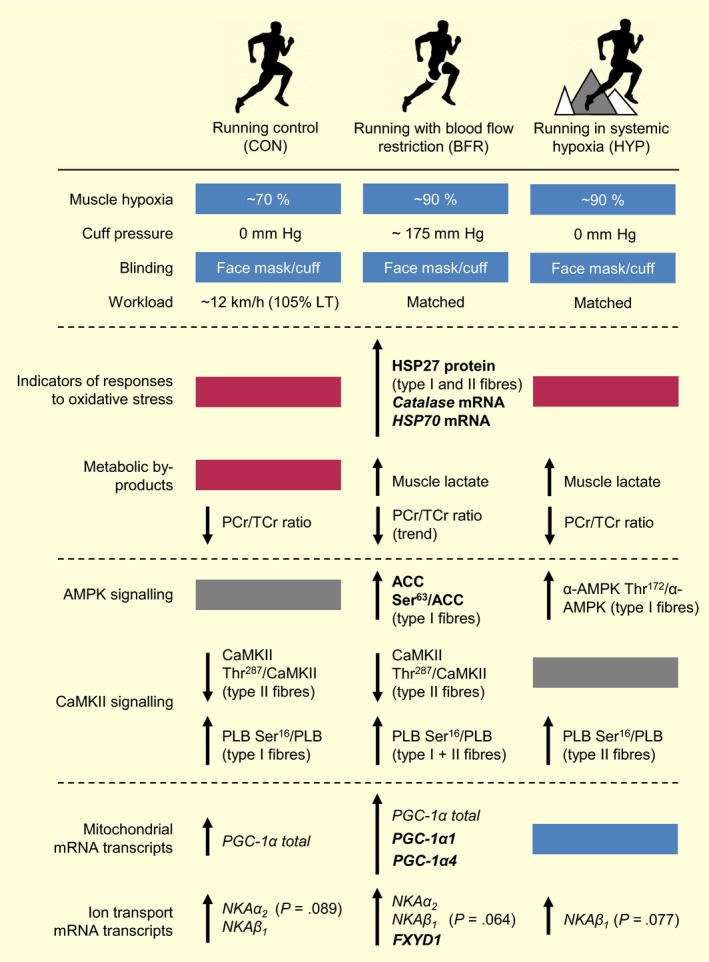
Summary of key findings. Effects of moderate‐intensity interval running without (CON) or with blood flow restriction (BFR), or in normobaric, systemic hypoxia (HYP) on the mRNA content of Na+,K+‐ATPase (NKAα1‐3, NKAβ1‐3, FXYD1) and PGC‐1α (total, 1α1, 1α4) isoforms, indicators of responses to oxidative stress (HSP27 protein content in type I and II muscle fibres, catalase and heat‐shock protein 70,HSP70, mRNA content), muscle hypoxia (ie deoxygenated haemoglobin as measured by near‐infrared spectroscopy), lactate concentration, and AMPK and CaMKII signalling in the skeletal muscle of men. “p‐” denotes phosphorylation; ACC, Acetyl‐CoA carboxylase; AMPK, 5′ AMP‐activated protein kinase; CaMKII, Ca2+/calmodulin‐dependent protein kinase II; PLB, phospholamban; LT, lactate threshold
3.1. Blood flow restriction augments increases in FXYD1 mRNA content after moderate‐intensity interval running in human skeletal muscle
A novel result was that FXYD1 mRNA content increased (2.7‐fold) due to BFR (Figure 2D). Despite similar increases in deoxygenated HHb between the BFR and HYP conditions (Figure 4A), systemic hypoxia was without impact on FXYD1 expression. This suggests the magnitude of muscle hypoxia was not important for the BFR‐induced increase in FXYD1. Nor may the increase be related to the severity of metabolic stress, as muscle lactate increased, whereas PCr content and PCr/Cr ratio decreased, to a similar level (+0 hour) in the BFR and HYP condition (Figure 5). In contrast, the induction of FXYD1 mRNA in BFR was accompanied by increases in indicators of responses to oxidative stress (Figure 4), implicating ROS in the regulation of FXYD1 mRNA by BFR exercise in human skeletal muscle. In agreement, FXYD1 overexpression has been shown to protect myocytes against ROS‐induced NKA dysfunction,49 highlighting a ROS‐protective effect of elevated FXYD1 content. In cell culture, AMPK can be activated by ROS,37 and this regulates FXYD transcription in mouse glycolytic skeletal muscles.50 In line with these observations, we found that the increases in FXYD1 mRNA and indicators of oxidative stress were paralleled by elevated AMPK downstream signalling, reflected by a higher ACC Ser79/ACC ratio. Taken together, FXYD1 mRNA content can be induced by a single session of BFR interval exercise in human skeletal muscle. This effect is likely related to greater oxidative stress and (or) AMPK activation. Moreover, the promoted FXYD1 mRNA content in BFR was dissociated from changes in phosphorylated CaMKII and PLB in type I and II fibres (Figure 9), suggesting transcriptional upregulation of FXYD1 mRNA content in human muscle does not require alterations in CaMKII autonomous activity.51
3.2. The effects of moderate‐intensity interval running on Na+,K+‐ATPase α1 and β3 mRNA content in human skeletal muscle are not influenced by blood flow restriction
The content of NKAα1 and β3 mRNA was unaffected by all exercise conditions (Figures 1A and 2C), despite pronounced differences among the conditions for changes in indicators of responses to oxidative stress, muscle hypoxia and lactate, and blood metabolites. This indicates the level of these mRNA transcripts are not severely affected by the nature of metabolic and ionic fluctuations, nor by the degree of hypoxia and oxidative stress, in human skeletal muscle. In support, raising the metabolic stress by performing simultaneous arm exercise was without effect on increases in muscle α1 and β3 mRNA content after isolated knee extensions.52 Based on the individual changes for α1 and β3 in the present study (Figures 1A and 2C), these isoforms seem to be similarly regulated at the mRNA level in human muscle. For example, the same 2 individuals who decreased their α1 mRNA content with BFR also reduced their β3 expression in the same condition. Further, parallel and selective increases in α1 and β3 mRNA levels have been observed following sprint interval cycling with or without cold‐water immersion in humans.11 In another human study, NKAα1 and β3 were the only mRNA transcripts of those investigated (α1‐3 and β1‐3) that remained unaltered in response to 45 minutes of cycling at 71% VO2max.10 Together, these results highlight α1 and β3 are likely regulated at the mRNA level by a similar pattern of cellular stress, which may be different from that (or those) important for changes in other NKA‐isoform mRNA transcripts (eg compare the individual changes for α1 and α2; Figure 1A,B).
3.3. The effects of blood flow restriction on changes in NKA α2, β1, β2, and α3 mRNA content in human skeletal muscle after moderate‐intensity running
The NKA α2 isoform is limiting for a muscle's contractile performance53 and forms up to 90% of NKA complexes in adult rat skeletal muscles.54 Together with the β1 isoform, it constitutes the largest NKA pool in this tissue. Understanding the cellular stressors regulating α2 and β1 expression is therefore fundamental. In the present study, BFR significantly elevated NKAα2 mRNA content (large effect), whereas it remained unaltered in CON and HYP. As the rise in the BFR condition was associated with increases in indicators of responses to oxidative stress, this could indicate ROS production may have been important for the potent effect of BFR on NKAα2 mRNA in the present study. In accordance, ROS have previously been shown to play a role in the transcriptional induction of this isoform in human muscle.10 However, the large effect size for CON and HYP (despite these conditions did not result in statistically significant gains) indicates the possibility of a statistical type II error for the change (Pre to +3 hours) in these conditions. Likewise, NKAβ1 mRNA was significantly elevated in CON (large effect), but not in BFR (P = .064) or in HYP (P = .077), despite a large effect (d = 0.8 and 1.1, respectively). These observations may have been due to the small sample size used and preclude us from unequivocally interpreting our data related to these isoforms. More research is required to clarify whether ROS production (or oxidative stress) is important for alterations in NKAα2 mRNA in response to a session of BFR exercise in humans.
No changes in the mRNA levels of NKAα3 and β2 were found for any condition (Figures 1C and 2B). We have previously observed no change in human muscle α3 mRNA content after sprint interval cycling of short duration (4‐seconds sprints).11 In contrast, exercise‐induced increases in α3 mRNA have been reported in other human studies. In these studies, induction of α3 mRNA occurred immediately after exercise, with the level returning to basal state after 3 hours of recovery.8, 10, 55 Thus, the time point of mRNA measurement may have influenced the current outcome and is a limitation of the present study. Another likely, at least contributing, explanation is the low expression of this transcript in human skeletal muscle55 and so the noise is much higher (CV 3‐fold higher vs other transcripts measured). The effect of a single session of exercise on β2 mRNA content is controversial with human studies reporting either increased, decreased, or unchanged, levels 3 hours after the end of exercise. In the present study, the level of β2 mRNA remained unchanged at the same time point. The reason for these conflicting findings is not clear,11 and further mechanistic studies are necessary to understand how the β2 isoform is regulated by exercise in human muscle.
