Abstract
Background
Flow disruption with the WEB is an innovative endovascular approach for treatment of wide-neck bifurcation aneurysms. Initial studies have shown a low complication rate with good efficacy.
Purpose
To report clinical and anatomical results of the WEB treatment in the cumulative population of three Good Clinical Practice (GCP) studies: WEBCAST (WEB Clinical Assessment of Intrasaccular Aneurysm), French Observatory, and WEBCAST-2.
Methods
WEBCAST, French Observatory, and WEBCAST-2 are single-arm, prospective, multicenter, GCP studies dedicated to the evaluation of WEB treatment. Clinical data were independently evaluated. Postoperative and 1-year aneurysm occlusion was independently evaluated using the 3-grade scale: complete occlusion, neck remnant, and aneurysm remnant.
Results
The cumulative population comprised 168 patients with 169 aneurysms, including 112 female subjects (66.7%). The patients' ages ranged between 27 and 77 years (mean 55.5±10.2 years). Aneurysm locations were middle cerebral artery in 86/169 aneurysms (50.9%), anterior communicating artery in 36/169 (21.3%), basilar artery in 30/169 (17.8%), and internal carotid artery terminus in 17/169 (10.1%). The aneurysm was ruptured in 14/169 (8.3%). There was no mortality at 1 month and procedure/device-related morbidity was 1.2% (2/168). At 1 year, complete aneurysm occlusion was observed in 81/153 aneurysms (52.9%), neck remnant in 40/153 aneurysms (26.1%), and aneurysm remnant in 32/153 aneurysms (20.9%). Re-treatment was carried out in 6.9%.
Conclusions
This series is at the moment the largest prospective, multicenter, GCP series of patients with aneurysms treated with WEB. It shows the high safety and good mid-term efficacy of this treatment.
Clinical trial registration
French Observatory: Unique identifier (NCT18069); WEBCAST and WEBCAST-2: Unique identifier (NCT01778322).
Keywords: aneurysm
Introduction
As wide-neck aneurysms are sometimes untreatable or difficult to treat with standard coiling, alternative techniques have been developed, including flow disruption.1–4 Flow disruption involves the placement of an intrasaccular cage that will disrupt the blood flow at the level of the neck and induce aneurysmal thrombosis. Two devices are currently available (Artisse: Medtronic, Minneapolis, Minnesota, USA, and WEB: Sequent Medical, Aliso Viejo, California, USA). Clinical evaluation data for the Artisse device are limited, but the WEB device has been evaluated in several retrospective and prospective series.5–10 Moreover Good Clinical Practice studies, including two European (WEBCAST (WEB Clinical Assessment of Intrasaccular Aneurysm), WEBCAST-2), 1 US (WEB-IT), and 1 French (French Observatory), have been conducted since the introduction of this new device in Europe in 2010.11–15
This paper reports the clinical and anatomical results, including mid-term (1-year) follow-up, of WEB aneurysm treatment in the accumulated population of the WEBCAST, French Observatory, and WEBCAST-2 series, which, to date, is the largest prospective and multicenter group of patients treated with WEB.
Materials and methods
The WEBCAST, WEBCAST-2, and French Observatory, are single-arm, prospective, consecutive, multicenter studies dedicated to the evaluation of WEB treatment for bifurcation aneurysms, conducted in Europe and France, respectively.
The three studies received national regulatory authorization, including in France CCTIRS (Consultative Committee of Information Processing in Healthcare Research programme) approval, Reims Institutional Review Board approval, and CNIL (National Commission for Data Processing and Freedom) approval. For WEBCAST and WEBCAST-2 centers outside France, national or institutional approval was obtained according to each country’s regulations. Written informed consent was obtained from all patients.
WEB devices
The WEB is a self-expanding, retrievable, electrothermally detachable, nitinol braided device, which is placed within the aneurysm sac. Several WEB device iterations were available over the course of the three studies.
The WEB DL was initially used and contains a second nitinol braid. From November 2013, WEB DL was no longer used, and was replaced by WEB SL and WEB SLS. WEB SL has a barrel shape and WEB SLS, a spherical shape.
The most recent evolution of the device has been enhanced visualization, WEB EV, which incorporates composite wire strands made from nitinol and platinum.
In parallel with this evolution, the microcatheters used to deliver these devices have changed. Initially, the WEB DL was delivered using Rebar-27 (Covidien, Irvine, California, USA), Headway 27 (Microvention, Tustin, California, USA), or DAC 038 (Stryker, Fremont, California, USA) according to the size of the device. Sequent Medical developed specific microcatheters for WEB treatment, including the VIA 27 and VIA 33 (Sequent Medical, Aliso Viejo, California, USA). Recently, the VIA 21 and 17 (Sequent Medical) were introduced for WEB sizes between 4 and 7 mm.
