Abstract
Background and objectives
There is a paucity of data available to describe drug dialyzability. Of the available information, most was obtained before implementation of modern hemodialysis membranes. Our study characterized dialyzability of the most commonly prescribed β-blockers in patients undergoing high-flux hemodialysis.
Design, setting, participants, & measurements
Patients on hemodialysis (n=8) were recruited to an open label, pharmacokinetic, four-way crossover trial. Single doses of atenolol, metoprolol, bisoprolol, and carvedilol were administered on separate days in random order to each patient. Plasma and dialysate drug concentrations were measured, and dialyzability was determined by the recovery clearance and arterial venous difference methods.
Results
Using the recovery clearance method, the dialytic clearance values for atenolol, metoprolol, bisoprolol, and carvedilol were 72, 87, 44, and 0.2 ml/min, respectively (P<0.001). Applying the arterial venous difference method, the dialytic clearance values of atenolol, metoprolol, bisoprolol, and carvedilol were 167, 114, 96, and 24 ml/min, respectively (P<0.001).
Conclusions
Atenolol and metoprolol are extensively cleared by hemodialysis compared with the negligible dialytic clearance of carvedilol. Contrary to estimates of dialyzability on the basis of previous literature, our data indicate that bisoprolol is also dialyzable. This finding highlights the importance of conducting dialyzability studies to definitively characterize drug dialytic clearance.
Keywords: beta blocker, hemodialysis, dialyzability, pharmacokinetics, dialytic clearance, Humans, carvedilol, Bisoprolol, Metoprolol, Atenolol, Cross-Over Studies, renal dialysis, Propanolamines, Carbazoles, Adrenergic beta-Antagonists, Dialysis Solutions
Introduction
The importance of cardiovascular disease among patients with CKD cannot be overstated. It accounts for nearly 45% of deaths among patients on hemodialysis, an incidence 10–20 times greater than in the general population (1–3). Over the past four decades, β-adrenergic receptor antagonists (β-blockers) have emerged as a fundamental component of treating cardiovascular disease. β-Blockers decrease BP, heart rate, myocardial oxygen demand, arrhythmia, and oxidative stress, and they improve left ventricular function (4). More importantly, their ability to reduce mortality has been shown in numerous randomized clinical trials (5–8). Although β-blockers are widely prescribed in patients on hemodialysis, this is on the basis of their proven efficacy in patients with normal kidney function. Extrapolating findings from the general population to patients with CKD has a number of caveats. The pharmacokinetics of many drugs are altered in CKD, including both kidney- and nonkidney-mediated elimination (9–11). Expectations that β-blockers will deliver similar therapeutic efficacy in patients on hemodialysis compared with the general population are based on very little evidence.
For drugs to be approved for use in patients, detailed pharmacokinetic analysis must be undertaken to characterize parameters, such as bioavailability, metabolic elimination, and kidney-mediated drug clearance. The majority of drugs are not tested in patients on dialysis during the drug development process, which results in a lack of appropriate dosage recommendations (12–15). Drug disposition is an especially important consideration in patients on hemodialysis because there is minimal to no residual kidney function for drug excretion. Despite requirements to characterize kidney-mediated elimination during drug development, there are very minimal data on drug clearance in patients on hemodialysis. Specifically, drug dialyzability—the efficiency of drug removal by dialysis—is likely to vary among β-blockers, which should be considered when they are prescribed to patients. The use of drugs that are highly dialyzed can result in subtherapeutic plasma concentrations during and after hemodialysis and increase the risk for adverse clinical outcomes (16). Currently, definitive data on drug dialyzability are only available for 10% of medications.
The dialyzability of β-blockers is presumed largely on the basis of physicochemical characteristics and not experimental evidence (17). Of the available information, most data were obtained before implementation of modern high-flux dialysis membranes, rendering many of the older studies irrelevant (18). Therefore, this study was conducted to define the modern dialyzability of the most commonly used β-blockers. We hypothesized, on the basis of physicochemical characteristics and previous literature (Table 1), that atenolol and metoprolol would be highly dialyzed, whereas bisoprolol and carvedilol would be minimally removed during hemodialysis (19–22).
Table 1.
Physicochemical properties and dialyzability statements for study β-blockers
| β-Blocker | Physicochemical Properties | Industry Statements | Review Articles | Expected Dialyzability | ||||
|---|---|---|---|---|---|---|---|---|
| Product Monographs | Dialysis of Drugs 2013a | Levin et al. (21) | Chazot and Jean (19) | Chen et al. (20) | Redon et al. (22) | |||
| Atenolol | Molecular mass: 266 D | Moderately dialyzable (20%–50%) | Conventional HD: yes; modern HD: likely | D | D | D | D | High dialyzability |
| Water solubility: 13,500 mg/L | ||||||||
| Protein binding: 10% | ||||||||
| VD: 4.2 L/kg | ||||||||
| Metoprolol | Molecular mass: 267 D | No statement | Conventional HD: yes; modern HD: likely | D | D | D | ND | High dialyzability |
| Water solubility: 16,900 mg/L | ||||||||
| Protein binding: 10% | ||||||||
| VD: 3.2 L/kg | ||||||||
| Bisoprolol | Molecular mass: 325 D | Not dialyzable | Conventional HD: yes; modern HD: no data | ND | ND | ND | ND | Low dialyzability |
| Water solubility: 2,240 mg/L | ||||||||
| Protein binding: 30% | ||||||||
| VD: 3.0 L/kg | ||||||||
| Carvedilol | Molecular mass: 406 D | Not dialyzable | Conventional HD: no; modern HD: unlikely | ND | ND | ND | ND | Low dialyzability |
| Water solubility: 0.583 mg/L | ||||||||
| Protein binding: >98% | ||||||||
| VD: 1.6 L/kg | ||||||||
Yes indicates that dialysis was found to enhance drug clearance from previously published studies. No indicates that dialysis was not found to enhance drug clearance from previously published studies. No data indicate that no data or assumptions from physicochemical properties exist to describe drug dialyzability. Likely drug is likely to be cleared by HD on the basis of physicochemical parameters, but no data exist. Unlikely drug is unlikely to be cleared by HD on the basis of physicochemical parameters, but no data exist. VD, volume of distribution; HD, hemodialysis; D, drug is listed as dialyzable in corresponding review article; ND, drug is listed as not dialyzable in corresponding review article.