3.4. Blood flow restriction augments increases in PGC‐1α total and isoform mRNA content after moderate‐intensity running
A single session of moderate‐intensity interval running raised the total muscle mRNA content of PGC‐1α by 1.8‐fold (Figure 3). This increase is small compared to those previously detected (8‐fold to 10‐fold) after endurance exercise sessions (eg 5 to 10 × 4 minutes at 90%‐95% of VO2max).56, 57, 58 However, the smaller gain in the present study is not surprising given our use of a low relative exercise intensity (105% LT, ~12 km h−1), the considerably high training status of our participants, and the positive relationship between exercise intensity and exercise‐induced increases in muscle PGC‐1α mRNA content previously reported.39, 59 Nevertheless, we chose this intensity as it was the highest tolerable mean speed, by which the exercise protocol could be performed with the chosen magnitude of BFR (cf. Materials and Methods). The small increase in PGC‐1α mRNA could also relate to a low exercise volume, as our participants spent substantially less time exercising compared to the protocols previously studied (eg 5 to 10 × 4 minutes or 1 hour of cycling).56, 57, 58
In the present study, BFR augmented the exercise‐induced increase in total PGC‐1α mRNA (4.3‐fold) and promoted the levels of the 1α1 (2.5‐fold) and 1α4 (6‐fold) transcripts (Figure 3). Consistent with these results, a reduction of ~15% to 20% in muscle blood flow during knee‐extensor exercise (45 min at 26% of one‐leg peak load) raised total PGC‐1α mRNA in human skeletal muscle.1, 26 These findings contrast with 2 previous human studies that reported either attenuated25 or unaltered27 effects of BFR on exercise‐induced change in skeletal muscle PGC‐1α mRNA. The changes in PGC‐1α levels in these studies may likely be explained by a low relative exercise intensity (40% of VO2max in BFR vs 70% in CON) or the timing of BFR (15 s into passive recovery from each cycling sprint), respectively, as these conditions seem suboptimal for sufficient facilitation of the cellular stressors and signalling proteins involved in PGC‐1α transcription.39, 59 In support, inductions of PGC‐1α mRNA transcripts in the present study were closely related to the degree of oxidative stress and downstream AMPK signalling, which is discussed in detail below.
3.5. Augmented PGC‐1α‐isoform mRNA content after blood flow‐restricted exercise is related to muscle oxidative stress and AMPK signalling
A novel result was that the BFR‐induced induction of PGC‐1α mRNA levels was temporally associated with increases in indicators of responses to oxidative stress. Specifically, HSP27 protein increased in both fibre types, whereas catalase and HSP70 mRNA was upregulated at the whole‐muscle level. As these indicators are particularly sensitive to increases in hydrogen peroxide (H2O2) levels in either astrocytes60 or myocytes,61, 62 these results indicate the effect of BFR on PGC‐1α transcripts may have been mediated, in part, by exacerbated ROS production or the resultant oxidative stress. This is consistent with a previous observation in young men of an attenuated exercise‐induced rise in PGC‐1α mRNA after oral consumption of antioxidants (vitamin C and E),63 and with those of a number of in vitro experiments where incubation of C2C12 cells with H2O2 promoted PGC‐1α mRNA content (1.4‐fold) and promoter activity,37 whereas pre‐incubation with the ROS scavenger, N‐acetylcysteine (NAC), abolished these effects.37, 64
Treatment of C2C12 cells with the AMPK activator, 5‐aminoimidazole‐4‐carboxamide‐1‐b‐D‐ribofuranoside (AICAR), has been found to increase both PGC‐1α mRNA (2.2‐fold) and PGC‐1α promoter activity (3.5‐fold), and several AICAR‐sensitive PGC‐1α promoter sites have been identified.65 In a more recent experiment, activation of PGC‐1α transcription by ROS coincided with promoted AMPK activation.37 In agreement with these in vitro observations, BFR provoked simultaneous exercise‐induced increases in PGC‐1α‐isoform mRNA, indicators of responses to oxidative stress, and ACC Ser79/ACC ratio (which strongly reflects AMPK activity66) in the present study. These findings support increased activation of AMPK could have been involved in the BFR‐induced upregulation of PGC‐1α‐isoform mRNA levels. Factors other than ROS could partly account for the increased AMPK downstream signalling with BFR in the current study. For example, circulating noradrenaline can stimulate AMPK activity in skeletal muscle cells,67 and BFR has been shown to exacerbate exercise‐induced increases in circulating noradrenaline.68 In addition, the increase in ACC Ser79/ACC ratio was most pronounced in type I fibres (Figure 8D), indicating BFR‐induced facilitation of AMPK signalling was fibre type‐dependent. Consistent with this result, circulatory occlusion (250 mm Hg) accelerated glycogenolysis in type I fibres during repeated contractions in humans,69 indicating altered metabolic activation of this fibre type during BFR exercise. Thus, future work should examine if modulation of exercise‐induced mRNA responses by BFR is fibre type‐specific. Moreover, inconsistent with the changes in ACC Ser79/ACC ratio, α‐AMPK Thr172/α‐AMPK was dissociated from increases in PGC‐1α mRNA levels (eg differences between BFR and HYP). This result is in agreement with a number of previous human studies.70, 71 Dissociation of ACC Ser79/ACC and α‐AMPK Thr172/α‐AMPK in the present study could relate to different exercise effects on total abundance of these proteins (eg compare the mean values for α‐AMPK protein after BFR vs HYP; Figure 8), and/or alternatively to the high sensitivity of ACC, in terms of phosphorylation, for (small) changes in AMPK activity.
3.6. The effects of blood flow‐restricted exercise on PGC‐1α‐isoform mRNA are unrelated to the severity of hypoxia, lactate accumulation, and modulation of fibre type‐dependent CaMKII signalling in human skeletal muscle
In a previous human study, 1 hour of moderate‐intensity cycling (60% of cycling peak power output) at simulated altitude (3000 m) had no effect on PGC‐1α mRNA in the skeletal muscle of recreationally active men.72 In agreement, PGC‐1α total and isoform mRNA levels were unaffected by systemic hypoxia (3250 m) in the present study, despite deoxygenated HHb was matched between the BFR and HYP condition (Figure 4A). Thus, the severity of exercise‐induced muscle hypoxia was not decisive for the effects of BFR on PGC‐1α levels in the present study. In cardiac myocytes, long‐term exposure to hypoxia (8 hours at 0.5% O2) elevated PGC‐1α mRNA by 3‐ to 6‐fold,73 indicating chronic exposure to hypoxia, at least in vitro, induces different mRNA responses compared to the intermittently hypoxic protocol applied in the present study. Given the comparable levels of muscle and blood metabolites after exercise with BFR or in systemic hypoxia (Figures 5 and 6), inadequate metabolic stress may also not account for the lack of an effect of systemic hypoxia. Rather, our results support the absence of an effect of systemic hypoxia was due, at least partly, to its insufficiency to substantially promote oxidative stress and activate AMPK compared to BFR. In addition, a novel result was that CaMKII Thr287/CaMKII ratio decreased in type II fibres after exercise with (P = .056) and without BFR, but not when systemic hypoxia was superimposed. This is the first indicator in humans that decreased arterial oxygen saturation may affect contraction‐stimulated CaMKII signalling in a fibre type‐dependent manner in skeletal muscle. CaMKII phosphorylation at Thr287 has been positively correlated (r 2 = .884) with CaMKII autonomous activity in this tissue.51 Given running in systemic hypoxia was without impact on PGC‐1α levels, this indicates increases in the mRNA levels of PGC‐1α isoforms in human muscle by a session of moderate‐intensity running with BFR does not involve changes in CaMKII autonomous activity. It should be noted our data are limited to the time point immediately after exercise, whereas a transient increase (0.7‐ to 1.5‐fold) in CaMKII autonomous, but not maximal activity has been detected early after the onset of moderate‐intensity (76% VO2max) cycling in human skeletal muscle.74
3.7. Conclusion and perspectives
In summary, a single session consisting of moderate‐intensity interval running with blood flow restriction augmented increases in the mRNA content of the NKA regulatory subunit, FXYD1, and of PGC‐1α (total) and its isoforms 1α1 and 1α4, in the skeletal muscle of men. These effects of BFR were associated with increased oxidative stress and fibre type‐specific AMPK downstream signalling, whereas the magnitude of muscle hypoxia, lactate accumulation, and fibre type‐dependent modulation of CaMKII signalling was unlikely involved. Thus, intermittent BFR exercise is a potent strategy to augment the acute signalling and gene response associated with ion transport and mitochondrial adaptation in human skeletal muscle. Based on this work, future research should examine whether the effects of repeated interval exercise sessions with BFR over time could translate to improvements in the muscle capacity for K+ handling and oxidative ATP production in humans.
4. MATERIALS AND METHODS
4.1. Ethical approval
This study was approved by the Human Research Ethics Committee of Victoria University, Melbourne, Australia (HRE14‐309), and was performed in accordance with the latest instructions in the Declaration of Helsinki. Participants provided oral and written informed consent before enrolment in the study.
4.2. Participants
Eight healthy men, engaged in team sports at a recreational level (5 in soccer, 2 in Australian‐rules football and one in basketball), participated in the study. Their physical characteristics are shown in Table 1. All participants were non‐smokers and engaged in their sport 2 to 4 times per week.
Table 1.
