Abstract
Background
We previously reported that the α-1 chain of type 11 collagen (COL11A1), not normally expressed in the colon, was up-regulated in stromal fibroblasts in most sporadic colorectal carcinomas. Patients with germline mutations in the APC gene show, besides colonic polyposis, symptoms of stromal fibroblast involvement, which could be related to COL11A1 expression. Most colorectal carcinomas are suggested to be a result of an activated Wnt- pathway, most often involving an inactivation of the APC gene or activation of β-catenin.
Methods
We used normal and polyp tissue samples from one FAP patient and a set of 37 sporadic colorectal carcinomas to find out if the up-regulation of COL11A1 was associated with an active APC/β-catenin pathway.
Results
In this study we found a statistically significant difference in COL11A1 expression between normal tissue and adenomas from one FAP patient, and all adenomas gave evidence for an active APC/β-catenin pathway. An active Wnt pathway has been suggested to involve stromal expression of WISP-1. We found a strong correlation between WISP-1 and COL11A1 expression in sporadic carcinomas.
Conclusions
Our results suggest that expression of COL11A1 in colorectal tumors could be associated with the APC/β-catenin pathway in FAP and sporadic colorectal cancer.
Background
The majority of colorectal carcinomas is sporadic. Mutations in the APC gene are somatically derived and prevalent in more than 80% of sporadic cases [1]. A minority of colorectal cancers is inherited. In familial adenomatosis polyposis (FAP), one allele is inactivated in germline, and the other one somatically [2,3]. In hereditary nonpolyposis colorectal cancer (HNPCC) tumor progression is evoked by an inherited deficiency in one of the DNA mismatch repair genes, and a somatic mutation in the APC gene is often a result of an increased mutation rate [4]. The APC protein normally binds to β-catenin in the cytoplasm and this leads to a rapid degradation of free β-catenin [5]. Inactivation of the APC gene results in a reduced degradation of β-catenin. β-catenin then accumulates in the nucleus, gets attached to Tcf-4/Lef, a transcription factor activating Wnt target genes [6,7]. β-catenin has also been suggested to activate the WISP-1 gene, a member of the connective tissue growth factor family, signaling oncogenic events downstream [8,9].
Collagen is the major component of the interstitial extracellular matrix (ECM). ECM is known to play an active role in numerous biological processes such as cell morphogenesis, proliferation, migration, differentiation, apoptosis as well as carcinogenesis [10,11]. In a previous study we found that colonic mucosa stromal cells in the majority of colorectal cancers expressed COL11A1, while COL11A1 was expressed only rarely in normal colon mucosa [12]. COL11A1 expression was also found in sporadic adenomas although the difference between normal and adenoma samples was not statistically significant (12). However, only a limited number of polyps were available for study [12].
A mutation in the APC gene constitutes an important event in colorectal carcinogenesis. The Wnt pathway has been suggested to regulate events also in connective tissue, such as activation of the WISP-1 gene. We therefore hypothesized that also the up regulation of COL11A1 could be a consequence of an activated Wnt pathway. The hypothesis fits well with the extra-colonic manifestations in FAP, such as skeleton- eye- and mesenteric (desmoids) fibromatosis, which could result from an over-expression of collagens [13]. It is also striking that an inactivating mutation in the COL11A1 gene involves the same organs, since affected individuals with Stickler's syndrome show skeleton and ophthalmic manifestations, such as osteochondrodysplasia, and retinal detachment and blindness [14].
In this study we investigated the possible up regulation of COL11A1 in FAP and sporadic colorectal cancer using tissue samples from one FAP patient and patients with sporadic colorectal carcinomas.
Materials and methods
Tumors
Twenty-six normal tissue samples from a FAP patient were collected from ceacum, ascending, transverse, and descending colon as well as rectum. Fourteen polyps were collected from the transverse and descending colon and rectum. The polyps were 2 mm in diameter. The FAP polyps and 37 sporadic carcinomas and 6 normal tissue samples were obtained immediately after surgery and frozen at -70°C. Samples were collected at Umeå University Hospital and Karolinska Hospital with ethical permission according guidelines in each hospital.
