Abstract
Background and objectives
Metabolomics is instrumental in identifying novel biomarkers of kidney function to aid in the prevention and management of CKD. However, data linking the metabolome to incident eGFR are sparse, particularly in Asian populations with different genetic backgrounds and environmental exposures. Therefore, we aimed to investigate the associations of amino acid and acylcarnitine profiles with change in eGFR in a Chinese cohort.
Design, setting, participants, & measurements
This study included 1765 community-living Chinese adults aged 50–70 years with baseline eGFR≥60 ml/min per 1.73 m2. At baseline, 22 amino acids and 34 acylcarnitines in plasma were quantified by gas or liquid chromatography coupled with mass spectrometry. Annual rate of change in eGFR was calculated, and incident eGFR decline was defined as eGFR<60 ml/min per 1.73 m2 by the end of 6 years of follow-up.
Results
The mean (SD) unadjusted annual change in eGFR was 2.2±2.0 ml/min per 1.73 m2 and the incidence of reduced eGFR was 16%. After Bonferroni correction, 13 of 56 metabolites were significantly associated with annual eGFR change. After multivariable adjustment of baseline covariates, including baseline eGFR, seven of the 13 metabolites, including cysteine, long-chain acylcarnitines (C14:1OH, C18, C18:2, and C20:4), and other acylcarnitines (C3DC and C10), were significantly associated with incident reduced eGFR (relative risks ranged from 1.16 to 1.25 per SD increment of metabolites; P<3.8E-03 after Bonferroni correction of multiple testing of the 13 metabolites). Moreover, principal component analysis identified two factors, consisting of cysteine and long-chain acylcarnitines, respectively, that were associated with incident reduced eGFR.
Conclusions
Elevated plasma levels of cysteine and a panel of acylcarnitines were associated with a higher incidence of reduced eGFR in Chinese adults, independent of baseline eGFR and other conventional risk factors.
Keywords: Adult; Incidence; Metabolomics; risk factors; acylcarnitine; Cysteine; Metabolome; Amino Acids; glomerular filtration rate; Principal Component Analysis; Follow-Up Studies; Genetic Background; Carnitine; Chromatography, Liquid; Renal Insufficiency, Chronic; Mass Spectrometry; Biomarkers; Environmental Exposure; Humans
Introduction
As a major public health challenge, CKD affects about 8%–16% of the world population and contributes to tremendous disease and socioeconomic burdens (1,2). The prevalence of CKD is expected to accelerate over the next few decades, particularly in countries undergoing rapid nutrition and lifestyle transitions, such as China, which has the largest population living with diabetes and hypertension in the world (3,4). Although CKD is preventable through early detection and intervention (5), the commonly used creatinine-based eGFR is not sensitive to detect incipient kidney dysfunction, and circulating concentrations of creatinine may be influenced by factors that are unrelated to kidney function (6,7). Therefore, it is necessary to identify novel biomarkers for early detection of the onset and progression of CKD.
With recent advances in metabolomics technology, it is now feasible to identify novel markers to predict eGFR change and therefore gain a deeper understanding of CKD pathophysiology (8). However, to date, data linking the metabolome to incident CKD are sparse (9–12). Moreover, most studies have investigated individuals of European or African descent, rather than Asians, who have different genetic backgrounds and environmental exposures and therefore different baseline risks of developing CKD.
To fill these knowledge gaps, we used a targeted and quantitative metabolomics approach to profile plasma levels of amino acids and multiple acylcarnitines in a well characterized Chinese cohort, and examined prospective associations between these metabolites and change in eGFR.
Materials and Methods
Study Population
The Nutrition and Health of Aging Population in China study is a prospective cohort study designed to investigate environmental and genetic factors of metabolic diseases (13). Briefly, the study was initiated in 2005 among 3289 residents living in Beijing or Shanghai, aged 50–70 years. After 6 years, 2529 participants completed the follow-up visit, and 760 persons were considered lost to follow-up due to loss of contact (n=554) or refusal to participate (n=206). Information on baseline and follow-up surveys is described elsewhere (13,14). In this analysis, we excluded participants without creatinine data at follow-up (n=261) or baseline metabolite data (n=459). When examining associations between metabolites and incident reduced eGFR, we further excluded 44 participants who had eGFR<60 ml/min per 1.73 m2 at baseline. The study protocol was approved by the Institutional Review Board of the Institute for Nutritional Sciences, Chinese Academy of Sciences. All participants provided written, informed consent.
Data Collection
Information on demographic, health status, dietary, and lifestyle factors was collected at baseline by trained staff using a standard questionnaire (13). Educational attainment (0–6, 7–9, or >10 years), current smoking (yes or no), current drinking (yes or no), antihypertensive medication use (yes or no), lipid-lowering medication use (yes or no), physical activity (low, moderate, or high), cardiovascular disease (yes or no), hypertension (yes or no), and type 2 diabetes (yes or no) were previously defined (13). All participants underwent a physical examination by trained staff. Body weight, height, waist circumference, and BP were measured following a standardized protocol (13). Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters.
At the time of baseline and follow-up surveys, fasting venous blood samples were collected after overnight fasting. Baseline plasma glucose, total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides, γ-glutamyltransferase, uric acid, and C-reactive protein were measured as previously described (13). Plasma creatinine levels at baseline and follow-up survey were measured by an alkaline picrate method (Roche Diagnostics, Mannheim, Germany) on an automatic analyzer (Hitachi 7080, Tokyo, Japan), with intra- and interassay coefficients of variation<3%, and then calibrated traceable to isotope dilution mass spectrometry standards (15).
