Abstract
Genetic analysis in model systems can provide a rich context for conceptual understanding of gene structure, regulation, and function. With an intent to create a rich learning experience in molecular genetics, we developed a semester-long course-based undergraduate research experience (CURE) using the CRISPR-Cas9 gene editing system to disrupt specific genes in the zebrafish. The course was offered to freshman students; nine students worked in four groups (two to three members per group) to design, synthesize, and test the nuclease activity of the CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats)/sgRNAs for targeted disruption of specific genes in the zebrafish. Each group worked with a gene with an already known mutant phenotype that can be visually scored and a gene that had not been studied in zebrafish previously. Embedded in the course were a series of workshop-styled units or tutorials, including tours to core facilities. The focus was on introducing and developing skills that could be accommodated within the span of a semester. Each group successfully cloned at least one plasmid-encoding CRISPR/sgRNA template, visually analyzed injected embryos, and performed genotyping assays to detect CRISPR-Cas9 activity. In-class discussions, a final end-of-semester written test, and group oral presentations were assessed for an understanding of the CRISPR-Cas9 system, application of the CRISPR-Cas9 system as a gene manipulation tool, and experimental methods used to create plasmid vectors and synthesize sgRNA. In addition, poster presentations were evaluated by faculty, graduate students, and senior undergraduate students at a University research exposition. Self-reflections in the form of group conversations were video recorded. All students (9/9) distinctly showed learning gains after completing the activity, but the extent of the gains was variable, as seen from results of a written test and poster presentation assessment. Qualitative analysis of evaluations and self-reporting data indicated several gains, suggesting that all students found many aspects of the CURE valuable and gained project-specific (conceptual) and transferrable skills (science process and science identity).
INTRODUCTION
Numerous studies have shown that undergraduate research experiences have many educational benefits ([1–5]). Largely, undergraduate students gain research experience by working in individual labs, mentored by faculty, post-docs, or senior graduate students. In contrast to mentored research experiences of individual students, course-based undergraduate research experiences (CUREs) are being effectively used to engage groups of students in short, authentic, research projects (6). CUREs for freshmen and sophomore students provide teachers with an opportunity to impact student learning and perception of research early in the students’ undergraduate training (7). CUREs facilitate student learning by engaging students and requiring them to think critically like scientists in a larger context of an experimental/research question (8).
Auchincloss et al. (6) identified five aspects of effective CUREs: 1) use of scientific practices, 2) discovery, 3) broadly relevant or important work, 4) collaboration, and 5) iteration. We have incorporated all five of these aspects into a CURE for freshman honors students. As part of the CURE in Molecular Genetics we developed, students designed experiments to test the role of specific genes implicated in human diseases during embryonic development of zebrafish (a model vertebrate organism). Students used the CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats)/Cas9 system to target orthologous human genes in zebrafish. Since the CRISPR/Cas9 technology is a novel and powerful gene editing tool to disrupt, add or change the sequence of a gene in many animal model systems (9), it can be effectively used to engage undergraduate students. Zebrafish is an ideal model system for an introductory CURE since they are simple to handle, produce a lot of eggs and can be visualized under a dissection microscope even by a novice. There are a number of studies showing the value and utility of zebrafish in engaging undergraduate students with content in genetics, molecular, cellular, and developmental biology (10–13).
In the CURE we developed, nine students worked in four groups (two to three members per group) to design, synthesize, and test the nuclease activity of CRISPR/sgRNAs for targeted disruption of specific zebrafish genes. Each group successfully cloned at least one plasmid-encoding CRISPR/sgRNA template, visually analyzed injected embryos, and performed genotyping assays to assess CRISPR-Cas9 nuclease activity. Students then communicated their findings by presenting them as a poster at a University-wide undergraduate research exposition.
We describe the format and various aspects of the CURE, which can be used to model similar courses involving both the CRISPR-Cas9 technology and the zebrafish system.