RNA and DNA preparation
Total RNA was extracted from frozen tissue by homogenizing with a power homogenizer (IKA Labortechnik) in Trizol Reagent according to manufacturer's protocol (Life Technologies) and used for RT-PCR. The RNA was treated with deoxyribonuclease I (Ambion). DNA was extracted from frozen tissue by homogenizing with a power homogenizer (IKA Labortechnik) in STAT 60 due to manufacturer's protocol (Tel-test "B") and used for PCR.
RT-PCR
Primers designed for COL11A1, COL5A2, WISP-1 and the internal control GAPDH were used on templates from tumors and normal tissue for comparison of expression. Total RNA (2 μg) was reverse transcribed in a total of 40 μl reaction mix, with the following conditions: 5 mM MgCl2, 50 mM KCL, 10 mM Tris-HCl, pH 8.3, 1 mM dNTP's, 1 U RNase inhibitor (Perkin Elmer), 2.5 U MuLV Reverse transcriptase (Perkin Elmer). PCR reaction volume was 25 μl containing 250 ng of each primer, 250 nM of dNTP's, and 2.5 U of ampliTaq (Gibco, Life Technologies). cDNA was diluted 1:2.5 in water and 10 μl was used for all samples and controls. The primers used were as follows: COL11A1 5'-AGG AGA GTT GAG AAT TGG GAA TC-3', 5'-TGG TGA TCA GAA TCA GAA GTT CG-3, for COL5A2 5'-AAA TGA TGG TGC AGG AGG TC-3', 5'-ATT GCC AGC TGG ACC TTC-3', for the control GAPDH2f 5'-GAA GGC TGG GGC TCA TTT G-3', 5'-GAT GGC ATG GAC TGT GGT CA-3. Thermocycler (PTC 225, Mj Research) was used with an initial denaturation step of 94°C for 5 min, followed by cycles of 94°C for 45 s, 59°C for 30 s, 72°C for 30 s, and a final elongation step of 72°C for 5 min. The PCR products were amplified 25, 30, and 36 cycles for linear range and separated on a 1.5% agarose gel containing ethidium bromide, experiments were repeated three times using the optimal cycle number. Each experiment was accompanied by amplification of GAPDH as a control and the intensities of the cDNA bands for the PCR product were normalized to the GAPDH band intensities. The intensity of the bands was estimated with the Image Gauge V3.41 software (Fuji Film Science).
Sequencing
The germline mutation in exon 15 of the FAP patient was determined using Big Dye kit sequencing on an ABI sequencer (Perkin Elmer).
Protein truncation test (PTT)
A 1.3 kb fragment of the APC exon 15 was amplified using the apcT7-modified primer 15eT7(5'GGA TCC TAA TAC GAC TCA CTA TAG GAA CAG ACC ACC ATG CTT AAA TAT TCA GAT GAG CAG TTG AA 3') and the antisens primer apcl5;3 (5'GAG CCT CAT CTG TAC TTC TGC 3') [15]. Approximately 200 ng genomic DNA and 350 ng of each PCR primer were dispensed in a 50 μl PCR reaction buffer (200 μM dNTPs, 1.5 mM MgCl2 and 2 U Taq polymerase). PCR conditions were 96°C for 1.30 min, followed by 35 cycles of (96°C for 30s, 60°C for 1.30 min, and 70°C for 1.30 mm), and a final elongation step at 70°C for 5 min. Protein truncation test (PTT) was performed according to the manufactures instructions (Promega). Incorporation of 35S-Metionin (Amersham) was used to detect the translation products after electrophoretic separation on a 5% SDS-polyacrylamid gel. The gel was then dried on a vacuum gel dryer and exposed to X-ray film (Cronex 4, Sterling Diagnostic Imageing).
LOH
Loss of the APC alleles was tested with PCR on normal colon mucosa, polyps and tumors. To a 25 μl reaction, 100 ng of DNA, 1.5 mM MgCl2, 200 μM dNTPs, 0.25 μM of flourochrome marked D5S346F, 0.25 μM D5S346R, and 1U Taq polymerase (Perkin Elmer), was applied. The PCR program was 96°C for 2 min, followed by 40 cycles of 96°C – 30 sec, 50°C – 30 sec, 72°C – 30 sec, and a final elongation step 72°C for 3 min. The PCR products were analyzed on a sequencing gel (ABI, Perkin Elmer).
