Abstract
Aims
To assess efficacy and safety of dulaglutide 1.5 mg combined with insulin, categorized by subgroups of baseline glycated haemoglobin (HbA1c; ≤9% and >9% [≤74.9 and >74.9 mmol/mol]), age (<65 and ≥65 years), and duration of diabetes (<10 and ≥10 years) at 6 months in patients with type 2 diabetes (T2D).
Materials and methods
This pooled analysis was conducted in a population of patients with T2D with similar baseline characteristics who were included in the AWARD‐4 and AWARD‐9 clinical trials and randomized to dulaglutide 1.5 mg (pooled mean baseline age 59 years, duration of diabetes 13 years, HbA1c 8.4% [68.3 mmol/mol]). Weight and hypoglycaemia were analysed by individual trial. In AWARD‐4, dulaglutide plus lispro three times daily was assessed against glargine plus lispro three times daily. In AWARD‐9, dulaglutide added to glargine was assessed against placebo added to glargine. Insulins were titrated to target in both trials.
Results
A total of 445 patients were included in this analysis (73% with HbA1c ≤9%, 27% [≤74.9 mmol/mol] with HbA1c >9% [>74.9 mmol/mol]; 70% aged <65 years, 30% aged ≥65 years; 36% with duration of diabetes <10 years, 64% with duration of diabetes ≥10 years). At 6 months, dulaglutide 1.5 mg significantly reduced HbA1c in all subgroups (P < .001), with the highest reduction observed in patients with baseline HbA1c >9% (>74.9 mmol/mol) (range − 1.3% to −2.5% [−14.2 to −27.3 mmol/mol]). The incidence rates of documented symptomatic and severe hypoglycaemia were similar in all subgroups in both trials. The most common adverse events observed in each trial were gastrointestinal in nature.
Conclusion
Dulaglutide 1.5 mg combined with basal or prandial insulin is efficacious for patients with T2D irrespective of age, duration of diabetes or baseline HbA1c.
Keywords: dulaglutide, GLP‐1 receptor agonist, type 2 diabetes
1. INTRODUCTION
Glucagon‐like peptide‐1 receptor agonists (GLP‐1RAs) are relevant agents for the management of type 2 diabetes (T2D) because of their multifactorial mechanism of action. These varied effects of the GLP‐1RA class translate into robust glycated haemoglobin (HbA1c) reduction and weight loss with a low risk of hypoglycaemia and, more recently, cardiovascular benefits, as observed with some of the long‐acting GLP‐1RAs.1, 2, 3 The use of GLP‐1RAs along with basal insulin has long been discussed, given their complementary effects on glycaemic control, opposite effects on weight (weight gain with insulin vs weight loss with GLP‐1RAs) and lower risk of hypoglycaemia with GLP‐1RAs as a result of their glucose‐dependent insulin secretion.4 In a recent meta‐analysis of 26 trials, the combination of GLP‐1RAs with basal insulin resulted in significantly greater reductions in HbA1c vs comparators. The HbA1c reduction efficacy was also similar in the pre‐specified subgroup analyses vs basal‐bolus regimens.5, 6
The once‐weekly GLP‐1RA, dulaglutide, has been assessed in 9 phase III clinical trials from the Assessment of Weekly Administration of LY2189265 in Diabetes (AWARD) clinical development programme in >5000 adult patients as monotherapy or as add‐on to 1 or 2 oral antihyperglycaemic medications, basal insulin or prandial insulin. Across these studies, the average HbA1c reduction and change in body weight from baseline at the primary endpoint was −0.78% to −1.64% (−8.52 to −17.92 mmol/mol) and −0.87 to −3.03 kg for the 1.5‐mg dose and −0.71% to −1.59% (−7.76 to −17.38 mmol/mol) and −2.06 to +0.20 kg for the 0.75‐mg dose, respectively.7, 8, 9, 10, 11, 12, 13, 14, 15 In the United States and the European Union, dulaglutide is indicated for use in combination with both basal or prandial insulin.16, 17 Dulaglutide 1.5 mg demonstrated superiority vs placebo when added to titrated insulin glargine for change in HbA1c from baseline (−1.44% [−15.7 mmol/mol] vs −0.67% [−7.32 mmol/mol] at 28 weeks), and demonstrated superiority against insulin glargine when combined with insulin lispro three times daily for change in HbA1c from baseline (−1.64% [−17.9 mmol/mol] vs −1.41% [−15.4 mmol/mol] at 26 weeks).7, 12
Because T2D is a progressive disease where almost 50% of β‐cell function is lost before diagnosis,18 the β‐cell‐dependent primary mechanism of action of GLP‐1RAs can cast doubts in the minds of physicians regarding its efficacy in patients with long‐standing disease or in elderly patients who have already resorted to insulin therapy. Despite having the highest prevalence of diabetes of any age group, the proportion of patients aged >65 years is often much smaller than other age groups in clinical trials, which warrants the need for pooled analyses to obtain data for this unique population.
In a recent integrated analysis of 7 AWARD studies, significant improvements in glycaemic control were observed after 6 months of dulaglutide treatment (1.5 mg or 0.75 mg) irrespective of gender, duration of diabetes (<5 years, ≥5 years and <10 years, ≥10 years), or baseline HbA1c (<8.5%, ≥8.5% [<69.4 mmol/mol, ≥69.4 mmol/mol]) in patients with T2D.19 In another post hoc analysis of 6 AWARD studies, both dulaglutide doses were well tolerated with similar efficacy, irrespective of age (≥65 years, <65 years).20 In a recent real‐world study, ~40% of patients initiating dulaglutide were already on insulin therapy.21 Considering such a large use of the combination of dulaglutide with insulin and its broad indication for use with basal or prandial insulins, this combination needs a more in‐depth assessment with regard to elderly patients with long‐standing and poorly controlled T2D.