Participant characteristics
| Age (y) | 26 ± 5 |
| Height (cm) | 177.3 ± 7.6 |
| Body mass (kg) | 74.3 ± 7.2 |
| Body mass index (kg m−2) | 23.6 ± 1.3 |
| Upper thigh circumference (relaxed/contracted; cm) | 57.5 ± 3.0/57.9 ± 2.8 |
| Upper thigh skinfold thickness (mm) | 8.2 ± 2.7 |
| VO2max (mL O2 min−1) | 4243 ± 408 |
| VO2max (mL O2 kg−1 min−1) | 57.4 ± 6.2 |
| Peak treadmill speed during the GXT (km h−1) | 14.9 ± 1.8 |
| Lactate threshold (running speed in km h−1) | 11.1 ± 1.6 |
GXT, graded exercise test.
Data are presented as mean ± SD. The lactate threshold was determined using the modified Dmax method. Skinfold thickness was measured over the vastus lateralis muscle belly and is the mean of 3 consecutive measurements. Peak treadmill speed was calculated as the sum of the last completed stage and the product of the fractional time at the last stage and the increment (1 km h−1).
4.3. Randomization and blinding
The study was a randomized, crossover experiment and took place in the Exercise Physiology Laboratory at the Institute of Sport, Exercise and Active Living (ISEAL), Victoria University, Melbourne, Australia. All sessions were performed on a Katana Sport XL treadmill (Lode, Groningen, Netherlands) in 21.4 ± 1.1°C and 40.8 ± 6.8% humidity. Participants completed 3 main trials matched for total work, duration (34 minutes) and work:rest ratio. These trials were separated by 1 week and consisted of interval running without (CON) or with blood flow restriction (BFR), or in normobaric, systemic hypoxia (HYP). Each participant was allocated a trial order using a random‐number generator (MS Excel 2013, Microsoft, Redmond, WA, USA). To minimize any perceived placebo effect (not to be confused with a true placebo effect),75 the participants were not informed about which trial was hypothesized to be of greatest value to the physiological response, and whether they were breathing hypoxic or normoxic air. A pneumatic tourniquet (Riester, Jungingen, Germany) was attached to the participant's preferred kicking (dominant) leg by adhesive tape in all trials, but it was only inflated in BFR. In addition, the participants were informed that the study purpose was to evaluate the effect of different degrees of BFR. Information about what trial was to be performed on each occasion was given on the day of execution.
4.4. Pre‐testing
Prior to the main trials, the participants visited the laboratory on 4 separate occasions interspersed by at least 48 hours. On the first visit, participants performed a graded exercise test (GXT). This test was used to assess the participant's lactate threshold (LT) and maximum oxygen consumption (VO2max). On the second visit, participants performed the BFR trial with near‐infrared spectroscopy (NIRS) probes placed over the vastus lateralis muscle belly of their dominant leg to assess muscle oxygen content (cf. section on Muscle deoxygenation), and to accustom the participants to BFR and the equipment. During the third visit, participants completed the same running protocol with NIRS probes attached. The first 3 exercise bouts during this visit were performed without BFR or systemic hypoxia. The remaining 6 bouts were completed in normobaric, systemic hypoxia to accustom the participants to HYP and to allow estimation of individual inspired oxygen fraction (FiO2) to be used in HYP to match the level of muscle hypoxia during ISC (detailed in BFR and systemic hypoxia). The tourniquet was worn during both the second and third visit. The fourth visit consisted of a GXT similar to the one performed during the first visit. The LT from the fourth visit was used to determine individual running speed during the main trials (ie ISC, CON and HYP).
4.5. Main trials
On the days of the main trials, the participants reported to the laboratory between 8 and 9 am after 7.3 ± 1.1 hours of sleep and after consuming a standardized dinner and breakfast (detailed in Diet and activity control) 15 and 2.5 hours, respectively, prior to arrival. After approx. 30 minutes of rest in the supine position, a catheter was inserted into an antecubital vein, allowing mixed‐venous blood to be sampled. After an additional 15 minutes of rest, blood and muscle were sampled, also in the supine position. Next, the participants moved to the treadmill where they were instrumented with one pair of NIRS optodes on the belly of the vastus lateralis muscle of their preferred kicking (dominant) leg to reliably and non‐invasively monitor muscle deoxygenation in vivo.76 A belt was placed around their chest to measure heart rate. In a sitting position with the dominant leg unloaded, muscle deoxygenation was measured for at least 2 minutes until a plateau was reached and a stable baseline reading was recorded. Next, participants were fitted with a facemask covering the mouth and nose to enable them to breathe normoxic or hypoxic air. A pneumatic tourniquet was attached to the participant's dominant leg by adhesive tape. The tourniquet was inflated only in BFR before each bout of exercise and deflated upon termination of each bout. A time‐aligned schematic representation of the experimental protocol is shown in Figure 11. Every trial commenced with a 5‐minutes warm‐up (WU) at 75% LT followed by 5 minutes of rest. At the third and fourth minute of the WU, a 5‐seconds acceleration to ~110% LT, followed by a 5‐seconds deceleration to 75% LT, was performed. Next, 3 series of three 2‐minutes running bouts were executed at a fixed relative intensity (105% LT, 11.6 ± 1.7 km h−1; no incline). The runs were separated by 1 minute, and the series by 5 minutes, of walking (~5 km h−1), respectively. This design was introduced to promote repeated periods of hypoxia‐reperfusion, which is a key stimulus for increases in NKA and PGC‐1α mRNA in cell culture.37, 77 The duration of the running bouts and the work:rest ratio were based on pilot work and balanced to achieve the highest tolerable mean speed by which the exercise regimen could be completed with the chosen magnitude of BFR (3.0 mm Hg cm−1). Antecubital venous blood was sampled at rest before exercise, prior to each series, immediately after each bout, and at 3 and 6 minutes after the end of exercise. Muscle was sampled at rest in the supine position before (Pre), immediately after (64 ± 28 seconds; +0 hour) and 3 hours post (+3 hours) exercise.
Figure 11.
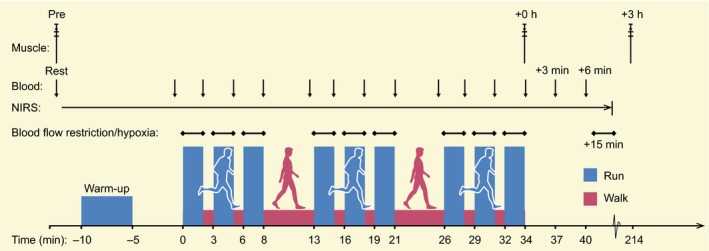
Time‐aligned, schematic representation of the experimental design. The participants performed 3 exercise trials separated by 1 week consisting of running without (control) or with the muscle blood flow partially occluded (blood flow restriction, BFR), or in normobaric, systemic hypoxia (hypoxia). The exercise intensity was set according to the participants’ individual lactate threshold (~12 km h−1). Muscle was sampled at rest before, immediately post (+0 hour) and after 3 hours (+3 hours) of recovery from each trial. Blood was sampled from an antecubital vein at the time points indicated. BFR was induced by inflation of a tourniquet (123 ± 12 to 226 ± 24 mm Hg during exercise and 320 mm Hg post‐exercise)
4.6. BFR and systemic hypoxia
In all trials, a pneumatic tourniquet made of nylon with a width of 13 cm (Riester) was externally applied to the most proximal part of the participant's preferred kicking leg. In BFR, 15 seconds prior to the onset of a run, the tourniquet was rapidly inflated over ~10 seconds to reach an end‐pressure of 3.0 mm Hg cm−1 (ie relative to thigh circumference, TCF; see below). The mean pressure was in the lower end of the range of pressures used in previous studies (~3‐5 mm Hg cm−1).28, 29, 78, 79, 80 The pressure during running ranged from (mean ± SD) 123 ± 12 (range: 109‐139) mm Hg in the float phase to 226 ± 24 (range: 200‐260) mm Hg in the landing phase. The difference between our predetermined (~175 mm Hg) and actual (mean ± SD) pressure during the trials was −1 ± 8.5 mm Hg. The tourniquet was deflated immediately after termination of exercise. After 15 minutes of recovery from exercise, the tourniquet was inflated to 320 mm Hg until there was a maximum plateau in muscle deoxygenation. TCF was measured before exercise as one‐third of the distance midline from the inguinal crease to the proximal border of patella. This represented the site of tourniquet application. In HYP, the participants executed the exercise bouts in normobaric, systemic hypoxia with a FiO2 of 14.0%, corresponding to an altitude of approx. 3250 m.
4.7. Muscle deoxygenation
Deoxygenation at the muscle level was measured by continuous‐wave, near‐infrared spectroscopy (NIRS), as described previously.76 A pair of NIRS optodes was positioned over the distal part of the vastus lateralis muscle ~15 cm above the proximal border of patella. Optodes were fixed in a plastic spacer, which was attached to the skin by double‐sided sticky discs to ensure direct contact between optodes and skin. A black bandage was placed over the optodes and around the leg for further fixation and to shield against extraneous light, and to minimize loss of transmitted near‐infrared light. The interoptode distance was 40 mm. Skinfold thickness was measured between the emitter and receiver optodes using a skinfold calliper (Harpenden). Skinfold thickness (8.2 ± 2.7 mm) was less than half the distance separating the optodes. Circumference of the plastic spacer was marked on the skin using an indelible pen, and pictures were taken to ensure that optodes were placed at the same position in all trials. Light absorption signals were converted to HHb deoxygenation changes using a differential pathlength factor (DPF) calculated according to participant's age. The DPF was the same across trials for each participant. Data were acquired at 10 Hz and subsequently filtered in R software (ver. x64 3.2.5, R Foundation for Statistical Computing, USA) using a 10th order zero‐lag, low‐pass Butterworth filter with a cut‐off frequency of 0.1. The optimal cut‐off frequency (ie reducing over‐ and underestimation of local means) was predetermined by an iterative analysis of root‐mean‐squared residuals derived from the application of multiple filters by use of a range of cut‐off frequencies (0.075‐0.150). Filtered data were used for the final analysis. Time alignment and normalization to the signal range between baseline (resting) and maximum (full occlusion) readings were completed in Excel (Ver. 2013, MS Office, Microsoft).
