Abstract
Background and objectives
Despite colistin’s longstanding reported association with nephrotoxicity, the attributable risk and timing of toxicity onset are still unknown. Whether substantial toxicity occurs during the initial 72 hours of exposure has important implications for early treatment decisions. The objective of this study was to compare colistin-exposed patients with a matched control group given other broad spectrum antibiotics.
Design, setting, participants, & measurements
We conducted a retrospective cohort study in patients treated for multidrug-resistant Pseudomonas, Klebsiella, or Acinetobacter spp. Colistin-exposed patients were matched to unexposed controls using propensity scores. AKI was defined according to the Kidney Disease Improving Global Outcomes creatinine criteria. Incidence rate ratios and risk differences of AKI in the matched cohort were estimated with the generalized estimating equation Poisson regression model. Risk factors for AKI were tested for effect modification in the matched cohort.
Results
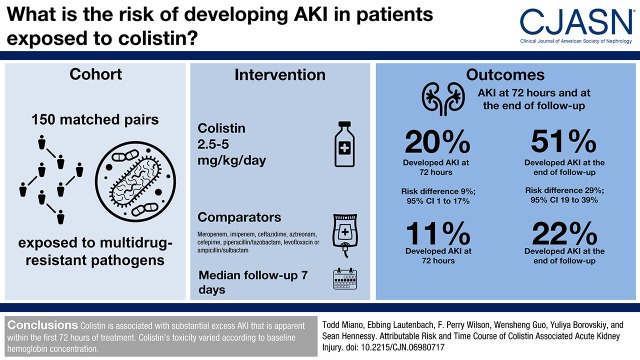
The study included 150 propensity-matched pairs with similar types of infection, similar delays to effective treatment, and similar baseline characteristics. Incidence of AKI was 77 of 150 (51%) in the colistin group versus 33 of 150 (22%) in matched controls (risk difference, 29%; 95% confidence interval, 19 to 39), corresponding to a number needed to harm of 3.5. Early toxicity was apparent, because AKI risk was higher in colistin-exposed patients at 72 hours of exposure (incidence rate ratio, 1.9; 95% confidence interval, 1.1 to 3.5). In both groups, hospital mortality in patients who experienced AKI was lower if kidney function returned to baseline during hospitalization. The effect of colistin exposure on AKI risk varied inversely according to baseline hemoglobin concentration.
Conclusions
Colistin is associated with substantial excess AKI that is apparent within the first 72 hours of treatment. Colistin’s toxicity varied according to baseline hemoglobin concentration.
Podcast
This article contains a podcast at https://www.asn-online.org/media/podcast/CJASN/2018_03_15_CJASNPodcast_18_4_M.mp3
Keywords: drug nephrotoxicity, acute renal failure, anemia, colistin, polymixin, acute kidney injury, sepsis, gram negative, multidrug resistant
Introduction
The rising prevalence of multidrug-resistant (MDR) and extensively drug-resistant (XDR) gram-negative infection is a major threat to human health worldwide (1,2). Of particular concern is the lack of novel antibiotics to combat XDR Pseudomonas, Acinetobacter, and Klebsiella species (2). This confluence of factors has led to the emergence of colistin as a mainstay of treatment for many MDR and XDR pathogens. These infections are associated with substantial mortality, resulting largely from inadequate empirical regimens and the attendant delays to effective treatment (3–5). The majority of colistin use in this setting is as directed therapy (i.e., after culture results are known). Outcomes might be improved by earlier empirical colistin treatment in high-risk patients (6–8). Importantly, decisions regarding the initiation of early empirical treatment require clear information regarding the time course of colistin’s adverse effects.
Despite colistin’s longstanding reported association with nephrotoxicity, the attributable risk and timing of toxicity onset are still unknown. Although previous uncontrolled studies show AKI rates of 40%–53% (9–14), meta-analyses of controlled studies have concluded that colistin does not increase AKI risk compared with β-lactam agents (15,16). The duration of treatment required to cause toxicity is also unknown. Colistin’s extensive tubular reabsorption (17) and high intrinsic toxicity (18) suggest that AKI might develop rapidly. However, clinical research data are conflicting, where the median time to AKI ranges from 5 to 12 days (11–14). These conflicting estimates may be related to differences in study design and AKI definition. Delayed-onset toxicity could have potentially life-saving implications for the role of colistin. If 48–72 hours of colistin exposure is below the toxicity threshold, empirical treatment in high-risk patients might be a rational approach to improve outcomes.
In this study, we compared the rate of AKI in colistin-exposed patients with that in controls in a cohort of patients with MDR gram-negative pathogens. Our goal was to measure the attributable risk of AKI in colistin-exposed patients and quantify the risk of early toxicity. We also sought to identify specific factors that modify colistin’s nephrotoxic effects.
Materials and Methods
Study Design and Source Population
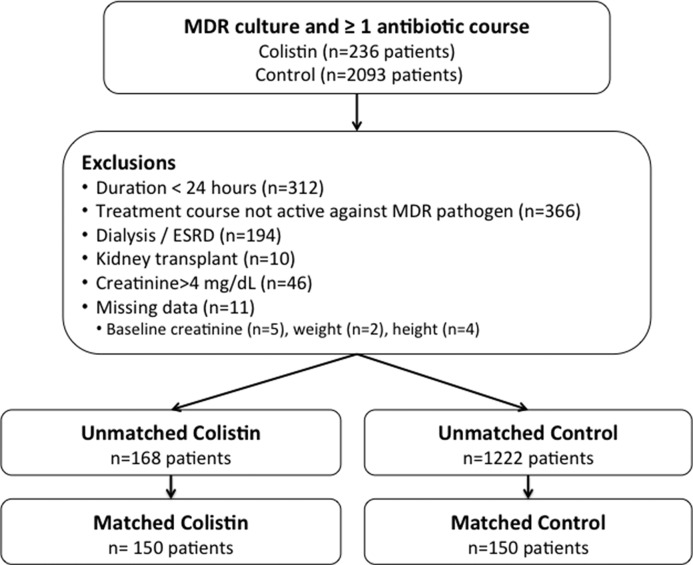
We conducted a retrospective cohort study of patients treated with colistin or a comparator agent at one of four hospitals within the University of Pennsylvania Health System from May 1, 2008 to September 30, 2014. Comparators were meropenem, imipenem, cefepime, piperacillin/tazobactam, levofloxacin, ceftazidime, aztreonam, or ampicillin/sulbactam (treatment of Acinetobacter spp. only). We included adults (age ≥18 years old) with cultures growing MDR Pseudomonas spp., Acinetobacter spp., or Klebsiella spp. MDR was defined as exhibiting nonsusceptibility to three or more antimicrobial classes (19). Patients were included if they received at least 24 hours of antibiotic treatment that was initiated on or after the day that an MDR culture was obtained. Patients were excluded if the antibiotic did not have in vitro activity against the MDR isolate; if there was a history of ESKD, dialysis dependence (RRT within 14 days of the index date), or kidney transplantation; if baseline creatinine was >4 mg/dl; or if there was missing baseline serum creatinine (within 48 hours of course initiation), lack of at least one follow-up creatinine, or missing baseline covariate data. The prevalence of missing data was low (n=11 patients). The Institutional Review Board of the University of Pennsylvania approved the study (protocol 819587).