The aim of the present post hoc analysis was to evaluate the efficacy and safety of dulaglutide 1.5 mg combined with basal or prandial insulin in patients with long‐standing T2D when categorized by subgroups of baseline HbA1c (≤9%, >9% [≤74.9 mmol/mol, >74.9 mmol/mol]), age (<65 years, ≥65 years) or duration of diabetes (<10 years, ≥10 years). The main hypothesis was that patients with long‐standing T2D treated with once‐weekly dulaglutide 1.5 mg combined with prandial or basal insulin significantly reduced HbA1c from baseline, irrespective of age, duration of diabetes or baseline HbA1c. Additionally, taking into account the American Diabetes Association recommendations of less stringent glycaemic goals for older adults with diabetes, glycaemic targets of HbA1c <7% (<53.0 mmol/mol) and <8% (<63.9 mmol/mol) were assessed.22
2. RESEARCH DESIGN AND METHODS
2.1. Individual trial design
The present analysis included patients from 2 randomized, phase III clinical trials from the AWARD programme (AWARD‐4 and AWARD‐9). Individual trial results were previously published.7, 12 Each trial was designed to evaluate the safety and efficacy of dulaglutide in adults with T2D with a primary endpoint of 26 weeks (AWARD‐4) or 28 weeks (AWARD‐9). In AWARD‐4, the primary objective was to assess the change from baseline in HbA1c with dulaglutide compared with insulin glargine, both when added to prandial insulin lispro three times daily.7 In AWARD‐9, the primary objective was to assess the change from baseline in HbA1c with dulaglutide compared with placebo, both when added to titrated insulin glargine. In AWARD‐4, the insulin lispro dose was titrated in each arm based on preprandial and bedtime glucose values using a lispro algorithm based on Bergenstal et al.23 In both studies, the insulin glargine dose was titrated to a fasting plasma glucose concentration <100 mg/dL (<5.6 mmol/L). Documented symptomatic hypoglycaemia was defined as plasma glucose ≤70 mg/dL (≤3.9 mmol/L) and severe hypoglycaemia was defined as an episode requiring the assistance of another person to actively administer carbohydrate or glucagon or to take other resuscitative actions. Both studies were conducted in accordance with the International Conference on Harmonization Guidelines for Good Clinical Practice and the Declaration of Helsinki. Participants provided signed informed consent and all protocols were approved by local ethical review boards.
2.2. Rationale for trial selection
In both AWARD‐4 and AWARD‐9, the majority of the patients enrolled were receiving basal insulin and metformin. The mean baseline HbA1c for participants in AWARD‐4 and AWARD‐9 was 8.5% and 8.4% (69.4 and 68.3 mmol/mol, respectively), the mean age was 58.9 and 60.2 years, and the mean duration of diabetes was 12.8 and 13.0 years, respectively.7, 12 This similarity in baseline characteristics between the 2 studies made them suitable for pooling to test the hypothesis of this post hoc analysis. Older patients were defined as those aged ≥65 years.24 Because AWARD‐9 assessed only the 1.5‐mg dose, only this dose was assessed and pooled in the present analysis.
2.3. Statistical analyses
In this analysis we assessed the efficacy and safety of dulaglutide 1.5 mg in patients with T2D according to baseline HbA1c level (≤9%, >9% [≤74.9 mmol/mol, >74.9 mmol/mol]), age (<65 years, ≥65 years), and duration of diabetes (<10 years, ≥10 years). Analyses were performed on the intention‐to‐treat population (efficacy measures) or safety population (safety measures). All analyses were conducted at 26 weeks in AWARD‐4 and 28 weeks in AWARD‐9 (hereafter, referred to as 6 months for the combined trials). Efficacy measures of HbA1c change from baseline and proportion of patients achieving HbA1c targets of <7% and <8% (<53.0 and <63.9 mmol/mol, respectively) on dulaglutide 1.5 mg were analysed using pooled data from the 2 trials. The pooled analysis for change in HbA1c was conducted using analysis of covariance (ANCOVA), with metformin use, country, study and subgroup as factors and baseline HbA1c, as a continuous covariate. Analyses of HbA1c did not include results obtained after post‐rescue therapy. Analyses of change in HbA1c and body weight were also conducted by individual study with dulaglutide 1.5 mg and active comparator arms using ANCOVA. Models included country, baseline metformin use, baseline value, treatment, subgroup and treatment‐by‐subgroup. Baseline value was not included as a covariate in the analysis of change in HbA1c for the subgroup baseline HbA1c (≤9%, >9% [≤74.9 mmol/mol, >74.9 mmol/mol]). The last observation was carried forward for missing data. Documented symptomatic and severe hypoglycaemia were analysed by individual study only and excluded events after post‐rescue therapy. Data are presented as least squares mean and 95% confidence interval (CI).
3. RESULTS
3.1. Baseline characteristics
The present analysis included data from 445 patients treated with dulagutide 1.5 mg. These patients were categorized into the following subgroups: baseline HbA1c ≤9% (≤74.9 mmol/mol) (n = 325; 73%); baseline HbA1c >9% (>74.9 mmol/mol) (n = 118; 27%); age <65 years (n = 313; 70%); age ≥65 years (n = 132; 30%); duration of diabetes <10 years (n = 158; 36%); and duration of diabetes ≥10 years (n = 287; 64% [Figure S1]). The mean ± SD baseline characteristics of all dulaglutide‐treated patients were: age 59.3 ± 9.5 years, HbA1c 8.4 ± 1.0% (68.3 ± 10.9 mmol/mol), body mass index (BMI) 32.3 ± 5.1 kg/m2 and duration of diabetes 12.9 ± 7.3 years, and 44.9% of the cohort (n = 200) were women. The pooled baseline characteristics of the patients treated with dulaglutide 1.5 mg stratified by subgroup are summarized in Table 1, as well as the baseline characteristics stratified by subgroup and individual trial for comparators. In AWARD‐4, the mean total daily insulin doses at baseline for the dulaglutide and insulin glargine arms were 55.2 IU and 53.9 IU, respectively.7 In AWARD‐9, the mean total daily insulin doses at baseline for the dulaglutide and placebo arms were 40.7 IU and 36.6 IU, respectively.12
Table 1.