Trial design and procedural modalities
Trial design and procedural modalities have been described in previous publications.16–19 Inclusion and exclusion criteria are presented in (table 1). Inclusion criteria for the three studies were ruptured (Hunt and Hess grade I, II, or III) and unruptured aneurysms located in the basilar artery (BA), middle cerebral artery (MCA) bifurcation, internal carotid artery terminus or anterior communicating artery (AComA) complex. In each center, endovascular treatment (EVT) was selected, for the patients in the studies, as the first-line treatment by a local multidisciplinary team that included neurosurgeons and neuroradiologists. The selection of aneurysms treated with the WEB device was performed autonomously in each center by the interventional neuroradiologists based on aneurysm characteristics.
Table 1.
Inclusion and exclusion criteria for French Observatory, WEBCAST, and WEBCAST-2
| Criteria | French Observatory | WEBCAST/WEBCAST2 |
| Inclusion | Similar criteria | |
|
||
| Inclusion | Distinctive criteria | |
|
|
|
| Exclusion | Similar criteria | |
|
||
| Exclusion | Distinctive criteria | |
| None |
|
|
ACA, anterior cerebral artery; AcomA, anterior communicating artery; AVM, arteriovenous malformation; BA, basilar artery; DN, Dome-to-Neck. ICH, intracranial hemorrhage; H&H, Hunt and Hess grade; ICAt, internal carotid artery terminus; MCA, middle cerebral artery; SAH, subarachnoid hemorrhage.
Pre-, intra-, and postoperative antiplatelet therapy was managed in each center as indicated for typical endovascular treatment with coils or stents and coils. Antiplatelet activity testing was not required in the study protocols. Triaxial access was recommended. Appropriate device sizing was determined based on 2D and 3D digital subtraction angiography (DSA). Depending on the size of the WEB device to be used, different microcatheters were used to catheterize the aneurysm (see above). Treatment with ancillary devices (balloon, coils and stents) could be performed in the French Observatory, if deemed necessary by the treating physician. In WEBCAST and WEBCAST-2 use of ancillary devices was authorized as a rescue treatment, not as an initial planned treatment strategy.
Data collection
Each center completed a patient file with the following data:
Demographic: patient’s age and gender;
Aneurysm: rupture status, location, size, and neck size;
Procedure: date, type of device used (DL or SL/SLS), perioperative antiplatelet medications, occurrence of complications during or after the procedure, and use of additional devices during the procedure (coils, remodeling balloons, stents, or flow diverters).
Preoperative Hunt and Hess grade was recorded for ruptured aneurysms. Modified Rankin Scale (mRS) score was collected before treatment (unruptured/recanalized aneurysms), at 30 days (±7 days), and at 12 months (±3 months). Finally, mid-term (before 15 months) vascular imaging data were collected. Re-treatment data were recorded.
Data analysis
The databases of the three studies were pooled after introduction of the more recent follow-up data. In the three studies, clinical data, including all adverse events, were independently monitored and analyzed by the same medical monitor (AM). All adverse events were recorded in this Good Clinical Practice (GCP) series even if no specific treatment was needed and they were not associated with clinical worsening. Thromboembolic events were diagnosed intraoperatively by angiography regardless of type (clotting near the neck of the aneurysm, clotting in the distal branches, and parent vessel occlusion). Postoperative thromboembolic events were diagnosed by MRI and/or DSA performed in cases of sudden neurological compromise. Intraoperative rupture was diagnosed by the exit of the tip of the coil or the microcatheter outside the limit of the aneurysmal sac and/or extravasation of contrast media. Morbidity was defined as mRS score >2 when preoperative mRS was ≤2 (or in the case of a ruptured aneurysm) and as an increase of 1 point when the preoperative mRS score was >2.
In the three studies, an expert interventional neuroradiologist (JB) independently evaluated aneurysm location on the initial angiogram, and aneurysm occlusion at the last angiographic follow-up, using the previously validated three-grade scale: complete occlusion, neck remnant, and aneurysm remnant. According to a previous publication, opacification of the proximal recess of the WEB device was considered as complete occlusion.9 Evolution of aneurysm occlusion between postoperative DSA and mid-term follow-up was also evaluated by the core laboratory using a three-grade scale: improved, stable, worse.
Statistical analysis
Continuous variables were described as mean±SD. Categorical data were described numerically as a categorical total and as a percentage of the analyzed population. Binomial data were described as a ratio of the true value and the analyzed population (x/n). Confidence intervals for binomial data were calculated by the Clopper-Pearson method, and p values were calculated by the Fisher exact test. Analyses were conducted using SPSS statistical software and ExactX Software for confidence intervals and p values.