Colistin Preparation and Dosing
Colistin used during the study period was colistimethate sodium. Each vial of colistimethate sodium contains 150 mg of colistin base activity, which is equivalent to approximately 5 million international units (20). Colistin dosing followed package insert recommendations for the entirety of the study period: 2.5–5 mg/kg per day colistin base activity divided into two to three doses for patients with normal kidney function (21). Dose adjustments for kidney function also followed the package insert. The standard at our institution is for all dosing regimens to be reviewed by a pharmacist before treatment initiation.
Outcome Definitions
The primary outcome was the occurrence of any stage of AKI during antibiotic exposure. AKI was defined according to the Kidney Disease Improving Global Outcomes (KDIGO) creatinine criteria (22), which are listed in Supplemental Table 1. We did not assess changes in urine output, because documentation of urine output is less reliable retrospectively and drug-induced AKI typically does not produce oliguria. Baseline creatinine was the last creatinine obtained before antibiotic initiation (as stated above, this value must have been obtained within 48 hours of antibiotic initiation). Follow-up continued until 48 hours after the last dose, occurrence of AKI, hospital discharge, death, or 30 days, whichever came first. Secondary outcomes include AKI during the first 72 hours of exposure, need for RRT, in-hospital mortality at 30 days, and the composite end point of AKI or in-hospital mortality at 30 days (to examine the effect of the competing risk of mortality) (23). We also examined the frequency of AKI resolution defined as a return of creatinine concentration to within 25% of the baseline value (24) by the time of hospital discharge. For patients who required RRT, we additionally required the patient to be free of RRT for >14 days.
Data Collection
All data were obtained from Penn Data Store (25), a data warehouse that contains medications, laboratory values, demographics, procedures, and discharge diagnosis data from patients admitted to the University of Pennsylvania Health System. Covariates included risk factors for AKI, measures of acute illness severity, and variables related to infection diagnosis (Supplemental Table 2, Table 1). Comorbidities were ascertained from discharge diagnosis codes following the algorithms of Quan et al. (26) and Elixhauser et al. (27). Pre-exposure AKI was defined by applying the KDIGO criteria from hospital admission up to the time of exposure, with baseline defined as the lowest creatinine value within the first 48 hours of admission (Supplemental Material). Concomitant medications and laboratory data were obtained during the 72-hour period before treatment. For each laboratory measure, the value most proximate to treatment initiation was collected. Baseline GFR was estimated using the Chronic Kidney Disease Epidemiology Collaboration equation (28) using the baseline creatinine concentration.
Table 1.
Baseline covariates
| Variable | Unmatched Cohort | Matched Cohort | ||||
|---|---|---|---|---|---|---|
| Colistin, n=168 | Control, n=1222 | Standardized Differencea | Colistin, n=150 | Control, n=150 | Standardized Differencea | |
| Demographics | ||||||
| Age, yr, median (IQR) | 61 (48–70) | 64 (52–74) | 0.22 | 61 (48–70) | 59 (47–69) | 0.09 |
| BMI, mean (SD) | 27 (9.7) | 27 (9.6) | −0.01 | 27 (9.7) | 27 (8.8) | 0.07 |
| Men, n (%) | 103 (61) | 687 (56) | 0.1 | 91 (61) | 83 (55) | 0.11 |
| Race, n (%) | ||||||
| White | 97 (57) | 679 (56) | 0.04 | 88 (59) | 86 (57) | 0.03 |
| Black | 50 (30) | 406 (33) | −0.08 | 43 (29) | 42 (28) | 0.02 |
| Otherb | 21 (13) | 137 (11) | 0.04 | 19 (13) | 22 (15) | −0.06 |
| Admission | ||||||
| Admit type, n (%) | ||||||
| Medical | 63 (38) | 528 (43) | −0.12 | 57 (38) | 58 (39) | −0.01 |
| Surgical | 77 (46) | 539 (44) | 0.04 | 68 (45) | 72 (48) | −0.05 |
| Long-term acute care | 28 (16) | 155 (13) | 0.11 | 25 (16) | 20 (13) | 0.09 |
| Center, n (%) | ||||||
| HUP | 112 (67) | 700 (57) | 0.19 | 99 (66) | 102 (68) | −0.04 |
| PMC | 17 (10) | 220 (18) | 0.23 | 15 (10) | 16 (11) | −0.03 |
| PAH | 11 (7) | 147 (12) | −0.19 | 11 (7) | 12 (8) | −0.02 |
| RIT | 28 (16) | 155 (13) | 0.11 | 25 (16) | 20 (13) | 0.09 |
| Prior length of stay, median (IQR) | 10 (3–28) | 6 (1–17) | 0.34 | 11 (3–29) | 10 (3–24) | 0.07 |
| Prior ABX,c median (IQR) | 9 (5–15) | 4 (2–7) | 0.55 | 9 (5–16) | 9 (5–17) | 0.03 |
| ICU admission, n (%) | 93 (55) | 360 (29) | 0.54 | 81 (54) | 84 (56) | −0.04 |
| Mechanical ventilation, n (%) | 78 (46) | 368 (30) | 0.34 | 66 (44) | 60 (40) | 0.08 |
| Comorbidities, n (%) | ||||||
| Heart failure | 73 (43) | 484 (39) | 0.08 | 66 (44) | 63 (42) | 0.04 |