Baseline demographics by subgroup for dulaglutide (pooled) and comparators (by study)
| HbA1c | Age | Duration of diabetes | ||||
|---|---|---|---|---|---|---|
| ≤9% | >9% | <65 y | ≥65 y | <10 y | ≥10 y | |
| All dulaglutide 1.5 mg + insulin lispro / insulin glargine (N = 445) | ||||||
| N = 325 | N = 118 | N = 313 | N = 132 | N = 158 | N = 287 | |
| Sex, female, % | 43.7 | 48.3 | 47.0 | 40.2 | 39.9 | 47.7 |
| Age, years | 60.4 ± 9.3 | 56.3 ± 9.6 | 54.8 ± 7.2 | 70.1 ± 4.1 | 55.1 ± 10.0 | 61.6 ± 8.4 |
| HbA1c, % | 8.0 ± 0.6 | 9.7 ± 0.6 | 8.5 ± 1.0 | 8.3 ± 0.9 | 8.5 ± 1.0 | 8.4 ± 1.0 |
| HbA1c, mmol/mol | 63.9 ± 6.6 | 82.5 ± 6.6 | 69.4 ± 10.9 | 67.2 ± 9.8 | 69.4 ± 10.9 | 68.3 ± 10.9 |
| FBG, mmol/L | 8.1 ± 2.5 | 10.3 ± 3.3 | 8.7 ± 2.9 | 8.9 ± 2.9 | 8.8 ± 3.0 | 8.7 ± 2.8 |
| Weight, kg | 91.5 ± 17.9 | 92.7 ± 18.4 | 92.8 ± 18.3 | 89.4 ± 17.0 | 95.6 ± 17.6 | 89.6 ± 17.9 |
| BMI, kg/m2 | 32.1 ± 4.9 | 32.7 ± 5.4 | 32.5 ± 5.2 | 31.6 ± 4.7 | 33.1 ± 4.9 | 31.8 ± 5.1 |
| Duration of diabetes, years | 13.3 ± 7.8 | 11.5 ± 5.4 | 11.6 ± 6.3 | 15.9 ± 8.5 | 5.9 ± 2.3 | 16.7 ± 6.2 |
| Insulin Glargine + Insulin Lispro (AWARD‐4; N = 296) | ||||||
| N = 204 | N = 92 | N = 206 | N = 90 | N = 97 | N = 199 | |
| Sex, female, % | 43.6 | 45.7 | 47.1 | 37.8 | 48.5 | 42.2 |
| Age, years | 60.6 ± 8.7 | 58.2 ± 9.8 | 55.4 ± 6.8 | 70.2 ± 3.9 | 57.3 ± 9.5 | 61.2 ± 8.6 |
| HbA1c, % | 8.0 ± 0.6 | 9.7 ± 0.6 | 8.6 ± 1.0 | 8.4 ± 1.1 | 8.5 ± 1.0 | 8.6 ± 1.0 |
| HbA1c, mmol/mol | 63.9 ± 6.6 | 82.5 ± 6.6 | 70.5 ± 10.9 | 68.3 ± 12.0 | 69.4 ± 10.9 | 70.5 ± 10.9 |
| FBG, mmol/L | 7.9 ± 2.6 | 10.1 ± 3.5 | 8.7 ± 3.1 | 8.3 ± 3.0 | 8.8 ± 3.3 | 8.5 ± 3.0 |
| Weight, kg | 90.0 ± 18.5 | 92.5 ± 19.7 | 91.1 ± 20.0 | 90.1 ± 16.2 | 93.4 ± 18.6 | 89.4 ± 18.9 |
| BMI, kg/m2 | 32.1 ± 5.3 | 33.0 ± 5.4 | 32.5 ± 5.5 | 32.2 ± 4.9 | 33.1 ± 5.2 | 32.1 ± 5.4 |
| Duration of diabetes, years | 13.3 ± 6.9 | 12.2 ± 6.6 | 12.0 ± 6.2 | 15.1 ± 7.5 | 5.9 ± 2.4 | 16.4 ± 5.5 |
| Placebo + insulin glargine (AWARD‐9; N = 150) | ||||||
| N = 122 | N = 28 | N = 92 | N = 58 | N = 54 | N = 96 | |
| Sex, female, % | 41.0 | 42.9 | 41.3 | 41.4 | 40.7 | 41.7 |
| Age, years | 61.0 ± 10.0 | 58.4 ± 12.1 | 54.5 ± 7.8 | 70.1 ± 3.9 | 56.7 ± 10.9 | 62.8 ± 8.9 |
| HbA1c, % | 8.0 ± 0.6 | 9.6 ± 0.5 | 8.4 ± 0.9 | 8.3 ± 0.8 | 8.4 ± 0.8 | 8.3 ± 0.9 |
| HbA1c, mmol/mol | 63.9 ± 6.6 | 81.4 ± 5.5 | 68.3 ± 9.8 | 67.2 ± 8.8 | 68.3 ± 8.8 | 67.2 ± 9.8 |
| FBG, mmol/L | 8.2 ± 2.3 | 10.6 ± 3.0 | 8.8 ± 2.5 | 8.5 ± 2.9 | 9.2 ± 2.7 | 8.4 ± 2.6 |
| Weight, kg | 91.7 ± 16.5 | 96.4 ± 19.7 | 95.8 ± 18.2 | 87.5 ± 14.0 | 94.1 ± 20.4 | 91.7 ± 15.1 |
| BMI, kg/m2 | 32.2 ± 4.8 | 34.0 ± 5.0 | 33.2 ± 5.3 | 31.6 ± 4.0 | 33.2 ± 5.8 | 32.2 ± 4.3 |
| Duration of diabetes, years | 13.6 ± 7.7 | 12.2 ± 7.5 | 11.9 ± 7.5 | 15.6 ± 7.4 | 5.6 ± 2.4 | 17.7 ± 6.0 |
Abbreviations: BMI, body mass index; FBG, fasting blood glucose; HbA1c, glycated haemoglobin.
Data are presented as mean ± SD or n (%). Insulin lispro was administered three times daily.
Dulaglutide‐treated patients with a duration of diabetes ≥10 years at baseline (mean duration 16.7 years) were older (mean age 61.6 years) than patients with <10 years duration of diabetes (mean age 55.1 years). Likewise, patients aged ≥65 years had longer duration of diabetes (mean duration 15.9 years) than patients aged <65 years (mean duration 11.6 years). Compared with the baseline HbA1c ≤9% (≤74.9 mmol/mol) subgroup, patients with baseline HbA1c >9% (>74.9 mmol/mol) were younger (mean age 56.3 years vs 60.4 years) and had a shorter duration of diabetes (mean duration 11.5 years vs 13.3 years). The mean baseline HbA1c in the HbA1c ≤9% and >9% subgroups (≤74.9 and >74.9 mmol/mol subgroups) was 8% (63.9 mmol/mol) and 9.7% (82.5 mmol/mol), respectively. Similar findings were observed in the comparator baseline characteristics by subgroup and by study (Table 1).
3.2. Pooled dulaglutide 1.5 mg‐treated patients glycaemic efficacy by subgroup
At 6 months, dulaglutide 1.5 mg significantly reduced HbA1c and fasting blood glucose (FBG) from baseline in all subgroups (P < .001). The greatest reduction in HbA1c and FBG was observed in the subgroup of patients with a baseline HbA1c >9% (>74.9 mmol/mol) (least squares mean −27.3 mmol/mol [95% CI −30.0, −24.4]), with 45.8% and 72.9% of these patients achieving an HbA1c target <7% (<53.0 mmol/mol) and <8% (<63.9 mmol/mol), respectively. The subgroup of patients with a baseline HbA1c ≤9% (≤74.9 mmol/mol) had a reduction in HbA1c of −14.2 mmol/mol (95% CI −16.4, −11.9), and 72.3% and 89.3% of these patients achieved HbA1c targets of <7% (<53.0 mmol/mol) and <8% (<63.9 mmol/mol), respectively.
Patients aged <65 years and ≥65 years had similar HbA1c reductions of −16.4 mmol/mol (95% CI −18.9, −14.8) and −17.5 mmol/mol (95% CI −20.1, −15.5), respectively, and similar reductions in FBG (Table S1). Additionally, similar proportions of patients achieved HbA1c targets (Figure 1).
Figure 1.
