Abstract
Background and objectives
Dysregulated mineral metabolism is a common and potentially maladaptive feature of critical illness, especially in patients with AKI, but its association with death has not been comprehensively investigated. We sought to determine whether elevated plasma levels of the osteocyte-derived, vitamin D–regulating hormone, fibroblast growth factor 23 (FGF23), are prospectively associated with death in critically ill patients with AKI requiring RRT, and in a general cohort of critically ill patients with and without AKI.
Design, setting, participants, & measurements
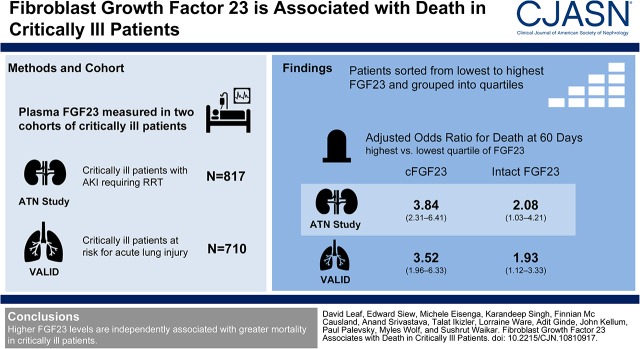
We measured plasma FGF23 and other mineral metabolite levels in two cohorts of critically ill patients (n=1527). We included 817 patients with AKI requiring RRT who enrolled in the ARF Trial Network (ATN) study, and 710 patients with and without AKI who enrolled in the Validating Acute Lung Injury biomarkers for Diagnosis (VALID) study. We hypothesized that higher FGF23 levels at enrollment are independently associated with higher 60-day mortality.
Results
In the ATN study, patients in the highest compared with lowest quartiles of C-terminal (cFGF23) and intact FGF23 (iFGF23) had 3.84 (95% confidence interval, 2.31 to 6.41) and 2.08 (95% confidence interval, 1.03 to 4.21) fold higher odds of death, respectively, after adjustment for demographics, comorbidities, and severity of illness. In contrast, plasma/serum levels of parathyroid hormone, vitamin D metabolites, calcium, and phosphate were not associated with 60-day mortality. In the VALID study, patients in the highest compared with lowest quartiles of cFGF23 and iFGF23 had 3.52 (95% confidence interval, 1.96 to 6.33) and 1.93 (95% confidence interval, 1.12 to 3.33) fold higher adjusted odds of death.
Conclusions
Higher FGF23 levels are independently associated with greater mortality in critically ill patients.
Keywords: acute renal failure; Vitamin D; renal dialysis; Humans; fibroblast growth factor 23; parathyroid hormone; Critical Illness; Osteocytes; Confidence Intervals; Renal Replacement Therapy; Fibroblast Growth Factors; Acute Kidney Injury; Calcium, Dietary; Biomarkers; Minerals; Comorbidity; Acute Lung Injury; Demography; Phosphates; Cohort Studies
Introduction
Dysregulated mineral metabolism, including hypocalcemia, hyperparathyroidism, and low circulating levels of 25-hydroxyvitamin D (25D) and 1,25-dihydroxyvitamin D (1,25D), is an important feature of critical illness (1,2), and is especially pronounced among patients with AKI (3). These mineral metabolite abnormalities may be causally associated with adverse outcomes in critical illness through a variety of pathways, including nonclassic effects of vitamin D metabolites on immunity and inflammation (4). However, the mechanisms responsible for dysregulated mineral metabolism in critical illness are poorly understood.
Fibroblast growth factor 23 (FGF23), an osteocyte-derived hormone initially discovered for its pathologic role in rare syndromes of urinary phosphate wasting (5), plays a critical role in regulating vitamin D and phosphate homeostasis. FGF23 inhibits the conversion of 25D to its biologically active form, 1,25D, while also stimulating the catabolism of both 25D and 1,25D (6).
Elevated FGF23 levels are now recognized as a key feature of dysregulated mineral metabolism in patients with CKD (7,8), and are one of the most robust predictors of cardiovascular disease (9) and death (10,11) in this patient population.
In contrast to CKD, few studies investigated FGF23 in critical illness. In a pilot study, we recently reported that higher plasma (n=113) and urinary (n=243) C-terminal FGF23 (cFGF23) levels are associated with an increased risk of the composite end point of AKI or death in critically ill patients (12). However, the study was focused on incident, rather than established, AKI, and thus included few patients with severe AKI at the time of FGF23 measurement; assessed only cFGF23, but not intact FGF23 (iFGF23) levels; and, most importantly, was underpowered to assess mortality.
We therefore analyzed plasma FGF23 levels in patients who enrolled in two large studies: the ARF Trial Network (ATN) study, which included patients with AKI requiring RRT, and the Validating Acute Lung Injury biomarkers for Diagnosis (VALID) study, which included patients with and without AKI. We hypothesized that in both cohorts, higher plasma cFGF23 and iFGF23 levels are independently and strongly associated with greater 60-day mortality.
Materials and Methods
Study Population
ATN Study.
The ATN study, described elsewhere in detail (13), enrolled 1124 critically ill patients with AKI requiring RRT from 27 major United States medical centers into a randomized trial of intensive versus less intensive RRT. Subject enrollment began in November of 2003 and was completed July of 2007. At the time of randomization, EDTA-plasma samples were archived in a biorepository and stored at −80°C. All patients who provided samples for the biorepository (n=817) were included in this study. These patients had similar baseline characteristics and outcomes compared with the overall cohort (n=1124) (Supplemental Table 1).
VALID Study.
The VALID study, described elsewhere in detail (14), is a single-center, prospective cohort study of patients admitted to intensive care units (ICUs) at Vanderbilt University Medical Center (VUMC; Nashville, TN). The VALID study began enrollment in 2006 and is ongoing. Eligible adult patients (≥18 years of age) were enrolled within 24 hours of ICU admission, and EDTA-plasma samples were obtained and stored at −80°C. Patients were excluded from the parent study if they had chronic lung disease requiring oxygen supplementation, pulmonary fibrosis, a history of cardiac arrest, transfer orders written or anticipated within 4 hours, or were admitted for an uncomplicated overdose. Additional exclusion criteria for this study were a history of kidney transplant (n=31), baseline eGFR<15 ml/min per 1.73 m2 (n=15), or receiving dialysis (n=150) at the time of enrollment.
Informed Consent.