Results
Patient and aneurysm population
The cumulative population comprised 168 patients (WEBCAST: 51; French Observatory: 62; WEBCAST-2: 55), including 112 female subjects (66.7%). The patients' ages ranged between 27 and 77 years (mean 55.5±10.2 years). All but one patient had one aneurysm, leading to a total number of aneurysms of 169. Aneurysm status was ruptured in 14/169 (8.3%), unruptured in 150/169 (88.8%), and recanalized in 5/169 (3.0%). In the five recanalized aneurysms, the initial aneurysm treatment was coiling. Aneurysm locations according to core laboratory analysis were MCA in 86/169 aneurysms (50.9%), AcomA in 36/169 (21.3%), BA in 30/169 (17.8%), and internal carotid artery terminus in 17/169 (10.1%).
Aneurysm size was between 2.8 and 17.0 mm (mean 7.6±2.5 mm).
Aneurysm neck size was between 2.4 and 13.8 mm (mean 5.2±1.6 mm). The neck was wide (≥4 mm) in 144/169 aneurysms (85.2%).
Antiplatelet treatment before and during the procedure is reported in (table 2). Antiplatelet activity testing was not performed in all participating centers and was not analyzed.
Table 2.
Antiplatelet treatment before, during, and after WEB procedure
| Antiplatelet treatment | Before (n=168) | During (n=168) | After (1-month follow-up) (n=167*) |
| 0 | 70 (41.7%) | 30 (17.9%) | 37 (22.2%) |
| 1 | 47 (28.0%) | 62 (36.9%) | 91 (54.5%) |
| 2 | 51 (30.4%) | 76 (45.2%) | 39 (23.4%) |
*One patient withdrew his consent after the procedure.
Treatment feasibility, adjunctive treatments, and adverse events
Treatment was successfully performed in 163/169 aneurysms (96.4%). Causes of failure were protrusion and subsequent retrieval of the device in two aneurysms, lack of appropriate device sizing in three aneurysms, and inability to deploy the WEB in one aneurysm.
Among aneurysms treated with WEB devices, adjunctive devices were used in 12/163 aneurysms treated with WEB (7.4%): coils alone in seven aneurysms (4.3%) and stents or flow diverters in five aneurysms (3.1%). A WEB DL was implanted in 78/163 aneurysms (47.9%) and WEB SL/SLS in 85/163 aneurysms (52.1%).
Adverse events are reported in (table 3). Intraoperative rupture (2/167 patient, 1.2%) and intracranial hemorrhage (related to antiplatelet treatment, 1/167 patients, 0.6%) were asymptomatic.
Table 3.
Adverse events, morbidity, and mortality at 1-month*
| Event type | All (n=167) | DL (n=80) | SL/SLS (n=87) | p Value |
| Thromboembolic events | 24 (14.4%) | 15 (18.8%) | 9 (10.3%) | 0.130 |
|
11 (6.6%) | 9 (11.3%) | 2 (2.3%) | 0.027 |
|
8 (4.8%) | 4 (5.0%) | 4 (4.6%) | 1.000 |
|
5 (3.0%) | 2 (2.5%) | 3 (3.4%) | 1.000 |
| Intraprocedural rupture | 2 (1.2%) | 1 (1.3%) | 1 (1.1%) | 1.000 |
| Intracranial hemorrhage | 1 (0.6%) | 1 (1.3%) | 0 (0.0%) | 0.479 |
| Morbidity† | 5 (3.0%) | 3 (3.8%) | 2 (2.3%) | 0.671 |
| Mortality | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | NA |
*One patient withdrew his consent between the procedure and the 1-month follow-up.
†Modified Rankin Scale (mRS) score >2 if baseline ≤2. mRS +1 or more if baseline >2. mRS >2 and ruptured at baseline.
Mortality/morbidity at 1 month
Mortality and morbidity at 1 month are shown in table 3 Clinical evaluation at 1 month was conducted in 167/168 patients (99.4%) (figure 1).
Figure 1.
Flow chart of the population included in the three studies for safety and efficacy.
There was no mortality at 1 month. Global morbidity was observed in 5/167 patients (3.0%): related to a thromboembolic event in two patients (mRS score 3) with device protrusion in one patient, to initial aneurysm rupture in two patients (mRS scores 3 and 4), and to worsening of pre-existing aneurysm mass effect in one patient (mRS score 3). Morbidity was related to the device in one patient (0.6%), to the procedure in one patient (0.6%), and to the disease in three patients (1.8%).
Mortality/morbidity at 1 year
At the 12-month follow-up (mean±SD 12.1±1.7 months), 153 of the 168 patients (91.1%) enrolled in the study were clinically evaluated with mRS scoring (figure 1).
Five out of 153 patients (3.3%) died between the 1-month and 1-year follow-up: three unrelated to aneurysm disease or treatment (cancer: two and cirrhosis: one), one from worsening of pre-existing mass effect described previously (this patient had a mRS score of 3 at 1 month), and one as a consequence and complication of a retroperitoneal hematoma during the index procedure. At 1 year all-cause, neuro-related, and procedure-related mortality are respectively, 5/153 (3.3%), 1/153 (0.7%), and 1/153 (0.7%).