Intended audience
Students in the Science and Technology Honors (STH) program at the University of Alabama, Birmingham, are required, in their senior year, to write a thesis based on a mentored research project—a two-year project on an authentic problem—in an established research laboratory. A series of courses is offered to prepare students for their honors thesis. In the second semester of the freshman year, the students have an opportunity to gain laboratory skills and research experience through Research Approaches (STH201) courses (in Biotechnology, Biomedical Engineering, Chemistry, and Molecular Genetics). The focus of these courses is typically on laboratory methods and experimental techniques, which would prepare students and help them with their two-year honors research project. The Molecular Genetics section was designed as a CURE with discovery as the central focus and introduced the techniques in the context of biological discovery. Since this section was being offered for the first time, we decided to limit the enrollment to ten students so that we could assess the factors that can contribute to the success of the CURE. Ten students registered for the CURE, and one student dropped due to a conflict in class schedule.
While the CURE we designed can be effectively offered to freshman students to familiarize them with authentic research practices, it can also be offered to impart technical skills in Molecular Genetics. We propose that it can be offered at any year of undergraduate collegiate training, with appropriate modifications. This CURE can also help kick start mentored research in a course-based format that will enable students to gain a broader perspective on laboratory research.
Prerequisite student knowledge
Students were required to have at least a high-school level knowledge in biology, with a basic understanding of the Central Dogma of molecular biology: (a) DNA as the genetic material, (b) flow of biological information from DNA to RNA to protein, (c) mutations causing changes in genetic information. The class activities were structured such that students who did not have a strong foundation in biology were still able to keep up with the pace of the class. Students did not possess a background in gene editing, the CRISPR/Cas9 system, or zebrafish handling. While there are no specific prerequisite lab skills, a few of the students had had limited exposure to the molecular biology techniques used in this course.
Learning time
Prior to the start of the course, students were asked to complete a set of online courses that are requirements to perform any research on campus (Appendix 1). The class was structured as a three credit-hour course. The class met twice a week for an hour and fifteen minutes each. The time spent by the students (in class) and the instructor (outside of class) on the various activities is summarized in Table 1. Students also spent some time outside of class hours to repeat experiments to achieve specific goals. This extra time varied between groups, based on their initial experimental results.
TABLE 1.
Time spent on planning various course activities and corresponding safety concerns.
| Activity | Out-of-Class “Planning” Timea | In-Class Time |
|---|---|---|
| Tutorials and tours | ||
| Assembling a PCR machine | 30 minutes | 3 hours |
| Working with 3D models of DNA | 30 minutes | 1.5 hours |
| Gene analysis and CRISPR/sgRNA design (CRISPR design tool at MIT) | — | 3 hours |
| CRISPR/sgRNA synthesis | ||
| 1. Order oligonucleotides | 1 hour | — |
| 2. pDR274 linearizationb | 2 hours | — |
| 3. Oligo annealing | — | 45 minutes |
| 3. Making bacterial platesb | 2 hour | — |
| 4. Competent cell (DH5α) preparationb | 4 hours | — |
| 5. Transformation and plating | 2 hours | 1.5 hours |
| 6. Colony PCR | — | 2 hours |
| 7. Analyze colony PCR products (polyacrylamide gels) | — | 2 hours |
| 8. Inoculation of colonies | — | 0.5 hours |
| 9. Plasmid extraction (alkaline lysis) | 1 hour | 2 hours |
| 10. Submission for Sanger sequencing | 0.5 hours | — |
| 11. Sequence analysis (using SnapGene) | — | 1 hour |
| 12. Plasmid linearization (HindIII) | — | 1 hours |
| 13. Purification of linearized plasmid | — | 1.5 hours |
| 14. In vitro transcription | — | 2 hours |
| 15. Injection of CRISPR/sgRNA-Cas9 mRNA mixture | 3 hours | — |
| 16. Collecting injected embryos and observing phenotypes | 1 hour | 2 hours |
| 17. Genomic DNA isolation | — | 1 hours |
| 18. PCR set up | — | 1 hours |
| 19. PCR product analysis | — | 2 hours |
| 20. Additional time outside of class hours for completion of lab work/experiments | 6 hours | |
| Total (approximately) | 17.5 hours | 33.75 hours |
Out-of-class “planning” time includes time spent by the instructor to prepare lab reagents and general housekeeping duties to ensure continuity of experiments from class to class.
Needs to be prepared by instructor ahead of time.
CRISPR = clustered regularly interspaced short palindromic repeats; PCR = polymerase chain reaction.
