Summary
Introduction
Vulnerability to psychiatric manifestations is achieved by the influence of genetic and environment including stress and cannabis consumption. Here, we used a psychosocial stress model based on resident‐intruder confrontations to study the brain corticostriatal‐function, since deregulation of corticostriatal circuitries has been reported in many psychiatric disorders. CB 1 receptors are widely expressed in the central nervous system and particularly, in both cortex and striatum brain structures.
Aims and methods
The investigation presented here is addressed to assess the impact of repeated stress following acute cannabinoid exposure on behavior and corticostriatal brain physiology by assessing mice behavior, the concentration of endocannabinoid and endocannabinoid‐like molecules and changes in the transcriptome.
Results
Stressed animals urinated frequently; showed exacerbated scratching activity, lower striatal N‐arachidonylethanolamine (AEA) levels and higher cortical expression of cholinergic receptor nicotinic alpha 6. The cannabinoid agonist WIN55212.2 diminished locomotor activity while the inverse agonist increased the distance travelled in the center of the open field. Upon CB 1 activation, N‐oleoylethanolamide and N‐palmitoylethanolamide, two AEA congeners that do not interact directly with cannabinoid receptors, were enhanced in the striatum. The co‐administration with both cannabinoids induced an up‐regulation of striatal FK506 binding protein 5. The inverse agonist in controls reversed the effects of WIN55212.2 on motor activity. When Rimonabant was injected under stress, the cortical levels of 2‐arachidonoylglycerol were maximum. The agonist and the antagonist influenced the cortical expression of cholinergic receptor nicotinic alpha 6 and serotonin transporter neurotransmitter type 4 in opposite directions, while their co‐administration tended to produce a null effect under stress.
Conclusions
The endocannabinoid system had a direct effect on serotoninergic neurotransmission and glucocorticoid signaling. Cholinergic receptor nicotinic alpha‐6 was shown to be deregulated in response to stress and following synthetic cannabinoid drugs thus could confer vulnerability to cannabis addiction and psychosis. Targeting the receptors of endocannabinoids and endocannabinoid‐like mediators might be a valuable option for treating stress‐related neuropsychiatric symptoms.
Keywords: CB1, Chrna6 and Slc6a4, Fkbp5, psychosocial stress
1. INTRODUCTION
Vulnerability to psychiatric disorders is determined by the interaction of genetic and environmental factors1 such as psychosocial stress and cannabis consumption. The dysregulation of corticostriatal circuitries is involved in the pathophysiology of psychotic disorders.2 Psychosocial stress has a remarkable influence on central nervous system (CNS) and animal behavior.3 When an organism is exposed to a stressor, biological mechanisms such as the hypothalamic‐pituitary‐adrenal axis (HPA) and cardiovascular readjustments come into play to restore homeostasis.4 Under standard conditions, HPA axis function is mainly influenced by stress, which enhances its activity, and the circadian rhythm.5 However, when stress is prolonged over the time and the activation exceeds the capacity to keep body's homeostasis, psychopathological sequelae can appear. Among the broad spectrum of brain structures closely involved in anxiety and stress disorders (for review see Ref. 6), we directed our efforts toward the dorsal striatum (dorsal CPu) and the prefrontal cortex (PFC) due to the fact that these areas are critically involved in social relationships.7, 8 There is a growing body of the literature that suggests that glucocorticoid receptor(s) involve G protein‐dependent mechanisms.9 In particular, corticostriatal activity is modulated by a variety of G protein‐coupled receptors such as the cannabinoid CB1 receptor, which is highly abundant in the CNS.10 CB1 receptors are the main target for endogenous endocannabinoids lipid signaling molecules and mediate both Δ9‐tetrahydrocannabinol (THC) and synthetic cannabinoid drugs pharmacological actions.11 The main endocannabinoids molecules for CB1 receptors are N‐arachidonylethanolamine (AEA)12 and 2‐arachidonoylglycerol (2‐AG).13 N‐arachidonoylphospatidylethanolamine‐specific phospholipase D (NAPE‐PLD) is responsible for the production of AEA, while 2‐AG is mostly synthesized by diacylglycerol lipase (DAGL‐α) in the CNS.14 After activating cannabinoid receptors, the endocannabinoid AEA and related endocannabinoid‐like NAEs PEA and OEA are enzymatically hydrolyzed by the fatty acid amide hydrolase (FAAH) while 2‐AG is primarily metabolized by monoacylglycerol lipase (MAGL).15
Interestingly, the endocannabinoid system has been described to have a direct effect on both neurotransmission16 and glucocorticoid signaling.17 Despite the lack of knowledge concerning the concrete corticostriatal mechanisms underlying anxiety, both pharmacologic (synthetic cannabinoid drugs) and behavioral testing have been applied. Thus, the aim of this study was to investigate the interplay between repeated long‐term exposure to psychosocial stress and acute challenge with cannabinoid drugs on mice corticostriatal circuitries. For this purpose, we evaluated the animals by behavioral testing and then quantified endocannabinoid and endocannabinoid‐like molecules and also changes in whole‐genome gene expression profile.
2. MATERIALS AND METHODS
All animal experimental procedures were approved by University of Göttingen Institutional Animal Care and Use Committee and also were in accordance with NIH guidelines for the use of animals in research and the European Communities Council Directive (86/609/EEC).
2.1. Animals
A total of 120 C57Bl6/J male mice aged 7‐8 weeks were purchased from Charles River Laboratories (Sulzfeld, Germany). On arrival, they were kept five per cage and maintained under standard conditions (12‐hour light/dark cycle with 6:00/18:00 lights on/off, a room temperature of 21 ± 2°C, and food and water ad libitum). After one‐week habituation period, mice were subjected to the experiment. One‐year‐old FVB/N male mice (Charles River Laboratories, Sulzfeld, Germany) were kept individually and used as residents. FVB/N mice were selected as residents because they are more offensive than the C57Bl/6 strain.3 The FVB/N strain was kept under the same protocol conditions as the C57Bl/6J colony but housed in a separate room to prevent the C57Bl/6J mice habituation to the odor of the residents.
2.2. Drugs
The CB1/CB2 receptor agonist WIN55212.2 (Sigma‐Aldrich, Seelze, Germany) and the selective cannabinoid CB1 receptor antagonist Rimonabant (Sequoia Research Products Ltd, Pangbourne, UK) were dissolved in a vehicle solution consisting of 10% DMSO (Sigma‐Aldrich, Seelze, Germany) and 0.1% Tween 80 (Sigma‐Aldrich, Seelze, Germany) in 0.9% saline and prepared on the 21 days of the experiment. A volume of 200 μL of drug and/or vehicle was administered intraperitoneally (WIN55212.2 and Rimonabant were administered at a concentration of 3 mg/kg) before the behavioral testing.
2.3. Experiment design and experimental groups
The C57Bl6/J male mice were sorted into two groups: Those exposed daily to psychosocial stress for 1 hour (stress) and those who were left undisturbed (control). The stress protocol was performed daily for 3 weeks. On day 21, the animals received an appropriate drug or vehicle injection and then evaluated by behavioral testing. Indeed, control and stressed mice were split into four subgroups each (15 animals per subgroup): mice treated twice with vehicle (Veh), mice subjected first to vehicle and then WIN55212.2 (WIN), mice treated first with Rimonabant and then with WIN55212.2 (Rim+WIN), and mice injected first with Rimonabant and then vehicle (Rim). Following the last injection with either vehicle or cannabinoid drug, the animals were tested by different behavioral paradigms during the rodent active phase (ie, after 8 pm).