Targeted Metabolomics
Plasma acylcarnitines were profiled using liquid chromatography–tandem mass spectrometry, as previously described (16). Briefly, chromatographic separation and mass spectrometric analysis of acylcarnitines were performed on an Agilent 1260 HPLC system and 6410B QQQ mass spectrometer (Agilent Technologies Inc., CA), respectively. Plasma amino acids were detected by gas chromatography–mass spectrometry on an Agilent 7890A gas chromatography system and 5975C inert MSD system (Agilent Technologies Inc., CA), with good linearity within a wide range, lower limit of quantitation, and better precision (Supplemental Methods, Supplemental Table 1). The validity of both metabolomic platforms has been previously described (16,17).
Outcome Ascertainment
eGFR was calculated using the Modification of Diet in Renal Disease (MDRD) study equation for Chinese individuals: eGFR (ml/min per 1.73 m2)=175×creatinine (mg/dl)−1.234 (Jaffe’s kinetic method) ×age−0.179×0.79 (if female), which was derived from native Chinese individuals and showed stronger correlation with measured GFR compared with other equations (18,19). The primary outcome was annual eGFR change, defined as the difference in eGFR between baseline and follow-up visits divided by the time between visits in years. Moreover, incident reduced eGFR was defined as the onset of eGFR<60 ml/min per 1.73 m2 during follow-up (20). In sensitivity analyses, a more stringent outcome, incident reduced eGFR plus annual eGFR decline>3%, was examined to reduce misclassification near the eGFR threshold (21). Additionally, eGFR was calculated using another definition, the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation (19,22).
Statistical Analyses
t tests or Wilcoxon’s Signed Rank tests were used for continuous variables, and chi-squared tests were used for categoric variables, for the comparison of baseline characteristics between participants with and without incident reduced eGFR. Spearman partial correlation coefficients (rs) were calculated to examine relationships among metabolites, as well as between the metabolites and covariates, after adjustment for age, sex, region, and residence. R package heatmap.2 was used to construct colored blocks representing correlation levels.
The cross-sectional associations of individual metabolites (scaled to SD of 1) with baseline eGFR were calculated using multivariable linear regression. Model 1 was adjusted for age, sex, region, and residence; model 2 was further adjusted for educational attainment, current smoking, current drinking, physical activity, BMI, lipid-lowering medication use, HDL, LDL, cardiovascular disease, hypertension, or type 2 diabetes. Longitudinal associations of metabolites with annual eGFR change were also assessed using multivariable linear regression, with additional adjustment for baseline eGFR. After Bonferroni correction, metabolites were considered significant at P<0.05/56=8.9E-04 corrected for multiple testing of the 56 metabolites in the fully adjusted model. Metabolites that were significantly associated with annual eGFR change were then analyzed with incident reduced eGFR using the log-Poisson model (23,24), with α=3.8E−03 after Bonforroni correction for multiple testing of 13 metabolites associated with annual eGFR change.
In secondary analyses, we performed a principal component analysis. The number of retained factors was determined by the scree plot. Varimax rotation was used to produce interpretable factors. Metabolites with a factor load ≥0.5 were reported as composing factors. Factor scores for each participant were calculated by summing the standardized values of metabolites weighted by their factor loadings. We then applied the abovementioned methods to examine associations of the factors with change in eGFR.
For metabolites significantly associated with incident reduced eGFR, analyses were stratified by age, sex, region, BMI, hypertension, type 2 diabetes, and eGFR at baseline. Potential interactions were examined by likelihood ratio test.
All analyses were conducted using R (version 3.1.1; www.r-project.org). A two-sided P value <0.05 was considered statistically significant, unless specified otherwise.
Results
Baseline Characteristics
During 6 years of follow-up, the incidence of reduced eGFR was 16% (274 of 1765). The mean (SD) annual eGFR change was −2.2±2.0 ml/min per 1.73 m2. Compared with noncases, participants with incident reduced eGFR were older, had lower educational attainment and higher uric acid levels, and were more likely to have hypertension and cardiovascular disease (P<0.05). The cases also had lower eGFR levels and higher loss of eGFR during follow-up (Table 1).
Table 1.
Baseline characteristics of 1765 participants in the Nutrition and Health of Aging Population in China study
| Characteristic | All (n=1765) | Incident Reduced eGFR | |
|---|---|---|---|
| No (n=1491) | Yes (n=274) | ||
| Age, yra | 58±6 | 58±6 | 61±6 |
| Male | 745 (42) | 631 (42) | 114 (42) |
| Beijing resident | 721 (41) | 608 (41) | 113 (41) |
| Urban resident | 725 (41) | 614 (41) | 111 (41) |
| Education level, yra | |||
| 0–6 | 828 (47) | 686 (46) | 142 (52) |
| 7–9 | 604 (34) | 528 (35) | 76 (28) |
| ≥10 | 333 (19) | 277 (19) | 56 (20) |
| Current smoker | 484 (27) | 406 (27) | 78 (28) |
| Current drinker | 436 (25) | 378 (25) | 58 (21) |
| Physical activity level | |||
| Low | 119 (7) | 106 (7) | 13 (5) |
| Medium | 699 (40) | 586 (39) | 113 (41) |
| High | 947 (54) | 799 (54) | 148 (54) |
| Cardiovascular diseaseab | 171 (10) | 133 (9) | 38 (14) |
| Type 2 diabetesc | 166 (9) | 144 (10) | 22 (8) |
| Hypertensionad | 915 (52) | 751 (50) | 164 (60) |
| Antihypertensive medication usea | 451 (26) | 365 (24) | 86 (31) |
| Lipid-lowering medication use | 101 (6) | 83 (6) | 18 (7) |
| Systolic BP, mmHga | 139±22 | 138±22 | 144±23 |
| Diastolic BP, mmHga | 80±11 | 80±11 | 81±11 |
| BMI, kg/m2 | 24.4±3.5 | 24.4±3.5 | 24.4±3.5 |
| Fasting glucose, mg/dl | 102±27 | 103±28 | 101±24 |
| Total cholesterol, mg/dl | 180±37 | 180±36 | 180±40 |
| HDL-cholesterol, mg/dl | 50±13 | 50±13 | 50±13 |
| LDL-cholesterol, mg/dl | 124±36.7 | 124±36 | 123±39 |
| Triacylglycerol, mg/dl | 94 (66–143) | 94 (65–143) | 94 (68–151) |
| Gamma-glutamyltransferase, IU/L | 23 (17–35) | 23 (17–35) | 23 (17–36) |
| C-reactive protein, mg/L | 0.62 (0.32–1.36) | 0.61 (0.32–1.38) | 0.70 (0.32–1.32) |
| Uric acid, mg/dla | 2.8 (2.3–3.3) | 2.7 (2.3–3.3) | 2.9 (2.3–3.5) |
| eGFR, ml/min per 1.73 m2a | 87±13 | 89±12 | 78±12 |
| eGFR change, ml/min per 1.73 m2 per yeara | −2.2±2.0 | −2.0±1.8 | −3.7±2.1 |
Data are presented as mean±SD or median (interquartile range) for continuous variables, and count (percentage) for categoric variables. Percentages may not add up to 100% because of rounding. There were no missing values. BMI, body mass index.