Among the five patients who had a mRS score of >2 at 1 month related to the procedure, 1 died between 1 month and 1 year (progressive brainstem compression), three had improved at 1 year (one patient was mRS score 1 and two patients were mRS score 2), and one was unchanged (mRS score 3 due to a thromboembolic event). Another patient had a mRS score 3 due to a thromboembolic event that occurred during re-treatment with a flow diverter (see below). At 1 year all-cause, neuro-related, and procedure-related morbidity are respectively, 2/153 (1.3%), 0/153 (0.0%), and 2/153 (1.3%).
Anatomical results at mid-term follow-up
Anatomical results at 1 year (mean±SD 12.1±1.7 months) were evaluated in 152/168 patients (90.5%) with 153/169 aneurysms (90.5%) (figure 1).
For patients re-treated before or at the 1-year follow-up, aneurysm occlusion was evaluated by DSA performed at the beginning of the re-treatment. The vascular imaging technique was DSA in 134/153 (87.6%) aneurysms, CT angiography in 4/153 aneurysms (2.6%), and MR angiography in 15/153 aneurysms (9.8%).
Aneurysm occlusion at mid-term and evolution of occlusion from immediate postoperative DSA to mid-term follow-up imaging is presented in (table 4) for the global population and patients treated with DL and SL/SLS. At mid-term, complete occlusion (figure 2) was seen in 81/153 aneurysms (52.9%), neck remnant (figure 3) in 40/153 aneurysms (26.1%), and aneurysm remnant (figure 4) in 32/153 aneurysms (20.9%). Adequate occlusion (complete occlusion or neck remnant) was observed in 121/153 aneurysms (79.1%).
Table 4.
Aneurysm occlusion at mid-term follow-up and evolution of aneurysm occlusion between postoperative DSA and mid-term follow-up imaging
| Aneurysm occlusion at mid-term follow-up | ||||
| All (n=153) | WEB DL (n=72) | WEB SL/SLS (n=81) | p Value | |
| Complete occlusion | 81 (52.9%) | 38 (52.8%) | 43 (53.1%) | 1.000 |
| Neck remnant | 40 (26.1%) | 19 (26.4%) | 21 (25.9%) | |
| Aneurysm remnant | 32 (20.9%) | 15 (20.8%) | 17 (21.0%) | |
| Evolution of aneurysm occlusion between postoperative DSA and mid-term follow-up imaging | ||||
| All (n=152) | WEB DL (n=72) | WEB SL/SLS* (n=80) | p Value | |
| Improved | 94 (61.8%) | 46 (63.9%) | 48 (60.0%) | 0.911 |
| Stable | 53 (34.9%) | 24 (33.3%) | 29 (36.3%) | |
| Worsened | 5 (3.3%) | 2 (2.8%) | 3 (3.8%) | |
*In one patient/one aneurysm, postoperative aneurysm occlusion was not evaluable.
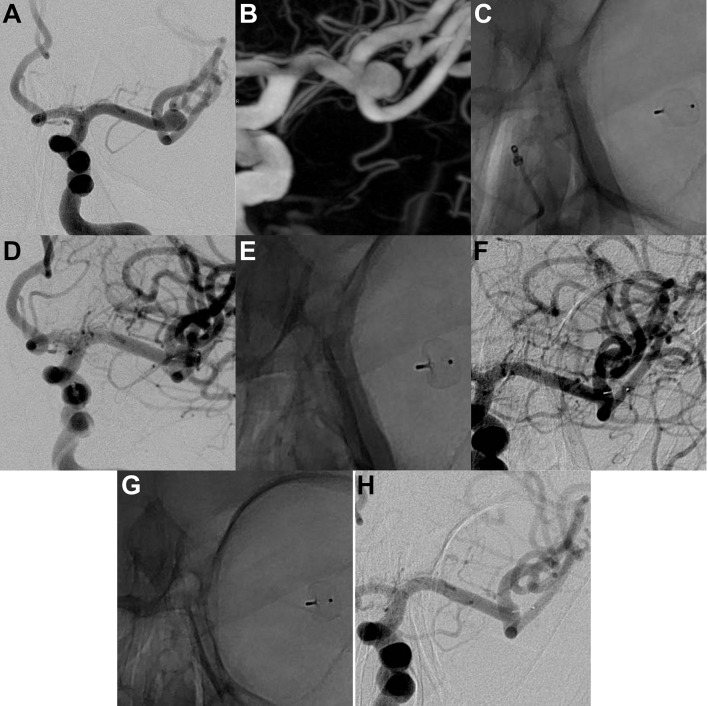
Figure 2.
