Abstract
Background and objectives
Patients on hemodialysis frequently experience pain and may be particularly vulnerable to opioid-related complications. However, data evaluating the risks of opioid use in patients on hemodialysis are limited.
Design, setting, participants, & measurements
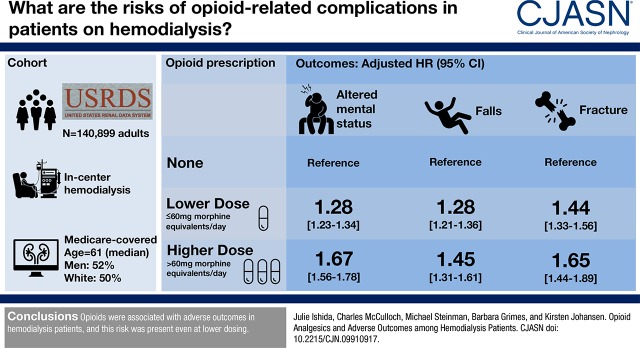
Using the US Renal Data System, we conducted a cohort study evaluating the association between opioid use (modeled as a time-varying exposure and expressed in standardized oral morphine equivalents) and time to first emergency room visit or hospitalization for altered mental status, fall, and fracture among 140,899 Medicare-covered adults receiving hemodialysis in 2011. We evaluated risk according to average daily total opioid dose (>60 mg, ≤60 mg, and per 60-mg dose increment) and specific agents (per 60-mg dose increment).
Results
The median age was 61 years old, 52% were men, and 50% were white. Sixty-four percent received opioids, and 17% had an episode of altered mental status (15,658 events), fall (7646 events), or fracture (4151 events) in 2011. Opioid use was associated with risk for all outcomes in a dose-dependent manner: altered mental status (lower dose: hazard ratio, 1.28; 95% confidence interval, 1.23 to 1.34; higher dose: hazard ratio, 1.67; 95% confidence interval, 1.56 to 1.78; hazard ratio, 1.29 per 60 mg; 95% confidence interval, 1.26 to 1.33), fall (lower dose: hazard ratio, 1.28; 95% confidence interval, 1.21 to 1.36; higher dose: hazard ratio, 1.45; 95% confidence interval, 1.31 to 1.61; hazard ratio, 1.04 per 60 mg; 95% confidence interval, 1.03 to 1.05), and fracture (lower dose: hazard ratio, 1.44; 95% confidence interval, 1.33 to 1.56; higher dose: hazard ratio, 1.65; 95% confidence interval, 1.44 to 1.89; hazard ratio, 1.04 per 60 mg; 95% confidence interval, 1.04 to 1.05). All agents were associated with a significantly higher hazard of altered mental status, and several agents were associated with a significantly higher hazard of fall and fracture.
Conclusions
Opioids were associated with adverse outcomes in patients on hemodialysis, and this risk was present even at lower dosing and for agents that guidelines have recommended for use.
Keywords: hemodialysis; clinical epidemiology; United States Renal Data System; opioid; Humans; United States; Middle Aged; Analgesics, Opioid; Cohort Studies; Opioid-Related Disorders; Pain; Morphine; Medicare; hospitalization; renal dialysis; Emergency Service, Hospital
Introduction
Pain is among the most commonly reported symptoms in patients on hemodialysis, with a prevalence of up to 81% (1–3), and patients on dialysis report more severe pain than the general population on average (4,5). Despite evidence of the benefit of opioid analgesics (6) and the availability of opioid prescribing guidelines in ESKD (6–9), several studies have suggested that pain is inadequately treated in patients on hemodialysis (10–16), and provider concern for adverse drug effects may present a barrier to effective pain management (10,13). However, it is plausible that patients on hemodialysis may be especially susceptible to opioid-related complications due to multiple comorbidities, polypharmacy, superimposed uremia, and reduced clearance by the kidney of active drug metabolites, and therefore, caution in their use may be warranted (10,17).
In the general population, opioids have been associated with mortality as well as altered mental status, falls, and fractures (18–22), which may be mediated by the depressive effects of opioids on the respiratory and central nervous system and their association with decreased bone mineral density (22–26). Epidemiologic data regarding the risks of these major opioid complications in patients on hemodialysis are limited to three cohort studies, in which opioid use was associated with all-cause mortality, dialysis discontinuation, hospitalization, and fracture (27,28); also, opioid use was higher among those who experienced a fall, but it was not an independent predictor of fall (29). However, there is a paucity of literature quantifying the risks of major adverse outcomes associated with opioid use in the United States hemodialysis population and examining their associations according to opioid dose or agent.
Our research objective was to investigate the association between opioid dose and agent with risk of major adverse outcomes (i.e., altered mental status, fall, and fracture) in patients on hemodialysis in the United States. We hypothesized that opioids would be associated with risk of these outcomes in a dose-dependent manner and that the risks associated with the individual opioid agents would align with guideline recommendations for opioid prescribing in patients on hemodialysis (i.e., agents recommended by guidelines would be associated with less harm than agents for which guidelines have recommended cautious use or avoidance) (8,9).