4.8. Graded exercise test (GXT)
Participants completed the GXT following a light, standardized meal ~3 hours prior to arrival. The test consisted of 4‐minutes runs punctuated by 1 minute of rest. The first run commenced at 5.0 km h−1, and the second at 8 km h−1. The speed was then increased by 1 km h−1 at the onset of each subsequent run until volitional exhaustion, defined as an inability to maintain the required speed. This progression in speed allowed a minimum of 7 running stages to be completed (range: 7‐11). After 5 minutes of rest, participants commenced running at the speed of the last completed run, after which the speed was increased by 1 km h−1 per minute until volitional exhaustion. This incremental bout was performed to ascertain attainment of a maximum 30‐seconds plateau in oxygen consumption. Before the test, a facemask was placed over the mouth and nose and connected to an online, gas‐analysing system for measurement of inspired and expired gases. To determine LT, blood was sampled at rest and immediately after each running stage from a 20‐gauge, antecubital venous catheter. The catheter was inserted at rest in a supine position on a laboratory bed at least 20 minutes prior to the test. The LT was calculated using the modified Dmax method as it has been shown to better discriminate between individuals in comparison with other methods.81 VO2max was determined as the mean of the 2 peak consecutive 15‐seconds values recorded during the test.
4.9. Diet and activity control
Participants consumed a standardized dinner (55 kJ kg−1 BM; 2.1 g carbohydrate kg−1 BM, 0.3 g fat kg−1 BM, and 0.6 g protein kg−1 BM) and breakfast (41 kJ kg−1 BM; 1.8 g carbohydrate kg−1 BM, 0.2 g fat kg−1 BM, and 0.3 g protein kg−1 BM) 15 and 3 hours, respectively, before every main trial. They recorded their dietary pattern within 48 hours prior to each laboratory visit and were asked to replicate the same nutritional intake as per before their first exercise trial. Participants were instructed to maintain their normal dietary pattern throughout the study and were free of anti‐inflammatory drugs and supplements, as well as medicine. The participants were instructed to replicate their weekly, routine physical activity throughout the study and to avoid activity beyond daily living in the 48 hours prior to each visit. In the 3‐hours period from termination of exercise to the +3 hours biopsy, oral consumption was limited to ad libitum water.
4.10. Muscle sampling
Vastus lateralis muscle biopsies were collected from the dominant leg in all trials for consistency using the Bergström needle biopsy technique with suction, amounting to 9 biopsies per participant. To minimize bleeding, the biopsy in ISC was obtained immediately after deflation of the tourniquet. In preparation for a muscle sample, a small incision was made under local anaesthesia (5 mL, 1% Xylocaine) through the skin, subcutaneous tissue and fascia of the muscle. Incisions were separated by approx. 1‐2 cm in 3 parallel lines of 3. Immediately after sampling, samples were rapidly blotted on filter paper to remove excessive blood and frozen in liquid nitrogen. The samples were stored at −80°C until being analysed. The incisions were covered with sterile Band‐Aid strips and a waterproof Tegaderm film dressing (3M, North Ryde, NSW, Australia).
4.11. Blood handling and analysis
To ensure blood samples accurately represented circulating blood, ~2 mL of blood was withdrawn and discarded before sampling of approx. 2 mL of blood per sample. After being drawn, samples were placed on ice until being analysed for lactate, pH and K+, concentrations after exercise on an ABL 800 Flex blood gas analyzer (Radiometer, Brønshøj, Denmark).
4.12. Arterial oxygen saturation (SaO2)
For safety reasons, adhesive optodes were placed on the tip of the left index finger to monitor arterial oxygen saturation during the HYP trial by pulse oximetry (Nellcor N‐600, Nellcor, Hayward, CA, USA). Data were recorded at rest in the standing position on the treadmill and during the final minute of each bout of running.
4.13. RNA isolation and reverse transcription
Muscle samples were homogenized (2 × 2 minutes at 30 Hz) in ~800 μL TRIzol reagent (Invitrogen, Carlsbad, CA, USA) using an electronic homogeniser (FastPrep FP120 Homogenizer, Thermo Savant, Thermo Fisher Scientific, Waltham, MA, USA). After homogenization, the supernatant was aspirated into a new, freshly autoclaved microfuge tube containing 250 μL chloroform (Sigma‐Aldrich, St. Louis, MO, USA). After few manual inversions and 5 minutes on ice, the mixture was centrifuged (15 minutes at 12 280 g) at 4°C. After centrifugation, the superior phase was pipetted into a new, autoclaved microfuge tube, and 400 μL 2‐isopropanol alcohol (Sigma‐Aldrich) and 10 μL of 5 mol L−1 NaCl were added. The samples were then stored at −20°C for 3 hours to precipitate the amount of RNA. After cooling, the samples were centrifuged (20 minutes at 12 280 g) at 4°C, and the isopropanol aspirated. The RNA pellet was rinsed with 75% ethanol made from DEPC‐treated H2O (Invitrogen Life Sciences) and centrifuged (8 minutes at 5890 g) at 4°C. After pipetting off the ethanol, the pellet was resuspended in 5 μL of heated (60°C) DEPC‐treated H2O. The samples were stored at −80°C until reverse transcription. RNA purity (mean ± SD, 1.96 ± 0.24; 260 nm/280 nm) and concentration (mean ± SD, 1.317 ± 1.311 μg μL−1) were determined spectrophotometrically on a NanoDrop 2000 (Thermo Fisher Scientific, Wilmington, DE, USA). In addition, RNA integrity was assessed in 6 randomly chosen samples on an electrophoresis station (Experion, Bio‐Rad, Hercules, CA, USA) using the manufacturer's RNA analysis kit (Experion RNA StdSens) and instructions. The RNA quality indicator (RQI) of the 6 samples was (mean ± SD) 8.1 ± 0.7. One microgram of RNA per sample was reverse‐transcribed into cDNA on a thermal cycler (S1000TM Thermal Cycler, Bio‐Rad) using a cDNA synthesis kit (iScript RT Supermix, #1708841; Bio‐Rad). The following incubation profile was used with random hexamers and oligo dTs in accordance with the manufacturer's instructions: 5 minutes at 25°C, 20 minutes at 46°C and 1 minute at 95°C. cDNA was stored at −20°C until real‐time PCR.
4.14. Real‐time RT‐PCR
Real‐time RT‐PCR was performed to determine the expression of target and reference genes. Reactions were prepared on a 384‐well plate using an automated pipetting system (epMotion 5073l, Eppendorf, Hamburg, Germany). One reaction was composed of 2 μL diluted cDNA, 0.15 μL forward and reverse primer (100 μmol L−1 concentration), 0.2 μL DEPC‐treated H2O and 2.5 μL iTaq universal SYBR Green Supermix (#1725125; Bio‐Rad). Real‐time RT‐PCR was performed on a QuantStudio 7 Flex Real‐Time PCR System (#4485701, Thermo Fisher Scientific) using the following protocol: denaturing at 95°C for 3 minutes, followed by 40 cycles of 95°C for 15 seconds, and 60°C for 60 seconds. Reactions were run in duplicate on the same plate with 4 template‐free and 4 RT‐negative controls. To account for variations in input RNA amounts and the efficiency of reverse transcription, target mRNA transcript levels were normalized to the geometric mean of 3 housekeeping genes using the method.82 This correction has been shown to yield reliable and valid mRNA data.83 Reference genes used were glyceraldehyde 3‐phosphate dehydrogenase (GAPDH), TATA‐binding protein (TBP) and β2 microglobulin (β2M). The mean (±SD) coefficient of variation (CV) of duplicate reactions (cycle threshold, C T), along with the forward and reverse sequences for the primers, is shown in Table 2. Criteria and procedure for the design of primers for NKA isoforms are presented elsewhere.11 Primers for PGC‐1α isoforms were identical to those previously used.18 Primer specificity was confirmed by performing a melt curve analysis at the end of each PCR run. The sample size for mRNA content was n = 8 for CON, n = 6 for BFR and n = 5 for HYP. Data points were excluded if contaminated (C T > 35, n = 4) or if unavailable due to a missed biopsy at +3 hours (n = 1). Limited amount of muscle precluded us from re‐analysing contaminated samples.
Table 2.