| Hypertension | 114 (68) | 854 (69) | −0.04 | 102 (68) | 102 (68) | 0 |
| Pulmonary disease | 83 (49) | 580 (47) | 0.04 | 76 (51) | 80 (53) | −0.05 |
| Liver disease | ||||||
| Mild | 28 (17) | 142 (12) | 0.15 | 22 (15) | 18 (12) | 0.08 |
| Severe | 10 (6) | 70 (6) | 0.01 | 10 (7) | 7 (5) | 0.09 |
| Diabetes | ||||||
| Uncomplicated | 35 (21) | 278 (23) | −0.05 | 32 (21) | 27 (18) | 0.08 |
| Complicated | 18 (11) | 111 (9) | 0.06 | 13 (9) | 13 (9) | 0 |
| CKD | 51 (30) | 379 (31) | −0.01 | 44 (29) | 45 (30) | −0.02 |
| Medications, n (%) | ||||||
| Aminoglycosides | 48 (29) | 95 (8) | 0.56 | 37 (25) | 31 (21) | 0.09 |
| Vancomycin | 75 (45) | 408 (33.4) | 0.23 | 68 (45) | 66 (44) | 0.03 |
| Inhaled antibioticsd | 13 (8) | 26 (2.1) | 0.26 | 12 (8) | 15 (10) | −0.07 |
| Intravenous acyclovir | 5 (3) | 10 (0.8) | 0.16 | 4 (3) | 3 (2) | 0.04 |
| ACEs/ARBs | 13 (8) | 133 (11) | −0.11 | 12 (8) | 12 (8) | 0 |
| NSAIDs | 5 (3) | 28 (2) | 0.04 | 4 (3) | 4 (3) | 0 |
| Loop diuretics | 48 (29) | 265 (22) | 0.16 | 43 (29) | 48 (32) | −0.07 |
| Calcineurin inhibitorse | 11 (7) | 74 (6) | 0.02 | 11 (7) | 10 (7) | 0.03 |
| Vasopressors | 27 (16) | 79 (6) | 0.31 | 21 (14) | 18 (12) | 0.06 |
| Iodinated contrast | 37 (22) | 221 (18) | 0.09 | 35 (23) | 39 (26) | −0.06 |
| Laboratory, median (IQR) | ||||||
| Hemoglobin, g/dl | 8.7 (7.9–9.8) | 9.4 (8.4–10.7) | −0.49 | 8.8 (7.9–9.8) | 8.7 (8.0–9.9) | 0 |
| WBC, ×108 cells per 1 L | 11.8 (7.4–18.0) | 10.5 (7.3–14.6) | 0.23 | 11.7 (7.2–18) | 11.4 (7.9–16.3) | 0.01 |
| Platelets, ×1011 cells per 1 L | 238 (111–351) | 250 (164–340) | −0.12 | 238 (113–361) | 248 (159–355) | −0.07 |
| Kidney function | ||||||
| Creatinine, mg/dl, median (IQR) | 0.7 (0.5–1.2) | 0.9 (0.6–1.3) | −0.23 | 0.7 (0.5–1.2) | 0.7 (0.5–1.1) | 0.03 |
| GFR, ml/min per 1.73 m2, median (IQR) | 86 (42–112) | 75 (46–104) | 0.23 | 86 (59–114) | 90 (58–123) | −0.06 |
| Prior AKI, n (%) | ||||||
| None | 118 (70) | 950 (78) | −0.17 | 106 (71) | 104 (69) | 0.03 |
| Yes: resolved | 32 (19) | 128 (10) | 0.24 | 28 (19) | 32 (21) | −0.07 |
| Yes: active | 18 (11) | 144 (12) | −0.03 | 16 (10) | 14 (9) | 0.04 |
| Microbiologic data | ||||||
| Organism, n (%) | ||||||
| Pseudomonas spp. | 69 (41) | 734 (60) | −0.38 | 68 (45) | 66 (44) | 0.03 |
| Klebsiella spp. | 72 (43) | 415 (34) | 0.18 | 60 (40) | 56 (37) | 0.06 |
| Acinetobacter spp. | 27 (16) | 73 (6) | 0.33 | 22 (15) | 28 (19) | −0.11 |
| Site, n (%) | ||||||
| Respiratory | 85 (51) | 460 (38) | 0.26 | 77 (51) | 76 (51) | 0.01 |
| Urine | 26 (15) | 485 (39) | −0.56 | 25 (17) | 23 (15) | 0.04 |
| Sterile sitef | 47 (28) | 173 (14) | 0.34 | 39 (26) | 41 (27) | −0.03 |
| Unknown | 10 (6) | 104 (9) | −0.09 | 9 (6) | 10 (7) | −0.03 |
| Time from culture to treatment, d, median (IQR) | 2.9 (1.0–4.6) | 1.1 (0.3–3.5) | 0.48 | 2.9 (0.9–4.7) | 2.9 (0.9–5.5) | −0.03 |
| Blood products,g n (%) | 44 (26) | 178 (15) | 0.29 | 37 (25) | 35 (23) | 0.03 |
Difference between groups refers to the standardized difference. IQR, interquartile range; BMI, body mass index; HUP, Hospital of the University of Pennsylvania; PMC, Presbyterian Medical Center; PAH, Pennsylvania Hospital; RIT, Penn Medicine at Rittenhouse; ABX, antibiotics; ICU, intensive care unit; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; NSAID, nonsteroidal anti-inflammatory drug; WBC, white blood cell.
Standardized difference is a measure of covariate balance that is not effected by sample size. Values >0.10 are considered evidence of meaningful imbalance.
Other includes Asian, American Indian or Alaskan Native, Native Hawaiian, or other Pacific Islander.
Previous courses of any duration whether given consecutively or in combination.
Tobramycin or colistin.
Tacrolimus or cyclosporin.
Blood cultures (colistin, n=17; control, n=25), tissue cultures (colistin, n=11; control, n=8), biopsy cultures (colistin, n=5; control, n=4), or ascites fluid cultures (colistin, n=6; control, n=4).
Red cells, platelets, or fresh frozen plasma
Data Analyses
Colistin-exposed patients were matched to controls using propensity scores. Propensity scores are defined as the predicted probabilities of receiving treatment (colistin) conditional on observed covariates (29). Propensity scores were estimated using logistic regression. Colistin exposure was the dependent variable, and all covariates listed in Supplemental Table 2 and Table 1 were independent variables. Colistin-exposed patients were matched 1:1 with controls using a greedy matching algorithm without replacement. The caliper width was set to 0.2 SDs of the logit of the propensity score (30). Covariate balance before and after matching was examined using standardized differences (31), with values >0.1 considered as evidence of meaningful differences (31).