Annual guidelines published by Renal Pharmacy Consultants, LLC (Saline, MI). Dialyzability is on the basis of scientific and industry data.
Materials and Methods
Study Design and Participant Eligibility
We conducted an open label, four-way crossover trial of the four most commonly prescribed β-blockers in Ontario, Canada—atenolol (50 mg), metoprolol (50 mg) bisoprolol (5 mg), and carvedilol (6.25 mg)—among eight patients receiving maintenance hemodialysis at the London Health Sciences Centre hemodialysis units. The sample size was determined by a power calculation using preliminary data and assuming an α of 0.05 and power of 0.8. Patients were at least 18 years of age and receiving thrice weekly hemodialysis for at least 90 days before the study. We excluded patients with significant gastrointestinal/liver disease, patients with body mass index >40 kg/m2, and those who could not safely receive a study β-blocker (treatment with contraindicated medications, prior adverse drug reactions to β-blockers, severe reactive airway disease, or hemodynamic instability during dialysis). Patients who met the eligibility criteria provided informed written consent in accordance with the Declaration of Helsinki. This study was approved by the Health Sciences Research Ethics Board at Western University (104909; registered at clinicaltrials.gov: NCT03361280).
Clinical Data Collection, Interventions, and Follow-Up
We randomly administered a single oral dose of one of the study β-blockers 4 hours before hemodialysis initiation. To achieve randomization, each β-blocker was assigned a number between one and four, and a random number generator was used to determine treatment order. A research assistant generated the randomization sequence, and the patient’s nephrologist or nurse provided the β-blocker on the study day. Because this was an open label study, there was no concealment of treatment. This process was repeated with each of the three remaining β-blockers during separate dialysis sessions, which were a minimum of 48 hours apart. During each hemodialysis treatment, blood samples were collected from the arterial and venous ports at six time points. During 4-hour dialysis treatments, we collected blood at 0, 0.5, 1, 2, 3, and 4 hours. During 3.5-hour dialysis treatments, we collected blood at hours 0, 0.5, 1, 1.5, 2.5, and 3.5. For 3-hour dialysis treatments, we collected blood at hours 0, 0.5, 1.5, 2, 2.5, and 3. Arterial blood samples were used to determine hematocrit. For all treatments, we also collected the total spent dialysate into a 200-L food-grade plastic barrel. We collected demographic information, health status, and dialysis prescription details at the time of study enrolment. The study period was from February 2015 to March 2016.
β-Blocker Extraction Analyses with Mass Spectrometry
β-Blockers were extracted from plasma and dialysate samples using solid-phase extraction cartridges (Phenomenex, Torrance, CA) conditioned according to the manufacturer’s instructions. β-Blocker concentrations in plasma (free plus protein bound) and dialysate were determined by ultraperformance liquid chromatography coupled to quadrupole time-of-flight mass spectrometry (Waters, Milford, MA). Methodologic details can be found in Supplemental Material.
Determining Dialytic Clearance
The two main methods to evaluate the clearance of medications during hemodialysis are (1) the arterial venous (A-V) difference method and (2) the recovery clearance method, and they are described by the following equations (18,23–25):
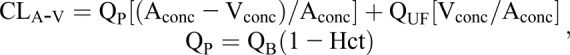 |
(1) |
where CLA-V is A-V difference clearance, QP is mean plasma flow rate, QB is mean blood flow rate, QUF is mean ultrafiltration rate, Hct is hematocrit, Aconc is arterial plasma drug concentration, and Vconc is venous plasma drug concentration. Also,
| (2) |
where CLR is dialyzer clearance, Rdrug is total amount of drug recovered in dialysate, and AUC0-T is the area under the plasma concentration-time curve during dialysis. The AUC0-T was calculated by the trapezoidal method using GraphPad Prism (version 6.01 for Windows; GraphPad Software, San Diego, CA).
Statistical Analyses
We performed all statistical analyses using GraphPad Prism (version 6.01 for Windows; GraphPad Software). We conducted hypothesis testing of atenolol, metoprolol, bisoprolol, and carvedilol clearance using one-way ANOVA. After rejection of the null hypothesis, multiple comparisons were evaluated by Tukey post hoc test. Results are presented as mean±SD. We considered a P value <0.05 statistically significant.
Results
Assay Validation and Performance
The recovery percentages of atenolol, metoprolol, bisoprolol, and carvedilol using solid-phase extraction were 101%, 112%, 93%, and 112%, respectively. The intraday accuracy and precision were assessed using five analytical replicates of the lowest concentration on the dialysate calibration curve. Accuracy, expressed as a bias percentage, was determined by comparing the mean measured concentration with the nominal concentration. Precision was determined by calculating the coefficient of variation percentage of the five replicates. Using a signal-to-noise ratio of at least 10:1, the lower limit of quantification of the calibration curve displayed acceptable accuracy (<10%) and precision (<10%) for all β-blockers, except for the precision of carvedilol, which was slightly above this limit. Specifically, the biases and coefficients of variation were 4.2% and 1.1% for atenolol, 1.9% and 1.5% for metoprolol, 2.0% and 2.7% for bisoprolol, and −6.0% and 12.5% for carvedilol, respectively.