Materials and Methods
Study Design and Population
We conducted a retrospective cohort study using data from the US Renal Data System (USRDS) 2013 standard analytic and Medicare payment files, which contain clinical and billing data from 2011, including the Part D prescription drug file. The University of California, San Francisco Committee on Human Research did not consider the study to involve human subjects research.
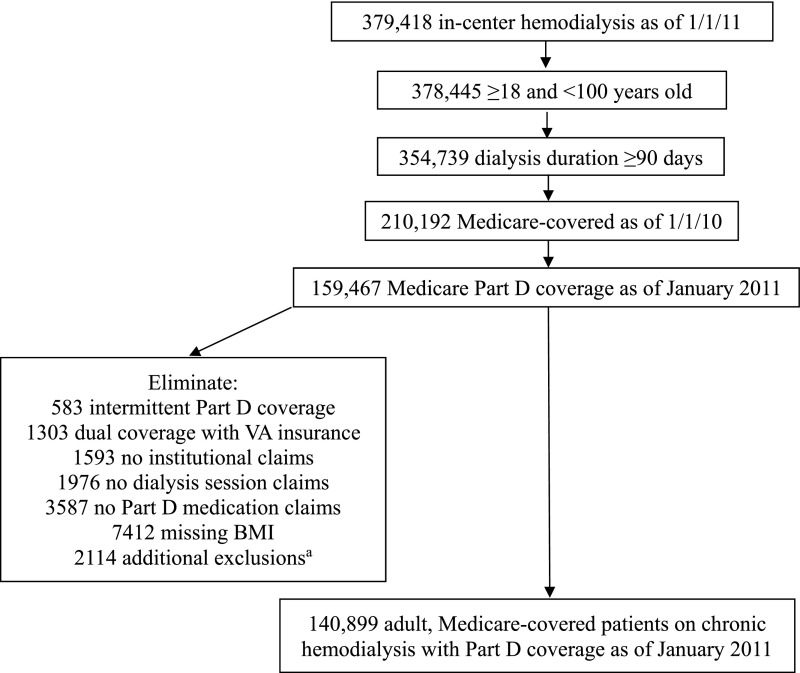
The study population consisted of prevalent adult Medicare-covered patients receiving chronic maintenance hemodialysis with Part D coverage as of January 2011 (Figure 1). We also required Medicare coverage during 2010 to capture claims data for determining comorbidity status. To maximize accuracy of exposure and outcome ascertainment, we eliminated those with intermittent Part D coverage; dual Veterans Affairs coverage; and no institutional, dialysis, or Part D medication claims in 2011. We also eliminated those with missing body mass index to have complete data for this potential confounding variable. Patients were also excluded from the cohort if any of the following occurred in January 2011: death, kidney transplant, change in modality away from in-center hemodialysis (e.g., home hemodialysis or peritoneal dialysis), uncertain/recovered function, loss to follow-up, withdrawal from dialysis, or loss of Part D coverage, which precluded our ability to ascertain exposure status as of February 2011.
Figure 1.
Flow diagram describing derivation of study cohort. BMI, body mass index; VA, Veterans Affairs. aPatients were additionally excluded from the cohort if any of the following occurred in January 2011: death, kidney transplant, change in modality away from in-center hemodialysis (e.g., home hemodialysis or peritoneal dialysis), uncertain/recovered function, loss to follow-up, withdrawal from dialysis, or loss of Part D coverage, which precluded our ability to ascertain exposure status as of February 2011.
Exposure Variables
Ascertainment of opioid exposure status was started in January 2011, and the timing of outcome ascertainment was delayed until February 2011 to allow for determination of prior opioid exposure. We used a time-varying definition of opioid exposure, in which a patient was considered to be exposed during periods of opioid medication possession. Our database was created such that there was a record for each person for each day of observation. Using this database structure, we were able to define continuous periods of medication possession as the period starting from the date of service of a prescription plus the number of days supplied (30). For each prescription, we calculated an average daily dose and converted this into standardized oral morphine equivalents to account for differences in potency among individual opioid agents (Supplemental Table 1) (31). Dosages of opioid and opioid/acetaminophen agents were combined during periods of overlapping possession, and we excluded values exceeding the 95th percentile of oral morphine equivalents. Periods of opioid exposure were then categorized as none (average daily total dose 0 mg), lower dose (0< average daily total dose ≤60 mg in oral morphine equivalents), or higher dose (average daily total dose >60 mg in oral morphine equivalents) (22).
The predictor variables were time-updated category of opioid dose use (none, lower, or higher), average daily total opioid dose (expressed as a continuous variable and scaled per 60 mg oral morphine equivalents), and receipt of individual opioid agents (i.e., hydrocodone, oxycodone, tramadol, codeine, hydromorphone, fentanyl, morphine, or methadone; expressed as a continuous variable and scaled per 60 mg oral morphine equivalents). We selected these agents for evaluation, because they were the most commonly used opioid agents in our cohort and represented a spectrum of opioids for which guidelines recommend use (e.g., hydromorphone, fentanyl, and methadone), use with caution (e.g., tramadol, hydrocodone, and oxycodone), or avoidance (e.g., morphine and codeine) (8,9).