We then fit a generalized estimating equation Poisson regression outcome model (32). We chose this model to account for the correlation among pairs induced by matching (32) and because it accounts for the person-time accrued during exposure. The primary effect measure was the incidence rate ratio (IRR). We also calculated attributable risk (risk difference). Covariates with residual imbalance (standardized difference >0.1) in the matched sample were controlled in the final adjusted outcome model in the matched sample. Time to AKI was depicted with Kaplan–Meier curves.
The following prespecified risk factors (9–14) were tested for effect modification: baseline GFR, vasopressor exposure, aminoglycoside exposure, vancomycin exposure, statin exposure (protective effect), and chloride concentration (33). In addition, we performed a post hoc risk factor analysis in colistin-exposed patients only. Independent risk factors in this step were then tested for effect modification in the entire matched sample. (Details of this analysis are in Supplemental Material.) Interaction terms were adjusted for confounding using a change in estimate approach (34). Variables were retained in the model if they changed the IRR by 10% or more (34). The rationale for adjustment is that propensity score matching ensures balance of covariates in colistin-exposed versus unexposed patients but that it may not guarantee balance within strata of a given interaction (35), which is particularly important when considering interactions with continuous variables. Additional details of the interaction modeling are in Supplemental Material.
We examined the robustness of our findings in a series of sensitivity analyses: (1) excluding courses with duration <72 hours, (2) excluding patients with pre-exposure AKI, (3) extending follow-up to 5 days after the last dose, (4) excluding patients with baseline CKD, (5) revising our primary end point to consider only KDIGO stage 2 or higher AKI, and (6) performing propensity score stratification analysis, where the propensity score is used to subclassify the base cohort into quintiles, estimates are derived within these strata, and stratum-specific estimates are averaged to produce the final effect estimate (36).
Results
Cohort Characteristics
The flow of patients through the study is shown in Figure 1. The unmatched population consisted of 168 colistin-exposed patients and 1222 control patients. The most common control antibiotics were cefepime, levofloxacin, and meropenem in both the unmatched and matched cohorts (Supplemental Table 3). AKI risk was similar among the various control antibiotics (Supplemental Table 4). The median colistin dose was 4.5 mg/kg per day (interquartile range [IQR], 3.4–5.3 mg/kg per day) (details are in Supplemental Table 5).
Figure 1.
Flow of patients through the study. ESRD, ESKD; MDR, multidrug resistant.
Baseline characteristics are shown in Supplemental Table 2 and Table 1. Notable differences in colistin-exposed patients versus controls before matching include a longer hospital stay before initiation, more previous antibiotic exposures, longer time to treatment initiation, higher frequency of intensive care unit admission, and more frequent vasopressor dependence. Propensity score matching resulted in 150 matched pairs. Baseline covariates were well balanced in the matched sample. Of note, the types of bacteria, culture sites, and time to effective treatment were similar in the matched sample. The median treatment duration was 7.0 days in both groups (P=0.79), and the frequency of creatinine monitoring was similar, with a median of 8 (IQR, 4–15) in colistin-exposed patients versus 8 (IQR, 3–13) in controls (P=0.21). Fifteen patients were discharged before day 30 while still receiving treatment (colistin, n=6; control, n=9). There were four variables with standardized differences >0.1 in the matched cohort: sex, hematologic malignancy, Acinetobacter pathogen, and concomitant treatment for vancomycin-resistant enterococcus. These variables were included in the final outcome model. Thirty-day mortality was higher in the colistin group (17 of 150 [11%]) versus the control group (seven of 150 [5%]; risk difference, 6%; 95% confidence interval [95% CI], 0.5 to 12.8). Mortality in the colistin group was lower in patients who received at least 4 mg/kg per day (six of 91 [7%]) versus those who received lower doses (11 of 59 [19%]; risk difference, 12%; 95% CI, 0.9 to 23.2).
Incidence Rates
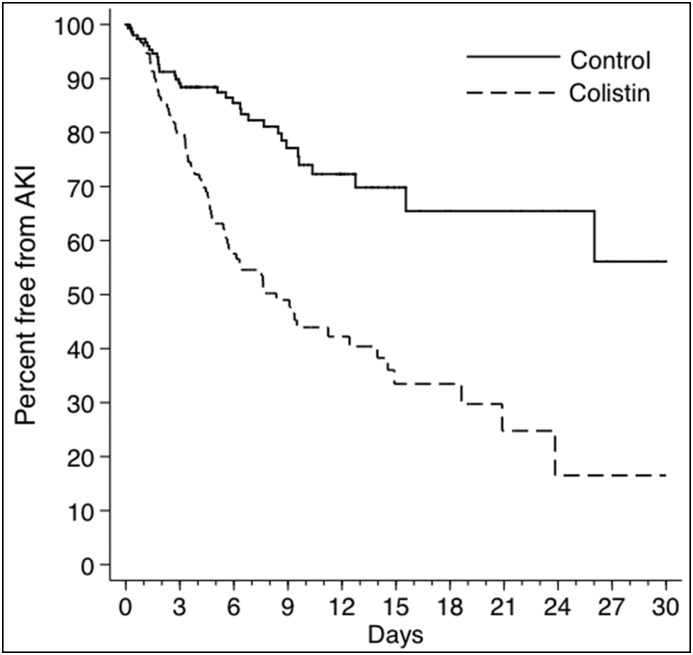
The rates and IRRs of study outcomes are presented in Table 2. The IRR for AKI was substantially increased at the end of follow-up (IRR, 2.9; 95% CI, 1.9 to 4.5), and it was already increased (IRR, 1.9; 95% CI, 1.1 to 3.5) at 72 hours. The rapid onset can be seen in Figure 2, which depicts Kaplan–Meier curves for AKI in colistin-exposed patients versus controls. Similar results were observed after excluding patients with prior AKI episodes (Supplemental Figure 1). Adjusting for the remaining unbalanced variables had negligible effects on the IRR estimates: IRR, 2.9; 95% CI, 1.9 to 4.5 at the end of follow-up and IRR, 1.9; 95% CI, 1.0 to 3.6 at 72 hours.