Clinical Characteristics of Subjects
All eight subjects completed the pharmacokinetic four-way crossover trial, and baseline clinical information is presented in Table 2. The mean age was 58 years old (ranging from 28 to 80 years old), mean height was 1.7 m, and mean weight was 95 kg. The mean body mass index was 32.6 kg/m2. There were four different dialyzers used in this study: FX600, FX800, FX1000, and Revaclear Max 400. The higher-value FX hemodiafilters have minor increases in their Kf and clearance of metabolites (e.g., urea and creatinine) (26). The Revaclear Max 400 dialyzer has a similar Kf but an elevated clearance of metabolites compared with the FX1000 (27).
Table 2.
Clinical characteristics of participants in a crossover trial assessing the dialyzability of four β-blockers
| Subject Identification | BB002 | BB003 | BB004 | BB005 | BB006 | BB007 | BB008 | BB009 |
|---|---|---|---|---|---|---|---|---|
| Sex | Man | Man | Man | Woman | Man | Man | Man | Man |
| Ethnicity | White | White | White | White | Aboriginal | Black Canadian | White | White |
| Age, yr | 42 | 73 | 66 | 28 | 47 | 72 | 80 | 53 |
| Weight, kg | 94 | 129 | 54 | 82 | 118 | 90 | 90 | 106 |
| Height, m | 1.7 | 1.9 | 1.7 | 1.5 | 1.7 | 1.6 | 1.7 | 1.8 |
| Body mass index, kg/m2 | 32.2 | 35.6 | 19.2 | 37.7 | 38.7 | 33.8 | 30.3 | 33.6 |
| Residual kidney output, ml/d | 300 | 425 | 260 | Anuric | Anuric | 500 | 300 | 300 |
| Cause of CKD | RPGN | DM and HTN | PCKD | Renux nephropath | DM and HTN | HTN | DM and HTN | DM |
| Dialysis duration, h | 4 | 4 | 3 | 3.5 | 4 | 4 | 3 | 4 |
| Dialysis frequency, sessions per week | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Dialyzer type | FX800 | FX1000 | FX600 | FX600 | FX1000 | FX800 | FX600 | Revaclear Max 400 |
| Mean blood flow rate, ml/mina | 382 | 323 | 354 | 397 | 287 | 385 | 325 | 313 |
| Dialysis adequacy, Kt/Va | 1.5±0.04 | 1.1±0.12 | 1.4±0.11 | 1.6±0.15 | 1.2±0.02 | 1.6±0.03 | 1.0±0.04 | 1.1±0.03 |
| Vascular access | Central catheter | Central catheter | Fistula | Fistula | Central catheter | Central catheter | Fistula | Fistula |
| Hematocrit, predialysisa | 0.27±0.01 | 0.30±0.01 | 0.33±0.01 | 0.29±0.01 | 0.27±0.01 | 0.28±0.01 | 0.29±0.02 | 0.35±0.01 |
| Hematocrit, postdialysisa | 0.24±0.01 | 0.34±0.02 | 0.36±0.01 | 0.33±0.01 | 0.28±0.01 | 0.30±0.01 | 0.32±0.03 | 0.37±0.01 |
RPGN, rapidly progressive GN; DM, diabetes mellitus; HTN, hypertension; PCKD, polycystic kidney disease.
Mean over the four dialysis sessions ±SD.
Safety
β-Blocker treatments were well tolerated by all study participants, and no serious adverse drug reactions occurred. No abnormalities in heart rate and BP were observed during the study.
Dialyzability of β-Blockers in Patients on Maintenance Hemodialysis
Recovery Method.