2.4. Social stress procedure
An intruder (C57Bl/6J mouse) was placed in the home cage of a resident (FVB/N mouse), and then they freely interacted until the first aggression was achieved. After the first attack, the C57Bl/6J mouse was isolated by the use of a small plastic wire‐mesh cage within the FVB/N mouse's cage protecting the intruder against direct aggression; however, olfactory, visual, and some vibrissae contact with residents were maintained. After 1 hour, the C57Bl/6J mouse was placed again in its home cage. To prevent habituation, every day intruder mice had encounters with different residents. The psychosocial stress protocol was performed once per day at a similar time (after 8 pm) to enhance the stress prediction factor in intruders. In contrast, controls were left undisturbed in an empty cage every day for 1 hour. Therefore, controls were subjected to the same experimental protocol in terms of handling and exposure to a new environment (different cage), but they did not interact with the residents.
2.5. Behavioral assessment
Mice were exposed to psychosocial stress or left undisturbed for 21 days. On day 21, they were acutely treated with different cannabinoid drugs or vehicle and finally evaluated by behavioral testing. The stress paradigm, the administration of drugs, and the behavioral assessment took place during the dark phase (active phase). We directed the behavioral analyses by measuring locomotor and anxiety‐like behavior in the open‐field (OF) test and testing also CNS activity and excitability by use of the functional observational battery (FOB).
2.5.1. Functional observational battery
The FOB has been widely used and adapted for general behavioral studies in mice.18 In this study, the FOB test comprised 28 parameters by which the investigator evaluated CNS activity and excitability by recording neuromuscular and autonomic effects, and sensorimotor reactivity. There were four consecutive testing situations: (i) in the home cage, (ii) in the observer's hand, (iii) in the OF, and (iv) manipulation tests. After a brief assessment in its home cage, each mouse was removed and handled by the observer to evaluate ease‐of‐removal, handling reactivity, and general appearance. Then, each mouse was assessed in an OF arena (60 cm × 90 cm) while the observer analyzed CNS activity, autonomic effects, muscle tone, equilibrium, and sensorimotor reactivity. Gait condition was scored from 1 to 8. The gait was scored as follows: normal gait, ataxia, splayed hind limbs, feet markedly splayed outward from the body, fore‐limb drag, walking on tiptoes, hunched body, and body drag. The severity of gait abnormality was also evaluated on a scale from 1 (normal) to 4 (severely abnormal).18
2.5.2. Open‐field activity
In the OF test, we evaluated the spontaneous locomotor and exploratory activity of mice. The experiment was conducted in a Plexiglass arena (45 × 45 × 55 cm), and each mouse was left in the same starting position. Animals were allowed to examine the OF for 10 minutes without habituation. We registered the total distance travelled, percent of time moving, time spent in the center (defined as 70% of area), hyperactivity (forward movement with speed greater than 20 cm/s), and the number of rearings by use of the ActiMot software (TSE, Bad Homburg, Germany) as described.3 The apparatus was cleaned with 70% ethanol p.a. between each test.
2.6. Brain sample collection and tissue evaluation
Animals were sacrificed immediately after OF task finished, and brain tissue was collected accordingly. All mice were deeply anesthetized by intraperitoneal injection of 2,2,2‐tribromoethanol (Sigma‐Aldrich, Hamburg, Germany) and then transcardially perfused with cold 0.1% phosphate buffer saline (PBS). Finally, the PFC and the dorsal CPu were freshly isolated and frozen in liquid nitrogen for LC‐MS, RNA seq, and quantitative RT‐PCR.
2.6.1. Extraction and measurement of AEA, 2‐AG, OEA, and PEA levels
The endogenous lipid signaling molecules AEA and 2‐AG, and endocannabinoid‐related molecules N‐palmitoylethanolamide (PEA), and N‐oleoylethanolamide (OEA) were purified from the PFC and the dorsal CPu and then quantified as described elsewhere.19 First, samples were dounce‐homogenized following with chloroform/methanol/Tris‐HCl 50 mmol/L pH 7.5 (2:1:1, v/v) containing internal deuterated controls solution for AEA, 2‐AG, PEA, and OEA measurement by isotope dilution ([2H]8AEA, [2H]52AG, [2H]4 PEA, [2H]4 OEA (Cayman Chemicals, MI, USA). The lipid organic phase was dried down, weighed, and prepurified on silica gel. Fractions were collected by eluting the column with 99:1, 90:10, and 50:50 (v/v) chloroform/methanol. The 90:10 fraction contained AEA, 2‐AG, PEA, and OEA, and it was used for isotopic dilution liquid chromatography‐atmospheric pressure chemical ionization‐mass spectrometry quantification (LC‐APCI‐MS), as previously described and using selected ion monitoring at M + 1 values for the four compounds and their deuterated homologues, as described in Ref. 20 N = 4 mice/group.
2.6.2. RNA extraction
Total RNA was extracted from 80 samples (control and stress; Veh, WIN, Rim+WIN, and Rim; PFC and dorsal CPu; N = 5 mice/group) using the TRIzol reagent according to the manufacturer's instructions (Invitrogen, NY, USA). The RNA was digested with RNase‐free DNase (Qiagen, Düsseldorf, Germany) and checked for integrity using capillary gel electrophoresis (Bioanalyzer, Agilent Technologies, Santa Clara, USA).
2.6.3. cDNA Library Preparation and RNA Seq
The library was prepared using the TruSeq RNA Sample Preparation Kit (Illumina, San Diego, USA) starting from 500 ng of total RNA. The cDNA libraries were quantified using the QuantiFluor dsDNA System (Promega, Madison, USA). The size range of the final cDNA libraries was determined by the Bioanalyzer 2100 (Agilent, Santa Clara, USA). The cDNA libraries were amplified and sequenced using cBot and HiSeq2000 (Illumina, San Diego, USA).
The sequence images were converted with the Illumina BaseCaller software to bcl files, which were demultiplexed to FASTQ files with CASAVA version 1.8.2. Quality was checked via FastQC (version 0.10.0, Babraham Bioinformatics).
2.6.4. Gene expression analyses
Sequences were aligned to the RefSeq21 mouse transcriptome using bwa,22 and raw “hits” per transcript were merged genewise. DESeq was used to analyze the counts per gene.23 The resulting P‐values were corrected using Benjamini‐Hochberg adjustment.24
2.6.5. Quantitative RT‐PCR
The most relevant top hits deregulated by stress and cannabinoid drugs were further evaluated by quantitative RT‐PCR. First‐strand cDNA was synthesized from 1 μg of total RNA using the High Capacity RNA‐to‐cDNA Kit (Applied Biosystems, Darmstadt, Germany). mRNA expression was quantified by RT‐PCR using the CXF96TM Real‐time PCR system (Bio‐Rad, Hercules, USA). We used GAPDH mRNA as an endogenous control. TaqMan assays for mouse Chrna6, Slc6a4, and Fkbp5 cDNAs were selected from validated and predesigned Assays‐on‐Demand (Applied Biosystems, Darmstadt, Germany) and used in real‐time PCR amplifications to detect the expressions of Chrna6, Slc6a4, and Fkbp5. The reactions were performed in triplicate using 2 μL of cDNA in a 10 μL reaction volume. mRNA expression was determined using the comparative cycle threshold (Ct) method with 2−ΔΔCt, according to the manufacturer's instructions (Applied Biosystems, Darmstadt, Germany). cDNAs were measured relative to a “calibrator” control sample. The 2−ΔΔCt for this “calibrator” was arbitrarily set to 1.