Forward and reverse primer sequences used in real‐time PCR, their amplification efficiency and the coefficient of variation (CV) of duplicates
| Gene | Forward sequence | Reverse sequence | Efficiency | CV (%) Mean ± SD |
|---|---|---|---|---|
| Na+,K+‐ATPase | ||||
| α1 | CGACAGAGAATCAGAGTGGTGT | GCCCTGTTACAAAGACCTGC | 1.79 | 0.7 ± 0.6 |
| α2 | ACATCTCCGTGTCTAAGCGG | AGCCACAGGAGAGCTCAATG | 2.25 | 0.7 ± 0.5 |
| α3 | ACTGAGGACCAGTCAGGGAC | CCTTGAAGACAGCGCGATTG | – | 3.4 ± 2.4 |
| β1 | CTGACCCGCCATCGCC | TAGAAGGATCTTAAACCAACTGCC | 1.76 | 0.5 ± 0.4 |
| β2 | TTCGCCCCAAGACTGAGAAC | AGAGTCGTTGTAAGGCTCCA | 1.83 | 1.4 ± 1.0 |
| β3 | TCATCTACAACCCGACCACC | GAAGAGCAAGATCAAACCCCAG | 1.90 | 0.8 ± 0.6 |
| FXYD1 | AGCGAGCAGAATTCCTCCAG | GCAGGGACTGGTAGTCGTAAG | 1.97 | 1.4 ± 1.6 |
| PGC‐1α | ||||
| Total | CAGCCTCTTTGCCCAGATCTT | TCACTGCACCACTTGAGTCCAC | 2.04 | 0.7 ± 0.5 |
| 1α1 | ATGGAGTGACATCGAGTGTGCT | GAGTCCACCCAGAAAGCTGT | 2.03 | 1.0 ± 1.1 |
| 1α4 | TCACACCAAACCCACAGAGA | CTGGAAGATATGGCACAT | 2.56 | 0.9 ± 0.8 |
| Oxidative stress | ||||
| Catalase | CTCAGGTGCGGGCATTCTAT | TCAGTGAAGTTCTTGACCGCT | 1.90 | 1.2 ± 1.0 |
| HSP70 | GGGCCTTTCCAAGATTGCTG | TGCAAACACAGGAAATTGAGAACT | 1.92 | 0.9 ± 0.5 |
| Housekeeping | ||||
| GAPDH | AATCCCATCACCATCTTCCA | TGGACTCCACGACGTACTCA | 2.12 | 1.0 ± 0.4 |
| β2M | TGCTGTCTCCATGTTTGATGTATCT | TCTCTGCTCCCCACCTCTAAGT | 2.08 | 0.9 ± 0.3 |
| TBP | CAGTGACCCAGCAGCATCACT | AGGCCAAGCCCTGAGCGTAA | 2.24 | 1.0 ± 0.7 |
Note that the primer set for the Na + ,K + ‐ATPase α3 (like several other sets we and other labs have tested) results in C T values of ~34 (ie upper end of detection range). This is likely due to the low expression of this isoform in human skeletal muscle.
4.15. Dissection and fibre typing of muscle fibres
All chemicals used for dot blotting and Western blotting were from Bio‐Rad unless otherwise stated. Antibodies are detailed in Table 3.
Table 3.
Primary antibodies used for dot blotting and Western blotting
| Protein | Primary antibody and supplier | Host species and isotype (antibody type) | Concentration | Molecular mass (kDa) |
|---|---|---|---|---|
| HSP27 | Thermo Fisher Scientific (#MA3‐015) | Mouse IgG (monoclonal) | 1:1000 | ~27 |
| p‐ACC Ser79 | Cell Signaling Technology (#3661S) | Rabbit IgG (polyclonal) | 1:1000 | ~257 |
| ACC | Cell Signaling Technology (#3676S) | Rabbit IgG (monoclonal) | 1:1000 | ~257 |
| p‐AMPK‐α Thr172 | Cell Signaling Technology (#2535) | Rabbit IgG (monoclonal) | 1:500 | ~63 |
| AMPK‐α | Cell Signaling Technology (#2603) | Rabbit IgG (monoclonal) | 1:1000 | ~63 |
| p‐CaMKII Thr287 | Cell Signaling Technology (#12716) | Rabbit IgG (monoclonal) | 1:1000 | ~50‐80 |
| CaMKII | Cell Signaling Technology (#4436) | Rabbit IgG (monoclonal) | 1:1000 | ~50‐80 |
| p‐PLB Ser16 | Merck Millipore (#07‐052) | Rabbit IgG (polyclonal) | 1:2000 | ~25 |
| PLB | Abcam (#ab2865) | Mouse IgG (monoclonal) | 1:1000 | ~25 |
| MHC I | Developmental Studies Hybridoma Bank, University of Iowa (#A4.840) | Mouse, IgM (monoclonal) | 1:200 | ~200 |
| MHC IIa | Developmental Studies Hybridoma Bank, University of Iowa (#A4.74) | Mouse, IgG (monoclonal) | 1:200 | ~200 |
| MHC IIx | Developmental Studies Hybridoma Bank, University of Iowa (#6H1) | Mouse, IgM (monoclonal) | 1:100 | ~200 |
Antibodies were diluted in 1% bovine serum albumin in 1× phosphate‐buffered saline with 0.02% sodium azide and 0.025% Tween.
One part of each muscle biopsy (50 ± 10 mg w.w.) was freeze‐dried for 40 hours, yielding 11.6 ± 2.7 mg d.w. muscle tissue. From these freeze‐dried portions, a minimum of 40 single‐fibre segments per sample (range: 40‐120; total n = 2750) were isolated under a dissecting microscope using fine jeweller's forceps. The segments were placed in individual microfuge tubes and incubated for 1 hour at room temperature in 10‐μL denaturing buffer (0.125 mol L−1 Tris‐HCl, 10% glycerol, 4% sodium dodecyl sulphate, 4 mol L−1 urea, 10% mercaptoethanol and 0.001% bromophenol blue, pH 6.8), in accordance with previous procedure.84 The denatured segments were stored at −80°C until future use.
Fibre type of individual segments was determined using dot blotting, as recently described (D Christiansen, MJ MacInnis, E Zacharewicz, BP Frankish, H Xu, RM Murphy, unpublished data). In brief, two 0.45‐μm PVDF membranes were activated in 95% ethanol and equilibrated for 2 minutes in cold transfer buffer (25 mmol L−1 Tris, 192 mmol L−1 glycine, pH 8.3, 20% methanol), after which a 1.5‐μL aliquot of denatured sample, corresponding to one‐seventh of a fibre segment, was spotted onto each membrane. The membranes were placed at room temperature on a dry piece of filter paper to dry completely (5‐10 minutes), after which they were reactivated in the ethanol and re‐equilibrated in transfer buffer. After a quick wash in Tris‐buffered saline‐Tween (TBST), membranes were blocked in 5% non‐fat milk in TBST (blocking buffer) for 5‐15 minutes. One of the blocked membranes was incubated (1 in 200 in 1% BSA with PBST) with myosin heavy chain I (MHCI) antibody, and the other membrane with myosin heavy chain IIa (MHCIIa) antibody for 2 hours at room temperature with gentle rocking. After a quick wash in blocking buffer, membranes were incubated (concentration: 1:20 000) with goat anti‐mouse IgG (MHCIIa, #PIE31430, ThermoFisher Scientific) or IgM (MHCI, #sc‐2064, Santa Cruz Biotechnology, Santa Cruz, CA, USA) horseradish peroxidase (HRP)‐conjugated secondary antibody for 1 hour at room temperature with rocking. Membranes were then quickly rinsed in TBST, exposed to Clarity enhanced chemiluminescence reagent (Bio‐Rad) and imaged on a ChemiDoc MP (Bio‐Rad). The membrane incubated with MHCIIa antibody was reprobed with MHCIIx antibody for 2 hours with rocking at room temperature, after which it was exposed to the same secondary antibody as MHCI (#sc‐2064, Santa Cruz Biotechnology) for 1 hour at room temperature and imaged accordingly. The difference in the host immunoglobulin species of the MHCIIa (IgG) and MHCIIx (IgM) antibodies allowed both isoforms to be quantified on the same membrane.
The remainder of each denatured fibre segment (7 μL) was grouped according to MHC expression to form samples of type I (MHCI) and type II (MHCIIa) fibres for each biopsy, in line with previous procedure.85 The number of fibre segments included in each group of muscle fibres per biopsy was (mean ± SD) n = 12 ± 6 (range: 5‐27) for type I, and n = 16 ± 5 (range 7‐33) for type IIa, fibres. Hybrid fibres (expressing multiple MHC isoforms) and type IIx fibres (classified by the absence of MHCI and MHCIIa, but the presence of MHCIIx protein), both constituting 3.1% of the total pool of fibres, were excluded from analysis.