Outcome Variables
The outcome variables (examined separately) were first episode of altered mental status, fall, and fracture (of the hip, femur, pelvis, leg, foot, arm, hand, or axial skeleton) requiring an emergency room visit or hospitalization during 2011. Using our continuous database structure, we were able to assign specific events dates to the outcomes of interest. We identified emergency room visits and hospitalizations using the revenue, physician/supplier, and institutional claims files. Outcome definitions were determined by International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis and Current Procedural Terminology codes (Supplemental Table 2). We included hospitalizations in which the outcomes of interest were designated as the primary diagnosis in the USRDS according to standard methodology (32) and emergency room visits in which there was a code for the outcomes of interest.
Statistical Analyses
Baseline characteristics of the study cohort are presented as mean±SD or median with 25th and 75th percentiles for continuous variables and percentages for categorical variables. In Table 1, patients were categorized into the following groups: no opioid use in 2011, lower dose (received only lower doses in 2011), and higher dose (received at least one higher dose in 2011). Rates of each of the outcomes were calculated for the overall follow-up time and with follow-up time stratified by opioid dose category (none, lower, or higher), and they were expressed as the number of events per 100 person-years.
Table 1.
Baseline characteristics of 140,899 adults receiving hemodialysis with Medicare Part D coverage in 2011 by opioid dose category
| Characteristic | None,a n=50,775 | Lower Dose,b n=57,411 | Higher Dose,c n=32,713 |
|---|---|---|---|
| Age, mean (SD), yr | 63 (15) | 61 (15) | 57 (15) |
| Duration of ESKD, median (25–75th percentiles), yr | 4 (2–7) | 4 (2–7) | 4 (2–7) |
| Sex, % | |||
| Men | 57 | 48 | 50 |
| Women | 43 | 52 | 50 |
| Race, % | |||
| White | 51 | 49 | 50 |
| Black | 40 | 45 | 46 |
| Other | 9 | 6 | 4 |
| Comorbidities, % | |||
| Cerebrovascular disease | 16 | 17 | 17 |
| Dementia | 4 | 3 | 3 |
| Depression | 7 | 10 | 16 |
| Seizures/epilepsy | 1 | 1 | 2 |
| Hypertension | 96 | 97 | 98 |
| Coronary artery disease | 21 | 22 | 22 |
| Congestive heart failure | 44 | 50 | 52 |
| Other cardiac disease | 17 | 18 | 19 |
| Dysrhythmia | 2 | 3 | 3 |
| Peripheral vascular disease | 27 | 32 | 35 |
| Chronic obstructive pulmonary disease | 15 | 20 | 25 |
| Diabetes mellitus | 62 | 66 | 63 |
| Liver disease | 10 | 12 | 16 |
| Cancer | 7 | 7 | 8 |
| Tobacco dependence | 8 | 13 | 21 |
| Alcohol dependence | 1 | 1 | 2 |
| Drug dependence | 2 | 3 | 6 |
| Opioid dependence | 0.1 | 0.1 | 1 |
| Inability to ambulate | 3 | 3 | 4 |
| Inability to transfer | 1 | 1 | 1 |
| Osteoporosis | 2 | 3 | 3 |
| Osteopenia | 1 | 1 | 2 |
| Body mass index, kg/m2, % | |||
| <20 | 8 | 7 | 7 |
| 20 to <25 | 28 | 24 | 22 |
| 25 to <30 | 29 | 27 | 26 |
| ≥30 | 35 | 42 | 45 |
No opioid use in 2011.
Received only lower doses (0< average daily total dose ≤60 mg oral morphine equivalents) in 2011.
Received at least one higher dose (average daily total dose >60 mg oral morphine equivalents) in 2011.
For each predictor and outcome pairing, we constructed a Cox model using a time-varying definition of opioid exposure to determine the hazard of each outcome during periods of lower dose compared with no opioid use and periods of higher dose compared with no opioid use. We also determined the hazard per 60-mg increment in average daily total opioid dose and individual opioid agent dose. We also performed an analysis of the associations of the number of concomitant opioid agents (one, two, and three or more versus none), accounting for dose, with outcomes. Exposure was time lagged (i.e., ascertained from the prior day) for fall and fracture to account for the possibility of effect/cause.
We accounted for potential confounders by adjusting for baseline demographic characteristics (i.e., age and duration on dialysis as continuous variables; sex, race [white, black, or other], geographic location of the ESKD network as defined by US Census geographic divisions, and body mass index [<20, 20 to <25, 25 to <30, or ≥30 kg/m2] as categorical variables), comorbidities (i.e., alcohol dependence, coronary artery disease, cancer, other cardiac disease, dysrhythmia, congestive heart failure, cerebrovascular disease, diabetes, drug dependence, opioid dependence, hypertension, inability to ambulate, inability to transfer, chronic obstructive pulmonary disease, peripheral vascular disease, tobacco dependence, liver disease, dementia, depression, seizures/epilepsy, osteoporosis, osteopenia, and skilled nursing facility resident as binary variables, indicating the presence or absence of each comorbidity), and number of total unique medications (zero to one, two to three, four to five, six to seven, or eight or more) and antihypertensives (zero, one, two, or three or more) prescribed at baseline as categorical variables. Comorbidities were determined to be present if they appeared on the USRDS Medical Evidence Report or if an appropriate ICD-9-CM diagnosis or procedure code was present on two outpatient claims (on different days) or one inpatient claim during 2010 in the institutional claims or physician/supplier files (Supplemental Table 3) (33–36). We also controlled for use of concomitant medications that might influence risk of the outcomes (e.g., sedative/hypnotics) as a time-varying covariate.