2.7. Statistical analysis
A two‐way ANOVA was used to examine the effects of stress and pharmacological treatment on our cohort of animals. The mean differences among the levels of one factor were determined by one‐way ANOVA or Brown‐Forsythe test when applicable. Bonferroni or nonparametric Tamhane post hoc test was applied for pairwise comparisons. Analysis of simple main effects was performed whether there was a significant interaction of the two main factors. Individual comparisons were made using the Student's t test. ANOVA for repeated measures was used to test the equality of means for mice body weight. Significance level was set to P < 0.05. Data are represented as mean ± SEM. Significant effects were identified by SPSS (IBM Corp, Chicago, USA).
3. RESULTS
Results are summarized in three main categories: (i) effects of stress, (ii) effects of drugs, and (iii) effects of stress under drug influence.
3.1. Effects of stress
We revealed a significant effect of psychosocial stress on body weight throughout the experimental period (F(1, 123) = 4.84, P < 0.05). Indeed, exposure to 2‐week social defeat paradigm significantly diminished body weight (t(122) = 2.78, P < 0.01), which returned to control levels by the end of the stress period (Figure 1, panel A). Social defeat animals displayed a pronounced scratching activity (F(1, 123) = 4.06, P < 0.05) (Figure 1, panel B), urinated more frequently (F(1, 123) = 4.97, P < 0.05) (Figure 1, panel C), and showed lower levels of AEA (F(1, 18) = 36.81, P < 0.01, in the dorsal CPu) (Figure 1, panel D). Transcriptome analysis of the selected brain structures revealed 62 top hits as deregulated genes by chronic psychosocial stress before Benjamini‐Hochberg adjustment (Figure S1). The cholinergic receptor nicotinic alpha 6 (Chrna6), as a candidate gene, was further analyzed by quantitative RT‐PCR. Thus, we determined that long‐term exposure to stress increased the expression of Chrna6 gene (F(1, 43) = 38.32, P < 0.01, in the PFC) (Figure 1, panel E) when compared to the nonstressed group.
Figure 1.
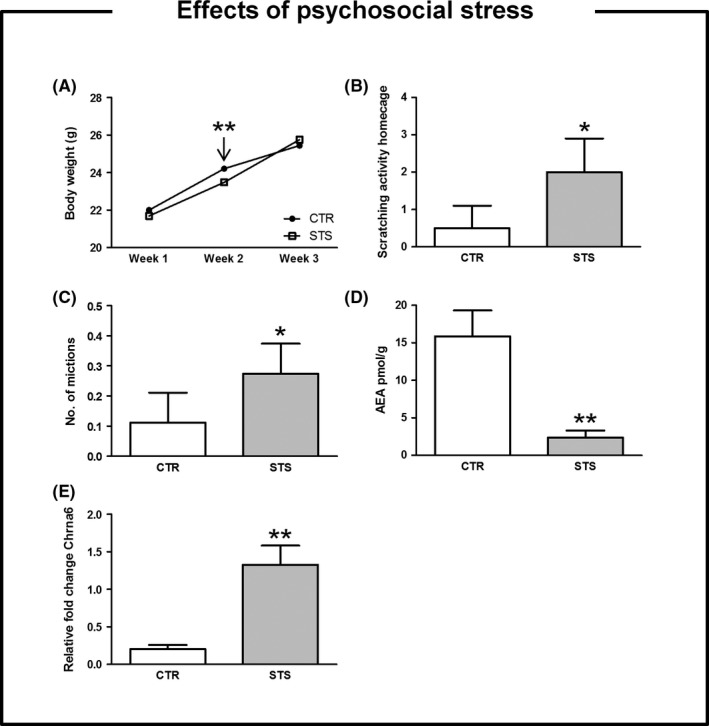
Effects of repeated exposure to psychosocial stress. Data are expressed as mean ± SEM. Exposure to 2‐wk social defeat paradigm significantly diminished body weight (P < 0.01), which returned to control levels by the end of the stress period (panel A). Social defeat animals displayed a pronounced scratching activity (P < 0.05) (panel B), urinated more frequently (P < 0.05) (panel C), and showed lower levels of AEA content (P < 0.01, in the dorsal CPu) (panel D). We determined that long‐term exposure to stress increased the expression of Chrna6 gene (P < 0.01, in the PFC) (panel E) when compared to controls. An * indicates significant differences between stress group and their respective control group. One or two symbols indicate P < 0.05; P < 0.01, respectively. N = 15 for behavioral testing, n = 4 for endocannabinoids quantification; n = 5 for whole‐genome gene expression. PFC, prefrontal cortex; dorsal CPu, dorsal striatum; CTR, control; STS, stress; AEA, N‐arachidonylethanolamine
3.2. Effects of drugs
Two‐way ANOVA showed a significant main effect of the drug treatment on total distance traveled (F(3, 121) = 8.36, P < 0.001), distance traveled in center (F(3, 121 = 3.72, P < 0.05), rearing activity (F(3, 121) = 3.07, P < 0.05), PEA levels (F(3, 17) = 7.72, P < 0.01, in the dorsal CPu), and OEA levels (F(3, 22) = 5.29, P < 0.01, in the dorsal CPu). Drug administration had a remarkable effect on the relative fold change expression for Fkbp5 (F(3, 44) = 11.44, P < 0.001, in the dorsal CPu). The main effects of drug treatment were further evaluated by one‐way ANOVA or Brown‐Forsythe test when applicable followed by multiple comparisons post hoc tests (Table S1). In the OF, the cannabinoid agonist WIN decreased the total distance traveled (P < 0.01) (Figure 2, panel A) while the administration of the inverse agonist Rim significantly antagonized such effects (P < 0.01) (Figure 2, panel A). Coadministration with both cannabinoid drugs clearly reduced the total distance traveled in comparison with those animals only treated with Rim (P < 0.05) (Figure 2, panel A). The distance traveled in center, a measure of anxiety‐like behavior, was statistically elevated in animals treated with Rim alone relative to mice only treated with the agonist (P < 0.01) (Figure 2, panel B). Animals were also observed in their homecage by registering the vertical movements (rearings). We found that animals treated simultaneously with both drugs showed more frequent rearing behavior than WIN‐treated animals (P < 0.05) (Figure 2, panel C). The quantification of the endocannabinoid‐like molecules, PEA and OEA, revealed the highest amounts for these lipids in mice subjected to acute administration with the agonist (P < 0.05, in the dorsal CPu) (Figure 2, panel D & E). Genomewide transcriptional profiling revealed two top hits as deregulated genes by WIN and 32 top hits as deregulated genes by Rim before Benjamini‐Hochberg adjustment (Figure S1). The candidate gene (Fkbp5) was validated by quantitative RT‐PCR, and main results are depicted in Figure 2 (panel F). We measured higher transcription rate for Fkbp5 after administration with Rim+WIN than vehicle (P < 0.01, in the dorsal CPu) or when compared to either WIN or Rim alone (P < 0.05, P < 0.01; respectively, in the dorsal CPu) (Figure 2, panel F).