4.16. Immunoblotting
Fibre type‐specific protein abundance and phosphorylation status of AMPK and CaMKII, and their downstream targets Acetyl‐CoA carboxylase (ACC) and phospholamban (PLB), respectively, and the protein content of heat‐shock protein 27 (HSP27) were determined by Western blotting. Fifteen micrograms of protein per sample (~5 μL) were separated (45 minutes at 200 V) on 26 wells, 4‐15% Criterion TGX stain‐free gels (Bio‐Rad). Each gel was loaded with all samples from one participant, 2 calibration curves (a 4‐ and a 3‐point) and 2 protein ladders (PageRuler, Thermo Fischer Scientific). Calibration curves were of human whole‐muscle crude homogenate with a known protein concentration, which was predetermined as described previously.7 After electrophoresis, gels were UV activated (5 minutes) on a Criterion stain‐free imager (Bio‐Rad). Proteins were wet‐transferred to 0.45 μm nitrocellulose membrane (30 minutes at 100 V) in a circulating bath at 4°C in transfer buffer (25 mmol L−1 Tris, 190 mmol L−1 glycine and 20% methanol). Membranes were then incubated (10 minutes) in antibody extender solution (Pierce Miser, Pierce, Rockford, IL, USA), washed in double‐distilled H2O and blocked for 2 hours in blocking buffer (5% non‐fat milk in Tris‐buffered saline‐Tween, TBST) at room temperature with rocking. To allow multiple proteins to be quantified on the same membrane, the membranes were cut horizontally at appropriate molecular masses using the 2 protein ladders as markers prior to probing with the primary antibodies overnight at 4°C, and for 2 hours at room temperature with constant, gentle rocking. Antibody details are presented in Table 3. Primary antibodies were diluted in 1% bovine serum albumin (BSA) in phosphate‐buffered saline with 0.025% Tween (PBST) and 0.02% NaN3. After washing in TBST and probing with appropriate horseradish peroxidase (HRP)‐conjugated secondary antibody (goat anti‐mouse immunoglobulins or goat anti‐rabbit immunoglobulins; Pierce) for 1 hour with rocking at room temperature, chemiluminescent images of membranes were captured on a ChemiDoc Touch (Bio‐Rad), followed by densitometry using Image Lab (Ver. 5.2.1, Bio‐Rad). Protein ladders were captured under white light prior to chemiluminescent imaging without moving the membranes. Band densities for proteins were quantified by reference to the mean of the 2 linear calibration curves loaded on the same gel and normalized to the total amount of protein in each lane on the stain‐free gel image. The same researcher with substantial experience with the techniques was responsible for performing all muscle analyses.
4.17. Muscle metabolites
A portion of each freeze‐dried muscle sample (2 mg d.w.) was dissected free of connective tissue, blood and fat before being powdered using a Teflon pestle. The content of ATP, PCr, creatine (Cr) and lactate in each sample was extracted using pre‐cooled perchloric acid/EDTA and KHCO3, and analysed fluorometrically using a modification of a method previously described,86 where samples are analysed in a 96‐well plate format. All samples from each participant, along with 2 standards of either ATP, PCr, Cr or lactate, a 4‐point NADH standard curve and blanks (ie double‐distilled H2O), were analysed in triplicate on the same plate. Absorbance readings of samples were normalized to the standards and subtracted blanks. The content of PCr, Cr and ATP was adjusted to Cr level across trials.
4.18. Statistics
Data were firstly assessed for normality using the Shapiro‐Wilk test. An appropriate transformation of data was applied, if necessary, to obtain a normal distribution prior to subsequent statistical analyses. Paired Student's t tests were applied to test the null hypotheses of no effects of time (Pre, +3 hours) within condition using the expression data, and to test for differences between conditions (CON, BFR, HYP), using the ΔmRNA values (ie difference between Pre and +3 hours). For blood and metabolite data, a 2‐way repeated‐measures (RM) ANOVA was used to test the null‐hypotheses of no effects of time (Pre, +3 hours) or condition (CON, BFR, HYP). The same test was used to assess the null hypotheses of no effects of time (Pre, +0 hour) or fibre type (type I and type II) within condition for the content of total and phosphorylated proteins and to evaluate conditional interactions with time (Pre, +0 hour) within fibre type. Data normalized to total protein, and not relative changes, were used for protein analyses. For the Butterworth‐filtered NIRS data, a 2‐way RM ANOVA was used to test the null hypothesis of no time and condition effects. Multiple pairwise, post hoc analyses used the Tukey test. Interpretation of effect size (d) was based on Cohen's conventions, where <0.2, 0.2‐0.5, >0.5‐0.8 and >0.8 were considered as trivial, small, moderate and large effect, respectively.87 Data are reported as means ± SEM unless otherwise stated. The α‐level was set at P ≤ .05. Statistical analyses were performed in Sigma Plot (Ver. 11.0, Systat Software, San Jose, CA, USA).
CONFLICT OF INTEREST
The authors have no conflict of interest that relates to the content of this article.
Supporting information
ACKNOWLEDGEMENTS
We thank the participants for being engaged throughout the study and for their donations of muscle tissue. We are also grateful for the help from Jujiao Kuang with re‐measuring the mRNA content in our samples. The monoclonal antibodies for the myosin heavy chain isoforms and NKA α1 isoform were obtained from the Development Studies Hybridoma Bank (DSHB) under the auspices of the NICHD and maintained by the University of Iowa, Department of Biological Sciences, Iowa City, IA 52242, USA. The antibodies directed against adult human MHC I and IIa isoforms (A4.840 and A4.74, respectively) were developed by Dr H. Blau, and the antibody directed against NKA α1 (a6F) was developed by Dr D.M. Fambrough. Danny Christiansen was supported by an International Postgraduate Research Scholarship from Victoria University, Melbourne, VIC 3011, Australia.
Christiansen D, Murphy RM, Bangsbo J, Stathis CG, Bishop DJ. Increased FXYD1 and PGC‐1α mRNA after blood flow‐restricted running is related to fibre type‐specific AMPK signalling and oxidative stress in human muscle. Acta Physiol. 2018;223:e13045 https://doi.org/10.1111/apha.13045
See Editorial Commentary: Ferguson, R. A. 2018. Blood flow‐restricted exercise: Providing more bang for buck in trained athletes? Acta Physiol 223, e13065.
REFERENCES
- 1. Eiken O, Bjurstedt H. Dynamic exercise in man as influenced by experimental restriction of blood flow in the working muscles. Acta Physiol Scand. 1987;131:339‐345. [DOI] [PubMed] [Google Scholar]
- 2. McKenna MJ. The roles of ionic processes in muscular fatigue during intense exercise. Sports Med. 1992;13:134‐145. [DOI] [PubMed] [Google Scholar]
- 3. Sjogaard G, Adams RP, Saltin B. Water and ion shifts in skeletal muscle of humans with intense dynamic knee extension. Am J Physiol. 1985;248:R190‐R196. [DOI] [PubMed] [Google Scholar]
- 4. Overgaard K, Nielsen OB, Clausen T. Effects of reduced electrochemical Na+ gradient on contractility in skeletal muscle: role of the Na+‐K+ pump. Pflugers Arch. 1997;434:457‐465. [DOI] [PubMed] [Google Scholar]
- 5. McKenna MJ. Effects of training on potassium homeostasis during exercise. J Mol Cell Cardiol. 1995;27:941‐949. [DOI] [PubMed] [Google Scholar]
- 6. McKenna MJ, Bangsbo J, Renaud JM. Muscle K+, Na+, and Cl disturbances and Na+‐K+ pump inactivation: implications for fatigue. J Appl Physiol. 2008;104:288‐295. [DOI] [PubMed] [Google Scholar]
- 7. Christiansen D, Murphy RM, Broatch JR, Bangsbo J, McKenna MJ, Bishop DJ. Regulation of Na+,K + ‐ATPase isoforms and phospholemman (FXYD1) in skeletal muscle fibre types by exercise training and cold‐water immersion in men. bioRxiv 151035. 2017. https://doi.org/10.1101/151035. [Google Scholar]
- 8. Murphy KT, Snow RJ, Petersen AC, et al. Intense exercise up‐regulates Na+, K + ‐ATPase isoform mRNA, but not protein expression in human skeletal muscle. J Physiol. 2004;556:507‐519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Murphy KT, Petersen AC, Goodman C, et al. Prolonged submaximal exercise induces isoform‐specific Na+‐K + ‐ATPase mRNA and protein responses in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2006;290:R414‐R424. [DOI] [PubMed] [Google Scholar]
- 10. Murphy KT, Medved I, Brown MJ, Cameron‐Smith D, McKenna MJ. Antioxidant treatment with N‐acetylcysteine regulates mammalian skeletal muscle Na+‐K + ‐ATPase alpha gene expression during repeated contractions. Exp Physiol. 2008;93:1239‐1248. [DOI] [PubMed] [Google Scholar]
- 11. Christiansen D, Murphy RM, Broatch JR, et al. Post‐exercise cold‐water immersion increases Na+,K + ‐ATPase α2‐isoform mRNA content in parallel with elevated Sp1 expression in human skeletal muscle. bioRxiv 151100. 2017. https://doi.org/10.1101/151100. [Google Scholar]