Patients were censored at the time of death, receipt of a kidney transplant, change in modality away from in-center hemodialysis (e.g., home hemodialysis or peritoneal dialysis), uncertain/recovered function, withdrawal from dialysis, loss to follow-up, discontinuation of Part D coverage, or end of the study period. During every calendar year, there is a Medicare Part D coverage gap (i.e., “donut hole”), which represents a gap in coverage that occurs after the initial coverage limit has been exceeded but before catastrophic coverage applies (37). The Part D program offers a low-income subsidy, which provides full or partial coverage during the coverage gap, and lack of a low-income subsidy has been associated with lower medication adherence and persistence in patients with ESKD (38). Thus, we performed a sensitivity analysis restricting to patients who received the low-income subsidy and would be incentivized to obtain all of their prescriptions with Medicare Part D (39). We also performed a sensitivity analysis using a new user approach (28), in which we excluded patients with an opioid prescription from January 1, 2011 to March 31, 2011 and evaluated associations between opioid use and outcomes during the remainder of 2011. We performed subgroup analyses by age, sex, race, and dialysis duration. Testing for statistical significance was two tailed, and P<0.05 was considered significant. Analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC).
Results
Demographics, Opioid Use, and Incidence of Outcomes
Our cohort consisted of 140,899 Medicare-covered adults receiving in-center hemodialysis with Part D coverage in 2011 (Figure 1). The median age of the cohort was 61 years old (25th–75th percentiles, 51–72 years old), 52% were men, and 50% were white. Opioid use was associated with younger age, women, black race, higher body mass index, and higher prevalence of comorbidities (Table 1).
Opioids were frequently prescribed, with 90,124 (64%) patients receiving opioid analgesics in 2011 and 32,730 (23%) receiving at least one high-dose prescription. The prevalence of use of the individual agents of interest was as follows: hydrocodone (43%), oxycodone (22%), tramadol (15%), codeine (7%), hydromorphone (3%), fentanyl (3%), morphine (2%), and methadone (1%).
Seventeen percent of patients (23,715) had an episode of altered mental status, fall, or fracture in 2011, and 11% (15,658 events), 5% (7,646 events), and 3% (4,151 events) had an episode of altered mental status, fall, and fracture in 2011, respectively. The overall rates of first episode of altered mental status, fall, and fracture in the cohort were 15, 7, and 4 per 100 person-years, respectively. The rates of these adverse outcomes were highest during periods of possession of higher doses of opioids followed by periods of possession of lower doses and then, periods of nonuse: altered mental status (29, 20, and 13 per 100 person-years), fall (12, 10, and 6 per 100 person-years), and fracture (6, 5, and 3 per 100 person-years).
Associations between Total Opioid Dose and Outcomes
Compared with no opioid use, lower- and higher-dose opioid use was associated with higher hazard of altered mental status (adjusted hazard ratio [HR], 1.28 for lower dose; 95% confidence interval [95% CI], 1.23 to 1.34; P<0.001 and HR, 1.67 for higher dose; 95% CI, 1.56 to 1.78; P<0.001), fall (HR, 1.28 for lower dose; 95% CI, 1.21 to 1.36; P<0.001 and HR, 1.45 for higher dose; 95% CI, 1.31 to 1.61; P<0.001), and fracture (HR, 1.44 for lower dose; 95% CI, 1.33 to 1.56; P<0.001 and HR, 1.65 for higher dose; 95% CI, 1.44 to 1.89; P<0.001) (Table 2). Each 60-mg increment in dose (in standardized oral morphine equivalents) of any opioid was associated with a 29% higher hazard of altered mental status (95% CI, 26% to 33%; P<0.001), 4% higher hazard of fall (3%–5%; P<0.001), and 4% higher hazard of fracture (4%–5%; P<0.001).
Table 2.