Figure 2.
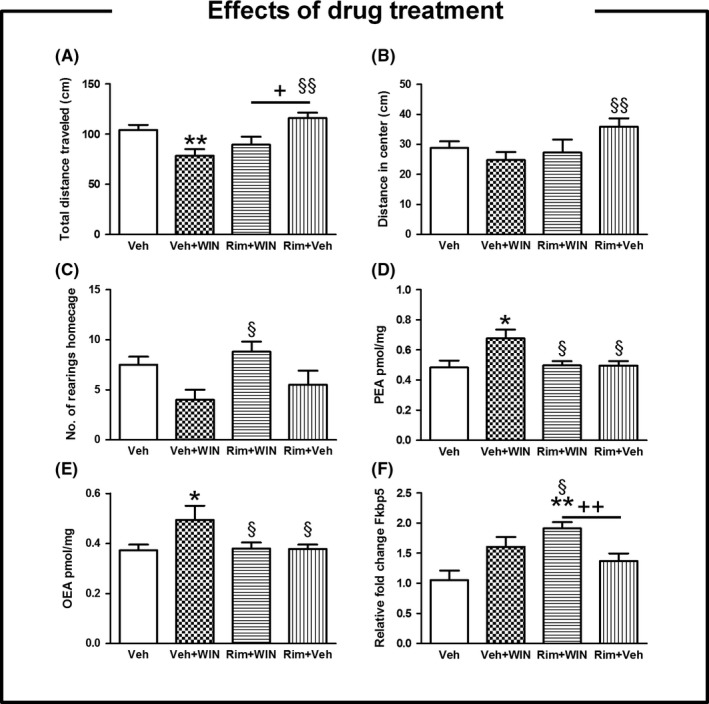
Effects of cannabinoid drugs administration. Data are expressed as mean ± SEM. In the OF, the cannabinoid agonist WIN decreased t and the total distance traveled (P < 0.01) (panel A) while the administration of the inverse agonist Rim significantly antagonized such effects (P < 0.01) (panel A). Coadministration with both cannabinoid drugs clearly reduced the total distance traveled in comparison to those animals single treated with Rim (P < 0.05) (panel A). The distance traveled in center was statistically higher in animals treated with Rim alone than those mice single treated with the agonist (P < 0.01) (panel B). We reported that animals treated simultaneously with both drugs showed more frequent rearing activity in their homecage than those animals treated with WIN alone (P < 0.05) (panel C). We found bigger amount of PEA and OEA in mice subjected to single administration with the agonist in comparison with the remaining drug‐treated groups (P < 0.05, in the dorsal CPu) (panel D & E). We measured higher transcription rate for Fkbp5 gene following administration with Rim+WIN than the vehicle group (P < 0.01, in the dorsal CPu) or when compared to either WIN or Rim alone (P < 0.05, P < 0.01; respectively, in the dorsal CPu) (panel F). An * indicates significant differences between drug‐treated mice and their respective vehicle group. Comparisons between WIN‐treated mice and remaining drug treatments are indicated by an §. Otherwise underlined + indicated other significant comparison intragroup. One or two symbols indicate P < 0.05; P < 0.01, respectively. N = 15 for behavioral testing, n = 4 for endocannabinoids quantification; n = 5 for whole‐genome gene expression. PFC, prefrontal cortex; dorsal CPu, dorsal striatum; OF, open field
3.3. Effects of stress under drug influence
A 2‐way ANOVA reported a significant interaction between the factors (stress × drug) on total distance traveled in the OF arena (F(3, 121) = 2.76, P < 0.05), 2‐AG content (F(3, 23) = 18.91, P < 0.001, in the PFC), and fold change expression for Chrna6 (F(3, 43) = 7.38, P < 0.001, in the PFC) and Slc6a4 gene (F(3, 43) = 3.18, P < 0.05, in the PFC). Simple main effects of the interaction between the factors are depicted in Table S2.
In an OF arena, repeated long‐term exposure to psychosocial stress exacerbated general motor activity following vehicle administration (Figure 3, panel A). Indeed, social defeat animals exposed to vehicle traveled longer distances than their controls (P < 0.01) (Figure 3, panel A). However, the pharmacological treatment with WIN reduced the total distance traveled in the OF when compared to Rim‐treated animals under either control or stress conditions (P < 0.01) (Figure 3, panel A). Likewise, psychosocially stressed mice exposed to the CB1 agonist WIN alone traveled smaller distances than the vehicle group (P < 0.01) (Figure 3, panel A). The administration of the inverse agonist in controls enhanced the total distance traveled when compared to vehicle‐exposed animals (P < 0.05) (Figure 3, panel A). Upon long‐lasting effects of stress, coadministration with Rim+WIN reduced the total distance traveled in comparison with vehicle (P < 0.01) and Rim‐treated animals (P < 0.01) (Figure 3, panel A).
Figure 3.
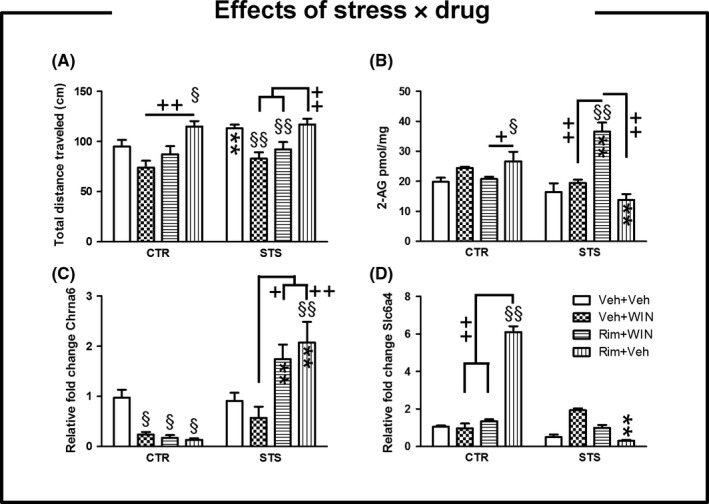
Effects of repeated exposure to psychosocial stress following acute cannabinoid drugs administration. Data are expressed as mean ± SEM. (1) Effects on mice behavior: In an open‐field arena, exposure to psychosocial stress exacerbated general motor activity following vehicle administration (panel A‐B). However, psychosocially stressed mice exposed to the agonist WIN alone traveled smaller distances than the vehicle group (P < 0.01) (panel A). The administration of inverse agonist Rim in controls enhanced the total distance traveled when compared to vehicle (P < 0.05) (panel A). (2) Effects on endocannabinoids levels: Controls treated with Rim alone underwent an increase in 2‐AG levels when compared to either vehicle or Rim+WIN‐treated mice (P < 0.05, in the PFC) (panel B). The administration of the inverse agonist Rim by the end of stress period reduced the levels of the endocannabinoid 2‐AG when compared to controls that received the same drug treatment (P < 0.01, in the PFC) (panel B). (3) Effects on gene expression: The use of synthetic cannabinoid drugs decreased the expression of Chrna6 in controls (P < 0.05, in the PFC) (panel C) and also did but not significantly when WIN was administered upon stress exposure while the inverse agonist Rim clearly antagonized such effects (P < 0.01, in the PFC) (panel C). Social defeat animals cotreated with Rim+WIN or treated with Rim alone underwent a remarkable upregulation of Chrna6 expression when compared to their nonstressed counterparts (P < 0.01, in the PFC) (panel C). We assessed a higher transcription rate for Slc6a4 gene in controls upon injection with the inverse agonist in comparison with the remaining control groups (P < 0.01, in the PFC) and also when this drug was administered in stressed animals (P < 0.01, in the PFC) (panel D). The remaining comparisons are depicted in the graph. An * indicates significant differences between stress groups and their respective control group. Control or stress intragroup comparisons between vehicle and treated mice are indicated by an §. Otherwise underlined + indicated intragroup comparisons between treatment groups in control or stress conditions. One or two symbols indicate P < 0.05; P < 0.01, respectively. N = 15 for behavioral testing, n = 4 for endocannabinoids quantification; n = 5 for whole‐genome gene expression. PFC, prefrontal cortex; CTR, control; STS, stress
On the other hand, controls only treated with Rim exhibited higher 2‐AG levels than either vehicle or Rim+WIN‐treated mice (P < 0.05, in the PFC) (Figure 3, panel B). However, social defeat mice treated with Rim+WIN displayed higher amounts of 2‐AG than their nonstressed counterparts (P < 0.01, in the PFC) but also when compared to either vehicle, WIN, or Rim alone under stress (P < 0.01, in the PFC) (Figure 3, panel B). The administration of the inverse agonist Rim by the end of the stress period reduced the levels of 2‐AG when compared to controls treated with the same drug (P < 0.01, in the PFC) (Figure 3, panel B).