Learning objectives
The broad goal of the course is to give students an opportunity to experience authentic research enabling them to gain science identity and science process skills. There are two sets of learning objectives: conceptual and hands-on. The conceptual learning objectives were based on understanding key ideas in molecular genetics and genome editing. Without a solid foundation in these concepts, students would not be able to proceed with the hands-on bench research. Once the students gained some familiarity with the main concepts, they proceeded to perform hands-on research on the bench. The conceptual and hands-on learning objectives and corresponding assessments are listed in Table 2.
TABLE 2.
Conceptual and hands-on learning objectives and corresponding assessments.
| Outcome | Assessment |
|---|---|
| Conceptual | |
| 1. Students will explain the nature of genes and how gene mutations help us get insights into gene function. | In-class group presentation Final in-class test |
| 2. Students will explain how the CRISPR-Cas9 nuclease system is used as a genome engineering tool. | Project outline—white board discussion Final in-class test |
| Hands-on | |
| 1. Students will assemble a thermocycler (OpenPCR) and gain an appreciation of its inner working for use in polymerase chain reactions (PCRs). | Blog Video documentary |
| 2. Students will search the zebrafish genome (using ENSEMBL genome browser), analyze gene sequences (using a sequence analysis software), and generate CRISPR-Cas9 nucleases to disrupt (knockout) genes in the zebrafish system. | Gene annotation and CRISPR/sgRNA design Annotated SnapGene Files |
| 3. Students will perform basic steps in molecular cloning. | Plasmid construction—sequence confirmation |
| 4. Students will communicate the knowledge they gained about gene modifying technologies to a scientific audience (on campus poster presentation). | Poster design and presentation |
CRISPR = clustered regularly interspaced short palindromic repeats; PCR = polymerase chain reaction.
PROCEDURE
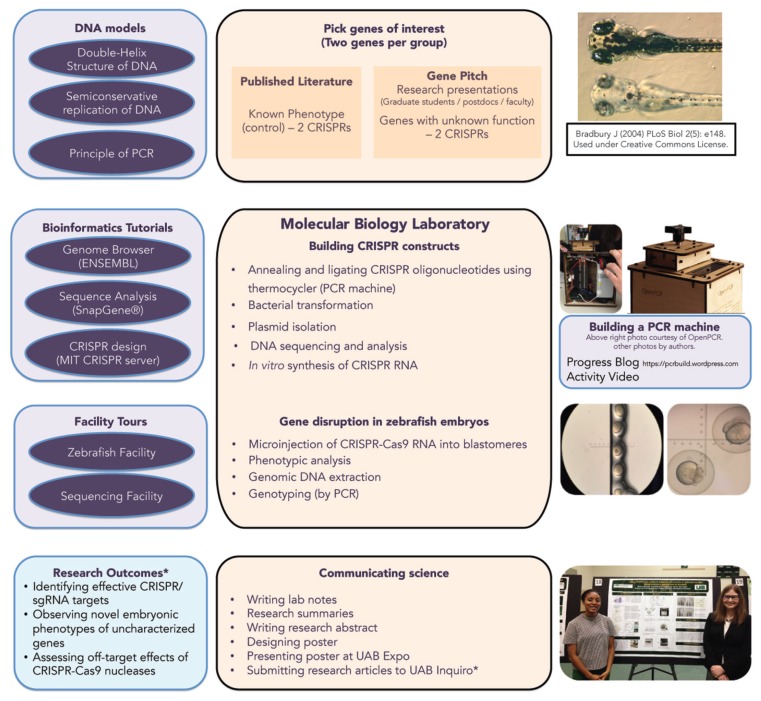
A series of workshop-styled units or tutorials (modules), including tours to core facilities, was integrated into the course (Fig. 1). These modules are relatively independent of the core molecular biology laboratory module, making them conducive to flexible scheduling during the semester. We briefly describe these modules and activities below.
FIGURE 1.
Course overview with modules, objectives, and outcomes. The orange boxes show the preparatory and core laboratory modules leading up to the poster presentations. The violet boxes show the modules that support the core laboratory modules.
Student and faculty instructions
1. Model building – DNA structure and transcription
Physical models have been shown to be valuable in teaching and learning molecular structures and their functions (14). To help students gain a molecular perspective of DNA structure and mechanisms of replication that are the foundations of molecular biology experiments like polymerase chain reaction (PCR), we used 3D models. We obtained physical DNA models on loan from the Walter Schroeder Library, Milwaukee School of Engineering, Milwaukee, WI (http://cbm.msoe.edu/teachRes/library/). Appendix 2 contains web links to detailed student and teacher notes used in the model-building exercise. Students are given the student handout from the “Guided Discovery Approach to investigating the structure of DNA” resource page. This activity allows students to build the double helix model in a way that mimics the model building effort of Watson and Crick in 1953.