Table 2.
Outcomes
| Outcome | Colistin, n (Rate per 100 d) | Control, n (Rate per 100 d) | IRR (95% CI) |
|---|---|---|---|
| AKI | |||
| End of follow-up | 77 (7.6) | 33 (2.6) | 2.9 (1.9 to 4.5) |
| 72 h | 30 (7.4) | 16 (3.8) | 1.9 (1.1 to 3.5) |
| Dialysis at end of follow-up | 4 (0.28) | 3 (0.20) | 1.4 (0.3 to 6.0) |
| AKI or death at end of follow-upa | 82 (8.0) | 39 (3.1) | 2.6 (1.8 to 3.9) |
IRR, incidence rate ratio; 95% CI, 95% confidence interval.
Seventeen patients died in the colistin group and seven patients died in the control group at the end of follow-up. Death was preceded by AKI in 12 and two patients in the colistin and control groups, respectively.
Figure 2.
Time to AKI in Colistin group versus control group.
Risk Difference, AKI Severity, and Sensitivity Analyses
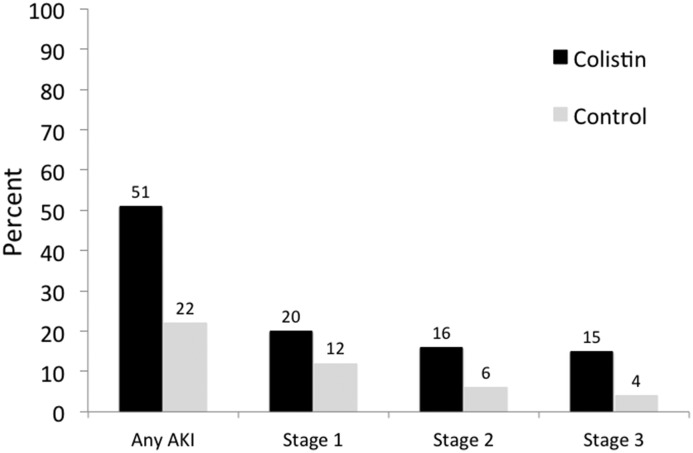
The cumulative incidence of AKI at the end of follow-up was 77 of 150 (51%) in the colistin group versus 33 of 150 (22%) in matched controls (risk difference, 29%; 95% CI, 19 to 39). A higher risk difference was also apparent at 72 hours: colistin: 30 of 150 (20%) versus control: 16 of 150 (11%; risk difference, 9%; 95% CI, 1 to 17). AKI severity in the matched cohort is depicted in Figure 3. Although the incidence of AKI was substantially increased during colistin exposure across all severity stages, the rate of dialysis was low and did not differ between groups. The results of sensitivity analyses are presented in Table 3. Results were consistent across all analyses (Supplemental Tables 6–9 have additional details).
Figure 3.
Cumulative Incidence and severity of AKI in Colistin group versus control group. AKI severity was staged according to the Kidney Disease Improving Global Outcomes creatinine criteria.
Table 3.
Sensitivity analyses for the association of colistin with AKI at the end of follow-up
| Sensitivity Analysis | IRR (95% CI) |
|---|---|
| Exclude course <72 h in durationa | 3.2 (1.9 to 5.4) |
| 5-d Postexposure follow-upb | 2.9 (1.9 to 4.4) |
| Exclude patients with pre-exposure AKIc | 5.7 (3.1 to 10.4) |
| Propensity score stratificationd | 2.9 (2.3 to 3.9) |
| Exclude patients with CKDe | 3.3 (1.9 to 6.0) |
| KDIGO stage 2 or higher AKIf | 3.7 (2.1 to 6.7) |
IRR, incidence rate ratio; 95% CI, 95% confidence interval; KDIGO, Kidney Disease Improving Global Outcomes.
Analysis included 117 matched pairs.
Analysis included 150 matched pairs.
Analysis included 101 matched pairs.
Stratified across propensity score quintile.
Analysis included 100 matched pairs.
AKI definition revised to include only KDIGO stage 2 or higher AKI events.
Risk Factors, Effect Modification, and Recovery
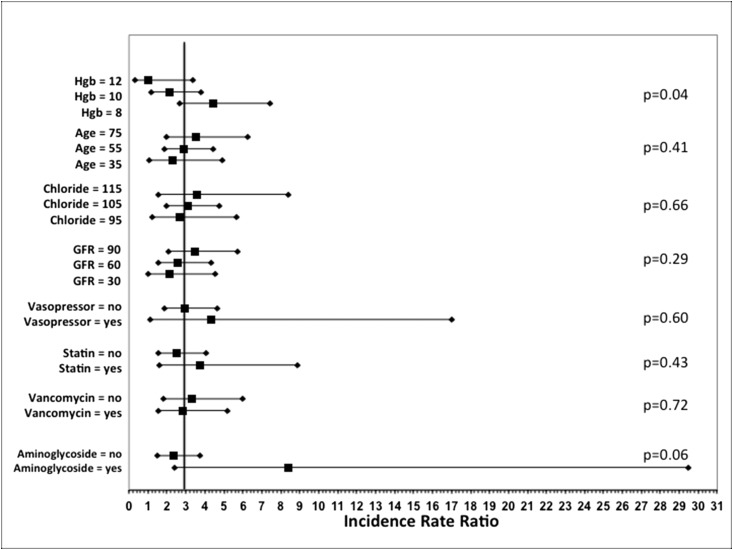
Risk factors for AKI in colistin-exposed patients are shown in Table 4. Independent risk factors include age, baseline hemoglobin concentration, liver disease, and baseline GFR. AKI incidence across colistin dosing strata is depicted in Supplemental Figure 2, which shows increasing risk with higher doses. However, the association between dose and AKI was not significant in the multivariable model. The estimates for effect modification are shown in Figure 4. Significant effect modification was found for baseline hemoglobin concentration (P=0.04). Of note, liver disease could not be tested because of zero events in one of the interaction strata.
Table 4.