- 12. Nordsborg N, Bangsbo J, Pilegaard H. Effect of high‐intensity training on exercise‐induced gene expression specific to ion homeostasis and metabolism. J Appl Physiol. 2003;95:1201‐1206. [DOI] [PubMed] [Google Scholar]
- 13. Nordsborg NB, Kusuhara K, Hellsten Y, et al. Contraction‐induced changes in skeletal muscle Na(+), K(+) pump mRNA expression ‐ importance of exercise intensity and Ca(2 + )‐mediated signalling. Acta Physiol. 2010;198:487‐498. [DOI] [PubMed] [Google Scholar]
- 14. Juel C. Na+‐K + ‐ATPase in rat skeletal muscle: muscle fiber‐specific differences in exercise‐induced changes in ion affinity and maximal activity. Am J Physiol Regul Integr Comp Physiol. 2009;296:R125‐R132. [DOI] [PubMed] [Google Scholar]
- 15. Nordsborg NB, Siebenmann C, Jacobs RA, et al. Four weeks of normobaric “live high‐train low” do not alter muscular or systemic capacity for maintaining pH and K(+) homeostasis during intense exercise. J Appl Physiol. 2012;112:2027‐2036. [DOI] [PubMed] [Google Scholar]
- 16. Thomas C, Sirvent P, Perrey S, Raynaud E, Mercier J. Relationships between maximal muscle oxidative capacity and blood lactate removal after supramaximal exercise and fatigue indexes in humans. J Appl Physiol. 2004;97:2132‐2138. [DOI] [PubMed] [Google Scholar]
- 17. Bishop DJ, Granata C, Eynon N. Can we optimise the exercise training prescription to maximise improvements in mitochondria function and content? Biochem Biophys Acta. 2014;1840:1266‐1275. [DOI] [PubMed] [Google Scholar]
- 18. Ruas JL, White JP, Rao RR, et al. A PGC‐1alpha isoform induced by resistance training regulates skeletal muscle hypertrophy. Cell. 2012;151:1319‐1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Popov DV, Lysenko EA, Kuzmin IV, Vinogradova V, Grigoriev AI. Regulation of PGC‐1alpha isoform expression in skeletal muscles. Acta Naturae. 2015;7:48‐59. [PMC free article] [PubMed] [Google Scholar]
- 20. Silvennoinen M, Ahtiainen JP, Hulmi JJ, et al. PGC‐1 isoforms and their target genes are expressed differently in human skeletal muscle following resistance and endurance exercise. Physiol Rep. 2015;3:e12563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Perez‐Schindler J, Summermatter S, Santos G, Zorzato F, Handschin C. The transcriptional coactivator PGC‐1alpha is dispensable for chronic overload‐induced skeletal muscle hypertrophy and metabolic remodeling. Proc Natl Acad Sci USA. 2013;110:20314‐20319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lundberg TR, Fernandez‐Gonzalo R, Norrbom J, Fischer H, Tesch PA, Gustafsson T. Truncated splice variant PGC‐1alpha4 is not associated with exercise‐induced human muscle hypertrophy. Acta Physiol. 2014;212:142‐151. [DOI] [PubMed] [Google Scholar]
- 23. Radzyukevich TL, Moseley AE, Shelly DA, et al. The Na(+)‐K(+)‐ATPase alpha2‐subunit isoform modulates contractility in the perinatal mouse diaphragm. Am J Physiol Cell Physiol. 2004;287:C1300‐C1310. [DOI] [PubMed] [Google Scholar]
- 24. Manoharan P, Radzyukevich TL, Hakim Javadi H, et al. Phospholemman is not required for the acute stimulation of Na, K‐ATPase alpha2 activity during skeletal muscle fatigue. Am J Physiol Cell Physiol. 2015;309:C813‐C822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Conceicao MS, Chacon‐Mikahil MP, Telles GD, et al. Attenuated PGC‐1alpha Isoforms following endurance exercise with blood flow restriction. Med Sci Sports Exerc. 2016;48:1699‐1707. [DOI] [PubMed] [Google Scholar]
- 26. Norrbom J, Sundberg CJ, Ameln H, Kraus WE, Jansson E, Gustafsson T. PGC‐1alpha mRNA expression is influenced by metabolic perturbation in exercising human skeletal muscle. J Appl Physiol. 2004;96:189‐194. [DOI] [PubMed] [Google Scholar]
- 27. Taylor CW, Ingham SA, Ferguson RA. Acute and chronic effect of sprint interval training combined with post‐exercise blood flow restriction in trained individuals. Exp Physiol. 2015;101:143‐154. [DOI] [PubMed] [Google Scholar]
- 28. Abe T, Kearns CF, Sato Y. Muscle size and strength are increased following walk training with restricted venous blood flow from the leg muscle. Kaatsu‐walk training. J Appl Physiol. 2006;100:1460‐1466. [DOI] [PubMed] [Google Scholar]
- 29. Abe T, Fujita S, Nakajima T, et al. Effects of low‐intensity cycle training with restricted leg blood flow on Thigh muscle volume and VO2MAX in young men. J Sports Sci Med. 2010;9:452‐458. [PMC free article] [PubMed] [Google Scholar]
- 30. Cook CJ, Kilduff LP, Beaven CM. Improving strength and power in trained athletes with 3 weeks of occlusion training. Int J Sports Physiol Perform. 2014;9:166‐172. [DOI] [PubMed] [Google Scholar]
- 31. Sundberg CJ, Kaijser L. Effects of graded restriction of perfusion on circulation and metabolism in the working leg; quantification of a human ischaemia‐model. Acta Physiol Scand. 1992;146:1‐9. [DOI] [PubMed] [Google Scholar]
- 32. Horiuchi M, Okita K. Blood flow restricted exercise and vascular function. Int J Vasc Med. 2012;2012:543218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gundermann DM, Fry CS, Dickinson JM, et al. Reactive hyperemia is not responsible for stimulating muscle protein synthesis following blood flow restriction exercise. J Appl Physiol. 2012;112:1520‐1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Clanton TL. Hypoxia‐induced reactive oxygen species formation in skeletal muscle. J Appl Physiol. 2007;102:2379‐2388. [DOI] [PubMed] [Google Scholar]
- 35. Slezak J, Tribulova N, Pristacova J, et al. Hydrogen peroxide changes in ischemic and reperfused heart. Cytochemistry and biochemical and X‐ray microanalysis. Am J Pathol. 1995;147:772‐781. [PMC free article] [PubMed] [Google Scholar]
- 36. Simpson PJ, Lucchesi BR. Free radicals and myocardial ischemia and reperfusion injury. J Lab Clin Med. 1987;110:13‐30. [PubMed] [Google Scholar]
- 37. Irrcher I, Ljubicic V, Hood DA. Interactions between ROS and AMP kinase activity in the regulation of PGC‐1alpha transcription in skeletal muscle cells. Am J Physiol Cell Physiol. 2009;296:C116‐C123. [DOI] [PubMed] [Google Scholar]
- 38. Gibala MJ, McGee SL, Garnham AP, Howlett KF, Snow RJ, Hargreaves M. Brief intense interval exercise activates AMPK and p38 MAPK signaling and increases the expression of PGC‐1alpha in human skeletal muscle. J Appl Physiol. 2009;106:929‐934. [DOI] [PubMed] [Google Scholar]
- 39. Egan B, Carson BP, Garcia‐Roves PM, et al. Exercise intensity‐dependent regulation of peroxisome proliferator‐activated receptor coactivator‐1 mRNA abundance is associated with differential activation of upstream signalling kinases in human skeletal muscle. J Physiol. 2010;588:1779‐1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bartlett JD, Hwa Joo C, Jeong TS, et al. Matched work high‐intensity interval and continuous running induce similar increases in PGC‐1alpha mRNA, AMPK, p38, and p53 phosphorylation in human skeletal muscle. J Appl Physiol. 2012;112:1135‐1143. [DOI] [PubMed] [Google Scholar]
- 41. Kourie JI. Interaction of reactive oxygen species with ion transport mechanisms. Am J Physiol. 1998;275:C1‐C24. [DOI] [PubMed] [Google Scholar]
- 42. Juel C, Hostrup M, Bangsbo J. The effect of exercise and beta2‐adrenergic stimulation on glutathionylation and function of the Na, K‐ATPase in human skeletal muscle. Physiol Rep. 2015;3:e12515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lundberg G, Olofsson P, Ungerstedt U, Jansson E, Sundberg CJ. Lactate concentrations in human skeletal muscle biopsy, microdialysate and venous blood during dynamic exercise under blood flow restriction. Pflugers Arch. 2002;443:458‐465. [DOI] [PubMed] [Google Scholar]
- 44. Murphy KT, Macdonald WA, McKenna MJ, Clausen T. Ionic mechanisms of excitation‐induced regulation of Na+‐K + ‐ATPase mRNA expression in isolated rat EDL muscle. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1397‐R1406. [DOI] [PubMed] [Google Scholar]
- 45. Kitaoka Y, Takeda K, Tamura Y, Hatta H. Lactate administration increases mRNA expression of PGC‐1alpha and UCP3 in mouse skeletal muscle. Appl Physiol Nutr Metab. 2016;41:695‐698. [DOI] [PubMed] [Google Scholar]
- 46. Kusuhara K, Madsen K, Jensen L, Hellsten Y, Pilegaard H. Calcium signalling in the regulation of PGC‐1alpha, PDK4 and HKII mRNA expression. Biol Chem. 2007;388:481‐488. [DOI] [PubMed] [Google Scholar]
- 47. Morales‐Alamo D, Ponce‐Gonzalez JG, Guadalupe‐Grau A, et al. Critical role for free radicals on sprint exercise‐induced CaMKII and AMPKalpha phosphorylation in human skeletal muscle. J Appl Physiol. 2013;114:566‐577. [DOI] [PubMed] [Google Scholar]
- 48. Kasai K, Yamashita T, Yamaguchi A, et al. Induction of mRNAs and proteins for Na/K ATPase alpha1 and beta1 subunits following hypoxia/reoxygenation in astrocytes. Brain Res Mol Brain Res. 2003;110:38‐44. [DOI] [PubMed] [Google Scholar]