P<0.05 for comparison between incident reduced eGFR group and noncase group.
Cardiovascular disease was defined as having self-reported coronary heart disease or stroke.
Type 2 diabetes was defined as fasting plasma glucose concentration >7.0 mmol/L, taking antidiabetic medications, or self-reported physician-diagnosed diabetes.
Hypertension was defined as systolic BP >140 mmHg, diastolic BP >90 mmHg, use of antihypertensive medications, or self-reported physician-diagnosed hypertension.
Of 56 metabolites measured, 22 were amino acids and 34 acylcarnitines (Supplemental Table 2). Strong correlations (rs>0.7) were observed within the same classes of metabolites, such as branched-chain amino acids and medium- and long-chain acylcarnitines (Supplemental Figure 1). Weak-to-modest correlations (rs<0.5) were observed between metabolites and covariates (Supplemental Figure 2).
Associations between Metabolites and eGFR at Baseline
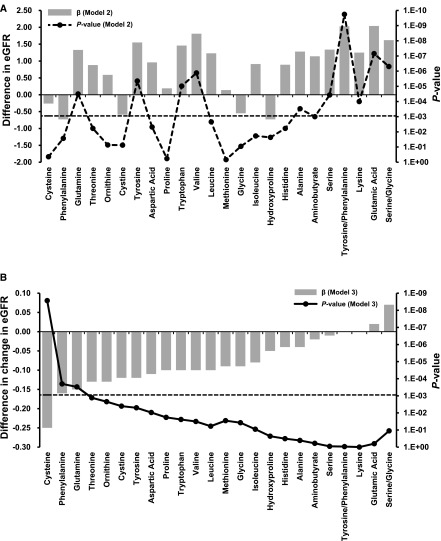
After adjusting for age, sex, region, and residence, 16 metabolites were significantly associated with baseline eGFR. The associations remained significant for 15 metabolites—eight amino acids (Figure 1A) and seven acylcarnitines (Figure 2A)—when further controlling for lifestyle, anthropometrics, and medical history (model 2). The eight amino acids were glutamic acid, valine, tyrosine, tryptophan, glutamine, serine, lysine, and alanine, with effect sizes per SD ranging from 1.25 to 2.04 ml/min per 1.73 m2 (all P<8.9E-04). The seven acylcarnitines were C3DC, C4, C5OH, C5:1, C6DC, C7DC, and C12OH, with effect sizes per SD ranging from −1.10 to −2.56 ml/min per 1.73 m2 (all P<8.9E-04; Supplemental Table 3).
Figure 1.
Associations of plasma amino acids and their ratios with baseline eGFR (eight amino acids significantly associated) and annual change in eGFR (three significantly associated) in the Nutrition and Health of Aging Population in China study. (A) Difference in eGFR per SD increment in metabolite (ml/min per 1.73 m2). (B) Difference in change in eGFR per SD increment in metabolite (ml/min per 1.73 m2 per year). Effect sizes (β) (left y axis), with corresponding P values (right y axis), for the amino acids arranged by ascending β (model 3) for annual eGFR change along the x axis are shown. The dotted horizontal line shows the cutoff for P=8.9E-04 after Bonferroni correction for multiple testing of the 56 metabolites. Model 2 was adjusted for age, sex, region, residence, educational attainment, physical activity, current smoking, current drinking, body mass index, lipid-lowering medication use, HDL, LDL, cardiovascular disease, hypertension, and type 2 diabetes. Model 3 was further adjusted for baseline eGFR only for outcome of annual eGFR change.
Figure 2.
Associations of plasma acylcarnitines (acylCNs) with baseline eGFR (seven acylCNs significantly associated) and annual change in eGFR (10 significantly associated) in the Nutrition and Health of Aging Population in China study. (A) Difference in eGFR per SD increment in metabolite (ml/min per 1.73 m2). (B) Difference in change in eGFR per SD increment in metabolite (ml/min per 1.73 m2 per year). Effect sizes (β) (left y axis), with corresponding P values (right y axis), for the acylCNs arranged by ascending acyl chain length along the x axis are shown. The dotted horizontal line shows the cutoff for P=8.9E-04 after Bonferroni correction for multiple testing of the 56 metabolites. Short-, medium-, and long-chain acylCN categories were calculated as Z scores of log-transformed acylCNs of carbon chains ≤6, 7–12, and ≥14, respectively. Model 2 was adjusted for age, sex, region, residence, educational attainment, physical activity, current smoking, current drinking, body mass index, lipid-lowering medication use, HDL, LDL, cardiovascular disease, hypertension, and type 2 diabetes. Model 3 was further adjusted for baseline eGFR only for outcome of annual eGFR change.