Risk factors for AKI in colistin-exposed patients
| Variable | IRR (95% CI) | P Value |
|---|---|---|
| Age,a yr | 1.2 (1.1 to 1.3) | <0.001 |
| Liver disease | 2.6 (1.4 to 4.8) | 0.01 |
| Hemoglobin, g/dl | 0.8 (0.7 to 0.9) | 0.01 |
| eGFR,b ml/min per 1.73 m2 | 1.2 (1.1 to 1.3) | 0.01 |
| NSAID exposure | 1.9 (0.9 to 4.2) | 0.09 |
| Race | 0.08 | |
| White | Reference | |
| Black | 0.7 (0.4 to 1.2) | |
| Other | 0.5 (0.2 to 1.0) |
Poisson regression statistical analysis methods are detailed in Supplemental Material. IRR, incidence rate ratio; 95% CI, 95% confidence interval; NSAID, nonsteroidal anti-inflammatory drug.
Per 5-year increase in age.
Per 20-ml/min per 1.73 m2 increase in eGFR.
Figure 4.
Analysis of factors that may modify the risk of AKI in the Colistin group versus control group. The reference line depicts the incidence rate ratio from the primary analysis. Interactions were adjusted for confounding in a multivariable Poisson regression model. Hemoglobin (Hgb; in units of grams per deciliter), age (years), chloride (in units of milliequivalents per liter), and GFR (in units of milliliters per minute per 1.73 m2 body surface area) were modeled as continuous variables. Effect estimates were derived by solving the regression equation for clinically relevant values. For example, the regression equation from the Hgb model was solved to identify the corresponding incidence rate ratio for colistin versus control at Hgb values of 8, 10, and 12 g/dl. Additional details of the interaction analyses can be found in Supplemental Material.
Resolution of AKI in colistin-exposed patients versus control patients is described in Supplemental Table 10. Recovery occurred in 58% of AKI cases in patients in the control group versus 44% of AKI cases in patients in the colistin group (risk difference, 13.5%; 95% CI, −33.6 to 6.7). In both groups, hospital mortality in patients who experienced AKI was lower if kidney function returned to baseline during hospitalization.
Discussion
Antimicrobial treatment decisions are complex. Clinicians must consider the patient’s risk of drug resistance, local susceptibility patterns, and the risk for adverse events among the available treatment options. Although colistin has been available for decades, there has remained substantial confusion regarding its true propensity to cause nephrotoxicity. Against this backdrop, we conducted a propensity score–matched analysis of colistin versus control antibiotics. Our study is the first to clearly define the attributable risk and timing of onset of colistin-associated AKI. The attributable risk of AKI through the end of follow-up was nearly 30%, corresponding to a number needed to harm of 3.5. More importantly, the onset of toxicity seems to be rapid. Kaplan–Meier estimates began to differ within the first 2 days of follow-up, and the IRR for AKI was significantly increased at 72 hours.
The incidence of AKI in the colistin courses was 51%, consistent with several recent studies showing the incidence to be 40%–53% (9–14). Notably, those studies used similar AKI definitions and dosing strategies (9–14). Results that suggest low toxicity risk were from other studies with more restrictive AKI definitions and less aggressive dosing regimens (15,16,37). Several studies have shown a strong relationship between daily dose and AKI risk (9–12), consistent with our findings. This is particularly relevant given that even higher doses may be required to ensure pharmacodynamic target attainment (20), which is consistent with our data that show an association between dose and mortality. Consequently, the low rates of AKI in studies using reduced doses may not be clinically relevant. The majority of AKI events in the colistin group were stage 1 or stage 2, and <3% of patients required dialysis. Moreover, we observed resolution of AKI during admission in 44% and 58% of patients in the colistin and control groups, respectively. The colistin resolution rate is similar to that in previous studies (9,38). Importantly, our data may be underestimating the true resolution rate given that many patients may have had kidney function return to baseline after hospital discharge.
Although previous uncontrolled studies have suggested delayed onset of toxicity (ranging from 5 to 12 days), the estimates may have been biased by the lack of a control group (11–14). Our data indicate that colistin exposure results in an appreciable excess of AKI events within the first few days of treatment. Rapid-onset toxicity is consistent with colistin’s high intrinsic toxicity and extensive accumulation in the cortical tissue of the kidney (17,18). This rapid onset has important clinical implications. Inadequate empirical treatment has been consistently linked to worse outcome in patients with MDR and XDR gram-negative infection (3–5). Although empirical colistin could minimize the delay to effective treatment (6–8), our findings suggest that such empirical use may be encumbered by considerable early nephrotoxicity. This unfavorable tradeoff highlights the importance of risk stratification. In regions where the prevalence of multidrug resistance is high, clinicians must be familiar with their local antibiograms, risk factors for resistant pathogens, and should consider routine consultation with infectious diseases specialists when making early treatment decisions in high-risk patients. The risk-to-benefit ratio of empirical colistin could also be improved by rapid diagnostic methods, which might facilitate identification of patients who require early colistin therapy (39). Given that most AKI events were mild to moderate severity and that they were frequently reversible, more aggressive use of colistin in the empirical setting may be prudent in high-risk patients to avoid the potentially devastating consequences of delayed treatment.
We observed higher mortality in patients treated with colistin. There are multiple potential explanations for this finding, which should be interpreted in the context that mortality was not our primary outcome. Although colistin is bactericidal, colistin resistance can develop rapidly, and the optimal dosing regimen remains unclear (20). It is also possible that infections in the colistin group were more difficult to treat, because the drug is often reserved to treat the most resistant pathogens (9–16). In addition, a portion of the higher mortality may be mediated through the increased AKI rate in the colistin group, which may be further modified by the degree of AKI reversibility (24). Mortality was lower in both groups for patients who experience resolution of AKI.
Previous studies of colistin-AKI risk factors were conducted in exposed-only cohorts, making it impossible to determine whether a factor predisposes to colistin toxicity versus whether a factor was a risk factor for AKI in general. We addressed this limitation by testing for effect modification. AKI risk factors in the colistin group included hemoglobin, age, liver disease, and baseline GFR. We hypothesize that the positive association between GFR and AKI rate may be driven by the fact that patients with higher GFR received larger doses (i.e., dose and GFR are collinear), and greater drug filtration at the glomerulus increases colistin exposure in the proximal tubule. Of the risk factors tested for interaction, we only observed significant effect modification with hemoglobin concentration. Anemia has been linked to AKI risk after cardiac surgery (40,41), which may be the result of reduced oxygen delivery to the kidney (42). In addition, a recent study found low hematocrit to be a risk factor for AKI during colistin treatment (11). Animal models have shown that ischemia increases the rate of gentamicin (another nephrotoxin) uptake into proximal tubular cells while also magnifying the degree of toxicity produced by any given level of proximal tubular cell accumulation (43). Taken together, the data support the hypothesis that colistin’s toxicity threshold may be reduced in patients with decreased oxygen delivery to the kidney.