2. Assembling the PCR machine
The thermocylcer/PCR machine being a workhorse of molecular biology, we felt that it would be a valuable experience to know about its construction and mechanism. Students were provided with a DIY kit from OpenPCR to assemble the thermocycler along with the manual (web link in Appendix 2). Two crucial aspects of the “Build your own PCR” activity were (a) tool building, and (b) collaboration. Two class periods were dedicated to this activity early on in the semester, which was sufficient to assemble a working PCR machine.
3. “Gene pitch.”
To make the experience of identifying genes and targeting them for mutagenesis engaging and authentic, we invited researchers from the UAB community (who do not use zebrafish as a model system in their research work) to present a gene of their interest to the class. Students could pick a gene that they found very interesting, on which they would work for the rest of the semester. Five researchers “pitched” their genes of interest and mentioned how creating mutations in the orthologous genes in zebrafish would be useful to their research study.
4. Bioinformatics tutorials leading to design of oligonucleotides
Students were introduced to the organization of the zebrafish genome and how to search for specific genes by using the ENSEMBL genome browser (Appendix 2, web link on how to use ENSEMBL). Once the students gained familiarity with ENSEMBL data, they were introduced to the CRISPR design tool using the MIT server, http://crispr.mit.edu/ (15). In addition to these two online resources, they also were introduced to SnapGeneViewer, a sequence-management software, to help them annotate various features, including CRISPR target sites and genotyping primers (Appendix 2, web link on how to use SnapGeneViewer).
Students used the ENSEMBL genome browser, MIT CRISPR design tool, and SnapGeneViewer to design oligonucleotides to construct CRISPR plasmids, and for genotyping (PCR) experiments. The instructor ordered lyophilized synthetic oligonucleotides from Integrated DNA Technologies (IDT). Several other companies offer custom synthesis of oligonucleotides.
5. Molecular cloning to generate CRISPR/sgRNA template plasmid
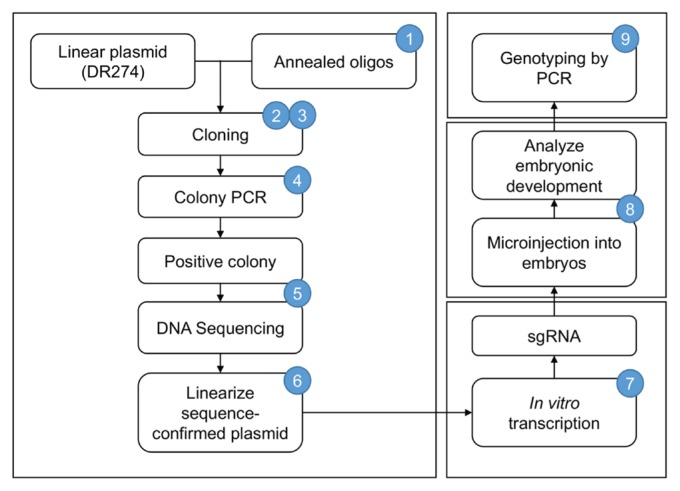
The core of the laboratory exercises included molecular cloning methods, with a goal of synthesizing CRISPR/sgRNA for embryo microinjections. A method described by Hwang et al. was used in the course (16). This method employs a series of simple steps to create a modified plasmid that is used as a template for in vitro transcription of sgRNA (Fig. 2; the steps involved in the preparation of a linearized template for in vitro transcription are described in Appendix 3).
FIGURE 2.
Workflow of the molecular biology lab module. Experiments included construction and linearization of a CRISPR/sgRNA template, in vitro transcription of sgRNA, injection of sgRNA with Cas9 protein, observation and analysis of development in injected embryos, and genotyping injected embryos by PCR to detect CRISPR-Cas9 nuclease activity. Numbers indicated in blue circles represent the week in which the activity/procedure was done. CRISPR = clustered regularly interspaced short palindromic repeats; PCR = polymerase chain reaction.