Unruptured left middle cerebral artery aneurysm. (A) DSA, working view and (B) 3D-DSA show the aneurysm (transverse diameter: 6.3 mm; height: 5.3 mm; neck: 5.4 mm). (C) and (D) DSA at the end of the procedure (unsubtracted and subtracted view, respectively) show the detached WEB device (WEB SL 7×3 mm) and residual flow in the aneurysm and the device. (E) and (F) DSA at 6 months (unsubtracted and subtracted views, respectively) show the WEB device and complete aneurysm occlusion. (G) and (H) DSA at 12 months (unsubtracted and subtracted views, respectively) shows the WEB device and stable complete aneurysm occlusion.
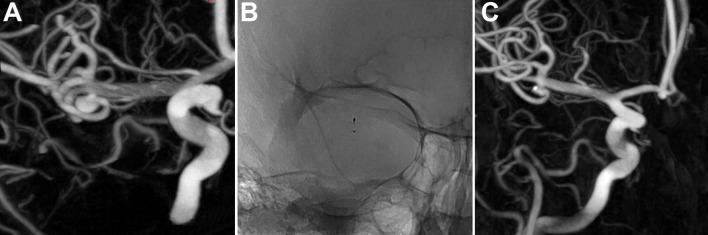
Figure 3.
Unruptured left middle cerebral artery aneurysm. (A) 3D DSA shows the aneurysm (transverse diameter: 3.8 mm; height: 4.2 mm; neck: 2.4 mm). (B) One-year DSA (unsubtracted view) shows the WEB device. (C) One-year 3D-DSA shows a neck remnant.
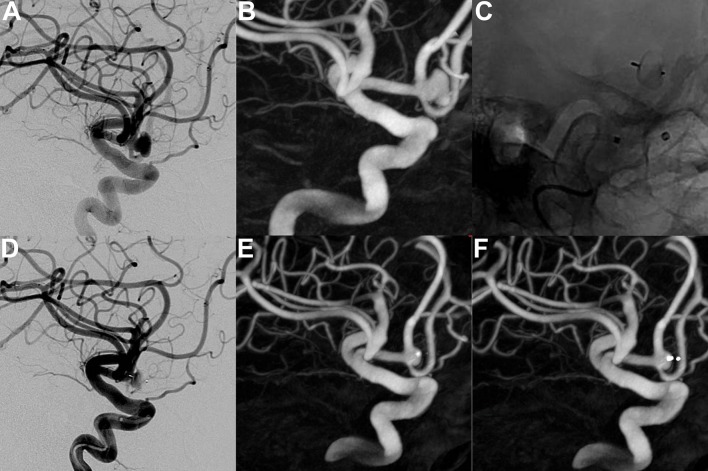
Figure 4.
Unruptured anterior communicating artery aneurysm. (A) DSA, working view and (B) 3D-DSA show the aneurysm (transverse diameter: 6.2 mm; height: 4.3 mm; neck: 4.6 mm). (C) and (D) DSA (unsubtracted and subtracted views, respectively) shows the detached WEB device (WEB SL 7×3 mm) and slow flow in the aneurysm and the device. (E) and (F) 3D-DSA at 12 months (with and without device subtraction, respectively) shows a small aneurysm remnant.
Importantly no neck or aneurysm remnant was associated with bleeding/rebleeding during the follow-up period.
Re-treatment
The re-treatment rate was evaluated in 160 aneurysms. Excluded from this analysis were the six aneurysms that were not treated with WEB, the aneurysms of the two patients who withdrew their consent, and one aneurysm in a patient lost to follow-up. Eleven aneurysms were re-treated (6.9%): with stent and coils in four aneurysms, flow diverters in four, stent in one patient, WEB and stent in one patient and WEB in one patient. One patient re-treated with a flow diverter 14 months after the initial procedure had a delayed parent artery occlusion with clinical worsening (mRS score 3). Re-treatment of one patient was attempted with a flow diverter, but it was not possible to place the diverter correctly for anatomical reasons (coverage of perforators).
Discussion
The cumulative population of the three European and French GCP studies is the largest prospective and multicenter cohort of patients with aneurysms treated with WEB with a completely independent evaluation of clinical and anatomical results.
Feasibility and safety of WEB aneurysm treatment
The treatment with WEB was highly feasible (96.4%) knowing that three of six failures were related to the lack of appropriate size availability. Adjunctive devices were used in a relatively limited percentage of cases (7.4%). In 4.3%, coils were used in addition to the WEB device on a planned or unplanned basis. For some aneurysms, their shape or size made it clear that treatment with the WEB alone was potentially difficult and adjunctive use of coils was foreseen at the beginning of the procedure. In other cases, the adjunctive placement of coils was decided after WEB detachment if the device was not properly deployed against the aneurysm wall or was not occluding the neck. This situation was mostly encountered at the beginning of the WEB experience, when the device was not routinely oversized. Stents and flow diverters were added in 3.1% of aneurysms in cases of WEB protrusion or when the neck was so wide that it was not possible to manage it without an intravascular device.