Whole‐genome gene expression revealed 10 top hits genes deregulated simultaneously by stress and cannabinoid administration before Benjamini‐Hochberg adjustment (Figure S1) (Table 1). Three of them (Fkbp5, Chrna6, and Slc6a4) were further analyzed by quantitative RT‐PCR. Two‐way ANOVA showed a significant effect of the interaction on Slc6a4 (F(3, 43) = 3.18, P < 0.05) and Chrna6 (F(3, 43) = 7.38, P < 0.001) in the PFC but did not reveal differences in the dorsal CPu. The use of synthetic cannabinoid drugs diminished the expression of Chrna6 in controls (P < 0.05, in the PFC) (Figure 3, panel C) and also did so, but not significantly, when WIN was administered in stressed animals. In contrast, the inverse agonist Rim clearly antagonized the effects of WIN on Chrna6 expression under stress (P < 0.01, in the PFC) (Figure 3, panel C). Furthermore, Rim+WIN administration in stressed mice slightly decreased the expression of Chrna6 when compared to Rim alone, although Chrna6 expression was higher than with single WIN treatment (P < 0.05, in the PFC) (Figure 3, panel C). By the end of the stress protocol, acute injection with Rim increased the expression of Chrna6 in contrast to vehicle (P < 0.01, in the PFC) (Figure 3, panel C). Social defeat animals exposed to either Rim+WIN or Rim alone underwent an upregulation of Chrna6 expression when compared to their nonstressed counterparts (P < 0.01, in the PFC) (Figure 3, panel C). It was generally observed that in the PFC, the expression of Slc6a4 transcripts was higher in the control group treated with the inverse agonist Rim compared to all the groups (P < 0.01, in the PFC, Figure 3, panel D).
Table 1.
Differentially deregulated top 10 genes in response to chronic psychosocial stress and acute cannabinoid treatment
| Genes | PFC | DS | FC Stress | FC Rim | FC WIN | q value Stress | q value Rim | q value WIN |
|---|---|---|---|---|---|---|---|---|
| Chrna6 | 33.8 | 45.6 | 13.5 | −13.2 | −15.2 | 6.46E‐11 | 0 | 0 |
| Slc6a4 | 64.2 | 102.3 | 31.7 | −34.1 | −32.5 | 1 | 1 | 0 |
| Ldlr | 7.5 | 7.7 | −0.2 | −0.3 | −0.1 | 0.7 | 0.01 | 1 |
| Sdf2l1 | 5.6 | 5.3 | 0.5 | 0.5 | 0.2 | 0.002 | 0.02 | 1 |
| Lrg1 | 4.1 | 2.5 | 0.5 | 0.7 | 0.5 | 0.1 | 0.02 | 1 |
| Fkbp5 | 7.8 | 8.0 | 0.5 | 0.4 | 0.2 | 4.81E‐06 | 0.03 | 1 |
| Dok3 | 5.2 | 4.9 | 0.4 | 0.5 | 0.1 | 0.1 | 0.03 | 1 |
| Hspa5 | 10.7 | 10.8 | 0.3 | 0.3 | 0.2 | 0.01 | 0.05 | 1 |
| Manf | 7.9 | 7.9 | 0.2 | 0.2 | 0.1 | 0.01 | 0.05 | 1 |
| Hspa1a | 6.8 | 6.0 | 0.3 | 0.4 | 0.2 | 0.03 | 0.05 | 1 |
Column titles from left to right: official gene symbol; PFC, prefrontal cortex; dorsal CPu, dorsal striatum; FC stress, fold change stress; FC Rim, fold change Rimonabant; FC WIN, fold change WIN55212.2; q value Stress, P adjust value Stress; q value Rim, P adjust value Rimonabant; q value WIN, P adjust value WIN55212.2.
4. DISCUSSION
Long‐term stress confers risk to develop psychiatric disorders and symptoms.25 In response to prolonged stress, glucocorticoid release has been shown to have deleterious effects on different brain regions and also is associated with the regression of synapses and dendritic spines.26 Remarkably, dysfunctions in corticostriatal connectivity have been involved in the pathophysiology of psychiatric disorders.27 Moreover, the G protein‐coupled CB1 is extremely abundant and a key modulator of the corticostriatal pathways.10
4.1. Effects of stress
After 2 weeks of daily psychosocial stress, animals showed loss of body weight in line with Iniguez et al.28 Our data revealed that chronic psychosocial stress exacerbates scratching activity.29 The endocannabinoid system has been described to have a direct effect on serotonergic neurotransmission which in turn, modulates several behaviors and physiological functions16 such as itch sensation.30 Moreover, the pronounced scratching phenotype reported here could also be attributable to the lower levels of AEA observed in the PFC.
In fact, pharmacological manipulation of the degrading enzymes leading to increased levels of AEA has shown a promising tool for reducing itch sensation.31 Additionally, mice with defective endocannabinoid signaling at CB1 receptors displayed a pronounced scratching behavior (for review see Ref. 32).
Stress is well known to affect urinary bladder function and exacerbates signs of urinary bladder dysfunction. We demonstrated that socially defeated animals urinated more frequently probably caused by reduced bladder capacity as described.33
A decrease in motor activity is among the changes most commonly noted in subordinates and defeated animals.3 However, we found that social defeat animals traveled longer distances in the OF arena. These discrepancies could be attributable to methodological differences such as: (i) the use of mice rather than rats; (ii) housing conditions (five mice to a cage instead of just one); and (iii) the time at testing, as here the stress protocol and drug administration were performed during the active phase (after 8 pm) instead of the sleep phase. Finally, we measured an overexpression of Chrna6 under stress. Studies of genetic linkage reported that the coding Chrna6 region confers risk to develop neuropsychiatric disorders.34 Likewise, Kimbrel et al35 reported that two SNPs linked to CHRNA6 conferred risk for developing post‐traumatic stress disorder. Thus, the results described here point out a plausible contribution of Chrna6 to the long‐lasting effects observed in social defeat animals.