Risk of altered mental status, fall, and fracture among adults receiving hemodialysis with Medicare Part D coverage in 2011 by opioid dose and agent
| Altered Mental Status | Fall | Fracture | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Opioid exposure | Events (n) | Rate (per 100 PY) | Crude HR (95% CI) | Adjusted HR (95% CI) | Events (n) | Rate (per 100 PY) | Crude HR (95% CI) | Adjusted HR (95% CI) | Events (n) | Rate (per 100 PY) | Crude HR (95% CI) | Adjusted HR (95% CI) |
| Opioid dose | ||||||||||||
| Categorical | ||||||||||||
| None | 11,453 | 13 | 1.00 (Reference) | 1.00 (Reference) | 5659 | 6 | 1.00 (Reference) | 1.00 (Reference) | 3052 | 3 | 1.00 (Reference) | 1.00 (Reference) |
| Lower dosea | 3131 | 20 | 1.52 (1.46 to 1.58)b | 1.28 (1.23 to 1.34)b | 1534 | 10 | 1.50 (1.42 to 1.59)b | 1.28 (1.21 to 1.36)b | 848 | 5 | 1.53 (1.42 to 1.65)b | 1.44 (1.33 to 1.56)b |
| Higher dosec | 1074 | 29 | 2.17 (2.04 to 2.31)b | 1.67 (1.56 to 1.78)b | 453 | 12 | 1.83 (1.67 to 2.02)b | 1.45 (1.31 to 1.61)b | 251 | 6 | 1.87 (1.64 to 2.12)b | 1.65 (1.44 to 1.89)b |
| Continuous | ||||||||||||
| Total opioid dose (per 60 mg) | 1.50 (1.42 to 1.50)b | 1.29 (1.26 to 1.33)b | 1.34 (1.34 to 1.42)b | 1.04 (1.03 to 1.05)b | 1.42 (1.34 to 1.50)b | 1.04 (1.04 to 1.05)b | ||||||
| Opioid agent (per 60 mg) | ||||||||||||
| Recommended | ||||||||||||
| None | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | ||||||
| Hydromorphone | 1.59 (1.34 to 1.77)b | 1.39 (1.23 to 1.57)b | 1.27 (1.06 to 1.59)b | 1.02 (1.01 to 1.04)b | 1.50 (1.13 to 1.87)b | 1.03 (1.02 to 1.04)b | ||||||
| Fentanyl | 1.42 (1.34 to 1.50)b | 1.18 (1.11 to 1.25)b | 1.27 (1.19 to 1.42)b | 1.06 (0.96 to 1.17) | 1.27 (1.13 to 1.50)b | 1.03 (0.90 to 1.18) | ||||||
| Methadone | 1.27 (1.13 to 1.42)b | 1.14 (1.01 to 1.29)b | 1.27 (1.00 to 1.50)b | 1.08 (0.92 to 1.28) | 1.13 (0.89 to 1.42) | 1.08 (0.87 to 1.34) | ||||||
| Use with caution | ||||||||||||
| Hydrocodone | 1.59 (1.50 to 1.68)b | 1.37 (1.29 to 1.47)b | 1.68 (1.59 to 1.87)b | 1.34 (1.27 to 1.41)b | 1.59 (1.42 to 1.77)b | 1.40 (1.32 to 1.48)b | ||||||
| Oxycodone | 1.34 (1.34 to 1.38)b | 1.31 (1.25 to 1.38)b | 1.19 (1.13 to 1.27)b | 1.13 (1.06 to 1.22)b | 1.34 (1.19 to 1.42)b | 1.25 (1.19 to 1.31)b | ||||||
| Tramadol | 1.97 (1.68 to 2.19)b | 1.48 (1.31 to 1.69)b | 1.87 (1.59 to 2.31)b | 1.42 (1.19 to 1.69)b | 2.19 (1.77 to 2.70)b | 1.65 (1.41 to 1.92)b | ||||||
| Avoid | ||||||||||||
| Codeine | 5.05 (3.14 to 8.20)b | 3.65 (2.12 to 6.30)b | 2.19 (0.94 to 5.05) | 1.57 (0.64 to 3.83) | 3.46 (1.27 to 9.69)b | 2.94 (1.00 to 8.66)b | ||||||
| Morphine | 1.59 (1.42 to 1.68)b | 1.40 (1.27 to 1.55)b | 1.19 (0.99 to 1.42)b | 1.15 (0.99 to 1.32) | 1.27 (0.94 to 1.59) | 1.11 (0.90 to 1.38) | ||||||
Results are adjusted for age, sex, race, duration on dialysis, network, body mass index, alcohol dependence, coronary artery disease, cancer, other cardiac disease, dysrhythmia, congestive heart failure, cerebrovascular disease, diabetes, drug dependence, opioid dependence, hypertension, inability to ambulate, inability to transfer, chronic obstructive pulmonary disease, peripheral vascular disease, tobacco dependence, liver disease, dementia, depression, seizures/epilepsy, osteoporosis, osteopenia, skilled nursing facility resident, medication burden, number of hypertensive medications, and concomitant medications. PY, person-year; HR, hazard ratio; 95% CI, 95% confidence interval.
Periods of lower-dose (0< average daily total dose ≤60 mg oral morphine equivalents) opioid exposure.
P value <0.05.
Periods of higher-dose (average daily total dose >60 mg oral morphine equivalents) opioid exposure.
Associations between Individual Opioid Agents and Outcomes
All individual agents were associated with a significantly higher hazard of altered mental status ranging from 14% to 365% per 60 mg (Table 2). Hydromorphone, hydrocodone, oxycodone, and tramadol were associated with a significantly higher risk of fall (2%–42% higher hazard per 60 mg), and hydromorphone, hydrocodone, oxycodone, tramadol, and codeine were associated with a significantly higher risk of fracture (3%–294% higher hazard per 60 mg). For altered mental status, fall, and fracture, the highest hazards were associated with codeine, a medication for which guidelines recommend avoidance.