Thromboembolic events, including the asymptomatic appearance of thrombus during the procedure, were seen in 14.4% of patients, but permanent deficit was found in only 3.0% of patients. This rate of thromboembolic events is slightly higher than that reported in a large coiling series (7.1% in ATENA/unruptured aneurysms and 13.3% in CLARITY/ruptured aneurysms).1 2 Importantly, those series included all kinds of aneurysms, whereas the present series is dealing with wide-neck bifurcation aneurysms, associated with a higher risk of thromboembolic events.20
Intraoperative rupture was reported in two patients (1.2%). This rate is lower than that reported in ATENA (2.0%) and CLARITY (3.7%). Moreover, both ruptures were asymptomatic. Finally, one delayed (48 hours) intracranial bleeding was observed (0.6%) with no clinical worsening.
Global morbidity and mortality at 1 month were, respectively, 3.0% and 0.0%. Treatment-related morbidity and mortality were, respectively, 1.2% and 0.0% and are comparable or better than the rates reported in coiling series like ATENA (morbidity: 1.7%; mortality: 1.4%) or CLARITY (morbidity: 3.7%; mortality: 1.5%). These percentages confirm the high degree of safety of the treatment, considering that the WEB treatment was used in difficult aneurysms and the studied population was partially included at the beginning of the clinical experience with WEB (learning curve).
A comparison of morbidity/mortality with that in surgical series is more difficult. In the largest meta-analysis dealing with unruptured aneurysms treated by clipping, the rate of death after surgery (1.7%) was higher than in the present series (0.0%) and the rate of unfavorable outcomes at 1 year was 6.7% compared with 4.6% in the present series (with procedure-related unfavorable outcomes of 2.0%).16 Similarly, in the International Study of Unruptured Intracranial Aneurysms (ISUIA), in a very large prospective group of patients treated by surgery, the rate of surgery-related death was 1.8% and morbidity (mRS score 3–5) 3.0%.17 At 1 year, surgery-related death was 2.7% and morbidity 1.4%. In one single-center series, dealing with ruptured and unruptured MCA aneurysms (the most common indication for WEB in the studied population), surgical mortality was 5.3% and at late follow-up permanent neurological morbidity was 4.6%.18 In patients with unruptured MCA aneurysms, poor outcomes were seen in 6.1%.
Efficacy of WEB aneurysm treatment
Complete aneurysm occlusion was observed in 52.9% of aneurysms with adequate occlusion (complete occlusion or neck remnant) in 79.1% of aneurysms. These results are very similar to results reported in a retrospective European series, with complete and adequate occlusion, respectively, of 69.0% and 89.7%, at mid-term follow-up (median: 13 months), and respectively, of 68.4% and 84.2%, at long-term follow-up (median: 27 months).9 10
Anatomical results observed in this cumulative population are difficult to compare with previous series dealing with other endovascular approaches, as WEB treatment is used in a relatively specific group of aneurysms (wide-neck bifurcation aneurysms that are prone to recurrence).19 In the Matrix and Platinum Science (MAPS) Trial, a subgroup analysis was conducted showing that in unruptured wide-neck aneurysms (not necessarily bifurcation), the rate of complete and adequate occlusion (at 12 months) was, respectively, 27.1% and 57.6% with coils.21 With stenting and coiling, higher rates of complete and adequate occlusion (respectively, 45.7% and 62.8%) were obtained, although accompanied by a higher rate of complications. Finally, it is very difficult to compare WEB treatment and clipping efficacy as surgical series reporting 1-year or long-term anatomical results evaluated with DSA by an independent core laboratory are lacking.16 18
Re-treatment was performed in a limited percentage of aneurysms treated with the WEB (6.3%). It is difficult to compare re-treatment rates from one study to another, as indications for re-treatment are not well established and vary considerably from one center to another.22 However, the very low re-treatment rate observed with WEB is comparable to the lowest rate of re-treatment reported in the largest coiling series: HELPS trial, 3.0% with both bare and hydrogel coils; CLARITY study, 3.3% for aneurysms treated with bare coils, but 9.5% in the matrix group; Cerecyte Coil Trial, 3.5% in the bare coils group and 7.7% in the Cerecyte coils group; MAPS wide-neck aneurysm subset, 13.7% for coils alone and 14.1% for stent and coils.19 21 23 24
Place of the WEB device in the EVT armamentarium
In the three GCP series, the WEB device was used in typical indications: wide-neck bifurcation aneurysms. Recent publications suggest that indications for WEB aneurysm treatment may increase in the future. In a recent, single-center series, WEB treatment was used in 49% of aneurysms treated with EVT.25 In another multicenter series, WEB was used in ‘atypical’ locations.26
Limitations
This study has two main limitations. First, it is not a randomized study, and comparison with other techniques is difficult. However, it is to date the largest GCP series of patients with aneurysms treated with WEB and demonstrates the good safety and efficacy (at mid-term follow-up) of this treatment. Second, long-term follow-up is needed to precisely evaluate the place of this treatment in the management of intracranial aneurysms. Follow-up at 3 and 5 years is foreseen in French Observatory, WEBCAST and WEBCAST-2.