4.2. Effects of drug administration
Single administration of the agonist WIN reduced total distance traveled while acute treatment with the inverse agonist Rim slightly increased it when compared to controls an observation corroborated by Brzozka et al.3
In the OF, mice treated with the inverse agonist Rim traveled longer distances in the center than the remaining groups, which support an anxiolytic effect mediated by the drug,36 even though CB1 antagonism can also produce anxiogenic actions.37 Animals treated with Rim+WIN showed more frequent rearing behavior than WIN‐treated animals. Indeed, cannabis smoke exposure induced lower rearing activity, while the use of Rimonabant prevented the smoke‐induced decrease in rearing.38
Upon CB1 activation by WIN, we observed an increase in the levels of both OEA and PEA, two mediators metabolically related to AEA. In line with our results, Bardou et al39 reported an increase in OEA and PEA contents after inhibiting the enzyme fatty acid amide hydrolase. Despite the fact that OEA and PEA levels often, but not always, change in a similar manner to those of AEA under physiological or pathological conditions, we did not observe here any similar changes in AEA levels following chronic psychosocial stress. This finding is compatible with the general concept that OEA and PEA are not eCBs in as much as they do not activate directly CB1 and CB2 receptors, but instead modulate noncannabinoid receptors, such as G protein‐coupled receptor 55 (GPR55), peroxisome proliferator‐activated receptor‐α (PPAR‐α), and transient receptor potential vanilloid type‐1 (TRPV1) channels. Recently, Musella et al reported that significant levels of PEA are present in the striatum, where this mediator enhances GABAergic neurotransmission via GPR55. Interestingly, PEA was previously reported to exhibit greater binding affinity for GPR55 than CB1, CB2, PPAR‐α, and TRPV1 (for review see Ref. 40). Thus, it is noteworthy to speculate that exposure to cannabinoid drugs, by altering PEA levels, modifies GPR55 receptor activity.
It is well known that the glucocorticoid receptor is regulated by several chaperones and cochaperones including the FKBP5 protein.41 In particular, we found that the administration of the CB1 inverse agonist Rimonabant potentiated the expression of Fkbp5 when compared to the remaining groups. This is in agreement with data showing that FKBP5 regulates glucocorticoid receptor sensitivity, increases its resistance, and decreases its efficiency at controlling the negative feedback in response to elevated levels of corticosterone,42 whereas Rimonabant can aggravate the hyperactivity of the HPA axis during stress.43 Several studies attest to the idea that FKBP5 is crucial for the development of stress‐related mental disorders 44, 45. However, it is currently unclear how the activation of the endocannabinoid system through the use of synthetic cannabinoid drugs may regulate either Fkbp5 or glucocorticoid signaling as pointed out by Wang et al.46 In conclusion, our understanding of FKBP5 functions is still incomplete and thus, further work must address this question.
4.3. Effects of stress under drug influence
The inverse agonist Rimonabant in nonstress conditions antagonized the effects of WIN55212.2 on total distance travelled.3 Under experimentally induced psychosocial stress, mice treated with either WIN or Rim+WIN showed less total distance traveled than the vehicle group, while single Rimonabant administration antagonized the effects of WIN.3 We also observed that stressed mice exposed to vehicle displayed an exacerbated total distance traveled when compared to their nonstressed counterparts. Against the present findings, Brzozka et al3 found that repeated exposure to social defeat paradigm reduced the total distance traveled in the OF. The differences reported here could be explained by the influence of the circadian rhythm and the housing conditions (5 mice per cage instead of just one). Indeed, mice are nocturnal animals mainly active during the dark period.5 Furthermore, the absence of social support (individual housing) amplifies the anxiety induced by psychosocial stress.47
It is widely accepted that upon HPA activation, the content of endocannabinoid lipid molecules varies according to the duration/type of stress stimuli and the brain structure. We found that social defeat animals exposed to vehicle showed similar 2‐AG levels than their controls in agreement with.48 In contrast, Gorzalka et al49 determined that after chronic stress, the endocannabinoid signaling was compromised because endocannabinoid levels were reduced in all limbic structures. Chronic restraint stress, for example, progressively increases 2‐AG content within distinct anatomical regions such as the medial PFC, limbic forebrain, amygdala, hippocampus, and hypothalamus.50 These differences could be explained by the influence of the circadian rhythm and also related to the stress protocol used. In fact, there is a strong evidence to suggest that in rodents circadian alterations occur in endocannabinoid brain levels,51 CB1 expression,52 and the activity of enzymes controlling the metabolism of endocannabinoids.51 The administration of Rimonabant in controls as well as the pharmacological blockade of MAGL enzyme has been reported in a powerful increase of 2‐AG levels in the CNS.53 Here, such increase was also observed when both cannabinoids were administered but not when Rim was injected alone. Thus, the use of synthetic cannabinoids in this model warrants further investigation in order to elucidate the underlying mechanisms associated with the metabolism of 2‐AG.
Not surprisingly, we found that the cannabinoid agonist WIN and the inverse agonist Rim influenced the expressions of Chrna6 and Slc6a4 in opposite directions, while their coadministration tended to produce a net null effect under experimentally induced psychosocial stress. Nicotinic receptor containing α6 subunit (CHRNA6) is expressed in distinct brain regions important for addiction behaviors and also is identified to confer susceptibility to neuropsychiatric disorders.34 In line with this report, emerging data point out that the coding region for CHRNA6 confers vulnerability to drugs of abuse and their related behavioral phenotypes.54 Interestingly, the present investigation is the first to demonstrate that simultaneous exposure to distinct environmental factors such as chronic stress and acute cannabinoid drug administration can regulate the expression of the Chrna6 gene which in turn and could be crucial for the development of cannabis addiction and psychosis. Long‐term exposure to stress constitutes a key environmental risk component for developing stress‐related disorders in susceptible individuals. The underlying mechanisms disrupted in these disorders are serotonergic55 and endocannabinoid56 dependent. Particularly, serotonin release inhibition is attributable to the lack of CB1 receptors which in turn, promotes higher concentration of this neurotransmitter in the synaptic cleft.57 Then, we can speculate that CB1 blockage by the administration of Rim could counteract the excessive serotonin neurotransmitter by increasing the expression of the serotonin transporter protein (Slc6a4).58 Furthermore, SLC6A4 single nucleotide polymorphisms have been associated with stress‐related psychiatric disorders,59 which are consistent with our findings.
5. CONCLUSIONS
In summary, the data described here highlight the effects of long‐term exposure to stress on mouse corticostriatal circuitries following acute challenge with distinct cannabinoid drugs (For further details see Figure S2). Particularly, we found that psychological stress played a role in the exacerbation of micturition frequency, anxiety‐related behavior,58 and scratching phenotype,29 while the use of synthetic cannabinoids drugs interfered with locomotor activity, rearing, and anxiety‐like behavior.38 The glucocorticoid receptor is regulated by several chaperones and cochaperones including the FKBP5 protein41 but also is influenced by endocannabinoid system.17 Additionally, we demonstrated that psychosocially stressed animals displayed changes in the serotonergic system upon acute exposure to synthetic cannabinoid drugs.55 The coding region for CHRNA6 confers vulnerability to drugs of abuse and their related behavioral phenotypes,54 which in turn, could be crucial for the development of cannabis addiction and psychosis. Our findings in both PFC and dorsal CPu need to be corroborated by further studies if we want to understand the role of CB1 and endocannabinoids and related mediators in corticostriatal signaling under psychosocial stress.