Associations between Metabolites and Change in eGFR
Longitudinal associations with annual eGFR change were detected among 12 metabolites after multivariable adjustment in model 2. When additionally controlling for baseline eGFR, the positive associations of amino acids were attenuated toward the null, whereas the inverse associations of several amino acids and short- and medium-chain acylcarnitines were strengthened and became significant (Figures 1B and 2B). After Bonferroni correction, 13 metabolites were significantly associated with annual eGFR change, including three amino acids (cysteine, glutamine, and phenylalanine) and ten acylcarnitines (C3DC, C5OH, C8, C10, C14:1OH, C16:2, C18, C18:2, C20, and C20:4). Higher levels of all of these metabolites were associated with larger annual eGFR reduction (effect sizes per SD ranged from −0.15 to −0.25 ml/min per 1.73 m2, all P<8.9E-04; Table 2). Effect sizes for all metabolites are shown in Supplemental Table 4.
Table 2.
Associations of plasma metabolites with change in eGFR in the Nutrition and Health of Aging Population in China study
| Metabolite | Change in eGFR (ml/min per 1.73 m2 per year) | Incident Reduced eGFR | ||
|---|---|---|---|---|
| β (95% Confidence Interval) | P Value | Relative Risk (95% Confidence Interval) | P Value | |
| Cysteine | −0.25 (−0.33 to −0.17) | 2.7E−09 | 1.25 (1.13 to 1.39) | 3.5E−05a |
| C3DC | −0.19 (−0.27 to −0.11) | 5.8E−06 | 1.18 (1.06 to 1.32) | 2.6E−03a |
| C10 | −0.17 (−0.25 to −0.10) | 1.6E−05 | 1.20 (1.07 to 1.33) | 1.1E−03a |
| C20 | −0.18 (−0.26 to −0.09) | 3.8E−05 | 1.12 (1.01 to 1.24) | 3.3E−02 |
| C14:1OH | −0.17 (−0.25 to −0.09) | 4.5E−05 | 1.17 (1.06 to 1.29) | 1.9E−03a |
| C18 | −0.17 (−0.26 to −0.09) | 5.6E−05 | 1.17 (1.06 to 1.30) | 2.0E−03a |
| C18:2 | −0.17 (−0.25 to −0.09) | 1.0E−04 | 1.18 (1.07 to 1.30) | 6.0E−04a |
| C16:2 | −0.16 (−0.24 to −0.07) | 2.0E−04 | 1.16 (1.05 to 1.28) | 4.1E−03 |
| Phenylalanine | −0.16 (−0.24 to −0.07) | 2.0E−04 | 1.16 (1.05 to 1.29) | 4.0E−03 |
| Glutamine | −0.15 (−0.23 to −0.07) | 3.0E−04 | 1.14 (1.02 to 1.27) | 1.6E−02 |
| C8 | −0.14 (−0.22 to −0.06) | 4.0E−04 | 1.15 (1.03 to 1.27) | 1.1E−02 |
| C20:4 | −0.15 (−0.23 to −0.07) | 4.0E−04 | 1.19 (1.08 to 1.31) | 4.0E−04a |
| C5OH | −0.15 (−0.23 to −0.06) | 4.0E−04 | 1.10 (1.01 to 1.21) | 3.7E−02 |
Data are annual change in eGFR or relative risk per SD increase in metabolite concentration, after adjustment for age, sex, region (Beijing/Shanghai), residence (urban/rural), education, current smoking, current drinking, physical activity, body mass index, lipid-lowering medication use, HDL, LDL, cardiovascular disease, hypertension, type 2 diabetes, and eGFR at baseline. Only metabolites associated with annual eGFR change after Bonferroni correction for multiple testing of the 56 metabolites (P<8.9E−04) are shown.
Significant association with incident reduced eGFR with P<3.8E−03 after Bonferroni correction for multiple testing of the 13 metabolites associated with annual eGFR change.
Regarding incident reduced eGFR, seven of these 13 metabolites, including cysteine, short- and medium-chain acylcarnitines (C3DC and C10), and long-chain acylcarnitines (C14:1OH, C18, C18:2 and C20:4), showed positive associations, with relative risks (RRs) ranging from 1.17 to 1.25 (all P<3.8E-03) per SD increment of metabolites (Table 2). In sensitivity analyses, when eGFR was calculated using the CKD-EPI equation or eGFR decline >3% per year were further considered to define reduced eGFR, the results remained similar (Supplemental Table 5). When C-reactive protein was included as a covariate, the associations were not materially changed (data not shown).
In addition, principal components analysis identified six factors according to the scree plot (Supplemental Figure 3). Consistent with the results for individual metabolites, factor 1 (composed mainly of long-chain acylcarnitines) and factor 6 (composed of cysteine and threonine) were positively associated with incident reduced eGFR (all P<0.05; Table 3).
Table 3.