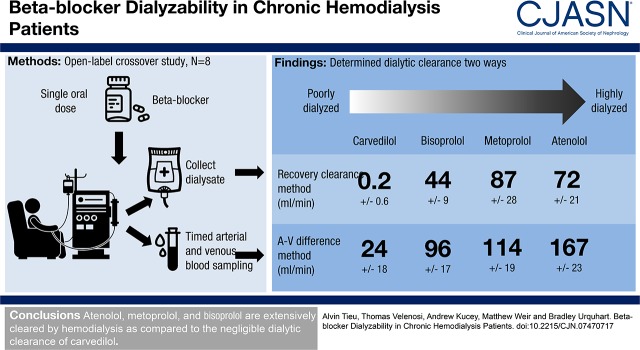
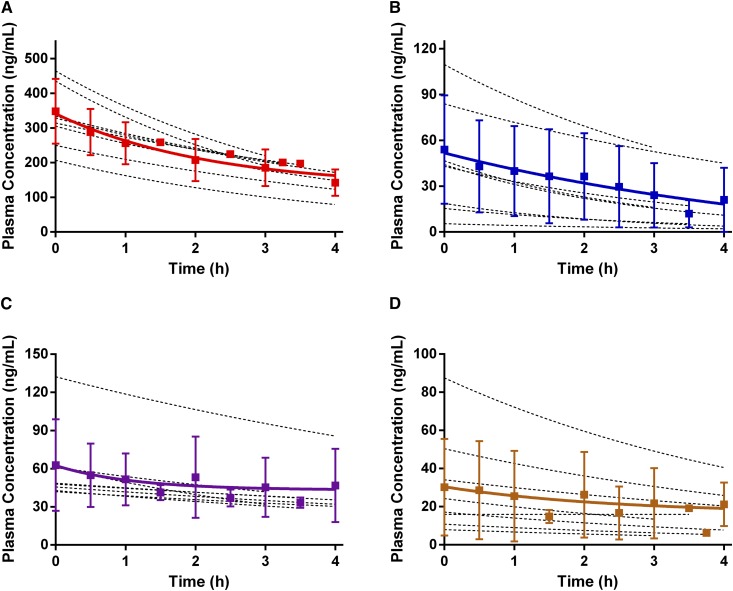
Collection and analysis of plasma samples over the duration of the hemodialysis session allowed us to create plasma concentration-time profiles (Figure 1) and determine β-blocker exposure for each subject (reported as AUC0−T). For atenolol, metoprolol, bisoprolol, and carvedilol, patient drug exposures were 827.9, 106.9, 180.7, and 150.5 ng∙h/ml. The amounts of β-blocker recovered in total spent dialysate were 3.7, 0.6, 0.5, and 0.0 mg, respectively. Drug exposure values and the total amounts of drug recovered in dialysate were applied to the recovery clearance method (Equation 2) to calculate dialytic clearance. The null hypothesis that there is no difference in clearance between any of the β-blockers was rejected (P<0.001). Atenolol was readily dialyzable with a dialytic clearance of 72±21 ml/min, which was similar to metoprolol’s clearance of 87±28 ml/min (Figure 2). The clearances of atenolol and metoprolol were considerably higher than that bisoprolol at 44±9 ml/min (P=0.02 compared with atenolol and P<0.001 compared with metoprolol). Carvedilol had a substantially lower clearance (0.2±0.6 ml/min; P<0.001) than all of the other study β-blockers.
Figure 1.
β-Blocker plasma concentration-time profiles of patients with ESKD during hemodialysis. Plasma concentration-time profiles of atenolol (A), metoprolol (B), bisoprolol (C), and carvedilol (D) during hemodialysis in patients with ESKD. Each subject received a single oral dose of a β-blocker 4 hours before dialysis onset. Plasma concentrations of β-blockers were determined using ultraperformance liquid chromatography coupled to a quadrupole time-of-flight mass spectrometer. Dashed curves represent individual pharmacokinetic profiles, and mean pharmacokinetic curves are displayed in color. Results are presented as mean±SD, with n=8 for all treatment groups.
Figure 2.
Applying the recovery clearance equation, the dialytic clearance of atenolol and metoprolol are significantly elevated as compared with bisoprolol, while carvedilol displayed negligible dialyzability. Dialytic clearance values for the four β-blockers (atenolol, metoprolol, bisoprolol, and carvedilol) during hemodialysis in patients with ESKD. Plasma concentrations of β-blockers were determined using ultraperformance liquid chromatography coupled to a quadrupole time-of-flight mass spectrometer, and dialyzability was calculated using the recovery clearance method. Results are presented as mean±SD, with n=8 for each treatment group. *P<0.01 for carvedilol relative to all other β-blockers; #P<0.05 relative to bisoprolol; ##P<0.01 relative to bisoprolol.
A-V Difference Method.
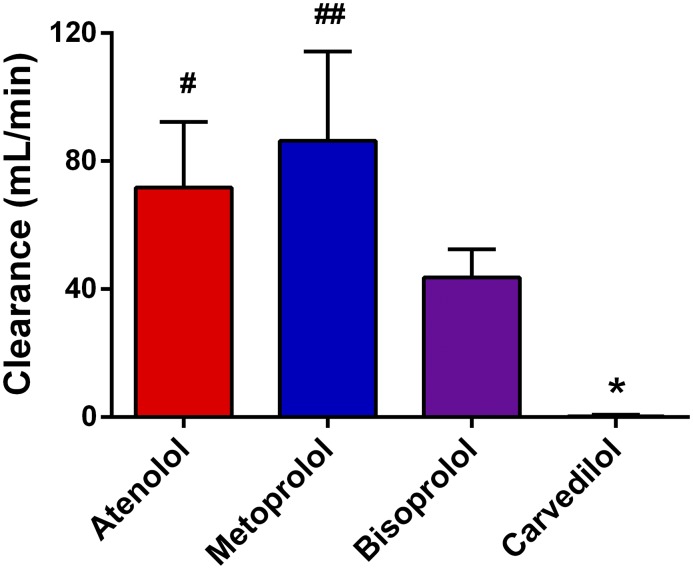
The blood flow and predialysis hematocrit for each subject are shown in Table 2. These two variables along with the difference in β-blocker concentrations between the arterial and venous ports were used to calculate dialyzability values by applying the A-V difference equation (Equation 1). The null hypothesis that there is no difference in clearance between any of the β blockers was rejected (P<0.001). We found atenolol to be the most highly dialyzed β-blocker, with a mean±SD dialytic clearance of 167±23 ml/min. The dialytic clearances for metoprolol and bisoprolol were 114±19 and 96±17 ml/min, respectively (Figure 3), which were both significantly lower than atenolol (P<0.001). The dialytic clearances of atenolol, metoprolol, and bisoprolol were all significantly higher than carvedilol’s clearance of 24±18 ml/min (P<0.001).
Figure 3.