In the ATN study, all patients or their surrogates provided written informed consent, and the study was approved by the Human Rights Committee at the West Haven Veterans Affairs Cooperative Studies Program Coordinating Center and by the institutional review boards at each of the participating sites. In the VALID study, the VUMC institutional review board granted a waiver of consent if patients were unable to participate in the consent process and no surrogate was available, due to the minimal risk of the study.
Study Design
We assessed the association between plasma FGF23 levels and death in the ATN (n=817) and VALID (n=710) studies using a prospective cohort and case-cohort study design, respectively. Cases were defined as patients who died within 60 days after enrollment. The case-cohort in VALID was selected from a source population of 1732 patients with samples available (Supplemental Figure 1). An advantage of the case-cohort design is that oversampling of cases is an efficient approach that provides nearly the same magnitude of statistical power compared with the full cohort (15).
Exposures, Measurements, and Outcomes
The primary exposure was plasma FGF23 level, measured on enrollment in both studies. The primary outcome was 60-day mortality. In the VALID study, we also assessed 1-year mortality as a secondary outcome.
FGF23 Measurements.
The immunometric assay for cFGF23 detects both the intact hormone and C-terminal cleavage products, whereas the assay for iFGF23 recognizes the intact, biologically active hormone only (16). Few studies of patients with critical illness have compared the results from both assays. Therefore, we measured both cFGF23 and iFGF23 levels using second generation ELISA kits (Immutopics, San Clemente, CA) in batched assays by investigators who were unaware of the outcomes. Specifically, in the ATN study we measured cFGF23 levels in all 817 participants, and we measured iFGF23 levels in a random subcohort (n=400). Characteristics among participants from the random subcohort (n=400) were similar to the full cohort (n=817) (data not shown). In the VALID study we measured both cFGF23 and iFGF23 in all 710 participants. Interassay coefficients of variation for cFGF23 and iFGF23, determined from blinded replicate samples, were <10%. We report additional performance characteristics for the cFGF23 and iFGF23 assays elsewhere (17).
Additional Measurements.
In the ATN study we also measured plasma levels of vitamin D metabolites, including 25D, 1,25D, and 24,25-dihydroxyvitamin D3, (24,25D3), and intact parathyroid hormone (PTH) in the same random subcohort (n=400) in which we measured iFGF23. Vitamin D metabolites were measured using immunoaffinity enrichment and liquid chromatography–tandem mass spectrometry (18). Serum levels of creatinine, calcium, phosphate, and albumin were measured for clinical purposes by the hospital laboratory in both the ATN and VALID studies. Plasma IL-6 levels were measured in the ATN study only (details reported elsewhere) (19). A summary of the measurements performed in both cohorts, along with assay performance characteristics, is provided in Supplemental Table 2.
Statistical Analyses
We performed the statistical analyses with SAS Version 9.4 (Cary, NC) and R Version 3.2.3 (Vienna, Austria). Baseline characteristics were compared between patients alive or dead at day 60 using Wilcoxon Rank Sum and chi-squared tests for continuous and categoric variables, respectively. In the ATN study, 1.7% of comorbidity and severity of illness data were missing and were imputed using regression switching with predictive mean matching (20). Five datasets were multiply-imputed, and results were pooled using Rubin’s rules (21). In VALID, <0.1% of data were missing and were not imputed. In both cohorts, the primary outcome variable (60-day mortality) was available in all patients.
We assessed the associations between FGF23 and other analyte levels using Spearman correlation coefficients and restricted cubic splines. We used univariable (model 1) and multivariable (models 2 and 3) logistic regression to assess the association between FGF23 and other mineral metabolite levels and 60-day mortality. We assessed FGF23 levels in quartiles to test for nonlinear associations and by natural log–transformation and normalization to 1 SD to allow comparison across biomarkers.
We adjusted for covariates in multivariable models (models 2 and 3) on the basis of univariable associations with 60-day mortality and biologic plausibility. In both cohorts, model 2 was adjusted for age, sex, race, baseline eGFR, diabetes, cancer, congestive heart failure, and chronic liver disease. In the ATN study, model 3 was further adjusted for the following severity of illness covariates assessed on enrollment: ICU type, mechanical ventilation, Acute Physiology and Chronic Health Evaluation (APACHE) II score (22), RRT before randomization, treatment group, type of RRT, oliguria, sepsis, hypotension, white blood cell count, hemoglobin, serum albumin, serum creatinine, and plasma IL-6 levels. In the VALID study, the severity of illness covariates included in model 3 were ICU type, mechanical ventilation, APACHE II score, sepsis, hypotension, white blood cell count, hemoglobin, serum creatinine, and AKI presence and severity, defined according to serum creatinine–based consensus criteria (23). The covariates included in model 3 differed slightly in the ATN study compared with the VALID study due to differences in the availability of covariates in the two cohorts as well as differences in univariable associations with mortality.
We depicted the association between FGF23 levels and 1-year survival in the VALID study using Kaplan–Meier curves, with differences assessed by the log-rank test. Multivariable adjusted associations were assessed using Cox proportional hazards models. Using Martingale residual plots and Kolmogorov-type supremum tests, we confirmed that the proportional hazards assumption was not violated. We used inverse probability weighting, using the Barlow weighting method (24), to account for the case-cohort design in the VALID study (15). All comparisons are two-tailed, with P<0.05 considered significant.
Results
Patient Characteristics
Demographic and clinical characteristics from participants in the ATN and VALID studies, dichotomized according to survival status at day 60, are shown in Table 1. Demographic and clinical characteristics according to quartiles of cFGF23 and iFGF23 are shown in Supplemental Table 3.
Table 1.