In vitro transcribed CRISPR/sgRNA samples (AMPLIS-CRIBE T7 High Yield Transcription Kit), along with Cas9 protein (GeneArt Platinum Cas9 Nuclease, ThermoFisher Scientific), were injected into zebrafish embryos by the instructor. Injected embryos were observed and analyzed for phenotypic changes by the students using bright field microscopy until five days post-fertilization (dpf). Subsequently, the injected embryos were processed for genomic DNA isolation that was used for genotyping by heteroduplex mobility assay (HMA) for the presence of insertions/deletions (indels) that involved PCR and polyacrylamide gel electrophoresis (17).
6. Facility tours
Students were given a guided tour of the zebrafish facility to expose them to husbandry and maintenance of zebrafish. They were also given a tour of the Genomics Core facility to enable them to understand the technologies used to obtain DNA sequence information. This tour was arranged after the students obtained the sequence files of the plasmids they constructed.
7. Documenting and communicating science
a) Lab notebook
Each group maintained a lab notebook with details of experimental methods, results and their analysis. The following prompts for the final report were given to students: (a) describe the utility of zebrafish as a model system (summary from your visit to the zebrafish facility), (b) describe the genes you are working with (trying to create mutant alleles using the CRISPR-Cas9 technology), (c) describe how you analyzed the gene sequences and designed the CRISPR guide sequences, (d) describe the methods you are using to create the CRISPR plasmid specific for each of the CRISPR/guides.
b) Poster abstracts
Students wrote abstracts for the posters to be presented at the UABExpo (University-wide student research exposition). Each group prepared and submitted one abstract with inputs and assistance from the instructor and teaching assistant (TA).
c) Preparing the poster, peer-evaluation and poster presentation
Students were given basic introductions and guidelines for making the poster. Students designed their posters as an in-class activity. Three copies of every group’s poster were printed out and distributed to the other groups for peer evaluation. Students were asked to evaluate the posters of the other groups and give feedback for improvements using the following questions: (1) “What would you change in their poster?” and (2) “Now that you have looked at the posters of your peers, what would you change about your poster?” Students used the feedback obtained from peer reviews to finalize their posters. In preparation for the UABExpo, each group presented their final poster to members of other groups and a small group of graduate students and postdocs. Finally, they presented the posters to a larger University-wide audience at the UABExpo (Appendices 5 and 7).
Safety concerns
Students were required to complete a set of online courses on the UAB Learning System (Appendix 1) that align with ASM Guidelines for Biosafety in Teaching Laboratories, specifically those for working with BSL-1 microorganisms. Students used E. coli DH5α strain cells that need to be handled with safe practices but do not pose a biohazard. Proper personal protection wear, including lab coats, gloves, closed-toed shoes, and protective eyewear, were worn by students while performing the experiments to avoid exposure to Kanamycin, acrylamide, TEMED, and ethidium bromide (when used to stain DNA). GELRED was used most of the time as a safer alternative to ethidium bromide. Bacteria and chemical reagents were properly contained and disposed of in appropriate biohazard bins within the laboratory. Bacterial solutions and plates marked for disposal were autoclaved or bleached. Students maintained a clean workspace and thoroughly washed their hands after completing each laboratory session in compliance with ASM guidelines.
DISCUSSION
Suggestions for assessment and evidence of student learning
We devised multiple assignments, both graded (Appendix 4) and ungraded, to give students a variety of learning opportunities. To have a strong formative assessment component in our course, we included activities that mandated in-class group discussions and peer review of experimental procedures. Apart from short discussions, there were two focused activities where every group had to discuss and write down their project outline and progress on the whiteboard. This was followed by making each group review the work of another group. The images in Appendix 6 show student work demonstrating their ability to keep up with the introduction of multiple layers of research process (background knowledge, conceptual understanding, technical skills, communication).
In terms of learning experimental technique-based skills, students were able to clone 14 of the 16 CRISPR constructs that they had set out to create. All plasmid constructs were sequence verified. This suggests that the students had a basic understanding of PCR and the structure of DNA as well as the competency to use PCR to perform molecular cloning within the context of the experiments that were conducted in class. Each group worked with a gene with an already known mutant phenotype that can be visually scored and a gene that had not been studied in zebrafish previously.