Statistical analysis
The difference between mRNA expression in normal samples and carcinomas in sporadic cases, and in normal tissue and polyps from the FAP patient was tested by a non-parametric method, Mann-Whitney U test, where p < 0.05 was considered systematic/statistically significant. Correlation between COL11A1 and WISP-1 expression was studied by linear correlation and calculated by Sperman rank order correlation coefficient. Experiments and analyses were made twice.
Results
Correlation between APC/β-catenin pathway and COL11A1 expression in FAP
First the APC germline mutation was determined using sequencing. It was a deletion of 4 base pairs at nt 4043 in exon 15, codon 1347, confirming the diagnosis of FAP in the patient. Twenty-six samples from normal colon tissue and 14 polyps from the FAP patient were tested for COL11A1 expression and inactivating mutations of the APC gene. RNA extracted from fresh frozen samples from the resected colon in the FAP patient were then studied using a semi-quantitative RT-PCR. Grossly normal biopsies from caecum, ascending, transverse, descending colon, and rectum, as well as polyps from ascending and descending colon, and rectum, were analyzed. Using a three fold reproduced RT-PCR experiment (examples shown in figure 1), it was shown that polyps expressed COL11A1 to a statistically significantly higher level than normal tissue samples (p = 0.001) (figure 2a). The relative lack of RNA made it possible only to make one northern blot experiment using a small number of samples. It was not possible to confirm the results in this experiment, since no signal for COL11A1 was obtained for normal tissues or polyps. The FAP adenomas showed a similar level of expression as the sporadic adenomas in our previous study [12]. A comparison of the levels of COL11A1 expression between FAP adenomas and sporadic colonic carcinomas was demonstrated in a RT-PCR experiment where FAP adenomas and sporadic colorectal tumors were run at the same time (figure 2b). The cancers expressed the RNA to a much higher degree than adenomas. In our previous study, COL11A1 and COL5A2 were co-expressed in sporadic colorectal cancer [12]. We therefore tested whether COL5A2 expression was also associated with COL11A1 in FAP adenomas. However, no expression of COL5A2 was found in FAP normal cells or FAP adenomas (examples shown in figure 1). Normal FAP tissue where cells have one APC-allele mutated in germline did not express COL11A1, why we hypothesized that a complete inactivation of the wild-type allele by a second mutation was necessary for the up-regulation of COL11A1. The 14 FAP adenomas were therefore studied for inactivating second mutations of the wild-type allele by mutation screening using protein truncation test (PTT) and loss of heterozygosity (LOH) studies. All polyps tested had mutations and/or LOH of the wild type allele (figure 3a). Thus, all FAP samples gave evidence for an inactivated wild-type allele. This supports the idea that all polyps were established through an inactivation of the APC gene on both alleles, which triggered the adenoma formation and maybe also the up-regulation of COL11A1 in FAP.
Figure 1.
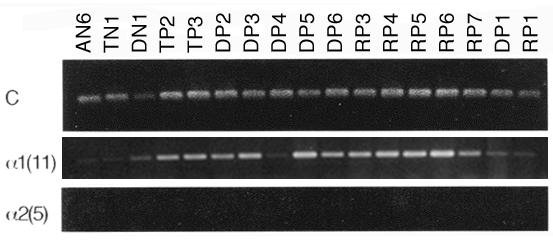
Example on RT-PCR comparing expression of GAPDH (C), COL11A1 (α1(11)), COL5A2 (α2(5)) in normal (N), and polyp tissues (P) from one FAP patient. AN, TN, DN, macroscopically normal tissue taken from ascending, transverse, descending colon respectively, TP, Polyp from transverse colon, DP, Polyp from descending colon, RP, Polyp from rectum.
Figure 2.