Enrollment characteristics
| Characteristic | ATN Study | VALID Study | ||||
|---|---|---|---|---|---|---|
| All (n=817) | Alive (n=402) | Dead (n=415) | All (n=710) | Alive (n=449) | Dead (n=261) | |
| Demographics | ||||||
| Age, yr, median (IQR) | 62 (51–72) | 59 (48–68) | 64 (54–76)a | 57 (45–68) | 54 (41–65) | 61 (51–73)a |
| Male sex, no. (%) | 568 (70) | 275 (68) | 293 (71) | 439 (62) | 271 (60) | 168 (64) |
| White, no. (%) | 628 (77) | 301 (75) | 327 (79) | 625 (88) | 381 (85) | 244 (93)a |
| Comorbidities, no. (%) | ||||||
| Diabetes mellitus | 199 (25) | 108 (28) | 91 (23) | 166 (23) | 103 (23) | 63 (24) |
| Congestive heart failure | 198 (25) | 93 (24) | 105 (26) | 90 (13) | 47 (10) | 43 (16) |
| Chronic liver disease | 95 (12) | 38 (10) | 57 (14) | 77 (11) | 46 (10) | 31 (12) |
| Chronic lung disease/COPDb | 103 (13) | 43 (11) | 60 (15) | 107 (15) | 68 (15) | 39 (15) |
| CKDc | 293 (38) | 137 (37) | 156 (39) | 183 (26) | 83 (18) | 100 (38)a |
| Malignancy | 158 (19) | 69 (17) | 89 (21) | 109 (15) | 58 (13) | 51 (20)d |
| Baseline kidney function | ||||||
| Creatinine, mg/dl, median (IQR)e | 1.1 (0.9–1.4) | 1.1 (0.8–1.4) | 1.1 (0.9–1.4) | 0.9 (0.7–1.2) | 0.8 (0.6–1.0) | 1.0 (0.8–1.4)a |
| eGFR, median (IQR)f | 71 (50–94) | 73 (52–96) | 70 (49–89) | 90 (60–112) | 98 (70–117) | 72 (50–98)a |
| Medical ICU, no. (%) | 361 (44) | 176 (44) | 185 (45) | 329 (46) | 181 (40) | 148 (57)a |
| Severity of illness | ||||||
| Sepsis, no. (%) | 527 (65) | 253 (63) | 274 (66) | 297 (42) | 162 (36) | 135 (52)a |
| APACHE II score, median (IQR)g | 26 (21–31) | 23 (19–29) | 28 (24–33)a | 26 (20–31) | 23 (19–29) | 29 (23–34)a |
| Mechanical ventilation, no. (%) | 661 (81) | 293 (73) | 368 (89)a | 512 (72) | 330 (74) | 182 (70) |
| Hypotension, no. (%)h | 515 (63) | 217 (54) | 298 (72)a | 450 (63) | 276 (61) | 174 (67) |
| AKI and RRT data on enrollment, no. (%) | ||||||
| AKIi | 817 (100) | 402 (100) | 415 (100) | 348 (49) | 228 (51) | 120 (46) |
| AKI severity | ||||||
| Stage 1 | 0 (0) | 0 (0) | 0 (0) | 238 (34) | 155 (35) | 83 (32) |
| Stage 2 | 0 (0) | 0 (0) | 0 (0) | 81 (11) | 54 (12) | 27 (10) |
| Stage 3 | 817 (100) | 402 (100) | 415 (100) | 29 (4) | 19 (4) | 10 (4) |
| Oliguriaj | 654 (80) | 300 (75) | 354 (85)a | |||
| RRT before enrollment | 561 (69) | 275 (68) | 286 (69) | |||
| RRT data in the ATN study, no. (%) | ||||||
| Treatment group | ||||||
| Intensive RRT | 403 (49) | 191 (48) | 212 (51) | |||
| Less intensive RRT | 414 (51) | 211 (52) | 203 (49) | |||
| Type of RRT | ||||||
| CRRT | 557 (68) | 237 (58) | 320 (77)a | |||
| Intermittent RRT | 266 (32) | 170 (42) | 96 (23)a | |||
Percentages are on the basis of the number of patients without missing data. ATN, ARF Trial Network; VALID, Validating Acute Lung Injury biomarkers for Diagnosis; IQR, interquartile range; COPD, chronic obstructive pulmonary disease; ICU, intensive care unit; APACHE II, Acute Physiology and Chronic Health Evaluation II; CRRT, continuous renal replacement therapy.
P<0.001, for comparison between patients alive versus dead at day 60.
Defined in the ATN study as chronic hypoxemia, hypercapnea, pulmonary hypertension, or ventilator dependence; defined in VALID as COPD.
Defined as baseline eGFR<60 ml/min per 1.73 m2.
P<0.05, for comparison between patients alive versus dead at day 60.
Defined in the ATN study as the premorbid serum creatinine (SCr) at the time of screening or, if unavailable, the lowest SCr within 4 d before screening; defined in VALID as the lowest SCr within 365 d before hospitalization. If no SCr values were available before hospitalization, the lowest SCr during hospitalization (excluding values obtained during RRT) was used as the baseline (44).
Reported in ml/min per 1.73 m2 and determined using the Chronic Kidney Disease Epidemiology Collaboration Equation (45).
An ICU severity of illness scoring system ranging from 0 to 71, with higher scores indicating more severe disease.
Defined in the ATN study as requirement for vasopressor support for >1 h; defined in VALID as systolic BP ≤90 mm Hg or need for vasopressor support.
All patients in the ATN study had AKI requiring RRT on enrollment; in VALID, AKI on enrollment was defined as an increase in SCr ≥0.3 mg/dl within 48 h or ≥50% within 7 d before enrollment (23).
Defined in the ATN study as an average urine output <20 ml/h for >24 h before enrollment.
Laboratory Values
Median cFGF23 levels were 3608 (interquartile range [IQR], 1079–12,983 RU/ml) and 603 (IQR, 483–1183 RU/ml), and median iFGF23 levels were 88 (IQR, 30–239 pg/ml) and 44 (24–87 pg/ml), among participants in the ATN and VALID studies, respectively. Compared with informal reference ranges (cFGF23, 21–82 RU/ml; iFGF23, 3.8–18.7 pg/ml) reported in elderly adults with normal kidney function (25), we observed markedly elevated cFGF23 levels and modestly elevated iFGF23 levels. Additional laboratory values are shown in Table 2.
Table 2.