Applying the arterial venous difference equation, atenolol was the most highly dialyzed β-blocker, while carvedilol displayed a dialytic clearance value significantly lower as compared with the other three β-blockers. Dialytic clearance values for the four β-blockers (atenolol, metoprolol, bisoprolol, and carvedilol) during hemodialysis in patients with ESKD. Plasma concentrations of β-blockers were determined using ultraperformance liquid chromatography coupled to a quadrupole time-of-flight mass spectrometer, and dialyzability was calculated using the arterial venous difference method. Results are presented as mean±SD, with n=8 for each treatment group. *P<0.01 for carvedilol relative to all other β-blockers; #P<0.01 relative to atenolol.
Discussion
We determined the dialyzability of four commonly prescribed β-blockers in patients receiving maintenance hemodialysis. We found atenolol and metoprolol to be highly dialyzable (72±21 and 87±28 ml/min) compared with bisoprolol (44±9 ml/min). All three of these β-blockers displayed dialytic clearance values markedly higher than that of carvedilol, which was found to have nearly negligible dialyzability (0.2±0.6 ml/min). With only 10% of currently marketed medications having definitive dialyzability information on the basis of experimental data (17), this is an important step in establishing how hemodialysis affects drug disposition.
Despite kidney excretion accounting for only 5% of metoprolol clearance (28), both atenolol and metoprolol have physicochemical properties that enable them to be extensively dialyzed (28–30). To our knowledge, no previous studies have determined the dialyzability of metoprolol. Metoprolol’s dialytic clearance (87 ml/min) is only 9% of its reported total clearance in patients with ESKD (31). Although this may not warrant a supplemental dose postdialysis, dialytic clearance shows an additional route of elimination for metoprolol that is not observed in healthy patients or patients with CKD. As for atenolol, the A-V difference method applied by Flouvat et al. (32) produced a clearance of 43 ml/min for patients with ESKD on a coil kidney dialysis with cuprophane membrane. Using the same equation, high-flux polysulfone dialyzers in our study revealed a substantially higher dialytic clearance of 167 ml/min. When the recovery clearance equation was applied, the dialyzability of atenolol at 72 ml/min was still higher than the value determined by Flouvat et al. (32). Furthermore, atenolol’s dialytic clearance (72 ml/min) is five- to nine-fold higher than its total clearance (8–14 ml/min) in patients with ESKD (33,34). Carvedilol’s larger volume of distribution, decreased water solubility, and extensive protein binding (98%) suggested minimal or low dialytic clearance (35,36). Similar to earlier findings by Miki et al. (37), we found that carvedilol displayed very minimal dialytic elimination. A low dialyzability was similarly expected for bisoprolol after consulting the dialysis of drugs guideline and peer-reviewed articles (Table 1) (19–22). Our study indicates that bisoprolol is cleared during hemodialysis, regardless of the calculation method used. Kanegae et al. (38) found a comparable dialytic clearance of 51 ml/min using the A-V method for patients who were also prescribed polysulfone-based dialyzer membranes. This finding is contrary to previous estimates of dialyzability for bisoprolol, but it is reflective of its intermediate physicochemical properties compared with carvedilol and atenolol (39,40). Although its total clearance ranges from 70 to 84 ml/min in patients with ESKD not on dialysis (38,41), the dialytic clearance of bisoprolol (44 ml/min) is still over 1.5-fold higher than its kidney-mediated excretion (28 ml/min) in these patients.
Only atenolol and metoprolol were expected to be dialyzable; however, the results of our study allow us to definitively state that bisoprolol is cleared during hemodialysis. If dosing is not carefully monitored, patients taking dialyzable drugs may have inadequate β-adrenergic receptor blockade, resulting in suboptimal treatment. Although the amounts of drugs that we recovered in dialysate may seem unsubstantial relative to their initial dose, the bioavailability of these β-blockers is an important consideration to determine the significance of hemodialysis on drug clearance. For example, approximately 50% of atenolol is absorbed systemically, with values as low as 12%–30% in patients with ESKD (33,34). Hence, the 3.7 mg of atenolol recovered in dialysate (7.5% of the initial dose) can be a significant amount and warrant changes for how atenolol is prescribed to patients on dialysis. Accordingly, atenolol, metoprolol, and bisoprolol may require postdialysis dosing to ensure that adequate plasma concentrations are maintained. Conversely, carvedilol removal by dialysis is negligible. Because kidney-mediated excretion accounts for <2% of its elimination, plasma levels of carvedilol do not accumulate in kidney impairment (42). These findings suggest that no dosage adjustments are required for carvedilol in patients on hemodialysis (37,43). Although current clinical practice guidelines outlined by the National Kidney Foundation make no recommendations on which β-blocker should be prescribed to patients with CKD (44), selection of carvedilol over other β-blockers may be considered.
The strongest evidence in support of carvedilol as opposed to other β-blockers is from its proven efficacy in patients with ESKD. Carvedilol is the only β-blocker that has been tested in prospective, randomized clinical trials in patients on hemodialysis. Cice et al. (45) showed that year-long administration of carvedilol reduces left ventricular volumes and improves overall cardiac function for patients on hemodialysis with dilated cardiomyopathy. This cohort was followed for another 12 months and showed a significant reduction in all-cause mortality, cardiovascular mortality, and all-cause hospitalizations compared with placebo-controlled patients (5). Additional analyses conducted in this observational study indicated that carvedilol use compared with placebo was associated with reductions in fatal myocardial infarctions and strokes—two main causes for cardiovascular death in patients with ESKD. Although carvedilol is typically reserved for patients with symptomatic heart failure, it may be worth expanding its use in patients on dialysis due to its pharmacokinetic properties and previous evidence indicating its strength to improve patient morbidity.
