Abstract
Publication bias occurs when studies with statistically significant results have increased likelihood of being published. Publication bias is commonly associated with inflated treatment effect which lowers the certainty of decision makers about the evidence. In this guide we propose that systematic reviewers and decision makers consider the direction and magnitude of publication bias, as opposed to just the binary determination of the presence of this bias, before lowering their certainty in the evidence. Direction of bias may not always exaggerate the treatment effect. The presence of bias with a trivial magnitude may not affect the decision at hand. Various statistical approaches are available to determine the direction and magnitude of publication bias.
Keywords: internal medicine, statistics & research methods
Empirical research has demonstrated that randomised controlled trials with positive findings are more likely to be submitted and published than trials with negative or null findings (OR of the likelihood of publication 3.90; 95% CI 2.68 to 5.68).1 Trials with positive findings are also published earlier (4–5 years vs 6–8 years)1 and are more likely to be published in English or in journals with higher impact.2 Thus, at any given time a meta-analysis is conducted, it will likely contain more trials with positive findings than those with negative findings and the estimated pooled effect size is likely to be exaggerated. This is a type of publication bias.
When decision makers make a recommendation based on evidence affected by publication bias, this action is supported by likely exaggerated benefit. Therefore, the balance of benefit and harm that led to the recommended action is likely distorted. Decision makers acknowledge this distortion by placing lower certainty in the evidence.3 Hence, they rate down their certainty in the evidence, which is a construct also called quality or confidence in the evidence.4
Presence of publication bias can be determined using various statistical tests. These tests have major limitations, require several assumptions that are difficult to ascertain and are commonly underpowered.5 In addition, these tests provide a binary outcome (bias is absent or present), which by itself, is a major challenge.
In this guide, we propose further evaluation of publication bias and how it impacts certainty in the evidence. We draw attention to the issues of magnitude and direction of publication bias.
Methods
We propose a framework to determine the impact of publication bias on certainty in the evidence. This framework is based on the empirical literature on publication bias, published guidance from the GRADE Working Group, and various epidemiological and statistical principles. Necessary definitions are provided in table 1.
Table 1.
Definitions
| Publication bias | The publication or non-publication of research findings, depending on the nature and direction of the results.22 |
| Certainty in the evidence | The certainty that a true effect lies on one side of a specified threshold or within a chosen range supporting a decision.4 |
| Selection model | A weight function of effect size or p value is used to model the probability of publication. This method highly depends on this weight and is usually recommended as a sensitivity analysis. |
| Begg test | A method that uses the rank test to examine the association between the observed effect sizes and their variances. However, it suffers from low statistical power. |
| Egger test | A method in which we regress the standardised effect size against the precision. The intercept is close to zero if no publication bias is present. This method may have inflated false-positive rates for ORs. |
| Trim and fill method | A method in which the missing studies are imputed to provide a bias-adjusted effect estimate. However, it requires the strong assumption that the missing studies have the most negative (or positive) effect sizes. |
| Skewness | A method that examines the asymmetry of residuals of the regression test. It has more statistical power than other tests. However, it may lose power if the available studies have a distribution that tends to have multiple modes. |
Direction of bias
A meta-analysis of 25 randomised controlled trials that evaluated the effects of exercise on depression has shown that exercise reduced depression symptoms (standardised mean difference (SMD) 0.98, 95% CI 0.68 to 1.28).6 The Begg test and the Egger test indicated potential publication bias (p values 0.001 and 0.004; respectively). However, it was the unpublished trials that might have shown a larger magnitude of improvement. Adjusting SMD using the trim and fill method would show a larger pooled effect of improvement (SMD 1.11; 95% CI 0.79 to 1.43).6 In other words, publication bias has led to an underestimated SMD (opposite to the typical publication bias that exaggerates benefits of interventions). This phenomenon, although not likely to be common, should intuitively prevent decision makers from reducing their certainty in the evidence when recommending exercise as a treatment for depression. This phenomenon is akin to when plausible bias and confounding suggests that the true association is stronger than the observed association.7 In the example of treating depression with exercise, the original rating of certainty in evidence would be reduced due to publication bias. However, evaluation of bias direction may prevent us from rating it down.
Visualisation of the funnel plot, although a crude and subjective approach with shapes possibly influenced by the statistic used to measure the effect size,8 9 can give a sense about the direction of bias (observation of the empty area of the funnel).
Magnitude of bias
Several methods have been proposed to determine the magnitude of bias or adjust the pooled estimate for bias. In sensitivity analysis, one can test whether the magnitude of bias is sufficiently small to the extent that it remains unimportant (will not change the decision at hand).