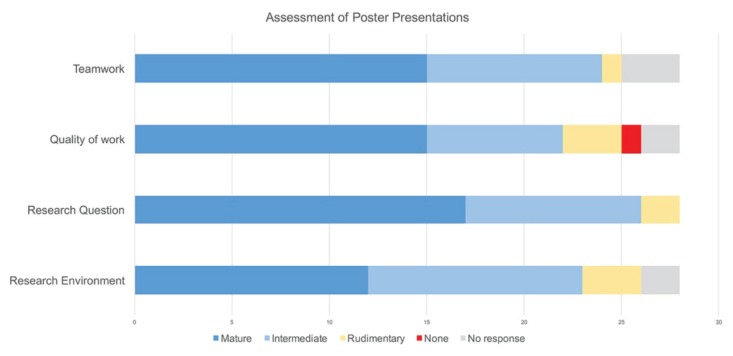
We included two summative assessment pieces to test student comprehension of course content and acquired skills. An in-class test focusing on problem solving and analytical skills, with predominantly higher Bloom’s level questions, was administered at the end of the semester (Appendix 4). An average of 15.4/23 (range: 8–22) was obtained by students in this test. All students demonstrated considerable understanding of the workflow and experimental procedures. The second summative assessment piece was administered at an undergraduate research exposition, where the students presented their research on posters (Appendix 7). We created a short questionnaire with five items (Appendix 5) and requested visiting junior and senior undergraduate students, graduate students, postdoctoral researchers, and faculty members to use it as a tool to assess the poster presentations. Feedback from 28 independent evaluators (volunteers) showed that the majority of students had a mature to intermediate understanding of the research environment, the specific research question they worked on, the quality and amount of work that goes into addressing research questions, and the importance of teamwork, collaboration, and communication in the research enterprise (Fig. 3). We believe that this form of assessment is valuable since student work was judged by a course-independent authentic audience, in an engaging setting. This exercise supplemented the in-class exam in reinforcing the learning goals of the course.
FIGURE 3.
Assessment of student learning from poster presentations at the undergraduate research exposition. Student presentations were assessed based on their demonstrated understanding in four areas. The x-axis represents the total number of poster evaluations.
Post-course feedback was obtained through two activities. At the end of the course, we interviewed students (in their respective groups) to obtain feedback about their overall learning experience. We used six questions described by Hanauer et al. (18) to obtain a general idea of each group’s experience with laboratory research. Student feedback interviews were video recorded and analyzed to identify main themes that emerged from the responses. All answers were transcribed, and the content was analyzed to construct the main themes of student experiences during the course (Box 1). Based on the student interviews, we could infer that the most exciting aspect of the class was using the CRISPR/Cas9 tool for the genome engineering (Box 1). Another critical dimension of the course was that the students were engaged in “broadly relevant work”— they were able to correlate knocking out genes in zebrafish with its relevance to biomedical research impacting human health. Students admitted that they had a very different idea of what a research career looks like before the course and that their perception of research was definitely transformed after the course. Students also found poster preparation and presentation to be a very useful activity.
BOX 1. Qualitative assessment questionnaire and student responses.
|
|
Description of student responses regarding their overall learning experience Theme 1. Engaging in broadly relevant and important work
“This class made me more aware about research than I was before to where I might consider doing research in the future (as a MD-PhD) as compared to just MD.” “I don’t want to be a PI who sits around and writes grants but it would be cool to see patients and to be at the front line of research in your field.” Theme 3. Communicating science (poster presentation) “The poster presentation was pretty cool because we created our own research project. It’s cool to culminate all of our work into one thing and look at all what we have done and presented to people. And having other people be really interested.” “Our poster presentation was definitely a big one (a memorable moment). Presenting to eight judges—I have never done that before.” |
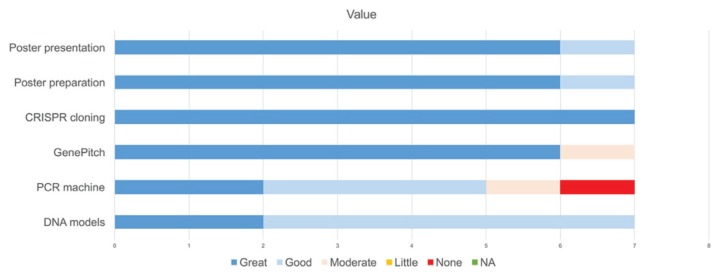
The second activity was a course survey. We surveyed students after the semester to obtain their views on the value of the class activities that were performed during the course (Fig. 4). Seven out of nine students enrolled in the course responded to the survey. All students found “great” value in cloning the CRISPR sgRNA. They found using DNA models, the gene pitch, and the poster preparation and presentation to be of “great” to “good” value.
