Parathyroid hormone (PTH) plays a key role in the pathogenesis of CKD mineral and bone disorder, which is associated with high morbidity and mortality in patients with CKD. Monitoring PTH in patients with CKD is thus recommended by international guideline committees to optimize patient care. However, measuring bioactive PTH is particularly tricky due to two simultaneously complicating circumstances. First, secreted PTH is degraded fast to N- and C-terminal fragments. Some of these fragments are PTH receptor–stimulating agonists other than the mature secreted PTH itself. Second, PTH and also its N-terminal bioactive fragments can be oxidized and will lose their PTH receptor–stimulating properties.
The active form of the hormone (PTH1–84) is produced by the sequential cleavage of the initially translated peptide, prepro-PTH (115 amino acids), via pro-PTH (90 amino acids) in the chief cells of the parathyroid glands. Neither prepro-PTH nor pro-PTH are detectable in plasma samples. PTH1–84 has a very short t1/2 and is degraded to PTH fragments. PTH exerts its effects through the interaction of its first N-terminal 34 amino acids with the PTH receptor. In the circulation, we usually find PTH1–84, C-terminal fragments, and N-terminal fragments (1,2).
PTH metabolism is altered in patients with CKD. Normally, most of PTH1–84 is transformed in the liver into the N-terminal PTH1–34 fragment and C-terminal fragments. The latter fragments are mainly catabolized in the kidney, and the degradation process involves solely glomerular filtration and tubular reabsorption, whereas the N-terminal PTH1–34 fragment undergoes both tubular reabsorption and peritubular uptake, like PTH1–84. Tubular reabsorption is mediated by megalin (1–3).
In CKD, both pathways of PTH degradation in the kidney are progressively impaired. This leads to a prolongation of the t1/2 of C-terminal PTH fragments in the circulation and therefore, accumulation. Moreover, there is no peritubular metabolism of PTH1–84 in uremic nonfiltering kidneys in contrast to peritubular uptake by normal filtering kidneys (1,2).
The ratio of mature PTH1–84 to C- and N-terminal PTH fragments is variable and depends on the degree of kidney impairment.
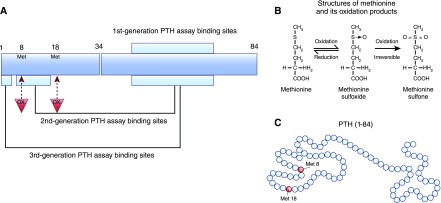
The first generation of PTH assays—RIAs—typically uses just one antibody directed toward the midregion of the PTH1–84 sequence and detects a mixture of mature PTH1–84, bioinactive C-terminal fragments, and N-terminal PTH fragments; it did, therefore, have major limitations (Figure 1). The discovery of truncated PTH fragments, particularly in patients with ESKD, in combination with the knowledge that the biologic activity of PTH1–84 is located within the N-terminal region (amino acids 1–34) stimulated the development of the next generation—second generation—of PTH assays: intact parathyroid hormone (iPTH) assays. These assays are two-site immunoassays, and they typically have a solid-phase capture antibody directed toward the C-terminal region of PTH (amino acids 26–32 or 39–84) and a detection antibody directed toward the N terminus (usually toward amino acids 12–24) (Figure 1) (1,2,4,5). This way of assay design increased specify of the PTH assays, because they avoided crossreactivity with C-terminal PTH fragments. Next, a third generation of PTH assays was developed, in which the detection antibody epitope was targeted farther toward the N terminus of PTH1–84 to amino acids 1–4. The solid-phase capture antibody directed toward the C-terminal region of PTH was the same as for second generation PTH assays (Figure 1). The clinical performance of second and third generation PTH assays seems to be comparable, and both are currently used (1,2).
Figure 1.
First to third generation parathyroid hormone (PTH) assays target different regions of the secreted PTH molecule and ignore PTH oxidation at Met8 and Met18. (A) The first generation of PTH assays typically uses just one antibody directed toward the midregion of the PTH1–84 sequence and detects a mixture of mature PTH1–84, bioinactive C-terminal fragments, and N-terminal PTH fragments. The second generation PTH assays (also called intact PTH assays) are two-site immunoassays and typically have a solid-phase capture antibody directed toward the C-terminal region of PTH (amino acids 26–32 or 39–84) and a detection antibody directed toward the N terminus (usually toward amino acids 12–24). In third generation PTH assays, the detection antibody epitope was targeted to amino acids 1–4. The solid-phase capture antibodies directed toward the C-terminal region of PTH were the same as for second generation PTH assays. Methionine residues that might be oxidized are marked with red triangles. (B) Amino acids, such as methionine, at positions 8 and 18 (red triangles in A) can be oxidized under conditions of oxidative stress. The first oxidation step is reversible, whereas the second one is not. (C) Amino acids 8 and 18 in the PTH molecule are belonging to the PTH receptor binding site of the hormone. An oxidation of these methionone residues alters the hormone receptor interaction. The nowadays used second and third generation PTH assays cannot distinguish between oxidized and nonoxidized PTH1–84 and/or N-terminal oxidized and nonoxidized PTH fragments.