One of the approaches depends on selection models using the weighted distribution theory. In this approach, the probability a study is published can be modelled using certain functions of its p value or effect size, and this probability is incorporated in a meta-analysis model to adjust the effect size, possibly along with other study level covariates as regression predictors. Although they may provide some benefits in correcting publication bias,10 11 selection models are not widely used because they require a large number of studies and because of their complexity and unavailability in standard meta-analysis software.12
The trim and fill method13 is based on the funnel plot in which missing studies are imputed by creating a mirror image of opposite corresponding studies. The adjusted effect size accounting for the missing studies can be used as a sensitivity analysis to determine the presence and magnitude of publication bias. It is important to note that this adjusted effect size is based on strong assumptions about the missing studies and should only be used for the purpose of sensitivity analysis (ie, should not be considered as a more accurate effect size to be used for decision making).14
The Begg rank test and the Egger regression test have been two popular methods for detecting publication bias.8 15 Both methods examine the association between the observed effect sizes and their precisions. However, the Begg test has been found to have very low power, and the Egger test may have inflated false-positive rates for binary outcomes.16 17
A more recent measure, the skewness of the standardised deviates, quantifies publication bias by describing the asymmetry of the distribution of included trials (values range from −∞ to +∞ and a value of 0 suggests no skewness).18 The skewness is roughly considered mild, noticeable and substantial if it’s absolute value is smaller than 0.5, between 0.5 and 1, and greater than 1, respectively. Like other measures, the skewness is recommended to be reported along with its 95% CI to reflect its variability. In addition, a positive skewness measure indicates that some studies on the left side in the funnel plot (ie, those with OR or relative risk <1.0) might be missing due to publication bias. Therefore, this approach can suggest a direction and magnitude of publication bias and can be more powerful than other tests in some situations.18 For example, in a meta-analysis that evaluated nicotine replacement therapy for smoking cessation,19 three commonly used publication bias tests yielded p values greater than 0.10. The skewness measure was 0.91 (95% CI 0.14 to 1.68) suggesting substantial publication bias18 and possible unpublished studies with relative risk <0.0. In this example of nicotine replacement therapy, the original rating of certainty in evidence would not be reduced unless the magnitude of publication bias is considered.
Proposed framework
Considering the importance of the direction and magnitude of bias on certainty in the evidence supporting a particular action, we propose a framework in which certainty in evidence is compromised by publication bias only if the bias was not trivial and if it had a direction that shifts the balance of net benefit in a way that would make the recommended action less compelling (figure 1). In other words, if unpublished studies are unlikely to change the net impact of the intervention, we would not rate it down. If the magnitude or directions of the bias are unknown or are likely to affect the net impact of the intervention, then we would rate it down.
Figure 1.
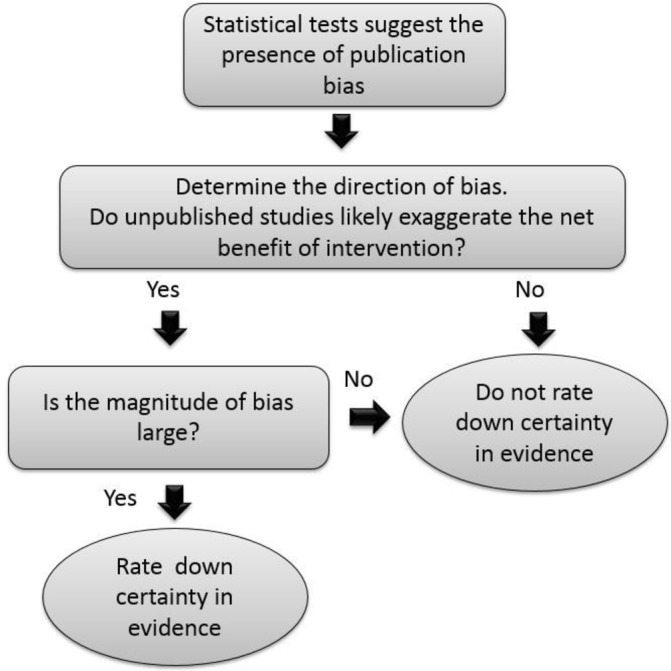
A proposed framework for determining the impact of publication bias on certainty in the evidence (incorporating the magnitude and direction of publication bias).
Discussion
Publication bias is one of the worst threats to the validity of scientific research. From an evidence synthesis perspective, we realise that the pooled estimates we produce may be based on studies exclusively showing that the treatment is effective (ie, we only see what works). This is a humbling fact. From a decision maker perspective, knowing that any decision we make is based on studies showing exaggerated benefit gives us very low certainty in our recommendations. Studies have shown that the likelihood of publication is often not associated with sample size, funding mechanism, investigator rank or gender.1 Therefore, publication bias remains unpredictable.
The available tests for publication bias lack statistical power; particularly when heterogeneity is high or the number of studies is low.20 Their validity depends on the assumptions often unmet in practice. At the present time, no single test can be recommended. Systematic reviewers should consistently use multiple publication bias detection methods, and non-statistical approaches such as comparing published evidence with data available in clinical trials registries, records of drugs or device approving agencies such as the Food and Drug Administration, and scientific conference proceedings. Searching trial registries is an important step that can reveal registered trials that remained unpublished. Three commonly searched registries are ClinicalTrials.gov (http://www.clinicaltrials.gov), International Standard Randomised Controlled Trial Number Register (http://isrctn.org) and Australian New Zealand Clinical Trials Registry (http://www.anzctr.org.au).21
Despite the low power and unreliability of the current methods, information about the direction and magnitude of the bias may inform judgements about the certainty of evidence better than the binary decision of a statistical test.