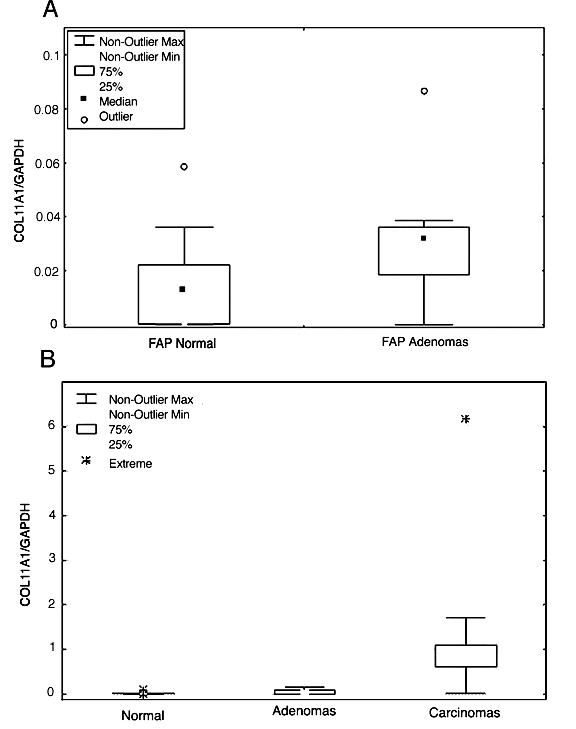
Box Plots illustrating the difference between expression of COL11A1 showing FAP normal tissue samples compared to FAP polyps (2a), and FAP polyps compared to sporadic colorectal carcinomas (2b). The boxes mark the interval between the 25th and 75th percentiles. The whiskers denote the interval between the 10th and 90th percentiles. An observation that lies more than 1.5 times the height of a box from the box is defined as outliner and when an observation lies more than 3 times the height of a box from the box, it is defined as an extreme value.
Figure 3.
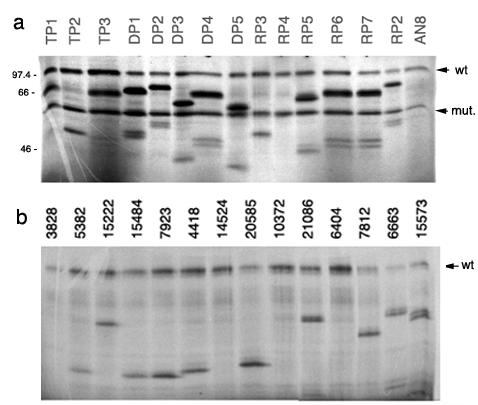
Protein truncation test (PTT) on a) FAP adenoma samples, wt, wild type allele, mut, germline mutated allele, other bands indicate secondary mutations of either the wild type or the germline mutated allele. TP, polyp from transverse colon, DP, polyp from descending colon, RP, polyp from rectum, AN, normal FAP tissue taken from ascending colon. The marker CFA756 was used to approximate the size in KD (left), b) examples of sporadic colorectal carcinomas.
Correlation between APC/β-catenin pathway and COL11A1 expression in sporadic colorectal cancer
In an attempt to relate the up-regulation of COL11A1 expression to the inactivation on the APC gene in sporadic cancer, a panel of 37 sporadic colorectal carcinomas was used.
These samples were already studied for COL11A1 expression by using RT-PCR in a previous study [12]. Mutations of the APC gene were searched for by using the protein truncation test (PTT) of exon 15, known to contain the majority of mutations in the so-called mutation cluster region (MCR) (figure 3b). In total 22 of 37 (59%) tumors had a truncated mutation in exon 15. There was no correlation between COL11A1 expression and a truncating mutation in exon 15 of the APC gene. However, the pathway can be activated by other mutations outside exon 15, by mutations not detected by our method, by mutations in other Wnt related genes or even epigenetic alterations. Therefore, we hypothesized that COL11A1 might be up-regulated in a similar fashion as and together with WISP-1. Using the expression of WISP-1 as an estimate of APC/β-catenin activation, a study was undertaken to find out if COL11A1 expression correlated with the expression of WISP-1 in the sporadic colorectal tumor samples. There was a statistically significant up-regulation of WISP-1 in sporadic carcinomas compared to the expression in normal tissue (p < 0.002). The expression of COL11A1 correlated well with the up-regulation of WISP-1 (correlation coefficient = 0.72, p < 0.001) in the tumors (Fig. 4). The expression of WISP-1 in FAP samples did not differ between normal tissue and polyps (p < 0.72) with the method used by us.