Enrollment laboratory values
| Variable | ATN Study | VALID Study | ||||||
|---|---|---|---|---|---|---|---|---|
| All (n=817) | Alive (n=402) | Dead (n=415) | P Valuea | All (n=710) | Alive (n=449) | Dead (n=261) | P Valuea | |
| Routine labs, median (IQR) | ||||||||
| White cell count, per mm3 | 13 (9–19) | 13 (9–18) | 14 (8–20) | 0.46 | 14 (9–19) | 13 (9–19) | 14 (9–19) | 0.51 |
| Hemoglobin, g/dl | 9.8 (8.9–10.8) | 9.7 (8.8–10.6) | 9.9 (9.0–10.9) | 0.04 | 9.8 (8.8–11.0) | 10.0 (8.9–11.1) | 9.7 (8.8–11.0) | 0.50 |
| Creatinine, mg/dl | 3.9 (2.9–5.1) | 4.3 (3.2–5.6) | 3.6 (2.7–4.6) | <0.001 | 1.1 (0.9–1.7) | 1.0 (0.8–1.4) | 1.4 (1.0–2.1) | <0.001 |
| Albumin, g/dl | 2.3 (1.9–2.8) | 2.4 (1.9–3.0) | 2.3 (1.8–2.8) | 0.03 | ||||
| cFGF23, RU/ml | ||||||||
| Median | 3608 | 1918 | 5819 | <0.001 | 603 | 483 | 1183 | <0.001 |
| IQR | 1079–12,983 | 740–7736 | 1656–18,731 | 212–1882 | 181–1218 | 366–4344 | ||
| iFGF23, pg/ml | ||||||||
| Median | 88 | 81 | 97 | 0.36 | 44 | 38 | 51 | <0.001 |
| IQR | 30–239 | 24–266 | 40–224 | 24–87 | 23–76 | 27–107 | ||
| Other markers, median (IQR)b | ||||||||
| Calcium, mg/dl | 7.7 (7.1–8.3) | 7.8 (7.2–8.4) | 7.7 (7.0–8.3) | 0.33 | 7.8 (7.4–8.3) | 7.8 (7.5–8.3) | 7.8 (7.4–8.3) | 0.52 |
| Phosphate, mg/dl | 5.2 (4.0–6.7) | 5.1 (4.0–6.7) | 5.2 (4.0–6.7) | 0.79 | 3.3 (2.5–4.3) | 3.2 (2.5–4.2) | 3.4 (2.4–4.7) | 0.44 |
| Parathyroid hormone, pg/ml | 304 (118–588) | 306 (118–567) | 301 (120–592) | 0.77 | ||||
| 25-hydroxyvitamin D, ng/ml | 10 (6–14) | 11 (7–14) | 9 (6–13) | 0.01 | ||||
| 1,25-dihydroxyvitamin D, pg/ml | 12 (8–18) | 11 (7–20) | 12 (8–18) | 0.73 | ||||
| 24,25-dihydroxyvitamin D3, ng/ml | 0.4 (0.3–0.7) | 0.5(0.3–0.7) | 0.4 (0.3–0.7) | 0.82 | ||||
| IL-6, pg/ml | 166 (74–539) | 125 (57–281) | 230 (104–911) | <0.001 | ||||
ATN, ARF Trial Network; VALID, Validating Acute Lung Injury biomarkers for Diagnosis; IQR, interquartile range; cFG;23, c-terminal fibroblast growth factor 23; RU/ml, reference units per millilter; iFGF23, intact fibroblast growth factor 23.
Comparison between patients alive versus dead at day 60.
Parathyroid hormone and vitamin D metabolite levels were available in a subcohort (n=400) of patients in the ATN study and were not available in VALID. IL-6 levels were available in all participants in the ATN study and were not available in VALID.
Association between FGF23 and Other Mineral Metabolite Levels
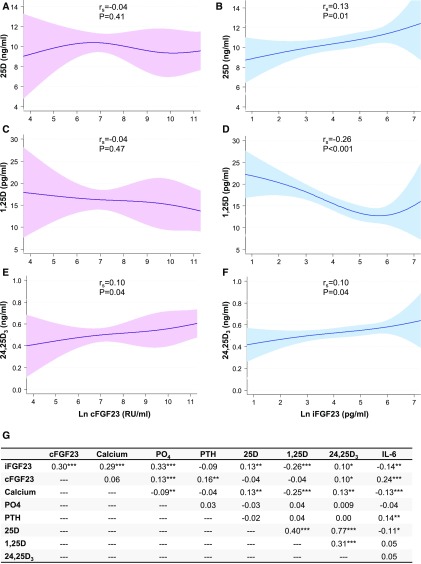
The associations between FGF23 and vitamin D metabolite levels are shown in Figure 1, A–F. The strongest association was between higher levels of iFGF23 and lower levels of 1,25D (rs=−0.26, P<0.001; Figure 1D), consistent with FGF23-mediated inhibition of 1-α hydroxylase. Additional correlations are shown in Figure 1G.
Figure 1.
Associations between FGF23 and other mineral metabolites in the ARF Trial Network study. Restricted cubic spline graphs depict the relationships between cFGF23, iFGF23, and (A and B) 25D, (C and D) 1,25D, and (E and F) 24,25D3 (n=394 for each graph). Four knots were specified at the fifth, 35th, 65th, and 95th FGF23 percentiles. The 95% confidence intervals are indicated by the shaded areas. (G) The Spearman correlation coefficient for each comparison. *P<0.05; **P<0.01; ***P<0.001. 25D, 25-hydroxyvitamin D; 1,25D, 1,25-dihydroxyvitamin D; 24,25D3, 24,25-dihydroxyvitamin D3; cFGF23, C-terminal fibroblast growth factor 23; iFGF23, intact fibroblast growth factor 23; Ln, natural log-transformed; PO4, phosphate; PTH, parathyroid hormone; rs, Spearman coefficient; RU/ml, reference units per milliliter.
FGF23 Levels and Mortality in the ATN Study
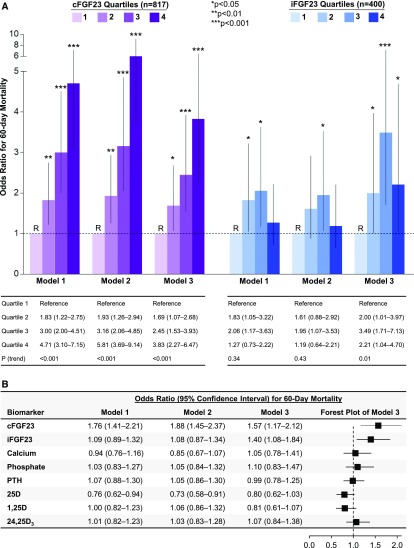
Higher quartiles of cFGF23 were associated with a monotonic increase in the risk of death in both unadjusted and adjusted analyses (adjusted odds ratio [OR], 3.83, for patients in the highest compared with lowest quartiles; 95% confidence interval (CI), 2.27 to 6.47; Figure 2A). Higher quartiles of iFGF23, by contrast, were associated with a nonlinear increase in the risk of death in unadjusted models, and were associated with a semilinear increase in the risk of death after adjustment for comorbidities and severity of illness (Figure 2A).
Figure 2.