Principal components analysis of plasma metabolites and change in eGFR in the Nutrition and Health of Aging Population in China study
| Factor | Description | Components | Eigenvalue | Change in eGFR (ml/min per 1.73 m2 per year) | Incident Reduced eGFR | ||
|---|---|---|---|---|---|---|---|
| β (95% Confidence Interval) | P Value | Relative Risk (95% Confidence Interval) | P Value | ||||
| 1 | Long-chain acylcarnitines | C18:2, C18, C14OH, C20:4, C20, C18:1, C16:2, C14:1OH, C16:1, C18OH | 11.39 | −0.13 (−0.21 to −0.05) | 0.002 | 1.14 (1.03 to 1.27) | 0.01 |
| 2 | Medium-chain acylcarnitines | C12, C12:1, C14, C12OH, C8, C10, C16, C6, C3DC, C2, C6OH, C10DC | 8.11 | −0.09 (−0.17 to −0.01) | 0.02 | 1.09 (0.98 to 1.21) | 0.12 |
| 3 | Branched-chain and aromatic amino acids | Leucine, valine, isoleucine, alanine, tyrosine, phenylalanine, tryptophan, glutamic acid, proline, methionine, lysine | 5.95 | −0.10 (−0.20 to 0.00) | 0.05 | 1.13 (0.99 to 1.28) | 0.06 |
| 4 | Short-chain acylcarnitines | C0, C3, C4, C5, C5OH | 3.02 | −0.05 (−0.14 to 0.03) | 0.22 | 1.00 (0.89 to 1.12) | 0.97 |
| 5 | Miscellaneous | Serine, glutamine, glycine, ornithine, histidine | 2.02 | −0.11 (−0.20 to −0.02) | 0.01 | 1.03 (0.91 to 1.16) | 0.67 |
| 6 | Miscellaneous | Cysteine, threonine | 1.94 | −0.23 (−0.31 to −0.15) | <0.001 | 1.32 (1.18 to 1.47) | <0.001 |
Six factors were retained according to the scree plot and varimax rotation performed to produce interpretable factors. Metabolites with a factor load ≥0.5 were reported as composing a factor. Data are annual change in eGFR or relative risk per SD increase in factor scores, after adjustment for age, sex, region (Beijing/Shanghai), residence (urban/rural), education, current smoking, current drinking, physical activity, body mass index, lipid-lowering medication use, HDL, LDL, cardiovascular disease, hypertension, type 2 diabetes, and eGFR at baseline.
In stratified analysis, the association of cysteine was more pronounced in participants with baseline eGFR>90 ml/min per 1.73 m2 (RR per SD, 2.71; 95% confidence interval [95% CI], 1.96 to 3.75), compared with the rest of participants with baseline eGFR 60–90 ml/min per 1.73 m2 (RR per SD, 1.14; 95% CI, 1.02 to 1.27) (Pinteraction<0.001). Moreover, the positive associations of C3DC and C10 with incident reduced eGFR were observed only among overweight/obese individuals, but not in normal-weight participants (both Pinteraction<0.05). Additionally, there was no significant interaction between region (Beijing/Shanghai) and the metabolites (all Pinteraction>0.4) (Supplemental Table 6).
Discussion
Using targeted metabolomic approaches, we found that plasma levels of cysteine and a panel of acylcarnitines, especially long-chain species, were significantly associated with change in eGFR in a Chinese cohort, independent of established risk factors including baseline eGFR.
Previously, most circulating short- and medium-chain acylcarnitines and amino acids, including tryptophan, ornithine, citrulline, and homocysteine, as well as ratios such as tyrosine-to-phenylalanine and serine-to-glycine, were reported to change with the progression of CKD or to correlate with eGFR in cross-sectional studies (25–28). Consistently, significant associations of the majority of these metabolites with eGFR were also indicated in our study. To date, only four longitudinal studies have investigated the link between metabolomic markers and change in eGFR in populations of European or African ancestry, with mixed findings (9–12). The null associations of cysteine and acylcarnitines with change in eGFR in these studies might be ascribed to ethnic diversities in genetic predisposition, diet, and lifestyle. In addition, only one study examined cysteine using a semiquantitative nontargeted method (9); however, three of these studies had a relatively narrow coverage of acylcarnitines (from <10 to 20) (9–11). In contrast, our study measured 34 acylcarnitines using a method involving chromatographic separation. This wider coverage of acylcarnitines apparently allowed us to discover more novel acylcarnitines and their associations. Besides the independent association with incident reduced eGFR, our earlier study previously demonstrated for the first time that a panel of acylcarnitines was associated with risk of incident type 2 diabetes in the same population (16).
Of all of the metabolites, plasma cysteine levels showed the strongest association with incident reduced eGFR, after multivariable adjustment including baseline eGFR. Cysteine and its oxidized form cystine are the most abundant small-molecular-weight thiol/disulfide couple in plasma, and their imbalance may amplify oxidative stress which is closely related to CKD risk (29,30). Previously, higher plasma levels of total cysteine, including free cystine, cysteine, and their protein-bound forms, were showed in patients with declined kidney function before receiving hemodialysis (31,32). Our study further suggested different associations of cysteine and cystine with CKD risk. In vitro studies have revealed detrimental effects of cysteine on various cell types, probably through generating reactive oxygen species or forming an adduct with nitric oxide (33–35). Thus, the cytotoxic properties of cysteine may impair endothelial function and induce kidney dysfunction (36). Another interesting finding of our study was that the association of cysteine with incident reduced eGFR was more pronounced in participants with normal kidney function (eGFR>90 ml/min per 1.73 m2) than those with mildly impaired kidney function (eGFR 60–90 ml/min per 1.73 m2) (20). The kidney has a compensatory ability to maintain GFR, and the creatinine-based eGFR may not be sensitive enough to indicate incipient kidney dysfunction (6). Collectively, cysteine might be a potential marker of incident kidney dysfunction, especially during the early-stages.
Our study also highlighted long-chain acylcarnitines as another important group of metabolites that might play a role in kidney dysfunction. Long-chain acylcarnitines transport cytosolic long-chain fatty acids across the inner mitochondrial membrane for β-oxidation (37). Accumulation of long-chain acylcarnitines, the initial metabolites of β-oxidation, might reflect mitochondrial dysfunction induced by lipotoxicity, which could drive the progression of kidney impairment (38,39). In fact, dramatic mitochondrial damage in multiple kidney cell types was found in mice fed a high-fat diet, possibly due to suppressing AMP kinase activity and subsequently hindering fatty acid oxidation (FAO) in the kidney (40,41). Nevertheless, future studies are needed to elucidate the role of long-chain acylcarnitines in the pathogenesis of kidney disease.