Additional Analyses
The magnitude of the associations was similar in the sensitivity analysis restricted to patients with the low-income subsidy (Supplemental Table 4). In the new user analysis, the magnitude of the associations was stronger (Supplemental Table 5). The associations were similar among users of one, two, and three or more concomitant opioid agents accounting for opioid dose (Supplemental Table 6). The relative risk of altered mental status associated with opioid use is greater for nonblacks than blacks (Supplemental Table 7).
Discussion
In our study of patients on hemodialysis with Medicare Part D coverage, we observed that opioid use was common and associated with risk for altered mental status, fall, and fracture in a dose-dependent manner, and the risk was present even when patients were not prescribed high doses. The relative risk of altered mental status associated with opioid use was higher among nonblacks than blacks. The magnitude of the associations was larger in the new user analysis, consistent with prior literature in which the risks of opioids were most pronounced in the time period immediately after onset of use (22,40). We also observed that all agents were associated with altered mental status, and several agents were associated with fall and fracture. Notably, even agents recommended for use in this population were associated with risk for adverse outcomes.
Our data are consistent with the limited available epidemiologic evidence that has suggested that patients on hemodialysis receiving opioids are at higher risk of major complications. In a study of approximately 150,000 United States patients on hemodialysis in the USRDS, opioid use was associated with all-cause mortality, dialysis discontinuation, and hospitalization (28). Our analysis, in which we focused on outcomes that could be temporally related to opioid use, is complementary to this study. Among patients on hemodialysis, opioid use has been associated with altered mental status in patient reports (41,42), and opioid medications were associated with a higher adjusted risk of hip and other fractures in an international study of 12,782 patients on hemodialysis (27). Our study observed that opioid use was associated with risk for fall in contrast to a study of 308 Belgian patients on hemodialysis, in which opioid use was higher among those who experienced a fall but was not independently associated with fall (29). The discrepant findings may be due to differences in study population, sample size, and methodology (e.g., exposure and outcome ascertainment or analytic approach).
Despite the high prevalence of pain among patients on hemodialysis (1–3), studies of the efficacy and safety of opioid medications in this population are scarce, and clinical practice guidelines for prescribing opioids in the setting of dialysis are on the basis of limited pharmacokinetic and anecdotal data (4,43). Nonetheless, recommendations consistently advise avoidance of morphine and codeine, which are converted to active metabolites that accumulate in the setting of kidney failure, leading to adverse effects, such as central nervous system and respiratory depression (8,9,43,44). In particular, both are metabolized to morphine-6-glucuronide, which is more potent than morphine itself, equilibrates slowly across the blood-brain barrier, and may result in prolonged sedation (4,43,45). Guidelines recommend that hydrocodone, oxycodone, and tramadol be used with caution given that their pharmacokinetic profile has not been well established in the setting of kidney failure (4,8,9,46,47). Fentanyl and methadone are thought to be relatively safe, because they are converted to inactive metabolites, but they require management and careful monitoring by experienced practitioners (44,48). Hydromorphone is thought to be better tolerated than morphine among those with kidney impairment (49), and although its active metabolite, hydromorphone-3-glucuronide, is excreted by the kidneys, it is rapidly dialyzed, which may make it a safe choice for patients on hemodialysis (4,43).
In our study, prescription of morphine was uncommon, suggesting that providers are following recommendations to avoid its use. However, despite the guidance to avoid codeine, its use was more common than recommended agents. Our results support the recommendation to avoid morphine and codeine, because receipt of these agents was associated with adverse outcomes. Hydrocodone, oxycodone, and tramadol were the most commonly used agents, and our results also support the recommendation to use these agents with caution, because they were each associated with all of the adverse outcomes. However, despite guidance recommending hydromorphone, fentanyl, and methadone, use of these agents was relatively uncommon, and they were also associated with adverse outcomes.
Our study has several limitations. Given that our study was observational, we cannot exclude the possibility of confounding by indication. However, we controlled for many important covariates, including demographics, comorbidities, medication burden, and potentially confounding concomitant medications (e.g., sedatives/hypnotics) to mitigate this concern. Opioid exposure was determined on the basis of Medicare Part D claims data, and previous work in the USRDS has shown the accuracy of the Medicare Part D claims to ascertain medication prescriptions (50). However, we do not know with certainty whether patients took their prescribed medications and the exact time period over which medications were consumed. Additionally, we were unable to distinguish scheduled versus as-needed dosing. Ascertainment of the outcomes was limited by the use of ICD-9-CM codes. However, it is unlikely that misclassification of opioid exposure would differ by outcome or that misclassification of the outcome would differ by opioid exposure. To the extent that nondifferential misclassification occurred, it would be expected to bias the results to the null, and therefore, our findings may represent a conservative estimate. Although we found that opioid prescription was common, we lacked information about the clinical context for the prescriptions (e.g., specific indications and level of pain), and therefore, we are unable to comment on the adequacy of pain management in our cohort. Finally, we are unable to determine whether the different associations by race are due to biologic factors, residual confounding, or chance, and these associations warrant further investigation. Strengths of our study are the large sample size and detailed medication data contained in the Medicare Part D claims, which enabled investigation of associations according to opioid dose and specific agents.