Figure 4.
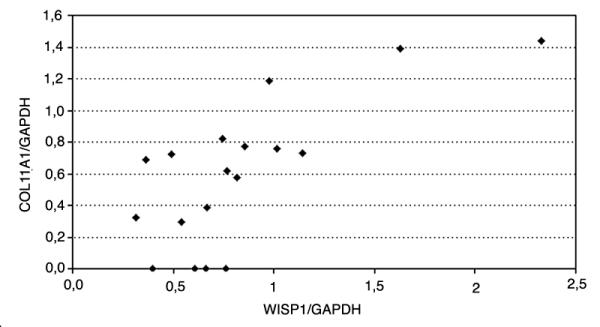
Diagram showing the correlation between COL11A1 and WISP-1 expression in sporadic colorectal carcinomas.
Discussion
In this study we found a statistically significant over-expression of COL11A1 in polyps from a patient with FAP. We have previously shown that COL11A1 was upregulated in the majority of sporadic colorectal cancer [12]. We hypothesized that the Wnt pathway could be directly involved in the up-regulation of COL11A1 in similarity to the extracellular molecule WISP-1. The Wnt pathway is involved in development of dorsal mesoderm induction and is also likely to be involved in development of the gut in humans. This is consistent with the expression of COL11A1 in human fetal colon as well as other human developing tissues, mainly skeleton and tendon [16,17].
COL11A1 is up-regulated in the APC/β-catenin pathway in FAP and sporadic colorectal cancer
COL11A1 was up-regulated in small colorectal polyps, from one FAP patient, but to a lower degree than seen in sporadic carcinomas. This supports the hypothesis that the APC/β-catenin pathway could influence COL11A1 expression in FAP. Not all sporadic colorectal carcinomas expressed COL11A1 in our previous study [12]. There was no association between an exon 15 truncating APC mutation and the expression of COL11A1 in the 37 sporadic colorectal tumors in this study. In FAP there are frequent examples of an inactivation of both APC-alleles in tumors, while in attenuated APC and sporadic colorectal cancer this is not equally frequent [18,19]. Still, the APC/β-catenin pathway is supposed to be involved in the vast majority of sporadic tumors, why we next used the expression of WISP-1 as an estimate of APC/β-catenin pathway activation in the sporadic tumors. WISP-1 is a member of the connective tissue growth factor family, shown to be over-expressed in 80% of colonic adenocarcinomas. It has been suggested to be activated by β-catenin[8], active in the Wnt pathway [20,21]. WISP-1 was up-regulated in sporadic tumors and there was a good correlation between COL11A1 and WISP-1 expression in the tumors. This correlation suggests that the over-expression of COL11A1 could be related to the APC/β-catenin pathway. However, some sporadic tumors with a truncating mutation in exon 15 did not show an up-regulation of the COL11A1, and there must be some explanation for that. One possible explanation could be the RT-PCR technique used, or that RNA for some samples could have been degraded for these particular genes even though RNA was not degraded for control genes. It is also possible that there is no association with the APC/β-catenin pathway
FAP adenomas showed no up-regulation of WISP-1 or COL5A2
WISP-1 was also expressed in normal tissue as well as in FAP, but no clear up-regulation of WISP-1 could be determined in FAP adenomas. However, if there is an association with COL11A1 expression, the much lower expression in adenomas may explain the lack of detectable up-regulation of WISP-1. The fact that COL5A2 was not expressed in the FAP adenomas, in contrast to sporadic carcinomas, suggests that COL5A2 could be involved in the transition from a benign to malignant state in colorectal cancer. Alternatively, the COL5A2 (as well as WISP-1) could be associated with a much higher expression of COL11A1, as seen in sporadic colorectal cancer.
Is the mechanism of COL11A1 transcription in FAP different from that in sporadic colorectal cancer?