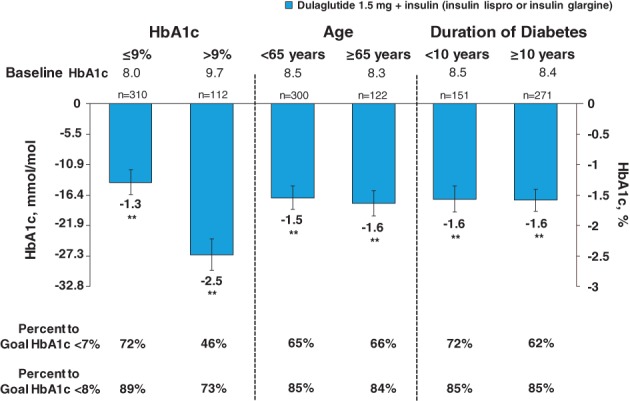
Change from baseline in glycated haemoglobin (HbA1c) by subgroups at 6 months. Data are presented as least squares mean (95% CI); **P < .001 vs baseline
Similar reductions in HbA1c were observed in the subgroups categorized by duration of diabetes (Figure 1). FBG reduction was slightly greater in patients with a duration of diabetes <10 years (Table S1). The proportion of patients achieving an HbA1c target <8% (<63.9 mmol/mol) was similar between the duration of diabetes subgroups, although fewer patients in the subgroup of ≥10 years reached the HbA1c target of <7% (<53.0 mmol/mol) compared with those with <10 years with T2D (61.6% vs 71.5%) (Figure 1).
3.3. Glycaemic efficacy with dulaglutide 1.5 mg and comparators for subgroups by individual study
For AWARD‐4, 295 dulaglutide 1.5 mg‐treated patients and 296 insulin glargine‐treated patients were included in the present analysis. HbA1c significantly decreased from baseline for both dulaglutide 1.5 mg plus insulin lispro and insulin glargine plus insulin lispro (hereafter, referred to as dulaglutide and insulin glargine, respectively) in all subgroups at 6 months (P < .001), with the highest reduction observed in the baseline HbA1c >9% (>74.9 mmol/mol) subgroup (mean HbA1c reductions −2.6% [−28.4 mmol/mol] and −2.3% [−25.1 mmol/mol], respectively). In this subgroup, 44% and 71% of dulaglutide‐treated patients and 40% and 65% of insulin glargine‐treated patients reached HbA1c targets <7% (<53.0 mmol/mol) and <8% (<63.9 mmol/mol), respectively (Figure 2A). Likewise, total daily insulin lispro dose in the dulaglutide arm and total daily insulin in the comparator arm were higher in patients with a baseline HbA1c >9% (Table 2). In contrast, patients from the baseline HbA1c ≤9% (≤74.9 mmol/mol) subgroup had the lowest reduction in HbA1c with dulaglutide and insulin glargine (mean HbA1c reduction −1.3% [−14.2 mmol/mol] and −1.2% [−13.1 mmol/mol], respectively); however, this subgroup also had a higher percentage of patients who achieved HbA1c targets (Figure 2A). In dulaglutide‐treated patients, HbA1c reductions were similar irrespective of age and duration of diabetes. A consistent pattern was observed in insulin glargine‐treated patients, with HbA1c reductions irrespective of age and duration of diabetes (Figure 2A).
Figure 2.
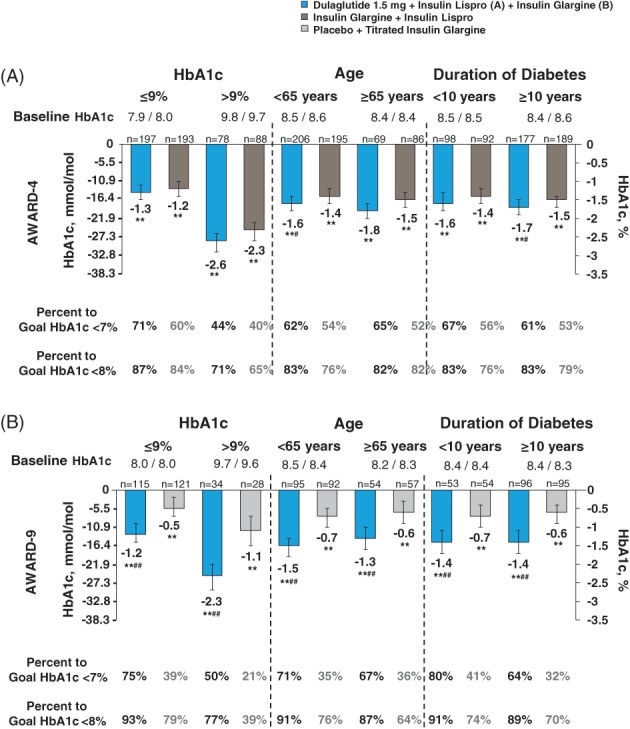
Change from baseline in glycated haemoglobin (HbA1c) at 6 months by individual study. Data are presented as LSM (95% CI); **P < 0.001 versus baseline; # P < 0.05 and ## P < 0.001 versus comparator. Insulin lispro was administered TID
Table 2.
Insulin doses (U/kg) for all dulaglutide 1.5 mg patients and all comparator patients by subgroup and individual study at 6 months
| HbA1c | Age | Duration of diabetes | ||||
|---|---|---|---|---|---|---|
| ≤9% | >9% | <65 y | ≥65 y | <10 y | ≥10 y | |
| AWARD‐4 | ||||||
| Dulaglutide 1.5 mg + insulin lispro | ||||||
| Lispro dose | 0.9 ± 0.7 | 1.1 ± 0.6 | 1.0 ± 0.6 | 1.0 ± 0.9 | 1.0 ± 0.6 | 1.0 ± 0.7 |
| Insulin glargine + insulin lispro | ||||||
| Total daily insulin dose | 1.3 ± 0.7 | 1.6 ± 0.8 | 1.4 ± 0.8 | 1.4 ± 0.7 | 1.5 ± 0.9 | 1.3 ± 0.7 |
| Glargine dose | 0.7 ± 0.4 | 0.8 ± 0.4 | 0.7 ± 0.4 | 0.7 ± 0.3 | 0.7 ± 0.5 | 0.7 ± 0.3 |
| Lispro dose | 0.7 ± 0.4 | 0.8 ± 0.5 | 0.7 ± 0.4 | 0.7 ± 0.4 | 0.8 ± 0.5 | 0.7 ± 0.4 |
| AWARD‐9 | ||||||
| Dulaglutide 1.5 mg + insulin glargine | ||||||
| Glargine dose | 0.6 ± 0.3 | 0.7 ± 0.3 | 0.6 ± 0.3 | 0.5 ± 0.3 | 0.6 ± 0.3 | 0.6 ± 0.3 |
| Placebo + insulin glargine | ||||||
| Glargine dose | 0.6 ± 0.3 | 0.9 ± 0.5 | 0.7 ± 0.4 | 0.6 ± 0.3 | 0.7 ± 0.4 | 0.7 ± 0.3 |
Abbreviation: HbA1c, glycated haemoglobin.
Data are presented as mean ± SD. Insulin lispro was administered three times daily.