Higher cFGF23 and iFGF23 levels associate with increased 60-day mortality in the ARF Trial Network study. (A) Odds ratios for 60-day mortality according to quartiles of cFGF23 and iFGF23 levels. Quartile 1 was the reference (R) group in all models. cFGF23 levels by quartile: quartile 1, <1072 RU/ml; quartile 2, 1078–3587 RU/ml; quartile 3, 3608–12,951 RU/ml; quartile 4, >12,983 RU/ml. iFGF23 levels by quartile: quartile 1, <30 pg/ml; quartile 2, 30–88 pg/ml; quartile 3, 88–240 pg/ml; quartile 4, >241 pg/ml. (B) Odds ratios for 60-day mortality according to natural log–transformed mineral metabolite levels standardized to 1 SD. Model 1 is unadjusted. Model 2 is adjusted for demographics and comorbidities (age, sex, race, baseline eGFR, diabetes, cancer, congestive heart failure, and chronic liver disease). Model 3 is further adjusted for severity of illness (intensive care unit type, mechanical ventilation, Acute Physiology and Chronic Health Evaluation II score, RRT before randomization, treatment group, type of RRT, oliguria, sepsis, hypotension, white blood cell count, hemoglobin, and serum/plasma levels of albumin, creatinine, and IL-6). 25D, 25-hydroxyvitamin D; 1,25D, 1,25-dihydroxyvitamin D; 24,25D3, 24,25-dihydroxyvitamin D3; cFGF23, C-terminal fibroblast growth factor 23; iFGF23, intact fibroblast growth factor 23; PTH, parathyroid hormone; RU/ml, reference units per milliliter.
Next, we assessed the magnitude of association between each mineral metabolite, expressed as a natural log–transformed continuous variable and normalized to 1 SD, with risk of death. In fully adjusted models, higher levels of cFGF23 and iFGF23 were each associated with an increased risk of death, whereas calcium, phosphate, parathyroid hormone, 25D, 1,25D, and 24,25D3 were not (Figure 2B).
Exploratory Analyses.
In exploratory analyses, the associations between cFGF23, iFGF23, and death remained significant after further adjustment for 25D, 1,25D, and serum phosphate levels (Supplemental Table 4). cFGF23 remained associated with death after adjustment for iFGF23 (Supplemental Table 5). The relationship between cFGF23 and death was consistent across a number of subgroups, including age (above versus below 65 years), sex, ICU type, sepsis, shock, APACHE II score (above versus below 25), RRT before randomization, and 25D and 1,25D levels above versus below the median (Supplemental Figure 2).
FGF23 Levels and Mortality in VALID
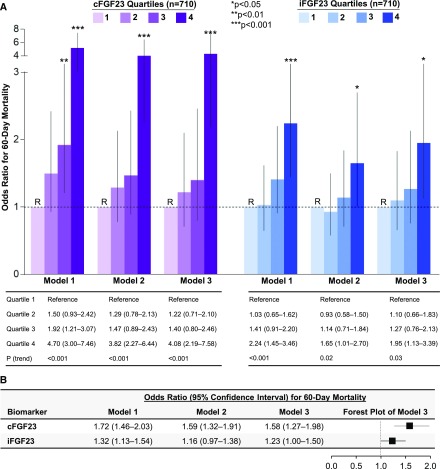
In the VALID study, higher quartiles of cFGF23 and iFGF23 were each associated with an increase in the risk of death in both unadjusted and adjusted analyses (adjusted OR, 4.08, for patients in the highest compared with lowest quartiles of cFGF23; 95% CI, 2.19 to 7.58; adjusted OR, 1.95, for patients in the highest compared with lowest quartiles of iFGF23; 95% CI, 1.13 to 3.39; Figure 3A). Higher cFGF23 and iFGF23 levels, assessed as natural log–transformed continuous variables, were also associated with increased 60-day mortality in both unadjusted and fully adjusted models (Figure 3B).
Figure 3.
Higher cFGF23 and iFGF23 levels associate with higher 60-day mortality in the Validating Acute Lung Injury biomarkers for Diagnosis (VALID) study. (A) Odds ratios for 60-day mortality according to quartiles of cFGF23 and iFGF23 levels. Quartile 1 was the reference (R) group in all models. cFGF23 levels by quartile: quartile 1, <211 RU/ml; quartile 2, 212–595 RU/ml; quartile 3, 602–1868 RU/ml; quartile 4, >1886 RU/ml. iFGF23 levels by quartile: quartile 1, <24 pg/ml; quartile 2, 24–44 pg/ml; quartile 3, 44–87 pg/ml; quartile 4, >87 pg/ml. (B) Odds ratios for 60-day mortality according to natural log–transformed FGF23 levels standardized to 1 SD. Model 1 is unadjusted. Model 2 is adjusted for demographics and comorbidities (age, sex, race, baseline eGFR, diabetes, cancer, congestive heart failure, and chronic liver disease). Model 3 is further adjusted for severity of illness (intensive care unit type, mechanical ventilation, Acute Physiology and Chronic Health Evaluation II score, sepsis, hypotension, white blood cell count, hemoglobin, serum creatinine, and AKI presence and severity on enrollment). cFGF23, C-terminal fibroblast growth factor 23; iFGF23, intact fibroblast growth factor 23; RU/ml, reference units per milliliter.
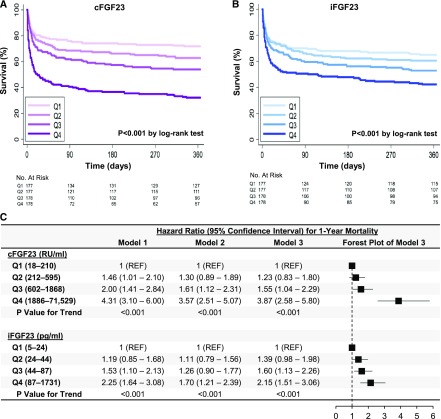
Higher quartiles of cFGF23 and iFGF23 were also associated with increased 1-year mortality in both univariable (Figure 4, A and B) and multivariable analyses (Figure 4C). In exploratory analyses, the associations between FGF23 levels and 1-year mortality were qualitatively similar when stratified by the presence or absence of AKI on enrollment (Supplemental Figure 3).
Figure 4.
Higher cFGF23 and iFGF23 quartiles associate with higher 1-year mortality in the Validating Acute Lung Injury biomarkers for Diagnosis (VALID) study. (A and B) Kaplan–Meier curves showing survival during the first year after enrollment, stratified according to quartiles of cFGF23 and iFGF23. (C) Hazard ratios for 1-year mortality according to cFGF23 and iFGF23 quartiles. Model 1 is unadjusted. Model 2 is adjusted for demographics and comorbidities (age, sex, race, baseline eGFR, diabetes, cancer, congestive heart failure, and chronic liver disease). Model 3 is further adjusted for severity of illness (intensive care unit type, mechanical ventilation, Acute Physiology and Chronic Health Evaluation II score, sepsis, hypotension, white blood cell count, hemoglobin, serum creatinine, and AKI presence and severity on enrollment). cFGF23, C-terminal fibroblast growth factor 23; iFGF23, intact fibroblast growth factor 23; Q, quartile; REF, reference group; RU/ml, reference units per milliliter.