There are other important considerations generated from our study. Atenolol overdose is effectively treated by hemodialysis (46). To our knowledge, it is unknown whether hemodialysis is effective for treating overdose of other β-blockers. Our study suggests that hemodialysis may be a consideration to treat metoprolol or bisoprolol overdose. The last important consideration pertains to factors that can contribute to interindividual variability in clearance. Hepatic drug metabolizing enzymes involved in clearance of metoprolol, bisoprolol, and carvedilol have genetic polymorphisms. For example, individuals can be categorized from poor to ultrarapid metabolizers of metoprolol on the basis of their CYP2D6 genotype (47). Hence, poor metabolizers of metoprolol may have a larger component of their dose available for removal by hemodialysis.
Our study has some limitations that should be considered. The recovery clearance method is accepted as the gold standard for determining dialyzability. This method requires a sensitive assay to measure low drug concentrations in large volumes of dialysate. Despite concentrating samples 100-fold, some dialysate samples from patients taking carvedilol were below our limit of quantification. However, the negligible dialytic clearance of carvedilol likely resulted in virtually no drug for detection. The precision of our carvedilol assay was slightly above our acceptable range. At very low concentrations of carvedilol, this will introduce a small amount of variability in the calculation of clearance. In addition, it is unknown whether β-blockers bind to dialysis membranes or the dialysate collection barrel, which can result in an underestimated recovery clearance value. When calculating AUC of β blockers, we determined total concentration (free plus protein bound). This may also result in an underestimated dialytic clearance. Another limitation of our study design is quantifying β-blockers after a single oral dose. Although a drug that is dialyzable after a single dose is also expected to be cleared when taken chronically, future studies should examine dialytic clearance for patients at steady state. Patients enrolled in our study used different dialyzers. Although the dialyzers were all modern high-flux dialyzers, the type of dialyzer may have introduced a small amount of variability in our clearance calculation. Lastly, our study did not investigate the interdialytic pharmacokinetics of the four β-blockers. Previous studies have shown that the t1/2 and kidney clearances of these drugs on nondialysis days return to values that are comparable with those of patients with ESKD not yet on RRT (32,37,48–50).
In conclusion, we have experimentally determined the dialytic clearance of the most commonly prescribed β-blockers. Our data show that atenolol and metoprolol are markedly dialyzed compared with carvedilol. Despite previous literature suggesting that bisoprolol would have little to no dialyzability, we show that it is, in fact, cleared during hemodialysis in patients using modern high-flux dialyzers. Definitive dialytic clearance data presented in this study can help design prospective trials to determine whether outcomes are superior with poorly dialyzable β-blockers compared with highly dialyzable β-blockers.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors would like to thank the staff, nurses, and biomedical technicians working at the London Health Sciences Centre dialysis clinics for their generosity and help in conducting this study. Images used to generate the graphic abstract were from Servier Medical Art (http://smart.servier.com).
This study was supported by the Canadian Institutes of Health Research, the Canadian Foundation for Innovation, and the Schulich School of Medicine and Dentistry at Western University.
Funders had no role in study design, data collection, or interpretation.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.07470717/-/DCSupplemental.
References
- 1.Cheung AK, Sarnak MJ, Yan G, Berkoben M, Heyka R, Kaufman A, Lewis J, Rocco M, Toto R, Windus D, Ornt D, Levey AS; HEMO Study Group : Cardiac diseases in maintenance hemodialysis patients: Results of the HEMO Study. Kidney Int 65: 2380–2389, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Foley RN, Parfrey PS, Sarnak MJ: Epidemiology of cardiovascular disease in chronic renal disease. J Am Soc Nephrol 9[12 Suppl]: S16–S23, 1998 [PubMed] [Google Scholar]
- 3.Collins AJ, Foley RN, Herzog C, Chavers BM, Gilbertson D, Ishani A, Kasiske BL, Liu J, Mau LW, McBean M, Murray A, St Peter W, Guo H, Li Q, Li S, Li S, Peng Y, Qiu Y, Roberts T, Skeans M, Snyder J, Solid C, Wang C, Weinhandl E, Zaun D, Arko C, Chen SC, Dalleska F, Daniels F, Dunning S, Ebben J, Frazier E, Hanzlik C, Johnson R, Sheets D, Wang X, Forrest B, Constantini E, Everson S, Eggers PW, Agodoa L: Excerpts from the US Renal Data System 2009 Annual Data Report. Am J Kidney Dis 55[1 Suppl 1]: S1–S420, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.López-Sendón J, Swedberg K, McMurray J, Tamargo J, Maggioni AP, Dargie H, Tendera M, Waagstein F, Kjekshus J, Lechat P, Torp-Pedersen C; Task ForceOn Beta-Blockers of the European Society of Cardiology : Expert consensus document on beta-adrenergic receptor blockers. Eur Heart J 25: 1341–1362, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Cice G, Ferrara L, D’Andrea A, D’Isa S, Di Benedetto A, Cittadini A, Russo PE, Golino P, Calabrò R: Carvedilol increases two-year survivalin dialysis patients with dilated cardiomyopathy: A prospective, placebo-controlled trial. J Am Coll Cardiol 41: 1438–1444, 2003 [DOI] [PubMed] [Google Scholar]