Interestingly, unlike long-chain acylcarnitines, the associations of medium- and short-chain species C10 (decanoylcarnitine) and C3DC (malonylcarnitine) with incident reduced eGFR became significant only after baseline eGFR was adjusted, implying that baseline eGFR might confound their associations. Indeed, medium- but not long-chain acylcarnitines were shown to decrease from kidney arterial to venous levels in individuals undergoing catheterization, suggesting that reduced eGFR could influence kidney uptake or elimination of these metabolites (42). The positive associations of C3DC and C10 with incident kidney dysfunction might be attributed to their effects on dysregulating FAO and mitochondrial stress, which subsequently impairs kidney function (43,44). It was also interesting that the associations of C3DC and C10 with future CKD risk were pronounced only in overweight/obese individuals. It is possible that obesity might increase FAO flux and induce incomplete FAO and accumulated acylcarnitines. Consequently, higher malonyl-CoA (indicated by C3DC) and C10 might inhibit CPT1 and FAO and counteract obesity-related mitochondrial overload (38). Nonetheless, whether C10 and C3DC are useful markers for CKD prevention and management needs to be addressed in future studies.
To our knowledge, this is the first relatively large-scale population-based cohort study investigating associations of amino acid and acylcarnitine profiles in relation to change in eGFR in Asians. Another strength of this study is that a multitude of covariates (including demographic factors, lifestyle, medication use, and medical history) were carefully controlled. Finally, a sensitive, targeted, quantitative metabolomics method was used to measure a broad spectrum of acylcarnitines, providing a unique opportunity to explore novel markers. There were also some limitations to our study. First, our findings warrant replication in an independent population. On the other hand, our study comprised two subpopulations from Beijing and Shanghai, with differences in diet, lifestyle, prevalence of obesity, hypertension, and diabetes (45,46), and the associations persisted in both subpopulations. Second, comparing all eligible participants, those lost to follow-up were more likely to be urban residents, and have higher levels of educational attainment, family income, and plasma triglycerides (14). Of note, the rate of loss to follow-up (23%) in this study was comparable with other studies (47). Third, we did not have direct measurement of kidney function, which is generally not feasible in large population studies. Instead, we used the modified MDRD study equation for Chinese individuals along with other definitions and obtained consistent results (18–21). Fourth, we could not evaluate the effect of proteinuria on associations of interest, because data for proteinuria were not available in our cohort. However, adjustment for proteinuria in a Japanese cohort study did not abolish the association between homocysteine and incident reduced eGFR (48). Homocysteine is a molecule sharing similar biologic properties with cysteine, implying that the associations between cysteine and incident reduced eGFR in our study might also be independent of proteinuria. Fifth, although plasma amino acid levels may be affected by diet and other modifiable factors, available evidence suggests that their levels are reproducible over 1–2 years (rs>0.4) (49). Sixth, cardiovascular disease assessment may have been subject to misclassification because it was exclusively on the basis of self-report. Finally, our study was conducted in a middle-aged and elderly Chinese population, and the results may not be generalizable to other ethnicities or younger populations. Moreover, the generalizability may be further limited by the fact that the rate of eGFR loss in our study was relatively faster than the apparently healthy population in Israel (−2.2 versus −1 ml/min per 1.73 m2 per year), which might be attributable to the high prevalence of CKD risk factors like type 2 diabetes and hypertension in our population at baseline (50).
In conclusion, our study showed that elevated plasma cysteine and a panel of acylcarnitines, especially long-chain acylcarnitines, were prospectively associated with change in eGFR, independent of baseline eGFR and other established and potential CKD risk factors in a Chinese population. More studies are merited to confirm our findings and also to elucidate relevant mechanisms.
Disclosures
None.
Supplementary Material
Acknowledgments
We are grateful to all participants of the Nutrition and Health of Aging Population in China study. We also thank Gang Liu, Qianlu Jin, He Zheng, Yao Hu, Pang Yao, Yiwei Ma, Quan Xiong, Shaofeng Huo, Zhenhua Niu, and Di Wang for their kind help at various stages of this study.
This work was supported by the Ministry of Science and Technology of China (2012CB524900, 2014CB910500, 2016YFC1304903, and 2017YFC0909700), the National Natural Science Foundation of China (30930081, 31470808, 81321062, 8151101068, 91539124, 81471013, and 81700700), and the Chinese Academy of Sciences (KJZD-EW-L14, KSCX2-EW-R-10, and ZDBS-SSW-DQC-02).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.07650717/-/DCSupplemental.