The development of the second and third generation PTH assays was clearly a big step toward the goal of developing PTH assays measuring just bioactive PTH. However, these efforts ignored a second biologic process altering the native PTH1–84 (other than PTH fragmentation): PTH oxidation. PTH1–84 has two methionine amino acids at positions 8 and 18—means within the receptor binding site—that can be oxidized in vivo (Figure 1) (3–7).
About 30 studies performed by independent leading research groups worldwide showed that oxidized parathyroid hormone (oxPTH) and nonoxidized parathyroid hormone (n-oxPTH) have completely different biologic properties (reviewed in refs. 3, 5, and 6). Initial studies using classic receptor binding assays showed that oxPTH has a much lower binding affinity to the PTH receptor. Other studies focused on the generation of the second messenger of PTH receptor cAMP. These studies indicated that oxPTH—in contrast to n-oxPTH—does not stimulate the PTH receptor to generate cAMP. In addition, it was shown that oxPTH loses its biologic action on smooth muscle cells contraction/vascular effects in tissues like trachea, aortic rings, and the uterus. Stimulation of alkaline phosphatase activity in cultured neonatal mouse calvarial bone cells by PTH was seen only after incubation with n-oxPTH but not with oxPTH. Other studies showed that only n-oxPTH (but not oxPTH) is able to regulate calcium and phosphate metabolism in vivo (reviews of the literature are in refs. 3, 6, and 7). These data clearly indicate that PTH oxidation is critical for the biologic activity of PTH. PTH oxidation, however, has been ignored so far in the development of first to third generation PTH assays. Therefore, an assay system was developed to measure just n-oxPTH. The new detection process for n-oxPTH consists of two steps. In the first step, any oxidized forms of oxPTH at positions Met8 and/or Met18 are removed from the sample by a specific affinity chromatography column. The column contains mAb raised against oxidized PTH1–34. In the second step, the remaining n-oxPTH is analyzed in a conventional second generation PTH system (8).
In this issue of the Clinical Journal of the American Society of Nephrology, Seiler-Mussler et al. (8) compared the association of iPTH and n-oxPTH measured in baseline samples of patients with CKD and an eGFR range between 89 and 15 ml/min per 1.73 m2. After correction for baseline eGFR and proteinuria, iPTH (but not n-oxPTH) was associated with atherosclerotic events, acute heart failure, CKD progression, and death from any cause. They concluded: “Implementation of n-oxPTH measurement into clinical routine is for nondialysis-dependent CKD patients of questionable value at this time, and the application of various PTH assays in clinical practice may confuse rather than inform the busy clinician. Thus, we advocate using second and third generation PTH assays as the methods of choice to determine PTH in nondialysis-dependent CKD patients” (8).
This conclusion, however, is misleading, because it ignores the complexity of PTH measurements. What the authors actually only showed is that oxidative stress is a more powerful predictor then biologically active n-oxPTH in patients with CKD stages 2–4 for atherosclerotic events, acute heart failure, CKD progression, and death from any cause. The key finding of this study that oxidative stress is an important independent risk factor in patients with CKD stages 2–4 for the above-mentioned outcomes is well established. The mean baseline iPTH concentration in this study was 60 pg/ml, and mean n-oxPTH was measured as 9 pg/ml. Because iPTH = oxPTH + n-oxPTH (3,5–7), mean (biologically inactive) oxPTH in this study was 51 pg/ml. In other words, the waste majority of the detected iPTH (85%) was actually oxPTH, reflecting oxidative stress and not bioactive PTH that is able to interact with the PTH receptor. This finding is in pretty good agreement with a previous study analyzing iPTH, n-oxPTH, and oxPTH in 620 children with CKD stages 2–4 (90% of iPTH is oxPTH), 342 adult patients with CKD on dialysis (89% of iPTH is oxPTH), and 602 kidney transplant recipients (89% of circulating iPTH is oxPTH) (3).