- 6.CIBIS-II Investigators and Committees : The cardiac insufficiency bisoprolol study II (CIBIS-II): A randomised trial. Lancet 353: 9–13, 1999 [PubMed] [Google Scholar]
- 7.Merit-HF Study Group : Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 353: 2001–2007, 1999 [PubMed] [Google Scholar]
- 8.Hansson L, Lindholm LH, Ekbom T, Dahlöf B, Lanke J, Scherstén B, Wester PO, Hedner T, de Faire U: Randomised trial of old and new antihypertensive drugs in elderly patients: Cardiovascular mortality and morbidity the Swedish Trial in Old Patients with Hypertension-2 study. Lancet 354: 1751–1756, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Nolin TD, Frye RF, Le P, Sadr H, Naud J, Leblond FA, Pichette V, Himmelfarb J: ESRD impairs nonrenal clearance of fexofenadine but not midazolam. J Am Soc Nephrol 20: 2269–2276, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Velenosi TJ, Fu AY, Luo S, Wang H, Urquhart BL: Down-regulation of hepatic CYP3A and CYP2C mediated metabolism in rats with moderate chronic kidney disease. Drug Metab Dispos 40: 1508–1514, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Thomson BK, Nolin TD, Velenosi TJ, Feere DA, Knauer MJ, Asher LJ, House AA, Urquhart BL: Effect of CKD and dialysis modality on exposure to drugs cleared by nonrenal mechanisms. Am J Kidney Dis 65: 574–582, 2015 [DOI] [PubMed] [Google Scholar]
- 12.Frankenfield DL, Weinhandl ED, Powers CA, Howell BL, Herzog CA, St Peter WL: Utilization and costs of cardiovascular disease medications in dialysis patients in Medicare part D. Am J Kidney Dis 59: 670–681, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Ishani A, Herzog CA, Collins AJ, Foley RN: Cardiac medications and their association with cardiovascular events in incident dialysis patients: Cause or effect? Kidney Int 65: 1017–1025, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Matzke GR, Dowling TC, Marks SA, Murphy JE: Influence of kidney disease on drug disposition: An assessment of industry studies submitted to the FDA for new chemical entities 1999-2010. J Clin Pharmacol 56:390–398, 2016 [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Zhang L, Abraham S, Apparaju S, Wu TC, Strong JM, Xiao S, Atkinson AJ Jr, Thummel KE, Leeder JS, Lee C, Burckart GJ, Lesko LJ, Huang SM: Assessment of the impact of renal impairment on systemic exposure of new molecular entities: Evaluation of recent new drug applications. Clin Pharmacol Ther 85: 305–311, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Weir MA, Dixon SN, Fleet JL, Roberts MA, Hackam DG, Oliver MJ, Suri RS, Quinn RR, Ozair S, Beyea MM, Kitchlu A, Garg AX: β-Blocker dialyzability and mortality in older patients receiving hemodialysis. J Am Soc Nephrol 26: 987–996, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Velenosi TJ, Urquhart BL: Pharmacokinetic considerations in chronic kidney disease and patients requiring dialysis. Expert Opin Drug Metab Toxicol 10: 1131–1143, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Tieu A, Leither M, Urquhart BL, Weir MA: Clearance of cardiovascular medications during hemodialysis. Curr Opin Nephrol Hypertens 25: 257–267, 2016 [DOI] [PubMed] [Google Scholar]
- 19.Chazot C, Jean G: Intradialytic hypertension: It is time to act. Nephron Clin Pract 115: c182–c188, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Chen J, Gul A, Sarnak MJ: Management of intradialytic hypertension: The ongoing challenge. Semin Dial 19: 141–145, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Levin NW, Kotanko P, Eckardt K-U, Kasiske BL, Chazot C, Cheung AK, Redon J, Wheeler DC, Zoccali C, London GM: Blood pressure in chronic kidney disease stage 5D-report from a kidney disease: Improving global outcomes controversies conference. Kidney Int 77: 273–284, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Redon J, Martinez F, Cheung AK: Special considerations for antihypertensive agents in dialysis patients. Blood Purif 29: 93–98, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Uehlinger DE, Schaedeli F, Kinzig M, Sörgel F, Frey FJ: Pharmacokinetics of fleroxacin after multiple oral dosing in patients receiving regular hemodialysis. Antimicrob Agents Chemother 40: 1903–1909, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee CS, Marbury TC, Benet LZ: Clearance calculations in hemodialysis: Application to blood, plasma, and dialysate measurements for ethambutol. J Pharmacokinet Biopharm 8: 69–81, 1980 [DOI] [PubMed] [Google Scholar]
- 25.Gotch FA, Panlilio F, Sergeyeva O, Rosales L, Folden T, Kaysen G, Levin N: Effective diffusion volume flow rates (Qe) for urea, creatinine, and inorganic phosphorous (Qeu, Qecr, QeiP) during hemodialysis. Semin Dial 16: 474–476, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Care FM: Dialysers and Filters Product Range. Available at: http://www.fmc ag.dk/files/Filterbrochure.pdf. Published in 2007. Accessed October 15, 2017.
- 27. Baxter: REVACLEAR Dialyzer Technology. Available at: https://www.baxter.com/assets/downloads/products_expertise/renal_therapies/Revaclear_Spec_Sheet_FINAL.pdf, 2013. Published in 2013. Accessed October 15, 2017.