FIGURE 4.
Post-course survey responses. Student responses (n=7 students) on the value of activities performed during the course.
CRISPR = clustered regularly interspaced short palindromic repeats; PCR = polymerase chain reaction.
Possible modifications
Several modifications to this course are possible, not only to make it widely useful but also effective and efficient. During subsequent offerings, the course was modified by employing alternative experimental methods to focus on different skills. For example, the module on cloning was completely eliminated by using a cloning-free approach to synthesizing sgRNA (19). This made it possible for students to focus more time on reading primary literature, identifying a research problem of their choice based on reading primary literature (e.g., role of evolutionary conserved non-coding sequences in regulation of known genes), and analyzing experimental results in greater depth. A modified version of the course was mirrored at a local four-year historically Black college for a small class (four students), with a focus on uncharacterized genes with known expression patterns, but without any known functions.
A well-designed rubric for the CURE would help in further enhancing student learning of course content and quality of student work from the outset. This rubric would be given to students at the beginning, and could be used as a guide throughout the course.
SUPPLEMENTAL MATERIALS
ACKNOWLEDGMENTS
We are thankful to the support provided by Dr. Diane Tucker and the Science & Technology Honors (STH) Program, Center for Integration of Research, Teaching and Learning (CIRTL), and Dr. Robert Kesterson, Director of the Transgenic & Genetically Engineered Models (TGEMs) Core. Financial support for this study was provided by TGEMs (supported by awards NIH P30CA13148, P30AR048311, P30DK074038, P30DK05336, and P60DK079626) and the Science and Technology Honors Program at UAB. The IRB protocol number for the Science & Technology Honors Program is X060619003 (PI: Dr. Diane Tucker). The authors declare that there are no conflicts of interest.
Footnotes
Supplemental materials available at http://asmscience.org/jmbe
REFERENCES
- 1.Bangera G, Brownell SE. Course-based undergraduate research experiences can make scientific research more inclusive. CBE Life Sci Educ. 2014;13(4):602–606. doi: 10.1187/cbe.14-06-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brownell SE, Hekmat-Scafe DS, Singla V, Chandler Seawell P, Conklin Imam JF, Eddy SL, Stearns T, Cyert MS. A high-enrollment course-based undergraduate research experience improves student conceptions of scientific thinking and ability to interpret data. CBE Life Sci Educ. 2015;14(2):14:ar21. doi: 10.1187/cbe.14-05-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elgin SC, Bangera G, Decatur SM, Dolan EL, Guertin L, Newstetter WC, San Juan EF, Smith MA, Weaver GC, Wessler SR, Brenner KA, Labov JB. Insights from a convocation: integrating discovery-based research into the undergraduate curriculum. CBE Life Sci Educ. 2016;15(2) doi: 10.1187/cbe.16-03-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laursen S, Hunter A-B, Seymour E, Thiry H, Melton G. Undergraduate research in the sciences: engaging students in real science. John Wiley & Sons; San Francisco, CA: 2010. [Google Scholar]
- 5.Lopatto D, Tobias S. Science in solution: the impact of undergraduate research on student learning. Council on Undergraduate Research; Washington DC: 2010. [Google Scholar]