References
- 1.Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, Saran R, Wang AY, Yang CW: Chronic kidney disease: Global dimension and perspectives. Lancet 382: 260–272, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Couser WG, Remuzzi G, Mendis S, Tonelli M: The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int 80: 1258–1270, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Zhang L, Wang F, Wang L, Wang W, Liu B, Liu J, Chen M, He Q, Liao Y, Yu X, Chen N, Zhang JE, Hu Z, Liu F, Hong D, Ma L, Liu H, Zhou X, Chen J, Pan L, Chen W, Wang W, Li X, Wang H: Prevalence of chronic kidney disease in China: A cross-sectional survey. Lancet 379: 815–822, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Yang G, Kong L, Zhao W, Wan X, Zhai Y, Chen LC, Koplan JP: Emergence of chronic non-communicable diseases in China. Lancet 372: 1697–1705, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Chen N, Hsu C-C, Yamagata K, Langham R: Challenging chronic kidney disease: Experience from chronic kidney disease prevention programs in Shanghai, Japan, Taiwan and Australia. Nephrology (Carlton) 15[Suppl 2]: 31–36, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Stevens LA, Coresh J, Greene T, Levey AS: Assessing kidney function--measured and estimated glomerular filtration rate. N Engl J Med 354: 2473–2483, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Levey AS, Perrone RD, Madias NE: Serum creatinine and renal function. Annu Rev Med 39: 465–490, 1988 [DOI] [PubMed] [Google Scholar]
- 8.Hocher B, Adamski J: Metabolomics for clinical use and research in chronic kidney disease. Nat Rev Nephrol 13: 269–284, 2017 [DOI] [PubMed] [Google Scholar]
- 9.Sekula P, Goek ON, Quaye L, Barrios C, Levey AS, Römisch-Margl W, Menni C, Yet I, Gieger C, Inker LA, Adamski J, Gronwald W, Illig T, Dettmer K, Krumsiek J, Oefner PJ, Valdes AM, Meisinger C, Coresh J, Spector TD, Mohney RP, Suhre K, Kastenmüller G, Köttgen A: A metabolome-wide association study of kidney function and disease in the general population. J Am Soc Nephrol 27: 1175–1188, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu B, Zheng Y, Nettleton JA, Alexander D, Coresh J, Boerwinkle E: Serum metabolomic profiling and incident CKD among African Americans. Clin J Am Soc Nephrol 9: 1410–1417, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goek ON, Prehn C, Sekula P, Römisch-Margl W, Döring A, Gieger C, Heier M, Koenig W, Wang-Sattler R, Illig T, Suhre K, Adamski J, Köttgen A, Meisinger C: Metabolites associate with kidney function decline and incident chronic kidney disease in the general population. Nephrol Dial Transplant 28: 2131–2138, 2013 [DOI] [PubMed] [Google Scholar]
- 12.Rhee EP, Clish CB, Ghorbani A, Larson MG, Elmariah S, McCabe E, Yang Q, Cheng S, Pierce K, Deik A, Souza AL, Farrell L, Domos C, Yeh RW, Palacios I, Rosenfield K, Vasan RS, Florez JC, Wang TJ, Fox CS, Gerszten RE: A combined epidemiologic and metabolomic approach improves CKD prediction. J Am Soc Nephrol 24: 1330–1338, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ye X, Yu Z, Li H, Franco OH, Liu Y, Lin X: Distributions of C-reactive protein and its association with metabolic syndrome in middle-aged and older Chinese people. J Am Coll Cardiol 49: 1798–1805, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Zong G, Zhu J, Sun L, Ye X, Lu L, Jin Q, Zheng H, Yu Z, Zhu Z, Li H, Sun Q, Lin X: Associations of erythrocyte fatty acids in the de novo lipogenesis pathway with risk of metabolic syndrome in a cohort study of middle-aged and older Chinese. Am J Clin Nutr 98: 319–326, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Liu G, Deng Y, Sun L, Ye X, Yao P, Hu Y, Wang F, Ma Y, Li H, Liu Y, Sun Q, Lin X: Elevated plasma tumor necrosis factor-α receptor 2 and resistin are associated with increased incidence of kidney function decline in Chinese adults. Endocrine 52: 541–549, 2016 [DOI] [PubMed] [Google Scholar]
- 16.Sun L, Liang L, Gao X, Zhang H, Yao P, Hu Y, Ma Y, Wang F, Jin Q, Li H, Li R, Liu Y, Hu FB, Zeng R, Lin X, Wu J: Early prediction of developing type 2 diabetes by plasma acylcarnitines: A population-based study. Diabetes Care 39: 1563–1570, 2016 [DOI] [PubMed] [Google Scholar]
- 17.Gao X, Pujos-Guillot E, Sébédio J-L: Development of a quantitative metabolomic approach to study clinical human fecal water metabolome based on trimethylsilylation derivatization and GC/MS analysis. Anal Chem 82: 6447–6456, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Ma Y-C, Zuo L, Chen J-H, Luo Q, Yu X-Q, Li Y, Xu JS, Huang SM, Wang LN, Huang W, Wang M, Xu GB, Wang HY: Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol 17: 2937–2944, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Kong X, Ma Y, Chen J, Luo Q, Yu X, Li Y, Xu J, Huang S, Wang L, Huang W, Wang M, Xu G, Zhang L, Zuo L, Wang H; Chinese eGFR Investigation Collaboration : Evaluation of the Chronic Kidney Disease Epidemiology Collaboration equation for estimating glomerular filtration rate in the Chinese population. Nephrol Dial Transplant 28: 641–651, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, de Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, Gansevoort RT, Kasiske BL, Eckardt KU: The definition, classification, and prognosis of chronic kidney disease: A KDIGO Controversies Conference report. Kidney Int 80: 17–28, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Bash LD, Coresh J, Köttgen A, Parekh RS, Fulop T, Wang Y, Astor BC: Defining incident chronic kidney disease in the research setting: The ARIC Study. Am J Epidemiol 170: 414–424, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zou G: A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 159: 702–706, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Skov T, Deddens J, Petersen MR, Endahl L: Prevalence proportion ratios: Estimation and hypothesis testing. Int J Epidemiol 27: 91–95, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Goek ON, Döring A, Gieger C, Heier M, Koenig W, Prehn C, Römisch-Margl W, Wang-Sattler R, Illig T, Suhre K, Sekula P, Zhai