- 28.Regårdh CG, Johnsson G: Clinical pharmacokinetics of metoprolol. Clin Pharmacokinet 5: 557–569, 1980 [DOI] [PubMed] [Google Scholar]
- 29.McAinsh J: Clinical pharmacokinetics of atenolol. Postgrad Med J 53[Suppl 3]: 74–78, 1977 [PubMed] [Google Scholar]
- 30.Cheung AK, Leypoldt JK: The hemodialysis membranes: A historical perspective, current state and future prospect. Semin Nephrol 17: 196–213, 1997 [PubMed] [Google Scholar]
- 31.Jordö L, Attman PO, Aurell M, Johansson L, Johnsson G, Regårdh CG: Pharmacokinetic and pharmacodynamic properties of metoprolol in patients with impaired renal function. Clin Pharmacokinet 5: 169–180, 1980 [DOI] [PubMed] [Google Scholar]
- 32.Flouvat B, Decourt S, Aubert P, Potaux L, Domart M, Goupil A, Baglin A: Pharmacokinetics of atenolol in patients with terminal renal failure and influence of haemodialysis. Br J Clin Pharmacol 9: 379–385, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kirch W, Köhler H, Mutschler E, Schäfer M: Pharmacokinetics of atenolol in relation to renal function. Eur J Clin Pharmacol 19: 65–71, 1981 [DOI] [PubMed] [Google Scholar]
- 34.McAinsh J, Holmes BF, Smith S, Hood D, Warren D: Atenolol kinetics in renal failure. Clin Pharmacol Ther 28: 302–309, 1980 [DOI] [PubMed] [Google Scholar]
- 35. GlaxoSmithKline Inc.: Carvedilol (Coreg) Product Monograph. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2005/020297s013lbl.pdf. Published in 2005. Accessed June 1, 2016.
- 36.Varin F, Cubeddu LX, Powell JR: Liquid chromatographic assay and disposition of carvedilol in healthy volunteers. J Pharm Sci 75: 1195–1197, 1986 [DOI] [PubMed] [Google Scholar]
- 37.Miki S, Masumura H, Kaifu Y, Yuasa S: Pharmacokinetics and efficacy of carvedilol in chronic hemodialysis patients with hypertension. J Cardiovasc Pharmacol 18[Suppl 4]: S62–S68, 1991 [PubMed] [Google Scholar]
- 38.Kanegae K, Hiroshige K, Suda T, Iwamoto M, Ohta T, Nakashima Y, Ohtani A: Pharmacokinetics of bisoprolol and its effect on dialysis refractory hypertension. Int J Artif Organs 22: 798–804, 1999 [PubMed] [Google Scholar]
- 39.Bühring KU, Sailer H, Faro HP, Leopold G, Pabst J, Garbe A: Pharmacokinetics and metabolism of bisoprolol-14C in three animal species and in humans. J Cardiovasc Pharmacol 8[Suppl 11]: S21–S28, 1986 [DOI] [PubMed] [Google Scholar]
- 40.Leopold G: Balanced pharmacokinetics and metabolism of bisoprolol. J Cardiovasc Pharmacol 8[Suppl 11]: S16–S20, 1986 [DOI] [PubMed] [Google Scholar]
- 41.Payton CD, Fox JG, Pauleau NF, Boulton-Jones JM, Ioannides C, Johnston A, Thomas P: The single dose pharmacokinetics of bisoprolol (10 mg) in renal insufficiency: The clinical significance of balanced clearance. Eur Heart J 8[Suppl M]: 15–22, 1987 [DOI] [PubMed] [Google Scholar]
- 42.Deetjen A, Heidland A, Pangerl A, Meyer-Sabellek W, Schaefer RM: Antihypertensive treatment with a vasodilating beta-blocker, carvedilol, in chronic hemodialysis patients. Clin Nephrol 43: 47–52, 1995 [PubMed] [Google Scholar]
- 43.Gehr TW, Tenero DM, Boyle DA, Qian Y, Sica DA, Shusterman NH: The pharmacokinetics of carvedilol and its metabolites after single and multiple dose oral administration in patients with hypertension and renal insufficiency. Eur J Clin Pharmacol 55: 269–277, 1999 [DOI] [PubMed] [Google Scholar]
- 44.Kidney Disease Outcomes Quality Initiative (K/DOQI) : K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis 43[5 Suppl 1]: S1–S290, 2004 [PubMed] [Google Scholar]
- 45.Cice G, Ferrara L, Di Benedetto A, Russo PE, Marinelli G, Pavese F, Iacono A: Dilated cardiomyopathy in dialysis patients--beneficial effects of carvedilol: A double-blind, placebo-controlled trial. J Am Coll Cardiol 37: 407–411, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Huang SH, Tirona RG, Ross C, Suri RS: Case report: Atenolol overdose successfully treated with hemodialysis. Hemodial Int 17: 652–655, 2013 [DOI] [PubMed] [Google Scholar]
- 47.Rau T, Heide R, Bergmann K, Wuttke H, Werner U, Feifel N, Eschenhagen T: Effect of the CYP2D6 genotype on metoprolol metabolism persists during long-term treatment. Pharmacogenetics 12: 465–472, 2002 [DOI] [PubMed] [Google Scholar]
- 48.Kirch W, Rose I, Demers HG, Leopold G, Pabst J, Ohnhaus EE: Pharmacokinetics of bisoprolol during repeated oral administration to healthy volunteers and patients with kidney or liver disease. Clin Pharmacokinet 13: 110–117, 1987 [DOI] [PubMed] [Google Scholar]
- 49.Masumura H, Miki S, Kaifu Y, Kitajima W, Abe Y: Pharmacokinetics and efficacy of carvedilol in hypertensive patients with chronic renal failure and hemodialysis patients. J Cardiovasc Pharmacol 19[Suppl 1]: S102–S107, 1992 [DOI] [PubMed] [Google Scholar]
- 50.Seiler KU, Schuster KJ, Meyer GJ, Niedermayer W, Wassermann O: The pharmacokinetics of metoprolol and its metabolites in dialysis patients. Clin Pharmacokinet 5: 192–198, 1980 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.