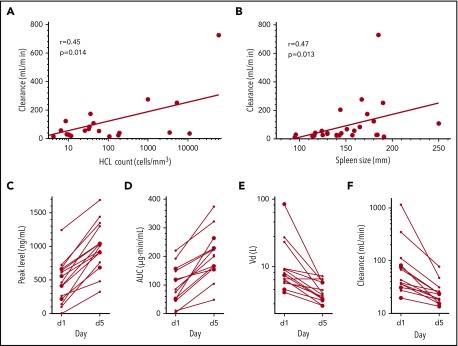
Figure 2.
Pharmacokinetic parameters, tumor burden, and immunogenicity. In patients receiving 50 µg/kg, clearance during cycle 1 day 5 was related to hairy cell count (A) and spleen size (B). At this dose level, peak levels (C), AUC (D), volumes of distribution (Vd; E), and clearance (F) on days 1 and 5 are shown for cycles given to 15 patients who had >50% neutralization (median, 93%) of 200 ng/mL documented from serum samples just before day 1. These patients were evaluated during cycles 2 (n = 3), 3 (n = 5), 4 (n = 4), 5 (n = 2), and 6 (n = 1) and received that cycle, because the result was known only after completion of the cycle, but were excluded from further retreatment. For the extension cohort enrolled at National Cancer Institute, immunogenicity was determined by in vitro cytotoxicity.1 Enrollment or retreatment for the extension cohort required ≤50% neutralization of 200 ng/mL of moxetumomab pasudotox. Approximately 17% of relapsed HCL/HCL variant patients have >50% neutralization of 200 ng/mL and would be ineligible for enrollment. Pharmacokinetic analyses were previously described.5-8