Discussion
In this study, which includes two prospective cohorts, we found that plasma FGF23 levels are elevated in critically ill patients, and that higher FGF23 levels are associated with higher mortality. This association was present for both cFGF23 and iFGF23, and was independent of other known risk factors. Additionally, we found that higher iFGF23 associates with lower 1,25D levels, consistent with FGF23-mediated inhibition of 25D-to-1,25D conversion. However, iFGF23 remained significantly associated with death independently of 25D and 1,25D. Cumulatively, these data identify elevated FGF23 as an important feature of dysregulated mineral metabolism in critical illness.
Our data are consistent with and expand on prior studies demonstrating an association between higher FGF23 levels and adverse outcomes in patients with CKD (9–11) and, to a lesser extent, critical illness and AKI (3,12,26). Prior studies on FGF23 in critical illness were limited by small sample sizes, resulting in lack of power to assess hard outcomes such as death. Additionally, prior studies included few patients with severe AKI at the time of FGF23 measurement, and assessed only cFGF23, but not iFGF23 levels. In contrast, this study is the first to report an independent association between higher plasma FGF23 levels and increased all-cause mortality in a large cohort of critically ill patients. Additionally, we confirmed these findings in critically ill patients both with and without AKI, and with measurement of both cFGF23 and iFGF23 levels.
The downstream consequences of FGF23 over-activity have not been fully elucidated. Specifically, whether cFGF23 fragments exert biologic activity is unknown, because conflicting findings have been reported (27–29). In contrast, iFGF23 unequivocally exerts biologic activity through binding to FGF receptors expressed throughout the body. Recent studies found that in addition to classic effects on kidney phosphate handling and vitamin D metabolism, iFGF23 also has myriad “off-target” effects. These include toxic effects on the cardiovascular system through promotion of left ventricular hypertrophy and endothelial dysfunction (30,31), profibrotic effects on the kidneys (32), proinflammatory effects demonstrated by upregulation of hepatic IL-6 production (33), and impairment of immune function (34,35). Specifically, iFGF23’s effects on the immune system include reduced monocyte mRNA expression of the antimicrobial peptide, LL37 (34), and impairment of neutrophil activation and recruitment into inflamed tissues (35). Impaired immunity could increase susceptibility to infection, a leading cause of death in critical illness, and could provide a biologic mechanism for our observations. An alternative mechanism relates to FGF23-mediated upregulation of superoxide, inhibition of nitric oxide bioavailability, and impairment of endothelium-dependent vasorelaxation (31). These effects could increase the risk of cardiovascular events, an important contributor to death in critical illness (36). Despite these negative consequences of FGF23 over-activity, neutralization of FGF23 with an mAb resulted in hyperphosphatemia, extensive arterial calcification, and increased mortality in a rat model of CKD (37).
We investigated FGF23’s effects on vitamin D metabolite activation and catabolism as a potential unifying mechanism for our findings, because lower levels of 25D and 1,25D have been associated with higher mortality in critically ill patients (38,39). We found that higher iFGF23 levels are associated with lower 1,25D levels, consistent with FGF23-mediated inhibition of 25D-to-1,25D conversion in the kidneys (6) and perhaps elsewhere (34). To investigate whether impairment of 25D-to-1,25D conversion could partially account for our findings with respect to FGF23 and mortality, we adjusted for both 25D and 1,25D levels and found that iFGF23 remained significantly associated with death in the ATN study. Accordingly, the association between FGF23 and mortality could be mediated by pathways independent of vitamin D metabolism. Alternatively, tissue-level paracrine effects of FGF23 on vitamin D metabolism can occur in the absence of major changes in circulating vitamin D levels (40).
An alternative explanation for our findings could be confounding due to inflammation, because inflammation upregulates FGF23 production (41). Interestingly, we found that IL-6 associates positively with cFGF23 and negatively with iFGF23 (Figure 1G), consistent with inflammation as a stimulus for both FGF23 production and cleavage. This pattern of increased FGF23 production matched by a near commensurate increase in FGF23 cleavage has also been observed in patients with iron deficiency anemia (41). In multivariable models in the ATN study, adjustment for IL-6 and other severity of illness covariates attenuated the association between cFGF23 and death, but strengthened the association between iFGF23 and death, consistent with inflammation acting as a positive and negative confounder of cFGF23 and iFGF23, respectively. Accordingly, confounding due to inflammation could be responsible for the differences we observed in the associations between cFGF23, iFGF23, and death in the ATN study.
Levels of cFGF23 showed a stronger association with death than levels of iFGF23 in both cohorts. Thus, future studies of FGF23 as a prognostic biomarker for adverse outcomes in critical illness could reasonably focus on cFGF23 alone. However, future studies aimed at understanding the biology of FGF23 in critical illness and other acute inflammatory conditions should continue to measure both cFGF23 and iFGF23 to fully characterize the nature of FGF23 production and cleavage in these settings.
Limitations of this study include its observational design, measurement of FGF23 levels at a single time point, and lack of cause-specific mortality. The contribution of changes in unmeasured variables, such as klotho, the coreceptor for FGF23, is unknown and requires additional study. Finally, although we adjusted for multiple covariates, we cannot exclude potential residual confounding by variables that may not have been ascertained. Although previous studies found that FGF23 levels increase in patients with AKI (12,42), we aimed to minimize confounding due to varying degrees of AKI severity by evaluating FGF23 levels in patients enrolled in the ATN study, all of whom had severe AKI requiring RRT. In VALID, we conducted sensitivity analyses stratified by the presence or absence of AKI on enrollment, and found similar results in both groups.