For AWARD‐9, 150 dulaglutide 1.5 mg‐treated patients and 150 placebo‐treated patients were included in the present analysis. HbA1c significantly decreased from baseline with both dulaglutide plus titrated insulin glargine and placebo plus titrated insulin glargine (hereafter, referred to as dulagutide and placebo, respectively) in all subgroups at 6 months (P < .001), with the highest reduction observed in patients with baseline HbA1c >9% (>74.9 mmol/mol) (dulaglutide, −2.3% [−25.1 mmol/mol]; placebo, −1.1% [−12.0 mmol/mol]). In this subgroup, 50% and 77% of dulaglutide‐treated patients and 21% and 39% of placebo‐treated patients reached HbA1c targets <7% (<53.0 mmol/mol) and <8% (<63.9 mmol/mol), respectively (Figure 2B). Similarly, the total insulin glargine dose in both dulaglutide and comparator arms was higher in the baseline HbA1c >9% (>74.9 mmol/mol) subgroup (Table 2). Conversely, the lowest reduction in HbA1c was observed in the baseline HbA1c ≤9% (≤74.9 mmol/mol) subgroup (dulaglutide −1.2% [−13.1 mmol/mol]; placebo −0.5% [−5.5 mmol/mol]); however, more patients from this subgroup achieved HbA1c targets of <7% (<53.0 mmol/mol) and <8% (<63.9 mmol/mol) (dulaglutide, 75% and 93%; placebo, 39% and 79% [Figure 2B]). In dulaglutide‐treated patients, HbA1c reductions were similar irrespective of age and duration of diabetes. A consistent pattern was observed in the placebo arm, with similar HbA1c reductions irrespective of age and duration of diabetes (Figure 2B).
Changes from baseline in FBG at 6 months according to subgroup and individual study are presented in Table S1.
3.4. Change in body weight with dulaglutide 1.5 mg and comparators according to individual study at 6 months
In AWARD‐4, dulaglutide plus insulin lispro significantly reduced body weight from baseline in all subgroups at 6 months (P ≤ .043), except for patients with a baseline HbA1c >9% (>74.9 mmol/mol) and duration of diabetes <10 years (mean body weight change 0.2 kg and −0.3 kg; mean insulin lispro dose 1.1 U/kg and 1.0 U/kg, respectively). The largest reduction was observed in patients with baseline HbA1c ≤9% (≤74.9 mmol/mol) (mean body weight reduction −1.3 kg [Figure 3A]). Insulin glargine plus insulin lispro significantly increased body weight from baseline in all subgroups at 6 months (P < .001), with the largest increase observed in patients with baseline HbA1c >9% (>74.9 mmol/mol) (mean body weight increase, 3.4 kg [Figure 3A]).
Figure 3.
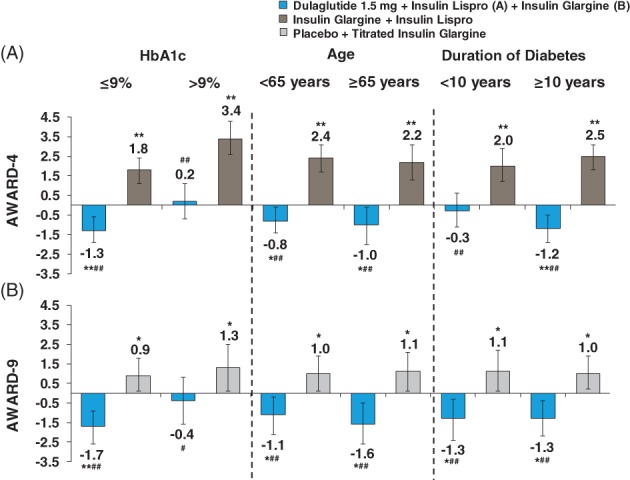
Change from baseline in body weight at 6 months by individual study. Data are presented as LSM (95% CI); *P < 0.05 and **P < 0.001 versus baseline, # P < 0.05 and ## P < 0.001 versus comparator. Insulin lispro was administered TID
In AWARD‐9, dulaglutide plus insulin glargine significantly reduced body weight from baseline in all subgroups at 6 months (P ≤ .024), except for patients with a baseline HbA1c >9% (>74.9 mmol/mol) (mean body weight reduction −0.4 kg; mean insulin glargine dose 0.7 U/kg). The largest reduction was observed in patients with baseline HbA1c ≤9% (≤74.9 mmol/mol) (mean body weight reduction −1.7 kg [Figure 3B]). Body weight significantly increased from baseline in all subgroups with placebo plus insulin glargine at 6 months (P ≤ .047). Similar to AWARD‐4, the largest increase was observed in patients with baseline HbA1c >9% (>74.9 mmol/mol) (mean body weight increase 1.3 kg).
3.5. Safety
3.5.1. Hypoglycaemia
The incidence and rate of documented symptomatic hypoglycaemia and number of severe hypoglycaemic events by individual study are shown in Table S2. Overall in AWARD‐4, the rate of documented symptomatic hypoglycaemia was high in both the dulaglutide and comparator arms, with numerically higher rates in the comparator arm25 (Table S2). The rate of documented symptomatic hypoglycaemia with dulaglutide plus insulin lispro was similar in all subgroups, except for patients with a duration of diabetes ≥10 years who had a numerically higher rate compared with patients with a duration <10 years (35.8 vs 25.4 events/patient/y). In AWARD‐9, the rate of documented symptomatic hypoglycaemia with dulaglutide 1.5 mg was generally similar within subgroups. The highest rate of documented symptomatic hypoglycaemia was observed in the subgroup with a baseline HbA1c >9% (>74.9 mmol/mol) (5.7 events/patient/y).
Overall, severe hypoglycaemic events were low in each subgroup in AWARD‐4 (Table S2), while there was only 1 event of severe hypoglycaemia in dulaglutide‐treated patients in AWARD‐9.
3.5.2. Other adverse events
The complete safety profile for each study was previously reported.7, 12 The most common adverse events in both studies were gastrointestinal in nature (including nausea, diarrhoea and vomiting) and were mild to moderate in severity and transient (data not shown).
4. DISCUSSION
In the present analysis, dulaglutide 1.5 mg combined with insulin in patients with T2D resulted in significant and clinically meaningful HbA1c reductions irrespective of baseline HbA1c, age and duration of diabetes. Results were consistent between both pooled and individual study analyses.