- 6.Auchincloss LC, Laursen SL, Branchaw JL, Eagan K, Graham M, Hanauer DI, Lawrie G, McLinn CM, Pelaez N, Rowland S, Towns M, Trautmann NM, Varma-Nelson P, Weston TJ, Dolan EL. Assessment of course-based undergraduate research experiences: a meeting report. CBE Life Sci Educ. 2014;13(1):29–40. doi: 10.1187/cbe.14-01-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodenbusch SE, Hernandez PR, Simmons SL, Dolan EL. Early engagement in course-based research increases graduation rates and completion of science, engineering, and mathematics degrees. CBE Life Sci Educ. 2016;15(2) doi: 10.1187/cbe.16-03-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jordan TC, Burnett SH, Carson S, Caruso SM, Clase K, DeJong RJ, Dennehy JJ, Denver DR, Dunbar D, Elgin SCR, Findley AM, Gissendanner CR, Golebiewska UP, Guild N, Hartzog GA, Grillo WH, Hollowell GP, Hughes LE, Johnson A, King RA, Lewis LO, Li W, Rosenzweig F, Rubin MR, Saha MS, Sandoz J, Shaffer CD, Taylor B, Temple L, Vazquez E, Ware VC, Barker LP, Bradley KW, Jacobs-Sera D, Pope WH, Russell DA, Cresawn SG, Lopatto D, Bailey CP, Hatfull GF. A broadly implementable research course in phage discovery and genomics for first-year undergraduate students. MBio. 2014;5(1):e01051–13. doi: 10.1128/mBio.01051-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riordan SM, Heruth DP, Zhang LQ, Ye SQ. Application of CRISPR/Cas9 for biomedical discoveries. Cell Biosci. 2015;5:33. doi: 10.1186/s13578-015-0027-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anorve-Andress K, Arcand AL, Borg BR, Brown JL, Chartrand CA, Frank ML, Jansen JN, Joyce MJ, Joyce MT, Kinney JA, Kruggel SL, Lecy AD, Ma P, Malecha KM, Melgaard K, Miller PL, Nelson KK, Nieto Robles M, Perosino TR, Peterson JM, Rollins AD, Scherkenbach WL, Smith AL, Sodergren KA, Stiller JJ, Wehber KR, Liang JO. Variation in spot and stripe patterns in original and regenerated zebrafish caudal fins. Zebrafish. 2016;13(4):256–265. doi: 10.1089/zeb.2015.1192. [DOI] [PubMed] [Google Scholar]
- 11.Felzien LK. Integration of a zebrafish research project into a molecular biology course to support critical thinking and course content goals. Biochem Mol Biol Educ. 2016;44(6):565–573. doi: 10.1002/bmb.20983. [DOI] [PubMed] [Google Scholar]
- 12.Marra MH, Tobias ZJ, Cohen HR, Glover G, Weissman TA. In vivo time-lapse imaging in the zebrafish lateral line: a flexible, open-ended research project for an undergraduate neurobiology laboratory course. J Undergrad Neurosci Educ. 2015;13(3):A215–A224. [PMC free article] [PubMed] [Google Scholar]
- 13.Sarmah S, Chism GW, 3rd, Vaughan MA, Muralidharan P, Marrs JA, Marrs KA. Using zebrafish to implement a course-based undergraduate research experience to study teratogenesis in two biology laboratory courses. Zebrafish. 2016;13(4):293–304. doi: 10.1089/zeb.2015.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roberts JR, Hagedorn E, Dillenburg P, Patrick M, Herman T. Physical models enhance molecular three-dimensional literacy in an introductory biochemistry course. Biochem Mol Biol Educ. 2005;33(2):105–110. doi: 10.1002/bmb.2005.494033022426. [DOI] [PubMed] [Google Scholar]
- 15.Hsu PD, Scott DA, Weinstein JA, Ran FA, Konermann S, Agarwala V, Li Y, Fine EJ, Wu X, Shalem O, Cradick TJ, Marraffini LA, Bao G, Zhang F. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat Biotechnol. 2013;31(9):827–832. doi: 10.1038/nbt.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hwang WY, Fu Y, Reyon D, Maeder ML, Tsai SQ, Sander JD, Peterson RT, Yeh JR, Joung JK. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat Biotechnol. 2013;31(3):227–229. doi: 10.1038/nbt.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Challa AK, Boitet ER, Turner AN, Johnson LW, Kennedy D, Downs ER, Hymel KM, Gross AK, Kesterson RA. Novel hypomorphic alleles of the mouse tyrosinase gene induced by CRISPR-Cas9 nucleases cause non-albino pigmentation phenotypes. PLoS ONE. 2016;11(5):e0155812. doi: 10.1371/journal.pone.0155812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanauer DI, Frederick J, Fotinakes B, Strobel SA. Linguistic analysis of project ownership for undergraduate research experiences. CBE Life Sci Educ. 2012;11(4):378–385. doi: 10.1187/cbe.12-04-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gagnon JA, Valen E, Thyme SB, Huang P, Akhmetova L, Pauli A, Montague TG, Zimmerman S, Richter C, Schier AF. Efficient mutagenesis by Cas9 protein-mediated oligonucleotide insertion and large-scale assessment of single-guide RNAs. PLoS ONE. 2014;9(5):e98186. doi: 10.1371/journal.pone.0098186. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.