The iPTH, n-oxPTH, and oxPTH data can be compared, because all of these studies used the same ways of determining these parameters (3,6,8,9). In patients with CKD stages 2–4 not on dialysis (8), oxidative stress assessed by iPTH measurements is obviously more important for outcomes, such as all-cause mortality, whereas in patients with CKD on dialysis, biologically active n-oxPTH is associated with cardiovascular outcomes (9). The effect of oxidative stress and bioactive n-oxPTH on cardiovascular outcomes seems to depend on the stage of kidney disease. This hypothesis, however, needs to be proven in larger study populations.
The information discussed is summarized below.
n-oxPTH can be measured by second (or third) generation PTH assays after prior removal of oxPTH1–84 and/or oxPTH fragments using a specific affinity chromatography column.
n-oxPTH = iPTH − oxPTH.
Only n-oxPTH1–84 or N-terminal n-oxPTH fragments (both are detectable with the n-oxPTH assay) are PTH receptor agonists.
About 85% of iPTH is oxidized in patients with CKD. iPTH measurements thus reflect mainly oxidative stress and to a minor degree, bioactive PTH being able to interact with the PTH receptor. Treatment guidelines for optimal PTH target values in patients with CKD can only be improved by dissecting oxidative stress from bioactive PTH.
Both oxidative stress and too high/too low PTH concentrations are harmful to patients with CKD, but they reflect independent pathways of disease progression in CKD, and hence, the resulting therapeutic consequences are different as well.
Because the currently used second and third generation PTH assays do only allow for an inaccurate assessment of the real PTH status of patients with CKD, measurements of bone-specific alkaline phosphatase might be a cheap and more reliable tool to estimate bone health.
n-oxPTH assay systems are not ready for clinical use, because we do not know n-oxPTH target values for patients with CKD.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related article, “Association of Nonoxidized Parathyroid Hormone with Cardiovascular and Kidney Disease Outcomes in Chronic Kidney Disease,” on pages 569–576.
References
- 1.Couchman L, Taylor DR, Krastins B, Lopez MF, Moniz CF: LC-MS candidate reference methods for the harmonisation of parathyroid hormone (PTH) measurement: A review of recent developments and future consideratios. Clin Chem Lab Med 52: 1251–1263, 2014 [DOI] [PubMed] [Google Scholar]
- 2.Cavalier E, Plebani M, Delanaye P, Souberbielle JC: Considerations in parathyroid hormone testing. Clin Chem Lab Med 53: 1913–1919, 2015 [DOI] [PubMed] [Google Scholar]
- 3.Hocher B, Oberthür D, Slowinski T, Querfeld U, Schaefer F, Doyon A, Tepel M, Roth HJ, Grön HJ, Reichetzeder C, Betzel C, Armbruster FP: Modeling of oxidized PTH (oxPTH) and non-oxidized PTH (n-oxPTH) receptor binding and relationship of oxidized to non-oxidized PTH in children with chronic renal failure, adult patients on hemodialysis and kidney transplant recipients. Kidney Blood Press Res 37: 240–251, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Ursem SR, Vervloet MG, Hillebrand JJG, de Jongh RT, Heijboer AC: Oxidation of PTH: In vivo feature or effect of preanalytical conditions? Clin Chem Lab Med 56: 249–255, 2018 [DOI] [PubMed] [Google Scholar]
- 5.Hocher B, Yin L: Why current PTH assays mislead clinical decision making in patients with secondary hyperparathyroidism. Nephron 136: 137–142, 2017 [DOI] [PubMed] [Google Scholar]
- 6.Hocher B, Pasch A: Hope for CKD-MBD patients: New diagnostic approaches for better treatment of CKD-MBD. Kidney Dis (Basel) 3: 8–14, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hocher B, Armbruster FP, Stoeva S, Reichetzeder C, Grön HJ, Lieker I, Khadzhynov D, Slowinski T, Roth HJ: Measuring parathyroid hormone (PTH) in patients with oxidative stress--do we need a fourth generation parathyroid hormone assay? PLoS One 7: e40242, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seiler-Mussler S, Limbach A, Emrich I, Pickering J, Roth H, Fliser D, et al. : Association of non-oxidized parathyroid hormone with cardiovascular and kidney disease outcomes in chronic kidney disease. Clin J Am Soc Nephrol 13: 569–576, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tepel M, Armbruster FP, Grön HJ, Scholze A, Reichetzeder C, Roth HJ, Hocher B: Nonoxidized, biologically active parathyroid hormone determines mortality in hemodialysis patients. J Clin Endocrinol Metab 98: 4744–4751, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Moorthi RN, Moe SM: CKD-mineral and bone disorder: Core curriculum 2011. Am J Kidney Dis 58: 1022–1036, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]