In FAP there may be a direct regulation of COL11A1 in the stromal cells since also the stromal cells have mutations in the APC gene. In sporadic colorectal cancer, the pathway must be activated and influence stromal cells by a somatic mutation in an epithelial cell. In the case of WISP-1, a model has been proposed where epithelial cells express WISP-1 to activate the Wnt pathway in stromal cells, which answer with secreting WISP-1, which in turn stimulates growth. A paracrine and then an autocrine production of WISP-1 might efficiently stimulate epithelial cells to continue unregulated proliferation and eventual tumor formation [9]. There may be a similar way for how the APC mutation in epithelial cells can induce a reaction in surrounding stromal fibroblasts in sporadic colorectal cancer.
Conclusion
The results from this study suggest that the up-regulation of COL11A1 in early adenomas in FAP and in sporadic colorectal carcinomas is associated with an activated APC/β-catenin pathway. The expression of COL11A1 could directly contribute to tumorigenesis in fibroblasts in FAP and explain osteomas and desmoids, or indirectly to polyp-formation and tumor progression in sporadic colorectal cancer.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgments
This work was supported by The Swedish Cancer Foundation, The Cancer Foundation in Stockholm, Åke Wiberg Foundation and Lion's Cancer Research Foundation in Umeå.
Contributor Information
Heléne Fischer, Email: helene.fischer@cmm.ki.se.
Sima Salahshor, Email: salahsho@uhnres.utoronto.ca.
Roger Stenling, Email: Roger.Stenling.us@vll.se.
Jan Björk, Email: jan.bjork@ks.se.
Gudrun Lindmark, Email: gudrun.linkmark@helsingborgslasarett.se.
Lennart Iselius, Email: lennart.iselius.@ks.se.
Carlos Rubio, Email: Carlos.Rubio@onkpat.ki.se.
Annika Lindblom, Email: annika.linkblom@cmm.ki.se.
References
- Laken SJ, Papadopoulos N, Petersen GM, Gruber SB, Hamilton SR, Giardiello FM, Brensinger JD, Vogelstein B, Kinzler KW. Analysis of masked mutations in familial adenomatous polyposis. Proc Natl Acad Sci USA. 1999;96:2322–2326. doi: 10.1073/pnas.96.5.2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamlum H, Ilyas M, Rowan A, dark S, Johnson V, Bell J, Fray ling I, Efstathiou J, Pack K, Payne S, Roylance R, Gorman P, Sheer D, Neale K, Phillips R, Talbot I, Bodmer W, Tomlinson I. The type of somatic mutation at APC in familial adenomatous polyposis is determined by the site of the germline mutation: a new facet to Knudson's 'two-hit' hypothesis. Nat Med. 1999;5:1071–1075. doi: 10.1038/12511. [DOI] [PubMed] [Google Scholar]
- Miyaki M, Tanaka K, Kikuchi-Yanoshita R, Muraoka M, Konishi M. Familial polyposis: recent advances. Critical Reviews in Oncology-Hematology. 1995;19:1–31. doi: 10.1016/1040-8428(94)00129-H. [DOI] [PubMed] [Google Scholar]
- Huang J, Papadopoulos N, McKinley AJ, Farrington SM, Curtis LJ, Wyllie AH, Zheng S, Willson JK, Markowitz SD, Morin P, Kinzler KW, Vogelstein B, Dunlop MG. APC mutations in colorectal tumors with mismatch repair deficiency. Proc Natl Acad Sci USA. 1996;93:9049–9054. doi: 10.1073/pnas.93.17.9049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC-/- colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- Bienz M, Clevers H. Linking colorectal cancer to Wnt signaling. Cell. 2000;103:311–320. doi: 10.1016/s0092-8674(00)00122-7. [DOI] [PubMed] [Google Scholar]
- Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837–1851. [PubMed] [Google Scholar]