Our study has limitations. The retrospective cohort design is susceptible to residual confounding. We minimized confounding by collecting and controlling for all available potential confounding variables with propensity score matching. In addition, we were unable to review patient charts to apply infection definitions or severity of illness measures. Importantly, we included a number of variables related to infection diagnosis, including white blood cell count, culture site, and infecting organism. In addition, we included other measures of illness severity, including admission to the intensive care unit and the need for mechanical ventilation. We also included baseline hemoglobin and platelet count, which are closely correlated with severity of illness and have been shown to predict mortality and AKI risk in a variety of populations (40,41,44–46). Nevertheless, as with any nonrandomized design, the possibility of residual confounding from acute illness severity cannot be eliminated. In addition, restricting our analysis to patients with culture-confirmed infection may limit the generalizability of our findings. However, the characteristics of our population are similar to those of populations in several other colistin AKI studies (9–16). Additional research is needed to understand whether the effects of colistin vary in other treatment scenarios (e.g., early empirical treatment with negative cultures). Lastly, we used diagnosis codes to define comorbid illness, which may misclassify some patients. We minimized this potential bias by using previously validated coding algorithms (26,27).
Colistin is associated with substantial excess AKI that is apparent within the first 72 hours of treatment. Colistin’s toxicity varied according to baseline hemoglobin concentration, a factor that may alter kidney tissue oxygenation.
Disclosures
None.
Supplementary Material
Acknowledgments
We would like to thank the Penn Data Store for their assistance in assembling the information used in this study.
This work was supported by National Institutes of Health grants 5F32HL124914 (to T.A.M.), T32GM075766 (to T.A.M. and S.H.), and 1K23DK097201 (to F.P.W.).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.06980717/-/DCSupplemental.
References
- 1.Centers for Disease Control and Prevention : Antibiotic Resistance Threats in the United States, 2013, Atlanta, GA, Centers for Disease Control and Prevention, 2013 [Google Scholar]
- 2.Boucher HW, Talbot GH, Benjamin DK Jr, Bradley J, Guidos RJ, Jones RN, Murray BE, Bonomo RA, Gilbert D; Infectious Diseases Society of America : 10 x ’20 Progress--development of new drugs active against gram-negative bacilli: An update from the Infectious Diseases Society of America. Clin Infect Dis 56: 1685–1694, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee H-Y, Chen C-L, Wu SR, Huang CW, Chiu CH: Risk factors and outcome analysis of acinetobacter baumannii complex bacteremia in critical patients. Crit Care Med 42: 1081–1088, 2014 [DOI] [PubMed] [Google Scholar]
- 4.Micek ST, Lloyd AE, Ritchie DJ, Reichley RM, Fraser VJ, Kollef MH: Pseudomonas aeruginosa bloodstream infection: Importance of appropriate initial antimicrobial treatment. Antimicrob Agents Chemother 49: 1306–1311, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tumbarello M, Viale P, Viscoli C, Trecarichi EM, Tumietto F, Marchese A, Spanu T, Ambretti S, Ginocchio F, Cristini F, Losito AR, Tedeschi S, Cauda R, Bassetti M: Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: Importance of combination therapy. Clin Infect Dis 55: 943–950, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Falagas ME, Rafailidis PI: When to include polymyxins in the empirical antibiotic regimen in critically ill patients with fever? A decision analysis approach. Shock 27: 605–609, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Tuon FF, Rymsza AM, Penteado-Filho SR, Pilonetto M, Arend LN, Levin AS: Should polymyxin be used empirically to treat infections in patients under high risk for carbapenem-resistant Acinetobacter? J Infect 62: 246–249, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Spellberg B, Bonomo RA: The deadly impact of extreme drug resistance in Acinetobacter baumannii. Crit Care Med 42: 1289–1291, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pogue JM, Lee J, Marchaim D, Yee V, Zhao JJ, Chopra T, Lephart P, Kaye KS: Incidence of and risk factors for colistin-associated nephrotoxicity in a large academic health system. Clin Infect Dis 53: 879–884, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Hartzell JD, Neff R, Ake J, Howard R, Olson S, Paolino K, Vishnepolsky M, Weintrob A, Wortmann G: Nephrotoxicity associated with intravenous colistin (colistimethate sodium) treatment at a tertiary care medical center. Clin Infect Dis 48: 1724–1728, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Lee YJ, Wi YM, Kwon YJ, Kim SR, Chang SH, Cho S: Association between colistin dose and development of nephrotoxicity. Crit Care Med 43: 1187–1193, 2015 [DOI] [PubMed] [Google Scholar]
- 12.Rattanaumpawan P, Ungprasert P, Thamlikitkul V: Risk factors for colistin-associated nephrotoxicity. J Infect 62: 187–190, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Gauthier TP, Wolowich WR, Reddy A, Cano E, Abbo L, Smith LB: Incidence and predictors of nephrotoxicity associated with intravenous colistin in overweight and obese patients. Antimicrob Agents Chemother 56: 2392–2396, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balkan II, Dogan M, Durdu B, Batirel A, Hakyemez IN, Cetin B, Karabay O, Gonen I, Ozkan AS, Uzun S, Demirkol ME, Akbas S, Kacmaz AB, Aras S, Mert A, Tabak F: Colistin nephrotoxicity increases with age. Scand J Infect Dis 46: 678–685, 2014 [DOI] [PubMed] [Google Scholar]
- 15.Gu WJ, Wang F, Tang L, Bakker J, Liu JC: Colistin for the treatment of ventilator-associated pneumonia caused by multidrug-resistant Gram-negative bacteria: A systematic review and meta-analysis. Int J Antimicrob Agents 44: 477–485, 2014 [DOI] [PubMed] [Google Scholar]