The greatest HbA1c reduction was observed in dulaglutide‐treated patients with baseline HbA1c >9% (>74.9 mmol/mol) in the pooled data and by individual study. In a broader analysis assessing key predictive factors that impact glycaemic efficacy with dulaglutide, baseline HbA1c was the most important predictor, which is consistent with the present results.26 In studies assessing other long‐acting GLP‐1RAs, such as semaglutide, albiglutide and liraglutide added to basal insulin, a larger HbA1c reduction was also observed in patients with higher baseline HbA1c.27, 28, 29 In a post hoc analysis of SUSTAIN‐5 that assessed semaglutide 0.5 mg and 1.0 mg added to basal insulin, a reduction in HbA1c of −2.2% and −2.5% (−24.0 and −27.3 mmol/mol, respectively) was observed in the subgroup with baseline HbA1c >9% (>74.9 mmol/mol), with 62% and 83% of patients achieving HbA1c target <8% (<63.9 mmol/mol), respectively.29 Taking into account that SUSTAIN‐5 had differences in study design and basal insulin titration schemes, these results mostly align with our subgroup analysis for baseline HbA1c >9% (>74.9 mmol/mol) in AWARD‐9. Furthermore, fixed‐ratio combinations of basal insulin and liraglutide or lixisenatide (ie, IDegLira and iGlarLixi) have also led to significant HbA1c reductions of >2% (>21.9 mmol/mol) when assessed in patients who were failing on basal insulin with baseline HbA1c concentrations >8.5% (>69.4 mmol/mol) or >9% (>74.9 mmol/mol), respectively.30, 31 These results were consistent with the present findings. Although fixed‐ratio combination therapies offer a gradual titration of the GLP‐1RA and overall have 1 less injection per week compared with dulaglutide combined with basal insulin, they are accompanied by certain limitations from a clinical practice perspective. Such limitations include chronic administration of sub‐therapeutic doses of GLP‐1RA in patients who require only a small dose of insulin and difficulties with insulin dose adjustments in patients who require a greater dose of insulin or have an elevated risk of hypoglycaemia.
In the present analysis, patients aged ≥65 years demonstrated robust and similar reductions in HbA1c, similar to those seen in the younger population. Consistent with the results of a previous pooled analysis of 6 AWARD trials, dulaglutide demonstrated similar efficacy and safety irrespective of age.20 Similar results were observed in other post hoc analyses assessing safety and efficacy of other GLP‐1RAs of fixed‐dose combination by age.32, 33, 34 As the American Diabetes Association recommends HbA1c targets of <8% (<63.9 mmol/mol) because of the multiple comorbidities that are prevalent in an older population, the 84% of dulaglutide‐treated patients reaching the goal is reassuring. Also, the lower weekly dulaglutide dose (0.75 mg) may be suitable for some elderly patients who may not need aggressive glycaemic control.20 Glycaemic efficacy should be balanced, however, with other potential benefits (eg, whether or not weight loss is needed for the patient) and safety issues (eg, declined renal function and the risk of hypoglycaemia), as individual treatment needs may differ for elderly patients.
Over time, glycaemic control decreases in patients with T2D because of the chronic and progressive nature of the disease.35 In the present analysis, clinically meaningful and similar HbA1c reductions were observed in both duration of diabetes subgroups (<10 years, ≥10 years) in the pooled dulaglutide analysis. This was also consistent for individual studies. In a previous post hoc analysis, dulaglutide showed significant HbA1c reductions irrespective of baseline β‐cell function measured by HOMA2‐%β.36 A recent study that assessed dulaglutide combined with insulin lispro in patients with chronic kidney disease (stages 3 and 4), and a mean duration of diabetes of 17 years showed similar efficacy to that in the basal‐bolus comparator arm.15 These data are supportive of the glucose‐lowering ability of dulaglutide, irrespective of the duration of diabetes when added to the insulin regimen. Similarly, patients with a mean duration of diabetes of 13 years had significant improvements in glycaemic control in long‐term cardiovascular outcome trials assessing the efficacy of long‐acting GLP‐1RAs, such as liraglutide and semaglutide.1, 2 Treatment with albiglutide resulted in similar glycaemic efficacy when assessed among categories of duration of diabetes of <5 years, ≥5 years, <10 years and ≥10 years, overall and in insulin studies.37 Additionally, treatment with the fixed‐ratio combination of lixisenatide plus insulin glargine resulted in a similar HbA1c effect in patients with a duration of diabetes <10 years and ≥10 years.30
Body weight data were not pooled because of the variable effects basal and prandial insulin have on weight and, therefore, were assessed only by individual study. At 6 months, dulaglutide decreased body weight in all subgroups except for a slight weight gain observed in patients with baseline HbA1c >9.0% (>74.9 mmol/mol) from AWARD‐4, in which dulaglutide was combined with high doses of insulin lispro three times daily. This subgroup specifically used the highest dose of insulin lispro, which may have prevented the reduction in weight associated with dulaglutide treatment. However, treatment differences in weight changes given the increase in body weight in the comparator arm were statistically and clinically significant. Similarly, in AWARD‐9 at 6 months, dulaglutide decreased body weight in all subgroups except for patients with baseline HbA1c >9.0% (>74.9 mmol/mol); however, treatment difference was significant vs the comparator. This subgroup was also receiving the highest dose of insulin, reflecting the impact of concomitant therapy on weight changes. Additionally, higher doses of concomitant insulin therapy may increase the risk of hypoglycaemia, as was the case in AWARD‐4. Still, patients receiving dulaglutide had numerically lower rates of hypoglycaemia compared with patients on a basal‐bolus regimen.
Several limitations should be considered when interpreting these results. First, this was a post hoc analysis with no adjustments for multiple testing and with imbalanced sample sizes in different subgroups. Although the cut‐off of ≥65 years was used for the elderly category, the percentage of patients aged ≥75 years was small. In addition, patients were on different types of insulin (either basal or prandial) with different titration schemes. Moreover, the durations of the studies in the present analysis were limited to 6 months, which may not represent the effects of long‐term use of dulaglutide. These data are intended to help clinicians take an individualized treatment approach for patients with T2D, providing additional insight into adding dulaglutide therapy in patients already treated with insulin.
In conclusion, dulaglutide 1.5 mg combined with basal or prandial insulin was found to be an efficacious option for elderly patients with long‐standing poorly controlled diabetes, and the overall safety profile was consistent with the GLP‐1RA class. The HbA1c reduction was greater in patients with higher baseline HbA1c.
Supporting information
Figure S1. Patient disposition for pooled dulaglutide 1.5 mg.
Table S1. Change from baseline in fasting blood glucose at 6 months by subgroup for pooled and individual studies (mmol/L).
Table S2. Incidence and rate of documented symptomatic hypoglycaemia and number of severe hypoglycaemia events by subgroup and individual study.
ACKNOWLEDGMENTS
This work is sponsored by Eli Lilly and Company. Additional details of these studies, “A Study in Patients with Type 2 Diabetes Mellitus (AWARD‐4)”, and “A Study of Dulaglutide (LY2189265) in Participants with Type II Diabetes (AWARD‐9)” can be found at http://clinicaltrials.gov (NCT01191268 and NCT02152371, respectively). The authors wish to thank Chrisanthi Karanikas, MS (Eli Lilly and Company) for writing and editorial assistance. Partial data from this study were presented at the American Diabetes Association 77th Scientific Sessions held June 9–13, 2017 in San Diego, California and the European Association for the Study of Diabetes 53rd Annual Meeting held September 11–15, 2017 in Lisbon, Portugal.