CONFLICTS OF INTEREST
The author reports no potential conflict of interests.
Supporting information
ACKNOWLEDGMENTS
We thank Prof. Lutz from University Medical Center of the Johannes Gutenberg University (Germany) for supplying the CB1 riboprobe. The authors would like to thank Prof. Colomina, Dr. Yang, and Mr. Ludewig for excellent technical support. Dr. Gil was supported by Juan de la Cierva postdoctoral fellowship of MINECO. Dr. Tomas‐Roig was supported by Deutsche Forschungsgemeinschaft fellowship (Grant TO 977/1‐1). The work was supported by DFG Research Center for Nanoscale Microscopy and Molecular Physiology of the Brain (CNMPB) and the German‐Research‐Foundation (DFG) grant CNMPB C1‐6 to Prof. Havemann‐Reinecke.
Tomas‐Roig J, Piscitelli F, Gil V, et al. Effects of repeated long‐term psychosocial stress and acute cannabinoid exposure on mouse corticostriatal circuitries: Implications for neuropsychiatric disorders. CNS Neurosci Ther. 2018;24:528–538. 10.1111/cns.12810
The first and last author share senior authorship.
The second and third author contributed equally to this work.
REFERENCES
- 1. van Os J, Kenis G, Rutten BPF. The environment and schizophrenia. Nature. 2010;468:203‐212. [DOI] [PubMed] [Google Scholar]
- 2. Fornito A, Harrison BJ, Goodby E, et al. Functional dysconnectivity of corticostriatal circuitry as a risk phenotype for psychosis. JAMA Psychiatry [Internet]. 2013;70:1143‐1151. [DOI] [PubMed] [Google Scholar]
- 3. Brzozka MM, Fischer A, Falkai P, Havemann‐Reinecke U. Acute treatment with cannabinoid receptor agonist WIN55212.2 improves prepulse inhibition in psychosocially stressed mice. Behav Brain Res. 2011;218:280‐287. [DOI] [PubMed] [Google Scholar]
- 4. Sapolsky R. Taming stress. Sci Am. 2003;289:86‐95. [DOI] [PubMed] [Google Scholar]
- 5. de Kloet ER. Stress in the brain. Eur J Pharmacol. 2000;405:187‐198. [DOI] [PubMed] [Google Scholar]
- 6. Duval ER, Javanbakht A, Liberzon I. Neural circuits in anxiety and stress disorders: a focused review. Ther Clin Risk Manag. 2015;11:115‐126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blair KS, Geraci M, Otero M, et al. Atypical modulation of medial prefrontal cortex to self‐referential comments in generalized social phobia. Psychiatry Res. 2011;193:38‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sripada C, Angstadt M, Liberzon I, McCabe K, Phan KL. Aberrant reward center response to partner reputation during a social exchange game in generalized social phobia. Depress Anxiety. 2013;30:353‐361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boychuk CR, Zsombok A, Tasker JG, Smith BN. Rapid glucocorticoid‐induced activation of TRP and CB1 receptors causes biphasic modulation of glutamate release in gastric‐related hypothalamic preautonomic neurons. Front Neurosci. 2013;7:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci [Internet]. 1991;11:563‐583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lovinger DM, Davis MI, Costa RM. Handbook of Basal Ganglia Structure and Function. Steiner H, Tseng K, eds. London: Academic Press; 2010:693. [Google Scholar]
- 12. Devane WA, Hanus L, Breuer A, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946‐1949. [DOI] [PubMed] [Google Scholar]
- 13. Sugiura T, Kondo S, Sukagawa A, et al. 2‐arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun [Internet]. 1995;215:89‐97. [DOI] [PubMed] [Google Scholar]
- 14. Basavarajappa BS. Critical enzymes involved in endocannabinoid metabolism. Protein Pept Lett. 2007;14:237‐246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Di Marzo V. Endocannabinoids: synthesis and degradation. Rev Physiol Biochem Pharmacol. 2008;160:1‐24. [DOI] [PubMed] [Google Scholar]
- 16. Lazary J, Juhasz G, Hunyady L, Bagdy G. Personalized medicine can pave the way for the safe use of CB 1 receptor antagonists. Trends Pharmacol Sci. 2011;32:270‐280. [DOI] [PubMed] [Google Scholar]
- 17. Tasker JG, Di S, Malcher‐Lopes R. Minireview: rapid glucocorticoid signaling via membrane‐associated receptors. Endocrinology. 2006;147:5549‐5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Golub MS, Germann SL, Lloyd KC. Behavioral characteristics of a nervous system‐specific erbB4 knock‐out mouse. Behav Brain Res. 2004;153:159‐170. [DOI] [PubMed] [Google Scholar]
- 19. Matias I, Petrosino S, Racioppi A, Capasso R, Izzo AA, Di Marzo V. Dysregulation of peripheral endocannabinoid levels in hyperglycemia and obesity: effect of high fat diets. Mol Cell Endocrinol. 2008;286(1–2 suppl 1):S66‐S78. [DOI] [PubMed] [Google Scholar]
- 20. Bisogno T, Martire A, Petrosino S, Popoli P, Di Marzo V. Symptom‐related changes of endocannabinoid and palmitoylethanolamide levels in brain areas of R6/2 mice, a transgenic model of Huntington's disease. Neurochem Int. 2008;52:307‐313. [DOI] [PubMed] [Google Scholar]
- 21. Pruitt KD, Brown GR, Hiatt SM, et al. RefSeq: an update on mammalian reference sequences. Nucleic Acids Res [Internet]. 2014;42:D756‐D763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li H, Durbin R. Fast and accurate short read alignment with Burrows‐Wheeler transform. Bioinformatics. 2009;25:1754‐1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11:R106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B [Internet]. 1995;57:289‐300. [Google Scholar]
- 25. Holtzman CW, Trotman HD, Goulding SM, et al. Stress and neurodevelopmental processes in the emergence of psychosis. Neuroscience. 2013;249:172‐191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McEwen BS. The ever‐changing brain: cellular and molecular mechanisms for the effects of stressful experiences. Dev Neurobiol. 2012;72:878‐890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shepherd GMG. Corticostriatal connectivity and its role in disease. Nat Rev Neurosci [Internet]. 2013;14:278‐291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Iñiguez SD, Riggs LM, Nieto SJ, et al. Social defeat stress induces a depression‐like phenotype in adolescent male c57BL/6 mice. Stress [Internet]. 2014;17:247‐255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhao P, Hiramoto T, Asano Y, Kubo C, Sudo N. Chronic psychological stress exaggerates the compound 48/80‐induced scratching behavior of mice. Pharmacol Biochem Behav. 2013. Apr;105:173‐176. [DOI] [PubMed] [Google Scholar]
- 30. Weisshaar E, Ziethen B, Röhl FW, Gollnick H. The antipruritic effect of a 5‐HT3 receptor antagonist (tropisetron) is dependent on mast cell depletion—an experimental study. Exp Dermatol [Internet]. 1999;8:254‐260. [DOI] [PubMed] [Google Scholar]