Pain is a highly prevalent symptom in patients on hemodialysis (1–3), and this population may be particularly vulnerable to complications related to opioid use. We observed that opioids were associated with a significantly higher risk of altered mental status, fall, and fracture in patients receiving hemodialysis, even at lower dosing and for agents recommended by guidelines. Thus, opioid use in patients on hemodialysis may not be as safe as guidelines suggest, and the benefit-to-risk ratio of their use in this population should be carefully considered. Future research and strategies to predict and mitigate the risks of opioid use in patients on hemodialysis are warranted.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases grants K23DK103963 (to J.H.I.) and K24DK085153 (to K.L.J.), National Institute on Aging grants K24AG049057 (to M.A.S.) and P30 AG044281 (to M.A.S.), and National Center for Advancing Translational Sciences, National Institutes of Health (NIH) through UCSF-Clinical & Translational Science Institute grants KL2 TR000143 and KL2 TR001870.
This study was presented as an oral abstract at the annual meeting of the American Society of Nephrology on November 4, 2017 in New Orleans, Louisiana.
The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. The data reported here have been supplied by the US Renal Data System. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US Government.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related Patient Voice, “Appropriate Use of Opioids in Patients with Kidney Diseases,” on pages 675–676.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.09910917/-/DCSupplemental.
References
- 1.Murtagh FE, Addington-Hall J, Higginson IJ: The prevalence of symptoms in end-stage renal disease: A systematic review. Adv Chronic Kidney Dis 14: 82–99, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Shayamsunder AK, Patel SS, Jain V, Peterson RA, Kimmel PL: Sleepiness, sleeplessness, and pain in end-stage renal disease: Distressing symptoms for patients. Semin Dial 18: 109–118, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Leinau L, Murphy TE, Bradley E, Fried T: Relationship between conditions addressed by hemodialysis guidelines and non-ESRD-specific conditions affecting quality of life. Clin J Am Soc Nephrol 4: 572–578, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davison SN: The prevalence and management of chronic pain in end-stage renal disease. J Palliat Med 10: 1277–1287, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Nahin RL: Estimates of pain prevalence and severity in adults: United States, 2012. J Pain 16: 769–780, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barakzoy AS, Moss AH: Efficacy of the world health organization analgesic ladder to treat pain in end-stage renal disease. J Am Soc Nephrol 17: 3198–3203, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Launay-Vacher V, Karie S, Fau JB, Izzedine H, Deray G: Treatment of pain in patients with renal insufficiency: The World Health Organization three-step ladder adapted. J Pain 6: 137–148, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Coalition for Supportive Care of Kidney Patients : Treating pain in advanced CKD and dialysis patients: Clinical algorithm and preferred medications, 2016. Available at http://www.kidneysupportivecare.org/Files/TreatingPaininLateCKD11-2016.aspx. Accessed May 21, 2017
- 9.Weisbord SD, Shields AM, Mor MK, Sevick MA, Homer M, Peternel J, Porter P, Rollman BL, Palevsky PM, Arnold RM, Fine MJ: Methodology of a randomized clinical trial of symptom management strategies in patients receiving chronic hemodialysis: The SMILE study. Contemp Clin Trials 31: 491–497, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Wyne A, Rai R, Cuerden M, Clark WF, Suri RS: Opioid and benzodiazepine use in end-stage renal disease: A systematic review. Clin J Am Soc Nephrol 6: 326–333, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davison SN: Pain in hemodialysis patients: Prevalence, cause, severity, and management. Am J Kidney Dis 42: 1239–1247, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Bailie GR, Mason NA, Bragg-Gresham JL, Gillespie BW, Young EW: Analgesic prescription patterns among hemodialysis patients in the DOPPS: Potential for underprescription. Kidney Int 65: 2419–2425, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Mercadante S, Ferrantelli A, Tortorici C, Lo Cascio A, Lo Cicero M, Cutaia I, Parrino I, Casuccio A: Incidence of chronic pain in patients with end-stage renal disease on dialysis. J Pain Symptom Manage 30: 302–304, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Carreon M, Fried LF, Palevsky PM, Kimmel PL, Arnold RM, Weisbord SD: Clinical correlates and treatment of bone/joint pain and difficulty with sexual arousal in patients on maintenance hemodialysis. Hemodial Int 12: 268–274, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Claxton RN, Blackhall L, Weisbord SD, Holley JL: Undertreatment of symptoms in patients on maintenance hemodialysis. J Pain Symptom Manage 39: 211–218, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Masajtis-Zagajewska A, Pietrasik P, Krawczyk J, Krakowska M, Jarzębski T, Pietrasiewicz B, Zbróg Z, Nowicki M: Similar prevalence but different characteristics of pain in kidney transplant recipients and chronic hemodialysis patients. Clin Transplant 25: E144–E151, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Manley HJ, McClaran ML, Overbay DK, Wright MA, Reid GM, Bender WL, Neufeld TK, Hebbar S, Muther RS: Factors associated with medication-related