Conflict of interest
K.P. has received research funding from Merck and Novo Nordisk, compensation for serving as a consultant from Eli Lilly, Novo Nordisk, Sanofi, and Merck, and honoraria (lecture bureau) from AstraZeneca, Novo Nordisk, Merck, and Sanofi. L.F.L., H.P. and M.Y. are employees and shareholders of Eli Lilly and Company.
Author contributions
M. Y. was responsible for the statistical considerations in the analysis. L. F. L., H. P. and M. Y. are the guarantors of this work and, as such, take responsibility for the integrity of the data and the accuracy of the data analysis. All authors contributed to the design of this post hoc analysis and participated in critical reviewing and interpreting the data for the manuscript. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Pantalone KM, Patel H, Yu M, Fernández Landó L. Dulaglutide 1.5 mg as an add‐on option for patients uncontrolled on insulin: Subgroup analysis by age, duration of diabetes and baseline glycated haemoglobin concentration. Diabetes Obes Metab. 2018;20:1461–1469. https://doi.org/10.1111/dom.13252
Funding information Eli Lilly and Company
REFERENCES
- 1. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834‐1844. [DOI] [PubMed] [Google Scholar]
- 2. Marso SP, Daniels GH, Brown‐Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bethel MA, Patel RA, Merrill P, et al. Cardiovascular outcomes with glucagon‐like peptide‐1 receptor agonists in patients with type 2 diabetes: a meta‐analysis. Lancet Diabetes Endocrinol. 2018;6(2):105‐113. [DOI] [PubMed] [Google Scholar]
- 4. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm ‐ 2017 executive summary. Endocr Pract. 2017;23(2):207‐238. [DOI] [PubMed] [Google Scholar]
- 5. Maiorino MI, Chiodini P, Bellastella G, Capuano A, Esposito K, Giugliano D. Insulin and glucagon‐like peptide 1 receptor agonist combination therapy in type 2 diabetes: a systematic review and meta‐analysis of randomized controlled trials. Diabetes Care. 2017;40(4):614‐624. [DOI] [PubMed] [Google Scholar]
- 6. Billings LK, Doshi A, Gouet D, et al. Efficacy and safety of insulin degludec/liraglutide (IDegLira) vs basal‐bolus therapy in patients with type 2 diabetes: DUAL VII trial. Poster presented at: the European Association for the Study of Diabetes 53rd Annual Meeting; September 11–15, 2017; Lisbon, Portugal. ePoster: #796. https://www.easd.org/virtualmeeting/home.html#!resources/efficacy-and-safety-of-insulin-degludec-liraglutide-ideglira-vs-basal-bolus-therapy-in-patients-with-type-2-diabetes-dual-vii-trial-27bfe216-a671-4fe5-9e43-7c1507dd09ad. Accessed November 15, 2017.
- 7. Blonde L, Jendle J, Gross J, et al. Once‐weekly dulaglutide versus bedtime insulin glargine, both in combination with prandial insulin lispro, in patients with type 2 diabetes (AWARD‐4): a randomised, open‐label, phase 3, non‐inferiority study. Lancet. 2015;385(9982):2057‐2066. [DOI] [PubMed] [Google Scholar]
- 8. Dungan KM, Povedano ST, Forst T, et al. Once‐weekly dulaglutide versus once‐daily liraglutide in metformin‐treated patients with type 2 diabetes (AWARD‐6): a randomised, open‐label, phase 3, non‐inferiority trial. Lancet. 2014;384(9951):1349‐1357. [DOI] [PubMed] [Google Scholar]
- 9. Dungan KM, Weitgasser R, Perez Manghi F, et al. A 24‐week study to evaluate the efficacy and safety of once‐weekly dulaglutide added on to glimepiride in type 2 diabetes (AWARD‐8). Diabetes Obes Metab. 2016;18(5):475‐482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Giorgino F, Benroubi M, Sun J‐H, Zimmermann AG, Pechtner V. Efficacy and safety of once weekly dulaglutide versus insulin glargine in patients with type 2 diabetes on metformin and glimepiride (AWARD‐2). Diabetes Care. 2015;38(12):2241‐2249. [DOI] [PubMed] [Google Scholar]
- 11. Nauck M, Weinstock RS, Umpierrez GE, Guerci B, Skrivanek Z, Milicevic Z. Efficacy and safety of dulaglutide versus sitagliptin after 52 weeks in type 2 diabetes in a randomized controlled trial (AWARD‐5). Diabetes Care. 2014;37(8):2149‐2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pozzilli P, Norwood P, Jódar E, et al. Placebo‐controlled, randomized trial of the addition of once‐weekly glucagon‐like peptide‐1 receptor agonist dulaglutide to titrated daily insulin glargine in patients with type 2 diabetes (AWARD‐9). Diabetes Obes Metab. 2017;19(7):1024‐1031. [DOI] [PubMed] [Google Scholar]
- 13. Umpierrez G, Povedano ST, Manghi FP, Shurzinske L, Pechtner V. Efficacy and safety of dulaglutide monotherapy versus metformin in type 2 diabetes in a randomized controlled trial (AWARD‐3). Diabetes Care. 2014;37(8):2168‐2176. [DOI] [PubMed] [Google Scholar]
- 14. Wysham C, Belvins T, Arakaki R, et al. Efficacy and safety of dulaglutide added on to pioglitazone and metformin versus exenatide in type 2 diabetes in a randomized controlled trial (AWARD‐1). Diabetes Care. 2014;37(8):2159‐2167. [DOI] [PubMed] [Google Scholar]
- 15. Tuttle KLM, Gross JL, Rayner B, et al. Comparable glycemic control, greater weight loss, and lower hypoglycemia with once weekly dulaglutide versus insulin glargine, both combined with Lispro, in type 2 diabetes and moderate to severe chronic kidney disease (AWARD‐7). Poster presented at: American Diabetes Association 74th Scientific Sessions; June 13–17, 2014; San Francisco, CA. Poster 138‐LB.