- 31. Tosun NC, Gunduz O, Ulugol A. Attenuation of serotonin‐induced itch responses by inhibition of endocannabinoid degradative enzymes, fatty acid amide hydrolase and monoacylglycerol lipase. J Neural Transm. 2014;122:363‐367. [DOI] [PubMed] [Google Scholar]
- 32. Caterina MJ. TRP channel cannabinoid receptors in skin sensation, homeostasis, and inflammation. ACS Chem Neurosci. 2014;5:1107‐1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mingin GC, Peterson A, Erickson CS, Nelson MT, Vizzard M. Social stress induces changes in urinary bladder function, bladder NGF content, and generalized bladder inflammation in mice. Am J Physiol Regul Integr Comp Physiol. 2014;307:R893‐R900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tabarés‐Seisdedos R, Rubenstein JLR. Chromosome 8p as a potential hub for developmental neuropsychiatric disorders: implications for schizophrenia, autism and cancer. Mol Psychiatry [Internet]. 2009;14:563‐589. [DOI] [PubMed] [Google Scholar]
- 35. Kimbrel NA, Garrett ME, Dennis MF, et al. Effect of genetic variation in the nicotinic receptor genes on risk for posttraumatic stress disorder. Psychiatry Res. 2015;229:326‐331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lafenêtre P, Chaouloff F, Marsicano G. The endocannabinoid system in the processing of anxiety and fear and how CB1 receptors may modulate fear extinction. Pharmacol Res. 2007;56:367‐381. [DOI] [PubMed] [Google Scholar]
- 37. Komaki A, Abdollahzadeh F, Sarihi A, Shahidi S, Salehi I. Interaction between antagonist of cannabinoid receptor and antagonist of adrenergic receptor on anxiety in male rat. Basic Clin Neurosci. 2014;5:218‐224. [PMC free article] [PubMed] [Google Scholar]
- 38. Bruijnzeel AW, Qi X, Guzhva LV, et al. Behavioral characterization of the effects of cannabis smoke and anandamide in rats. PLoS ONE. 2016;11:e0153327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bardou I, DiPatrizio N, Brothers HM, et al. Pharmacological manipulation of cannabinoid neurotransmission reduces neuroinflammation associated with normal aging. Health (Irvine Calif) [Internet]. 2012;4:679‐684. [Google Scholar]
- 40. Musella A, Fresegna D, Rizzo FR, et al. A novel crosstalk within the endocannabinoid system controls GABA transmission in the striatum. Sci Rep. 2017;7:7363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wochnik GM, Rüegg J, Abel GA, Schmidt U, Holsboer F, Rein T. FK506‐binding proteins 51 and 52 differentially regulate dynein interaction and nuclear translocation of the glucocorticoid receptor in mammalian cells. J Biol Chem. 2005;280:4609‐4616. [DOI] [PubMed] [Google Scholar]
- 42. Binder EB. The role of FKBP5, a co‐chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology. 2009;34(suppl 1):S186‐S195. [DOI] [PubMed] [Google Scholar]
- 43. Blasio A, Iemolo A, Sabino V, et al. Rimonabant precipitates anxiety in rats withdrawn from palatable food: role of the central amygdala. Neuropsychopharmacology [Internet]. 2013;38:2498‐2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xie P, Kranzler HR, Poling J, et al. Interaction of FKBP5 with childhood adversity on risk for post‐traumatic stress disorder. Neuropsychopharmacology. 2010;35:1684‐1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zimmermann P, Brückl T, Nocon A, et al. Interaction of FKBP5 gene variants and adverse life events in predicting depression onset: results from a 10 ‐year prospective community study. Am J Psychiatry. 2011;168:1107‐1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang M, Hill MN, Zhang L, Gorzalka BB, Hillard CJ, Alger BE. Acute restraint stress enhances hippocampal endocannabinoid function via glucocorticoid receptor activation. J Psychopharmacol. 2012;26:56‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Adamcio B, Havemann‐Reinecke U, Ehrenreich H. Chronic psychosocial stress in the absence of social support induces pathological pre‐pulse inhibition in mice. Behav Brain Res. 2009;204:246‐249. [DOI] [PubMed] [Google Scholar]
- 48. Wang W, Sun D, Pan B, et al. Deficiency in endocannabinoid signaling in the nucleus accumbens induced by chronic unpredictable stress. Neuropsychopharmacology. 2010;35:2249‐2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gorzalka BB, Hill MN, Hillard CJ. Regulation of endocannabinoid signaling by stress: implications for stress‐related affective disorders. Neurosci Biobehav Rev. 2008;32:1152‐1160. [DOI] [PubMed] [Google Scholar]
- 50. Patel S, Kingsley PJ, Mackie K, Marnett LJ, Winder DG. Repeated homotypic stress elevates 2‐arachidonoylglycerol levels and enhances short‐term endocannabinoid signaling at inhibitory synapses in basolateral amygdala. Neuropsychopharmacology. 2009;34:2699‐2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Valenti M, Viganò D, Casico MG, et al. Differential diurnal variations of anandamide and 2‐arachidonoyl‐glycerol levels in rat brain. Cell Mol Life Sci. 2004;61:945‐950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rueda‐Orozco PE, Soria‐Gomez E, Montes‐Rodriguez CJ, et al. A potential function of endocannabinoids in the selection of a navigation strategy by rats. Psychopharmacology. 2008;198:565‐576. [DOI] [PubMed] [Google Scholar]
- 53. Chanda PK, Gao Y, Mark L, et al. Monoacylglycerol lipase activity is a critical modulator of the tone and integrity of the endocannabinoid system. Mol Pharmacol [Internet]. 2010;78:996‐1003. [DOI] [PubMed] [Google Scholar]
- 54. Sadler B, Haller G, Agrawal A, et al. Variants near CHRNB3‐CHRNA6 are associated with DSM‐5 cocaine use disorder: evidence for pleiotropy. Sci Rep [Internet]. 2014;4:4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Inoue T, Tsuchiya K, Koyama T. Regional changes in dopamine and serotonin activation with various intensity of physical and psychological stress in the rat brain. Pharmacol Biochem Behav. 1994;49:911‐920. [DOI] [PubMed] [Google Scholar]
- 56. Burokas A, Martín‐García E, Gutiérrez‐Cuesta J, et al. Relationships between serotonergic and cannabinoid system in depressive‐like behavior: a PET study with [11C]‐DASB. J Neurochem. 2014;130:126‐135. [DOI] [PubMed] [Google Scholar]
- 57. Rudnick G. Serotonin transporters—structure and function. J Membr Biol. 2006;213:101‐110. [DOI] [PubMed] [Google Scholar]
- 58. Karg K, Burmeister M, Shedden K, Sen S. The serotonin transporter promoter variant (5‐HTTLPR), stress, and depression meta‐analysis revisited: evidence of genetic moderation. Arch Gen Psychiatry. 2011;68:444‐454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Smith AL, Leung J, Kun S, et al. The effects of acute and chronic psychological stress on bladder function in a rodent model. Urology. 2011;78:967.e1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