In conclusion, we found that higher plasma FGF23 levels are strongly and independently associated with an increased risk of death in critically ill patients. These associations could be mediated through local effects of FGF23-mediated inhibition of 25D activation, which may not be reflected by plasma vitamin D metabolite levels, off-target effects independent of vitamin D signaling, or confounding by inflammation, which may not have been fully accounted for in multivariable models. Given substantial interest in vitamin D administration as a therapeutic strategy for improving outcomes in critical illness (43), future studies are needed to determine whether elevated FGF23 levels could identify patients less likely to respond to vitamin D precursors due to impaired 25D-to-1,25D conversion. Future studies are also needed to determine the physiologic regulators of FGF23 homeostasis in critical illness, whether FGF23 could serve as a useful prognostic tool, and whether FGF23 could be targeted for therapeutic benefit in interventional studies.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Leonard Stern, from the Division of Nephrology, New York Presbyterian Hospital–Columbia University Medical Center, for providing much of the inspiration for this avenue of research. We also thank Ilja Nolte, from University Medical Center Groningen, Department of Epidemiology, and Marta Christov, from the Division of Nephrology, New York Medical College, for their invaluable assistance.
This work was supported by the following grants from the National Institute of Diabetes and Digestive Kidney Diseases (NIDDK): K23DK106448 (to D.E.L.), K23DK088964 (to E.D.S.), R01DK070910 (to J.A.K.), R21DK100754 and K24DK093723 (to M.W.), and U01DK085660 and R01DK093574 (to S.S.W.), and P30-DK079337 (pilot and feasibility award to D.E.L.) from the University of Alabama at Birmingham–University of California, San Diego O’Brien Center for AKI Research. E.D.S. was supported by the Vanderbilt Center for Kidney Disease and Integrated Program for AKI. L.B.W. was supported by K24HL103836 from the National Heart, Lung, and Blood Institute. The Veterans Affairs/National Institutes of Health ATN study was supported by the Cooperative Studies Program of the Department of Veterans Affairs Office of Research and Development (CSP #530) and by the NIDDK through interagency agreement Y1-DK-3508.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.10810917/-/DCSupplemental.
References
- 1.Zivin JR, Gooley T, Zager RA, Ryan MJ: Hypocalcemia: A pervasive metabolic abnormality in the critically ill. Am J Kidney Dis 37: 689–698, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Lee P, Eisman JA, Center JR: Vitamin D deficiency in critically ill patients. N Engl J Med 360: 1912–1914, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Leaf DE, Wolf M, Waikar SS, Chase H, Christov M, Cremers S, Stern L: FGF-23 levels in patients with AKI and risk of adverse outcomes. Clin J Am Soc Nephrol 7: 1217–1223, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hewison M: Antibacterial effects of vitamin D. Nat Rev Endocrinol 7: 337–345, 2011 [DOI] [PubMed] [Google Scholar]
- 5.ADHR Consortium : Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet 26: 345–348, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T: FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res 19: 429–435, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Gutierrez O, Isakova T, Rhee E, Shah A, Holmes J, Collerone G, Jüppner H, Wolf M: Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol 16: 2205–2215, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Hasegawa H, Nagano N, Urakawa I, Yamazaki Y, Iijima K, Fujita T, Yamashita T, Fukumoto S, Shimada T: Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease. Kidney Int 78: 975–980, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Scialla JJ, Xie H, Rahman M, Anderson AH, Isakova T, Ojo A, Zhang X, Nessel L, Hamano T, Grunwald JE, Raj DS, Yang W, He J, Lash JP, Go AS, Kusek JW, Feldman H, Wolf M; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators : Fibroblast growth factor-23 and cardiovascular events in CKD. J Am Soc Nephrol 25: 349–360, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Isakova T, Xie H, Yang W, Xie D, Anderson AH, Scialla J, Wahl P, Gutiérrez OM, Steigerwalt S, He J, Schwartz S, Lo J, Ojo A, Sondheimer J, Hsu CY, Lash J, Leonard M, Kusek JW, Feldman HI, Wolf M; Chronic Renal Insufficiency Cohort (CRIC) Study Group : Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA 305: 2432–2439, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gutiérrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, Smith K, Lee H, Thadhani R, Jüppner H, Wolf M: Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 359: 584–592, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leaf DE, Jacob KA, Srivastava A, Chen ME, Christov M, Jüppner H, Sabbisetti VS, Martin A, Wolf M, Waikar SS: Fibroblast growth factor 23 levels associate with aki and death in critical illness. J Am Soc Nephrol 28: 1877–1885, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palevsky PM, Zhang JH, O’Connor TZ, Chertow GM, Crowley ST, Choudhury D, Finkel K, Kellum JA, Paganini E, Schein RM, Smith MW, Swanson KM, Thompson BT, Vijayan A, Watnick S, Star RA, Peduzzi P; VA/NIH Acute Renal Failure Trial Network : Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med 359: 7–20, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen W, Janz DR, Shaver CM, Bernard GR, Bastarache JA, Ware LB: Clinical characteristics and outcomes are similar in ards diagnosed by oxygen saturation/fio2 ratio compared with pao2/fio2 ratio. Chest 148: 1477–1483, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barlow WE, Ichikawa L, Rosner D, Izumi S: Analysis of case-cohort designs. J Clin Epidemiol 52: 1165–1172, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Wolf M, White KE: Coupling fibroblast growth factor 23 production and cleavage: Iron deficiency, rickets, and kidney disease. Curr Opin Nephrol Hypertens 23: 411–419, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cui S, Vaingankar SM, Stenger A, Waikar SS, Leaf DE: Stability of fibroblast growth factor 23 in human plasma. J Appl Lab Med 1: 729–734, 2017 [DOI] [PubMed] [Google Scholar]