- Pennica D, Swanson TA, Welsh JW, Roy MA, Lawrence DA, Lee J, Brush J, Taneyhill LA, Deuel B, Lew M, Watanabe C, Cohen RL, Melhem MF, Finley GG, Quirke P, Goddard AD, Hillan KJ, Gurney AL, Botstein D, Levine AJ. WISP genes are members of the connective tissue growth factor family that are up-regulated in wnt-1-transformed cells and aberrantly expressed in human colon tumors. Proc Natl Acad Sci USA. 1998;95:14717–14722. doi: 10.1073/pnas.95.25.14717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Corcoran RB, Welsh JW, Pennica D, Levine AJ. WISP-1 is a Wnt-1- and beta-catenin-responsive oncogene. Genes Dev. 2000;14:585–595. [PMC free article] [PubMed] [Google Scholar]
- Boudreau N, Bissell MJ. Extracellular matrix signaling: integration of form and function in normal and malignant cells. Curr Opin Cell Biol. 1998;10:640–646. doi: 10.1016/S0955-0674(98)80040-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stracke ML, Murata J, Aznavoorian S, Liotta LA. The role of the extracellular matrix in tumor cell metastasis. In Vivo. 1994;8:49–58. [PubMed] [Google Scholar]
- Fischer H, Stenling R, Rubio C, Lindblom A. Colorectal carcinogenesis is associated with stromal expression of COL11A1 and COL5A2. Carcinogenesis. 2001:875–878. doi: 10.1093/carcin/22.6.875. [DOI] [PubMed] [Google Scholar]
- Klemmer S, Pascoe L, DeCosse J. Occurrence of desmoids in patients with familial adenomatous polyposis of the colon. 1987:385–392. doi: 10.1002/ajmg.1320280217. [DOI] [PubMed] [Google Scholar]
- Richards AJ, Yates JR, Williams R, Payne SJ, Pope FM, Scott JD, Snead MP. A family with Stickler syndrome type 2 has a mutation in the COL11A1 gene resulting in the substitution of glycine 97 by valine in alpha 1 (XI) collagen. Human Molecular Genetics. 1996;5:1339–1343. doi: 10.1093/hmg/5.9.1339. [DOI] [PubMed] [Google Scholar]
- Groden J, Thliveris A, Samowitz W, Carlson M, Gelbert L, Albertsen H, Joslyn G, Stevens J, Spirio L, Robertson M, et al. Identification and characterization of the familial adenomatous polyposis coli gene. Cell. 1991;66:589–600. doi: 10.1016/0092-8674(81)90021-0. [DOI] [PubMed] [Google Scholar]
- Lui VC, Kong RY, Nicholls J, Cheung AN, Cheah KS. The mRNAs for the three chains of human collagen type XI are widely distributed but not necessarily co-expressed: implications for homotrimeric, heterotrimeric and heterotypic collagen molecules. Biochemical Journal. 1995;311:511–516. doi: 10.1042/bj3110511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg MM, Hirvonen HE, Elima KJ, Vuorio El. Co-expression of collagens II and XI and alternative splicing of exon 2 of collagen II in several developing human tissues. Biochemical Journal. 1993;294:595–602. doi: 10.1042/bj2940595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyaki M, Konishi M, Kikuchi-Yanoshita R, Enomoto M, Igari T, Tanaka K, Muraoka M, Takahashi H, Amada Y, Fukayama M, et al. Characteristics of somatic mutation of the adenomatous polyposis coli gene in colorectal tumors. 1994:3011–3020. [PubMed] [Google Scholar]
- Spirio LN, Samowitz W, Robertson J, Robertson M, Burt RW, Leppert M, White R. Alleles of APC modulate the frequency and classes of mutations that lead to colon polyps. Nat Genet. 1998;20:385–388. doi: 10.1038/3865. [DOI] [PubMed] [Google Scholar]
- Iwao K, Nakamori S, Kameyama M, Imaoka S, Kinoshita M, Fukui T, Ishiguro S, Nakamura Y, Miyoshi Y. Activation of the beta-catenin gene by interstitial deletions involving exon 3 in primary colorectal carcinomas without adenomatous polyposis coli mutations. Cancer Res. 1998;58:1021–1026. [PubMed] [Google Scholar]
- Sparks AB, Morin PJ, Vogelstein B, Kinzler KW. Mutational analysis of the APC/beta-catenin/Tcf pathway in colorectal cancer. Cancer Res. 1998;58:1130–1134. [PubMed] [Google Scholar]