Conclusion
This analysis of the cumulative population of three GCP studies dealing with wide-neck bifurcation aneurysm treatment with the WEB device (WEBCAST, French Observatory, and WEBCAST-2) confirmed the high safety of this treatment with no mortality and low morbidity at 1 month. This treatment is associated with a good efficacy at 1 year with a complete and adequate occlusion rate of 52.9% and 79.1%, respectively.
neurintsurg-2017-013448supp001.docx (93.5KB, docx)
Footnotes
Contributors: All authors have provided a substantial contribution to the conception and design of the studies and/or the acquisition and/or the analysis of the data and/or the interpretation of the data; drafted the work or revised it for significant intellectual content; approved the final version of the manuscript; agree to be accountable for all aspects of the work, including its accuracy and integrity.
Funding: WEBCAST, WEBCAST-2, and French Observatory have been funded by Sequent.
Competing interests: LP: consultant for Balt, Microvention, Neuravi, and Penumbra. JM: consultant for Medtronic, Microvention, Stryker, and Balt. XB: consultant for Microvention and Stryker. ISI: consultant for Codman, Medtronic, Sequent, and Stryker. VC: consultant for Microvention and Balt and receives educational grants from Medtronic and Stryker. JF: has received fees as consultant or lecturer from Acandis, Bayer, Boehringer-Ingelheim, Codman, Covidien, MicroVention, Penumbra, Philips, Sequent, Siemens, and Stryker; his institution received funding from MicroVention, Medtronic, BMBF, BMWi, DFG, EU. JK: consultant for Microvention/Sequent. WW: consultant for Microvention, Phenox, and Medtronic. TL: consultant for Medtronic, Mentice, Microvention, and Route92. AM: consultant for Microvention/Sequent and Cerus Endovascular. JB: consultant and shareholder for Oxford Endovascular Ltd; his institution received funding from MicroVention. LS: consultant for Stryker, MicroVention, Medtronic, Balt. J-YG, HD, and SV have no disclosures.
Patient consent: Obtained.
Ethics approval: Reims institutional review board.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Cognard C, Pierot L, Anxionnat R, et al. Results of embolization used as the first treatment choice in a consecutive nonselected population of ruptured aneurysms: clinical results of the Clarity GDC study. Neurosurgery 2011;69:837–42. 10.1227/NEU.0b013e3182257b30 [DOI] [PubMed] [Google Scholar]
- 2.Pierot L, Spelle L, Vitry F. ATENA Investigators. Immediate clinical outcome of patients harboring unruptured intracranial aneurysms treated by endovascular approach: results of the ATENA study. Stroke 2008;39:2497–504. 10.1161/STROKEAHA.107.512756 [DOI] [PubMed] [Google Scholar]
- 3.Pierot L, Wakhloo AK. Endovascular treatment of intracranial aneurysms: current status. Stroke 2013;44:2046–54. 10.1161/STROKEAHA.113.000733 [DOI] [PubMed] [Google Scholar]
- 4.Pierot L, Biondi A. Endovascular techniques for the management of wide-neck intracranial bifurcation aneurysms: a critical review of the literature. J Neuroradiol 2016;43:167–75. 10.1016/j.neurad.2016.02.001 [DOI] [PubMed] [Google Scholar]
- 5.Pierot L, Liebig T, Sychra V, et al. Intrasaccular flow disruption: a new endovascular approach for the treatment of intracranial aneurysms. Results of a preliminary clinical evaluation in a multicenter series. AJNR Am J Neuroradiol 2012;33:1232–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pierot L, Klisch J, Cognard C, et al. Endovascular WEB flow disruption in middle cerebral artery aneurysms: preliminary feasibility, clinical, and anatomical results in a multicenter study. Neurosurgery 2013;73:27–35. 10.1227/01.neu.0000429860.04276.c1 [DOI] [PubMed] [Google Scholar]
- 7.Papagiannaki C, Spelle L, Januel AC, et al. Flow disruption with WEB device: report of a prospective, multicenter series of 83 patients with 85 aneurysms. AJNR Am J Neuroradiol 2014;35:2006–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mine B, Pierot L, Lubicz B. Intrasaccular flow-diversion for treatment of intracranial aneurysms: the Woven EndoBridge. Expert Rev Med Devices 2014;11:315–25. 10.1586/17434440.2014.907741 [DOI] [PubMed] [Google Scholar]