- 18.Strathmann FG, Laha TJ, Hoofnagle AN: Quantification of 1α,25-dihydroxy vitamin D by immunoextraction and liquid chromatography-tandem mass spectrometry. Clin Chem 57: 1279–1285, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murugan R, Wen X, Shah N, Lee M, Kong L, Pike F, Keener C, Unruh M, Finkel K, Vijayan A, Palevsky PM, Paganini E, Carter M, Elder M, Kellum JA; Biological Markers for Recovery of Kidney (BioMaRK) Study Investigators : Plasma inflammatory and apoptosis markers are associated with dialysis dependence and death among critically ill patients receiving renal replacement therapy. Nephrol Dial Transplant 29: 1854–1864, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.White IR, Royston P, Wood AM: Multiple imputation using chained equations: Issues and guidance for practice. Stat Med 30: 377–399, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Rubin DB: Multiple Imputation for Nonresponse in Surveys, New York, NY, Wiley, 1987 [Google Scholar]
- 22.Knaus WA, Draper EA, Wagner DP, Zimmerman JE: APACHE II: A severity of disease classification system. Crit Care Med 13: 818–829, 1985 [PubMed] [Google Scholar]
- 23.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group : Kdigo clinical practice guideline for acute kidney injury. Kidney Int Suppl 2: 1–138, 2012 [Google Scholar]
- 24.Barlow WE: Robust variance estimation for the case-cohort design. Biometrics 50: 1064–1072, 1994 [PubMed] [Google Scholar]
- 25.Chudek J, Kocełak P, Owczarek A, Bożentowicz-Wikarek M, Mossakowska M, Olszanecka-Glinianowicz M, Wiecek A: Fibroblast growth factor 23 (FGF23) and early chronic kidney disease in the elderly. Nephrol Dial Transplant 29: 1757–1763, 2014 [DOI] [PubMed] [Google Scholar]
- 26.Zhang M, Hsu R, Hsu CY, Kordesch K, Nicasio E, Cortez A, McAlpine I, Brady S, Zhuo H, Kangelaris KN, Stein J, Calfee CS, Liu KD: FGF-23 and PTH levels in patients with acute kidney injury: A cross-sectional case series study. Ann Intensive Care 1: 21, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goetz R, Nakada Y, Hu MC, Kurosu H, Wang L, Nakatani T, Shi M, Eliseenkova AV, Razzaque MS, Moe OW, Kuro-o M, Mohammadi M: Isolated C-terminal tail of FGF23 alleviates hypophosphatemia by inhibiting FGF23-FGFR-Klotho complex formation. Proc Natl Acad Sci U S A 107: 407–412, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berndt TJ, Craig TA, McCormick DJ, Lanske B, Sitara D, Razzaque MS, Pragnell M, Bowe AE, O'Brien SP, Schiavi SC, Kumar R: Biological activity of FGF-23 fragments. Pflugers Arch 454: 615–623, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson K, Levine K, Sergi J, Chamoun J, Roach R, Vekich J, Favis M, Horn M, Cao X, Miller B, Snyder W, Aivazian D, Reagan W, Berryman E, Colangelo J, Markiewicz V, Bagi CM, Brown TP, Coyle A, Mohammadi M, Magram J: Therapeutic effects of fgf23 c-tail fc in a murine pre-clinical model of x-linked hypophosphatemia via the selective modulation of phosphate reabsorption. J Bone Miner Res 32: 2062–2073, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutiérrez OM, Aguillon-Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, St John Sutton M, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Kuro-O M, Kusek JW, Keane MG, Wolf M: FGF23 induces left ventricular hypertrophy. J Clin Invest 121: 4393–4408, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silswal N, Touchberry CD, Daniel DR, McCarthy DL, Zhang S, Andresen J, Stubbs JR, Wacker MJ: FGF23 directly impairs endothelium-dependent vasorelaxation by increasing superoxide levels and reducing nitric oxide bioavailability. Am J Physiol Endocrinol Metab 307: E426–E436, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith ER, Tan SJ, Holt SG, Hewitson TD: FGF23 is synthesised locally by renal tubules and activates injury-primed fibroblasts. Sci Rep 7: 3345, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh S, Grabner A, Yanucil C, Schramm K, Czaya B, Krick S, Czaja MJ, Bartz R, Abraham R, Di Marco GS, Brand M, Wolf M, Faul C: Fibroblast growth factor 23 directly targets hepatocytes to promote inflammation in chronic kidney disease. Kidney Int 90: 985–996, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bacchetta J, Sea JL, Chun RF, Lisse TS, Wesseling-Perry K, Gales B, Adams JS, Salusky IB, Hewison M: Fibroblast growth factor 23 inhibits extrarenal synthesis of 1,25-dihydroxyvitamin D in human monocytes. J Bone Miner Res 28: 46–55, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rossaint J, Oehmichen J, Van Aken H, Reuter S, Pavenstädt HJ, Meersch M, Unruh M, Zarbock A: FGF23 signaling impairs neutrophil recruitment and host defense during CKD. J Clin Invest 126: 962–974, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mayr VD, Dünser MW, Greil V, Jochberger S, Luckner G, Ulmer H, Friesenecker BE, Takala J, Hasibeder WR: Causes of death and determinants of outcome in critically ill patients. Crit Care 10: R154, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shalhoub V, Shatzen EM, Ward SC, Davis J, Stevens J, Bi V, Renshaw L, Hawkins N, Wang W, Chen C, Tsai MM, Cattley RC, Wronski TJ, Xia X, Li X, Henley C, Eschenberg M, Richards WG: FGF23 neutralization improves chronic kidney disease-associated hyperparathyroidism yet increases mortality. J Clin Invest 122: 2543–2553, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Braun A, Chang D, Mahadevappa K, Gibbons FK, Liu Y, Giovannucci E, Christopher KB: Association of low serum 25-hydroxyvitamin D levels and mortality in the critically ill. Crit Care Med 39: 671–677, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nguyen HB, Eshete B, Lau KH, Sai A, Villarin M, Baylink D: Serum 1,25-dihydroxyvitamin D: An outcome prognosticator in human sepsis. PLoS One 8: e64348, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bai X, Miao D, Xiao S, Qiu D, St-Arnaud R, Petkovich M, Gupta A, Goltzman D, Karaplis AC: CYP24 inhibition as a therapeutic target in FGF23-mediated renal phosphate wasting disorders. J Clin Invest 126: 667–680, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.David V, Martin A, Isakova T, Spaulding C, Qi L, Ramirez V, Zumbrennen-Bullough KB, Sun CC, Lin HY, Babitt JL, Wolf M: Inflammation and functional iron deficiency regulate fibroblast growth factor 23 production. Kidney Int 89: 135–146, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leaf DE, Christov M, Jüppner H, Siew E, Ikizler TA, Bian A, Chen G, Sabbisetti VS, Bonventre JV, Cai X, Wolf M, Waikar SS: Fibroblast growth factor 23 levels are elevated and associated with severe acute kidney injury and death following cardiac surgery. Kidney Int 89: 939–948, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amrein K, Schnedl C, Holl A, Riedl R, Christopher KB, Pachler C, Urbanic Purkart T, Waltensdorfer A, Münch A, Warnkross H, Stojakovic T, Bisping E, Toller W, Smolle KH, Berghold A, Pieber TR, Dobnig H: Effect of high-dose vitamin D3 on hospital length of stay in critically ill patients with vitamin D deficiency: The VITdAL-ICU randomized clinical trial. JAMA 312: 1520–1530, 2014 [DOI] [PubMed] [Google Scholar]
- 44.Siew ED, Matheny ME: Choice of reference serum creatinine in defining acute kidney injury. Nephron 131: 107–112, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.