- 49. Liu CC, Karimi Galougahi K, Weisbrod RM, et al. Oxidative inhibition of the vascular Na+‐K+ pump via NADPH oxidase‐dependent beta1‐subunit glutathionylation: implications for angiotensin II‐induced vascular dysfunction. Free Radic Biol Med. 2013;65:563‐572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nilsson EC, Long YC, Martinsson S, et al. Opposite transcriptional regulation in skeletal muscle of AMP‐activated protein kinase gamma3 R225Q transgenic versus knock‐out mice. J Biol Chem. 2006;281:7244‐7252. [DOI] [PubMed] [Google Scholar]
- 51. Rose AJ, Kiens B, Richter EA. Ca(2 + )–calmodulin‐dependent protein kinase expression and signalling in skeletal muscle during exercise. J Physiol. 2006;574:889‐903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nordsborg N, Thomassen M, Lundby C, Pilegaard H, Bangsbo J. Contraction‐induced increases in Na+‐K + ‐ATPase mRNA levels in human skeletal muscle are not amplified by activation of additional muscle mass. Am J Physiol Regul Integr Comp Physiol. 2005;289:R84‐R91. [DOI] [PubMed] [Google Scholar]
- 53. Radzyukevich TL, Neumann JC, Rindler TN, et al. Tissue‐specific role of the Na, K‐ATPase alpha2 isozyme in skeletal muscle. J Biol Chem. 2013;288:1226‐1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Orlowski J, Lingrel JB. Tissue‐specific and developmental regulation of rat Na, K‐ATPase catalytic alpha isoform and beta subunit mRNAs. J Biol Chem. 1988;263:10436‐10442. [PubMed] [Google Scholar]
- 55. Aughey RJ, Murphy KT, Clark SA, et al. Muscle Na+‐K + ‐ATPase activity and isoform adaptations to intense interval exercise and training in well‐trained athletes. J Appl Physiol. 2007;103:39‐47. [DOI] [PubMed] [Google Scholar]
- 56. Wang L, Mascher H, Psilander N, Blomstrand E, Sahlin K. Resistance exercise enhances the molecular signaling of mitochondrial biogenesis induced by endurance exercise in human skeletal muscle. J Appl Physiol. 2011;111:1335‐1344. [DOI] [PubMed] [Google Scholar]
- 57. Perry CG, Lally J, Holloway GP, Heigenhauser GJ, Bonen A, Spriet LL. Repeated transient mRNA bursts precede increases in transcriptional and mitochondrial proteins during training in human skeletal muscle. J Physiol. 2010;588:4795‐4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cochran AJ, Little JP, Tarnopolsky MA, Gibala MJ. Carbohydrate feeding during recovery alters the skeletal muscle metabolic response to repeated sessions of high‐intensity interval exercise in humans. J Appl Physiol. 2010;108:628‐636. [DOI] [PubMed] [Google Scholar]
- 59. Nordsborg NB, Lundby C, Leick L, Pilegaard H. Relative workload determines exercise‐induced increases in PGC‐1alpha mRNA. Med Sci Sports Exerc. 2010;42:1477‐1484. [DOI] [PubMed] [Google Scholar]
- 60. Yu AL, Fuchshofer R, Birke M, Kampik A, Bloemendal H, Welge‐Lussen U. Oxidative stress and TGF‐beta2 increase heat shock protein 27 expression in human optic nerve head astrocytes. Invest Ophthalmol Vis Sci. 2008;49:5403‐5411. [DOI] [PubMed] [Google Scholar]
- 61. Zhong RZ, Zhou DW, Tan CY, et al. Effect of tea catechins on regulation of antioxidant enzyme expression in H2O2‐induced skeletal muscle cells of goat in vitro. J Agric Food Chem. 2011;59:11338‐11343. [DOI] [PubMed] [Google Scholar]
- 62. Kemp TJ, Causton HC, Clerk A. Changes in gene expression induced by H(2)O(2) in cardiac myocytes. Biochem Biophys Res Comm. 2003;307:416‐421. [DOI] [PubMed] [Google Scholar]
- 63. Ristow M, Zarse K, Oberbach A, et al. Antioxidants prevent health‐promoting effects of physical exercise in humans. Proc Natl Acad Sci USA. 2009;106:8665‐8670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhang Y, Uguccioni G, Ljubicic V, et al. Multiple signaling pathways regulate contractile activity‐mediated PGC‐1alpha gene expression and activity in skeletal muscle cells. Physiol Rep. 2014;2:e12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Irrcher I, Ljubicic V, Kirwan AF, Hood DA. AMP‐activated protein kinase‐regulated activation of the PGC‐1alpha promoter in skeletal muscle cells. PLoS ONE. 2008;3:e3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chen ZP, McConell GK, Michell BJ, Snow RJ, Canny BJ, Kemp BE. AMPK signaling in contracting human skeletal muscle: acetyl‐CoA carboxylase and NO synthase phosphorylation. Am J Physiol Endocrinol Metab. 2000;279:E1202‐E1206. [DOI] [PubMed] [Google Scholar]
- 67. Hutchinson DS, Bengtsson T. AMP‐activated protein kinase activation by adrenoceptors in L6 skeletal muscle cells: mediation by alpha1‐adrenoceptors causing glucose uptake. Diabetes. 2006;55:682‐690. [DOI] [PubMed] [Google Scholar]
- 68. Sundberg CJ. Exercise and training during graded leg ischaemia in healthy man with special reference to effects on skeletal muscle. Acta Physiol Scand Suppl. 1994;615:1‐50. [PubMed] [Google Scholar]
- 69. Greenhaff PL, Soderlund K, Ren JM, Hultman E. Energy metabolism in single human muscle fibres during intermittent contraction with occluded circulation. J Physiol. 1993;460:443‐453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Taylor CW, Ingham SA, Hunt JE, Martin NR, Pringle JS, Ferguson RA. Exercise duration‐matched interval and continuous sprint cycling induce similar increases in AMPK phosphorylation, PGC‐1alpha and VEGF mRNA expression in trained individuals. Eur J Appl Physiol. 2016;116:1445‐1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Brandt N, Gunnarsson TP, Hostrup M, et al. Impact of adrenaline and metabolic stress on exercise‐induced intracellular signaling and PGC‐1alpha mRNA response in human skeletal muscle. Physiol Rep. 2016;4:e12844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Slivka DR, Heesch MW, Dumke CL, Cuddy JS, Hailes WS, Ruby BC. Human skeletal muscle mRNAResponse to a single hypoxic exercise bout. Wilderness Environ Med. 2014;25:462‐465. [DOI] [PubMed] [Google Scholar]
- 73. Cunningham KF, Beeson GC, Beeson CC, McDermott PJ. Increased expression of estrogen‐related receptor beta during adaptation of adult cardiomyocytes to sustained hypoxia. Am J Cardiovasc Dis. 2016;6:46‐54. [PMC free article] [PubMed] [Google Scholar]
- 74. Rose AJ, Hargreaves M. Exercise increases Ca2 + ‐calmodulin‐dependent protein kinase II activity in human skeletal muscle. J Physiol. 2003;553:303‐309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ernst E, Resch KL. Concept of true and perceived placebo effects. BMJ. 1995;311:551‐553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Van Beekvelt MC, Colier WN, Wevers RA, Van Engelen BG. Performance of near‐infrared spectroscopy in measuring local O(2) consumption and blood flow in skeletal muscle. J Appl Physiol. 2001;90:511‐519. [DOI] [PubMed] [Google Scholar]
- 77. Silva E, Soares‐da‐Silva P. Reactive oxygen species and the regulation of renal Na+‐K + ‐ATPase in opossum kidney cells. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1764‐R1770. [DOI] [PubMed] [Google Scholar]
- 78. Fujita T, Brechue WF, Kurita K, Sato Y, Abe T. Increased muscle volume and strength following six days of low‐intensity resistance training with restricted muscle blood flow. Int J KAATSU Training Res. 2008;4:1‐8. [Google Scholar]
- 79. Abe T, Yasuda T, Midorikawa T, et al. Skeletal muscle size and circulating IGF‐1 are increased after two weeks of twice daily” KAATSU” resistance training. Int J KAATSU Training Res. 2005;1:6‐12. [Google Scholar]
- 80. Kubota A, Sakuraba K, Koh S, Ogura Y, Tamura Y. Blood flow restriction by low compressive force prevents disuse muscular weakness. J Sci Med Sport. 2011;14:95‐99. [DOI] [PubMed] [Google Scholar]
- 81. Bishop D, Jenkins DG, Mackinnon LT. The relationship between plasma lactate parameters, Wpeak and 1‐h cycling performance in women. Med Sci Sports Exerc. 1998;30:1270‐1275. [DOI] [PubMed] [Google Scholar]
- 82. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real‐time quantitative PCR and the 2(‐Delta Delta C(T)) Method. Methods. 2001;25:402‐408. [DOI] [PubMed] [Google Scholar]
- 83. Vandesompele J, De Preter K, Pattyn F, et al. Accurate normalization of real‐time quantitative RT‐PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:Research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Murphy RM. Enhanced technique to measure proteins in single segments of human skeletal muscle fibers: fiber‐type dependence of AMPK‐alpha1 and ‐beta1. J Appl Physiol. 2011;110:820‐825. [DOI] [PubMed] [Google Scholar]
- 85. Kristensen DE, Albers PH, Prats C, Baba O, Birk JB, Wojtaszewski JF. Human muscle fibre type‐specific regulation of AMPK and downstream targets by exercise. J Physiol. 2015;593:2053‐2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Harris RC, Hultman E, Nordesjo LO. Glycogen, glycolytic intermediates and high‐energy phosphates determined in biopsy samples of musculus quadriceps femoris of man at rest. Methods and variance of values. Scand J Clin Lab Invest. 1974;33:109‐120. [PubMed] [Google Scholar]
- 87. Cohen J. Statistical Power Analysis for the Behavioral Sciences, 2nd edn Abingdon: Routledge; 1988. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