- 9.Lubicz B, Klisch J, Gauvrit JY, et al. Short-term and mid-term follow-ups in patients with wide-neck bifurcation aneurysms treated with the WEB device: a retrospective European study. AJNR Am J Neuroradiol 2014;35:432–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pierot L, Klisch J, Liebig T, et al. WEB-DL endovascular treatment of wide-neck bifurcation aneurysms: long-term results in a European series. AJNR Am J Neuroradiol 2015;36:2314–9. 10.3174/ajnr.A4445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fiorella D, Molyneux A, Coon A, et al. Demographic, procedural and 30-day safety results from the WEB Intra-saccular Therapy Study (WEB-IT). J Neurointerv Surg 2017;012841. [DOI] [PubMed] [Google Scholar]
- 12.Pierot L, Moret J, Turjman F, et al. WEB treatment of intracranial aneurysms: feasibility, complications, and 1-month safety results with the WEB DL and WEB SL/SLS in the French Observatory. AJNR Am J Neuroradiol 2015;36:922–7. 10.3174/ajnr.A4230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pierot L, Costalat V, Moret J, et al. Safety and efficacy of aneurysm treatment with WEB: results of the WEBCAST study. J Neurosurg 2016;124:1250–6. 10.3171/2015.2.JNS142634 [DOI] [PubMed] [Google Scholar]
- 14.Pierot L, Moret J, Turjman F, et al. WEB treatment of intracranial aneurysms: clinical and anatomic results in the French observatory. AJNR Am J Neuroradiol 2016;37:655–9. 10.3174/ajnr.A4578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pierot L, Gubucz I, Buhk JH, et al. Safety and efficacy of aneurysm Treatment with the WEB: results of the WEBCAST 2 study. AJNR Am J Neuroradiol 2017;38:1151–5. 10.3174/ajnr.A5178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kotowski M, Naggara O, Darsaut TE, et al. Safety and occlusion rates of surgical treatment of unruptured intracranial aneurysms: a systematic review and meta-analysis of the literature from 1990 to 2011. J Neurol Neurosurg Psychiatry 2013;84:42–8. 10.1136/jnnp-2011-302068 [DOI] [PubMed] [Google Scholar]
- 17.Wiebers DO, Whisnant JP, Huston J, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet 2003;362:103–10. [DOI] [PubMed] [Google Scholar]
- 18.Rodríguez-Hernández A, Sughrue ME, Akhavan S, et al. Current management of middle cerebral artery aneurysms: surgical results with a "clip first" policy. Neurosurgery 2013;72:415–27. 10.1227/NEU.0b013e3182804aa2 [DOI] [PubMed] [Google Scholar]
- 19.Pierot L, Cognard C, Anxionnat R, et al. Endovascular treatment of ruptured intracranial aneurysms: factors affecting midterm quality anatomic results: analysis in a prospective, multicenter series of patients (CLARITY). AJNR Am J Neuroradiol 2012;33:1475–80. 10.3174/ajnr.A3003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pierot L, Cognard C, Anxionnat R, et al. Ruptured intracranial aneurysms: factors affecting the rate and outcome of endovascular treatment complications in a series of 782 patients (CLARITY study). Radiology 2010;256:916–23. 10.1148/radiol.10092209 [DOI] [PubMed] [Google Scholar]
- 21.Hetts SW, Turk A, English JD, et al. Stent-assisted coiling versus coiling alone in unruptured intracranial aneurysms in the matrix and platinum science trial: safety, efficacy, and mid-term outcomes. AJNR Am J Neuroradiol 2014;35:698–705. 10.3174/ajnr.A3755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pierot L, Fiehler J, White P. Point-TAR: a useful index to follow-up coiled intracranial aneurysms? AJNR Am J Neuroradiol 2015;36:2–4. 10.3174/ajnr.A4119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.White PM, Lewis SC, Gholkar A, et al. Hydrogel-coated coils versus bare platinum coils for the endovascular treatment of intracranial aneurysms (HELPS): a randomised controlled trial. Lancet 2011;377:1655–62. 10.1016/S0140-6736(11)60408-X [DOI] [PubMed] [Google Scholar]
- 24.Molyneux AJ, Clarke A, Sneade M, et al. Cerecyte coil trial: angiographic outcomes of a prospective randomized trial comparing endovascular coiling of cerebral aneurysms with either cerecyte or bare platinum coils. Stroke 2012;43:2544–50. 10.1161/STROKEAHA.112.657254 [DOI] [PubMed] [Google Scholar]
- 25.van Rooij WJ, Peluso JP, Bechan RS, et al. WEB treatment of ruptured intracranial aneurysms. AJNR Am J Neuroradiol 2016;37:1679–83. 10.3174/ajnr.A4811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pierot L, Biondi A, Narata AP, et al. Should indications for WEB aneurysm treatment be enlarged? Report of a series of 20 patients with aneurysms in "atypical" locations for WEB treatment. J Neuroradiol 2017;44:203–9. 10.1016/j.neurad.2016.12.011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
neurintsurg-2017-013448supp001.docx (93.5KB, docx)