G, Adamski J, Köttgen A, Meisinger C: Serum metabolite concentrations and decreased GFR in the general population. Am J Kidney Dis 60: 197–206, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Duranton F, Lundin U, Gayrard N, Mischak H, Aparicio M, Mourad G, Daurès JP, Weinberger KM, Argilés A: Plasma and urinary amino acid metabolomic profiling in patients with different levels of kidney function. Clin J Am Soc Nephrol 9: 37–45, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shah VO, Townsend RR, Feldman HI, Pappan KL, Kensicki E, Vander Jagt DL: Plasma metabolomic profiles in different stages of CKD. Clin J Am Soc Nephrol 8: 363–370, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen DQ, Cao G, Chen H, Liu D, Su W, Yu XY, Vaziri ND, Liu XH, Bai X, Zhang L, Zhao YY: Gene and protein expressions and metabolomics exhibit activated redox signaling and wnt/β-catenin pathway are associated with metabolite dysfunction in patients with chronic kidney disease. Redox Biol 12: 505–521, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kao MP, Ang DS, Pall A, Struthers AD: Oxidative stress in renal dysfunction: Mechanisms, clinical sequelae and therapeutic options. J Hum Hypertens 24: 1–8, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Moriarty-Craige SE, Jones DP: Extracellular thiols and thiol/disulfide redox in metabolism. Annu Rev Nutr 24: 481–509, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Arnadottir M, Hultberg B, Nilsson-Ehle P, Thysell H: The effect of reduced glomerular filtration rate on plasma total homocysteine concentration. Scand J Clin Lab Invest 56: 41–46, 1996 [DOI] [PubMed] [Google Scholar]
- 32.Hultberg B, Andersson A, Arnadottir M: Reduced, free and total fractions of homocysteine and other thiol compounds in plasma from patients with renal failure. Nephron 70: 62–67, 1995 [DOI] [PubMed] [Google Scholar]
- 33.Nishiuch Y, Sasaki M, Nakayasu M, Oikawa A: Cytotoxicity of cysteine in culture media. In Vitro 12: 635–638, 1976 [DOI] [PubMed] [Google Scholar]
- 34.Saez G, Thornalley PJ, Hill HAO, Hems R, Bannister JV: The production of free radicals during the autoxidation of cysteine and their effect on isolated rat hepatocytes. Biochim Biophys Acta 719: 24–31, 1982 [DOI] [PubMed] [Google Scholar]
- 35.Sheu F-S, Zhu W, Fung PC: Direct observation of trapping and release of nitric oxide by glutathione and cysteine with electron paramagnetic resonance spectroscopy. Biophys J 78: 1216–1226, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jia L, Furchgott RF: Inhibition by sulfhydryl compounds of vascular relaxation induced by nitric oxide and endothelium-derived relaxing factor. J Pharmacol Exp Ther 267: 371–378, 1993 [PubMed] [Google Scholar]
- 37.Steiber A, Kerner J, Hoppel CL: Carnitine: A nutritional, biosynthetic, and functional perspective. Mol Aspects Med 25: 455–473, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JR, Newgard CB, Lopaschuk GD, Muoio DM: Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 7: 45–56, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Weinberg JM: Lipotoxicity. Kidney Int 70: 1560–1566, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Szeto HH, Liu S, Soong Y, Alam N, Prusky GT, Seshan SV: Protection of mitochondria prevents high-fat diet-induced glomerulopathy and proximal tubular injury. Kidney Int 90: 997–1011, 2016 [DOI] [PubMed] [Google Scholar]
- 41.Declèves AE, Mathew AV, Cunard R, Sharma K: AMPK mediates the initiation of kidney disease induced by a high-fat diet. J Am Soc Nephrol 22: 1846–1855, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kalim S, Clish CB, Wenger J, Elmariah S, Yeh RW, Deferio JJ, Pierce K, Deik A, Gerszten RE, Thadhani R, Rhee EP: A plasma long-chain acylcarnitine predicts cardiovascular mortality in incident dialysis patients. J Am Heart Assoc 2: e000542, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ruderman NB, Saha AK, Vavvas D, Witters LA: Malonyl-CoA, fuel sensing, and insulin resistance. Am J Physiol 276: E1–E18, 1999 [DOI] [PubMed] [Google Scholar]
- 44.Williamson JR, Browning ET, Scholz R, Kreisberg RA, Fritz IB: Inhibition of fatty acid stimulation of gluconeogenesis by (+)-decanoylcarnitine in perfused rat liver. Diabetes 17: 194–208, 1968 [DOI] [PubMed] [Google Scholar]
- 45.Xu S, Yin X, Li S, Jin W, Lou H, Yang L, Gong X, Wang H, Shen Y, Pan X, He Y, Yang Y, Wang Y, Fu W, An Y, Wang J, Tan J, Qian J, Chen X, Zhang X, Sun Y, Zhang X, Wu B, Jin L: Genomic dissection of population substructure of Han Chinese and its implication in association studies. Am J Hum Genet 85: 762–774, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu Z, Lin X, Haas JD, Franco OH, Rennie KL, Li H, Xu H, Pang X, Liu H, Zhang Z, Zou S, Jiao S; Nutrition and Health of Ageing Population in China Study Research Group : Obesity related metabolic abnormalities: Distribution and geographic differences among middle-aged and older Chinese populations. Prev Med 48: 272–278, 2009 [DOI] [PubMed] [Google Scholar]
- 47.Vega S, Benito-León J, Bermejo-Pareja F, Medrano MJ, Vega-Valderrama LM, Rodríguez C, Louis ED: Several factors influenced attrition in a population-based elderly cohort: Neurological disorders in Central Spain Study. J Clin Epidemiol 63: 215–222, 2010 [DOI] [PubMed] [Google Scholar]
- 48.Ninomiya T, Kiyohara Y, Kubo M, Tanizaki Y, Tanaka K, Okubo K, Nakamura H, Hata J, Oishi Y, Kato I, Hirakata H, Iida M: Hyperhomocysteinemia and the development of chronic kidney disease in a general population: The Hisayama study. Am J Kidney Dis 44: 437–445, 2004 [PubMed] [Google Scholar]
- 49.Townsend MK, Clish CB, Kraft P, Wu C, Souza AL, Deik AA, Tworoger SS, Wolpin BM: Reproducibility of metabolomic profiles among men and women in 2 large cohort studies. Clin Chem 59: 1657–1667, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cohen E, Nardi Y, Krause I, Goldberg E, Milo G, Garty M, Krause I: A longitudinal assessment of the natural rate of decline in renal function with age. J Nephrol 27: 635–641, 2014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.