Footnotes
Contributors: MHM conceived the idea and drafted the manuscript. HC, LL and ZW critically revised the manuscript and approved the final submission.
Competing interests: None declared.
Patient consent: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Hopewell S, Loudon K, Clarke MJ, et al. Publication bias in clinical trials due to statistical significance or direction of trial results. Cochrane Database Syst Rev 2009;1:MR000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Siontis KC, Evangelou E, Ioannidis JP. Magnitude of effects in clinical trials published in high-impact general medical journals. Int J Epidemiol 2011;40:1280–91. 10.1093/ije/dyr095 [DOI] [PubMed] [Google Scholar]
- 3. Guyatt GH, Oxman AD, Montori V, et al. GRADE guidelines: 5. Rating the quality of evidence--publication bias. J Clin Epidemiol 2011;64:1277–82. 10.1016/j.jclinepi.2011.01.011 [DOI] [PubMed] [Google Scholar]
- 4. Hultcrantz M, Rind D, Akl EA, et al. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol 2017;87:4–13. 10.1016/j.jclinepi.2017.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jin ZC, Zhou XH, He J. Statistical methods for dealing with publication bias in meta-analysis. Stat Med 2015;34:343–60. 10.1002/sim.6342 [DOI] [PubMed] [Google Scholar]
- 6. Schuch FB, Vancampfort D, Richards J, et al. Exercise as a treatment for depression: A meta-analysis adjusting for publication bias. J Psychiatr Res 2016;77:42–51. 10.1016/j.jpsychires.2016.02.023 [DOI] [PubMed] [Google Scholar]
- 7. Guyatt GH, Oxman AD, Sultan S, et al. GRADE guidelines: 9. Rating up the quality of evidence. J Clin Epidemiol 2011;64:1311–6. 10.1016/j.jclinepi.2011.06.004 [DOI] [PubMed] [Google Scholar]
- 8. Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol 2001;54:1046–55. [DOI] [PubMed] [Google Scholar]
- 10. Carpenter JR, Schwarzer G, Rücker G, et al. Empirical evaluation showed that the Copas selection model provided a useful summary in 80% of meta-analyses. J Clin Epidemiol 2009;62:624–31. 10.1016/j.jclinepi.2008.12.002 [DOI] [PubMed] [Google Scholar]
- 11. Schwarzer G, Carpenter J, Rücker G. Empirical evaluation suggests Copas selection model preferable to trim-and-fill method for selection bias in meta-analysis. J Clin Epidemiol 2010;63:282–8. 10.1016/j.jclinepi.2009.05.008 [DOI] [PubMed] [Google Scholar]
- 12. Sutton AJ, Song F, Gilbody SM, et al. Modelling publication bias in meta-analysis: a review. Stat Methods Med Res 2000;9:421–45. 10.1177/096228020000900503 [DOI] [PubMed] [Google Scholar]
- 13. Duval S. Tweedie R. A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. Journal of the American Statistical Association 2000;95:89–98. [Google Scholar]
- 14. Peters JL, Sutton AJ, Jones DR, et al. Performance of the trim and fill method in the presence of publication bias and between-study heterogeneity. Stat Med 2007;26:4544–62. 10.1002/sim.2889 [DOI] [PubMed] [Google Scholar]
- 15. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. 10.2307/2533446 [DOI] [PubMed] [Google Scholar]
- 16. Sterne JAC, Gavaghan D, Egger M. Publication and related bias in meta-analysis. J Clin Epidemiol 2000;53:1119–29. 10.1016/S0895-4356(00)00242-0 [DOI] [PubMed] [Google Scholar]
- 17. Peters JL, Sutton AJ, Jones DR, et al. Comparison of two methods to detect publication bias in meta-analysis. JAMA 2006;295:676–80. 10.1001/jama.295.6.676 [DOI] [PubMed] [Google Scholar]
- 18. Lin L, Chu H. Quantifying publication bias in meta-analysis. Biometrics 2017;287 10.1111/biom.12817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stead LF, Perera R, Bullen C, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev 2012;11:CD000146 10.1002/14651858.CD000146.pub4 [DOI] [PubMed] [Google Scholar]
- 20. Lau J, Ioannidis JP, Terrin N, et al. The case of the misleading funnel plot. BMJ 2006;333:597–600. 10.1136/bmj.333.7568.597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gandhi GY, Murad MH, Fujiyoshi A, et al. Patient-important outcomes in registered diabetes trials. JAMA 2008;299:2543–9. 10.1001/jama.299.21.2543 [DOI] [PubMed] [Google Scholar]
- 22. Cochrane handbook for systematic reviews of interventions. Version 5.1.0: The Cochrane Collaboration, 2011. [Google Scholar]