- 16. Trulicity [Prescribing Information]. Indianapolis, IN: Eli Lilly and Company; 2017. http://pi.lilly.com/us/trulicity-uspi.pdf. Accessed November 13, 2017. [Google Scholar]
- 17. Trulicity [Summary of Product Characteristics]. Houten: Eli Lilly and Company; 2017. http://ec.europa.eu/health/documents/community-register/2014/20141121130063/anx_130063_en.pdf. Accessed January 26, 2018. [Google Scholar]
- 18. U.K. prospective diabetes study 16. Overview of 6 years' therapy of type II diabetes: a progressive disease. U.K. Prospective Diabetes Study Group. Diabetes. 1995;44(11):1249‐1258. [PubMed] [Google Scholar]
- 19. Gallwitz B, Dagogo‐Jack S, Thieu V, et al. Effect of once‐weekly dulaglutide on HbA1c and fasting blood glucose in patient subpopulations by gender, duration of diabetes, and baseline HbA1c. Diabetes Obes Metab. 2018;20(2):409‐418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boustani MA, Pittman It YM, Thieu VT, Varnado OJ, Juneja R. Similar efficacy and safety of once‐weekly dulaglutide in patients with type 2 diabetes aged ≥65 and <65 years. Diabetes Obes Metab. 2016;18(8):820‐828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Alatorre C, Fernández Landó L, Yu M, et al. Treatment patterns in patients with type 2 diabetes mellitus treated with glucagon‐like peptide‐1 receptor agonists: higher adherence and persistence with dulaglutide compared with once‐weekly exenatide and liraglutide. Diabetes Obes Metab. 2017;19(7):953‐961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. American Diabetes Association . Standards of medical care in diabetes‐2017. Diabetes Care. 2017;40(Suppl 1):S1‐S135.27979885 [Google Scholar]
- 23. Bergenstal R, Johnson MA, Powers M, et al. Adjust to target in Type 2 Diabetes: comparison of a simple algorithm with carbohydrate counting for adjustment of mealtime insulin glulisine. Diabetes Care. 2008;31(7):1305‐1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kirkman MS, Briscoe VJ, Clark N, et al. Consensus development conference on diabetes and older adults. diabetes in older adults: a consensus report. J Am Geriatr Soc. 2012;60(12):2342‐2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Data on file, Eli Lilly and Company and/or one of its subsidiaries. Eli Lilly and Company, Lilly Corporate Center: Indianapolis.
- 26. Wysham C, Guerci B, D'Alessio D, Jia N, Botros FT. Baseline factors associated with glycaemic response to treatment with once‐weekly dulaglutide in patients with type 2 diabetes. Diabetes Obes Metab. 2016;18(11):1138‐1142. [DOI] [PubMed] [Google Scholar]
- 27. Rosenstock J, Fonseca VA, Gross JL, et al. Advancing basal insulin replacement in type 2 diabetes inadequately controlled with insulin glargine plus oral agents: a comparison of adding albiglutide, a weekly GLP‐1 receptor agonist, versus thrice‐daily prandial insulin lispro. Diabetes Care. 2014;37(8):2317‐2325. [DOI] [PubMed] [Google Scholar]
- 28. Ahmann A, Rodbard HW, Rosenstock J, et al. Efficacy and safety of liraglutide versus placebo added to basal insulin analogues (with or without metformin) in patients with type 2 diabetes: a randomized, placebo‐controlled trial. Diabetes Obes Metab. 2015;17(11):1056‐1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bain S, Araki E, Desouza C, et al. Semaglutide reduces HbA1c and body weight across baseline HbA1c subgroups in the SUSTAIN 1‐5 clinical trials. Poster presented at the European Association for the Study of Diabetes 53rd Annual Meeting; September 11–15, 2017; Lisbon, Portugal. ePoster: #813. https://www.easd.org/myeasd/home.html#!resources/semaglutide-reduces-hba-sub-1c-sub-and-body-weight-across-baseline-hba-sub-1c-sub-subgroups-in-the-sustain-1-5-clinical-trials-aec5455d-8e8c-4639-ac67-6ef66569494a.
- 30. Niemoeller E, Souhami E, Wu Y, Jensen KH. iGlarLixi reduces A1C to a greater extent than basal insulin therapy regardless of A1C levels at screening. Poster presented at: 77th American Diabetes Association Scientific Sessions; June 9–13, 2017, San Diego, CA. 1079‐P. https://ada.scientificposters.com/epsAbstractADA.cfm?id=1. Accessed November 15, 2017.
- 31. Lingvay I, Pérez Manghi F, García‐Hernández P, et al. Effect of insulin glargine up‐titration vs insulin degludec/liraglutide on glycated hemoglobin levels in patients with uncontrolled type 2 diabetes: The DUAL V randomized clinical trial. JAMA. 2016;315(9):898‐907. [DOI] [PubMed] [Google Scholar]
- 32. Bode BW, Brett J, Falahati A, Pratley RE. Comparison of the efficacy and tolerability profile of liraglutide, a once‐daily human GLP‐1 analog, in patients with type 2 diabetes ≥65 and <65 years of age: a pooled analysis from phase III studies. Am J Geriatr Pharmacother. 2011;9(6):423‐433. [DOI] [PubMed] [Google Scholar]
- 33. Sorli CH, Harris SB, Jodar E, Lingvay I, Chandarana K, Langer J, Jaeckel E. IDegLira Is efficacious across baseline A1c categories in subjects with type 2 diabetes uncontrolled on SU, GLP‐1RA, or insulin glargine: analyses from completed phase 3b trials. Poster presented at: the American Diabetes Association American Diabetes Association 76th Scientific Sessions; June 10–14, 2016; New Orleans. Abstract # 837.
- 34. M Warren, Chaykin L, Trachtenbarg D, Nayak G, Wijayasinghe N, Cariou B. Efficacy and safety of once‐weekly semaglutide in elderly subjects with type 2 diabetes: post hoc analysis of SUSTAIN 1‐5 trials. 2017 EASD. Poster presented at: the 53rd European Association for the Study of Diabetes Annual Meeting; September 11–15, 2017; Lisbon, Portugal. ePoster #819. https://www.easd.org/myeasd/home.html#!resources/efficacy-and-safety-of-once-weekly-semaglutide-in-elderly-subjects-with-type-2-diabetes-post-hoc-analysis-of-sustain-1-5-trials-0020aab5-8057-400f-b2e2-4ce0ad4b79f7. Accessed November 15, 2017.
- 35. King P, Peacock I, Donnelly R. The UK prospective diabetes study (UKPDS): clinical and therapeutic implications for type 2 diabetes. Br J Clin Pharmacol. 1999;48(9):643‐648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mathieu C, Botros FT, Thieu V, Jia N, Zhang N, Garcia‐Perez L‐E. Effect of once weekly dulaglutide by baseline β‐cell function in the AWARD Program. Poster presented at: the American Diabetes Association American Diabetes Association 76th Scientific Sessions; June 10–14, 2016; New Orleans. Poster #1032‐P.
- 37. Home P, Miller D, Carr MC. Albiglutide provides effective glycasemic lowering across diabetes duration subgroups. Poster presented at: the 51st European Association for the Study of Diabetes Annual Meeting; September 2015; Stockholm, Sweden. Poster 785. https://hwww.easd.org/virtualmeeting/home.html#!resources/albiglutide-provides-effective-glycaemic-lowering-across-diabetes-duration-subgroups–3. Accessed November 15, 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Patient disposition for pooled dulaglutide 1.5 mg.
Table S1. Change from baseline in fasting blood glucose at 6 months by subgroup for pooled and individual studies (mmol/L).
Table S2. Incidence and rate of documented symptomatic hypoglycaemia and number of severe hypoglycaemia events by subgroup and individual study.