- 16.Liu Q, Li W, Feng Y, Tao C: Efficacy and safety of polymyxins for the treatment of Acinectobacter baumannii infection: A systematic review and meta-analysis. PLoS One 9: e98091, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma Z, Wang J, Nation RL, Li J, Turnidge JD, Coulthard K, Milne RW: Renal disposition of colistin in the isolated perfused rat kidney. Antimicrob Agents Chemother 53: 2857–2864, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phe K, Lee Y, McDaneld PM, Prasad N, Yin T, Figueroa DA, Musick WL, Cottreau JM, Hu M, Tam VH: In vitro assessment and multicenter cohort study of comparative nephrotoxicity rates associated with colistimethate versus polymyxin B therapy. Antimicrob Agents Chemother 58: 2740–2746, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL: Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18: 268–281, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Garonzik SM, Li J, Thamlikitkul V, Paterson DL, Shoham S, Jacob J, Silveira FP, Forrest A, Nation RL: Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob Agents Chemother 55: 3284–3294, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.X-Gen Pharmaceuticals, Inc. : Colistimethate (Colistin) Package Insert, Big Flats, NY, X-Gen Pharmaceuticals, Inc., 2010 [Google Scholar]
- 22.KDIGO Clinical Practice Guidelines for Acute Kidney Injury 2012. Available at: http://www.kdigo.org/clinical_practice_guidelines/AKI.php. Accessed May 13, 2017 [DOI] [PubMed]
- 23.Berry SD, Ngo L, Samelson EJ, Kiel DP: Competing risk of death: An important consideration in studies of older adults. J Am Geriatr Soc 58: 783–787, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pannu N, James M, Hemmelgarn B, Klarenbach S; Alberta Kidney Disease Network : Association between AKI, recovery of renal function, and long-term outcomes after hospital discharge. Clin J Am Soc Nephrol 8: 194–202, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Penn Data Store: Penn Data Store Overview. Available at: http://www.med.upenn.edu/dac/penn-data-store-warehouse.html. Accessed November 8, 2015
- 26.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA: Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 43: 1130–1139, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Elixhauser A, Steiner C, Harris DR, Coffey RM: Comorbidity measures for use with administrative data. Med Care 36: 8–27, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenbaum PR, Rubin DB: The central role of the propensity score in observational studies for causal effects. Biometrika 70: 41–55, 1983 [Google Scholar]
- 30.Austin PC: Some methods of propensity-score matching had superior performance to others: Results of an empirical investigation and Monte Carlo simulations. Biom J 51: 171–184, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Austin PC: Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput 38: 1228–1234, 2009 [Google Scholar]
- 32.Fitzmaurice GM, Laird NM, Ware JH: Applied Longitudinal Analysis, 2nd Ed., New York, Wiley, 2011 [Google Scholar]
- 33.Lobo DN, Awad S: Should chloride-rich crystalloids remain the mainstay of fluid resuscitation to prevent ‘pre-renal’ acute kidney injury?: Con. Kidney Int 86: 1096–1105, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maldonado G, Greenland S: Simulation study of confounder-selection strategies. Am J Epidemiol 138: 923–936, 1993 [DOI] [PubMed] [Google Scholar]
- 35.Rassen JA, Glynn RJ, Rothman KJ, Setoguchi S, Schneeweiss S: Applying propensity scores estimated in a full cohort to adjust for confounding in subgroup analyses. Pharmacoepidemiol Drug Saf 21: 697–709, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosenbaum PR, Rubin DB: Reducing bias in observational studies using subclassification on the propensity score. J Am Stat Assoc 79: 516–524, 1984 [Google Scholar]
- 37.Falagas ME, Kasiakou SK: Toxicity of polymyxins: A systematic review of the evidence from old and recent studies. Crit Care 10: R27, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kwon JA, Lee JE, Huh W, Peck KR, Kim YG, Kim DJ, Oh HY: Predictors of acute kidney injury associated with intravenous colistin treatment. Int J Antimicrob Agents 35: 473–477, 2010 [DOI] [PubMed] [Google Scholar]
- 39.Perez KK, Olsen RJ, Musick WL, Cernoch PL, Davis JR, Peterson LE, Musser JM: Integrating rapid diagnostics and antimicrobial stewardship improves outcomes in patients with antibiotic-resistant Gram-negative bacteremia. J Infect 69: 216–225, 2014 [DOI] [PubMed] [Google Scholar]
- 40.Habib RH, Zacharias A, Schwann TA, Riordan CJ, Engoren M, Durham SJ, Shah A: Role of hemodilutional anemia and transfusion during cardiopulmonary bypass in renal injury after coronary revascularization: Implications on operative outcome. Crit Care Med 33: 1749–1756, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Karkouti K, Grocott HP, Hall R, Jessen ME, Kruger C, Lerner AB, MacAdams C, Mazer CD, de Medicis É, Myles P, Ralley F, Rheault MR, Rochon A, Slaughter MS, Sternlicht A, Syed S, Waters T: Interrelationship of preoperative anemia, intraoperative anemia, and red blood cell transfusion as potentially modifiable risk factors for acute kidney injury in cardiac surgery: A historical multicentre cohort study. Can J Anaesth 62: 377–384, 2015 [DOI] [PubMed] [Google Scholar]
- 42.Johannes T, Mik EG, Nohé B, Unertl KE, Ince C: Acute decrease in renal microvascular PO2 during acute normovolemic hemodilution. Am J Physiol Renal Physiol 292: F796–F803, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Zager RA: Gentamicin effects on renal ischemia/reperfusion injury. Circ Res 70: 20–28, 1992 [DOI] [PubMed] [Google Scholar]
- 44.Cooke CR, Shah CV, Gallop R, Bellamy S, Ancukiewicz M, Eisner MD, Lanken PN, Localio AR, Christie JD; National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome Network : A simple clinical predictive index for objective estimates of mortality in acute lung injury. Crit Care Med 37: 1913–1920, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kertai MD, Zhou S, Karhausen JA, Cooter M, Jooste E, Li YJ, White WD, Aronson S, Podgoreanu MV, Gaca J, Welsby IJ, Levy JH, Stafford-Smith M, Mathew JP, Fontes ML: Platelet counts, acute kidney injury, and mortality after coronary artery bypass grafting surgery. Anesthesiology 124: 339–352, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Couto-Alves A, Wright VJ, Perumal K, Binder A, Carrol ED, Emonts M, de Groot R, Hazelzet J, Kuijpers T, Nadel S, Zenz W, Ramnarayan P, Levin M, Coin L, Inwald DP: A new scoring system derived from base excess and platelet count at presentation predicts mortality in paediatric meningococcal sepsis. Crit Care 17: R68, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.