problems in ambulatory hemodialysis patients. Am J Kidney Dis 41: 386–393, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Wang PS, Schneeweiss S, Glynn RJ, Mogun H, Avorn J: Use of the case-crossover design to study prolonged drug exposures and insidious outcomes. Ann Epidemiol 14: 296–303, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Söderberg KC, Laflamme L, Möller J: Newly initiated opioid treatment and the risk of fall-related injuries. A nationwide, register-based, case-crossover study in Sweden. CNS Drugs 27: 155–161, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Teng Z, Zhu Y, Wu F, Zhu Y, Zhang X, Zhang C, Wang S, Zhang L: Opioids contribute to fracture risk: A meta-analysis of 8 cohort studies. PLoS One 10: e0128232, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vestergaard P, Rejnmark L, Mosekilde L: Fracture risk associated with the use of morphine and opiates. J Intern Med 260: 76–87, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Ray WA, Chung CP, Murray KT, Hall K, Stein CM: Prescription of long-acting opioids and mortality in patients with chronic noncancer pain. JAMA 315: 2415–2423, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurella M, Bennett WM, Chertow GM: Analgesia in patients with ESRD: A review of available evidence. Am J Kidney Dis 42: 217–228, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Labianca R, Sarzi-Puttini P, Zuccaro SM, Cherubino P, Vellucci R, Fornasari D: Adverse effects associated with non-opioid and opioid treatment in patients with chronic pain. Clin Drug Investig 32[Suppl 1]: 53–63, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Kinjo M, Setoguchi S, Schneeweiss S, Solomon DH: Bone mineral density in subjects using central nervous system-active medications. Am J Med 118: 1414, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Johnell O, Kanis JA, Oden A, Johansson H, De Laet C, Delmas P, Eisman JA, Fujiwara S, Kroger H, Mellstrom D, Meunier PJ, Melton LJ 3rd, O’Neill T, Pols H, Reeve J, Silman A, Tenenhouse A: Predictive value of BMD for hip and other fractures. J Bone Miner Res 20: 1185–1194, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Jadoul M, Albert JM, Akiba T, Akizawa T, Arab L, Bragg-Gresham JL, Mason N, Prutz KG, Young EW, Pisoni RL: Incidence and risk factors for hip or other bone fractures among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study. Kidney Int 70: 1358–1366, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Kimmel PL, Fwu CW, Abbott KC, Eggers AW, Kline PP, Eggers PW: Opioid prescription, morbidity, and mortality in United States dialysis patients. J Am Soc Nephrol 28: 3658–3670, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Desmet C, Beguin C, Swine C, Jadoul M; Université Catholique de Louvain Collaborative Group : Falls in hemodialysis patients: Prospective study of incidence, risk factors, and complications. Am J Kidney Dis 45: 148–153, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Andrade SE, Kahler KH, Frech F, Chan KA: Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf 15: 565–574, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Nielsen S, Degenhardt L, Hoban B, Gisev N: A synthesis of oral morphine equivalents (OME) for opioid utilisation studies. Pharmacoepidemiol Drug Saf 25: 733–737, 2016 [DOI] [PubMed] [Google Scholar]
- 32.US Renal Data System Coordinating Center : USRDS 2013 researcher’s guide to the USRDS database., 2013
- 33.Nair SS, Lenihan CR, Montez-Rath ME, Lowenberg DW, Chertow GM, Winkelmayer WC: Temporal trends in the incidence, treatment and outcomes of hip fracture after first kidney transplantation in the United States. Am J Transplant 14: 943–951, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jetté N, Reid AY, Quan H, Hill MD, Wiebe S: How accurate is ICD coding for epilepsy? Epilepsia 51: 62–69, 2010 [DOI] [PubMed] [Google Scholar]
- 35.Jette N, Beghi E, Hesdorffer D, Moshé SL, Zuberi SM, Medina MT, Bergen D: ICD coding for epilepsy: Past, present, and future--a report by the International League Against Epilepsy Task Force on ICD codes in epilepsy. Epilepsia 56: 348–355, 2015 [DOI] [PubMed] [Google Scholar]
- 36.St Germaine-Smith C, Metcalfe A, Pringsheim T, Roberts JI, Beck CA, Hemmelgarn BR, McChesney J, Quan H, Jette N: Recommendations for optimal ICD codes to study neurologic conditions: A systematic review. Neurology 79: 1049–1055, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.US Renal Data System : USRDS 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End-stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2013 [Google Scholar]
- 38.Park H, Rascati KL, Lawson KA, Barner JC, Richards KM, Malone DC: Adherence and persistence to prescribed medication therapy among Medicare part D beneficiaries on dialysis: Comparisons of benefit type and benefit phase. J Manag Care Spec Pharm 20: 862–876, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yusuf AA, Howell BL, Powers CA, St Peter WL: Utilization and costs of medications associated with CKD mineral and bone disorder in dialysis patients enrolled in Medicare Part D. Am J Kidney Dis 64: 770–780, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller M, Barber CW, Leatherman S, Fonda J, Hermos JA, Cho K, Gagnon DR: Prescription opioid duration of action and the risk of unintentional overdose among patients receiving opioid therapy. JAMA Intern Med 175: 608–615, 2015 [DOI] [PubMed] [Google Scholar]
- 41.Don HF, Dieppa RA, Taylor P: Narcotic analgesics in anuric patients. Anesthesiology 42: 745–747, 1975 [DOI] [PubMed] [Google Scholar]
- 42.Tran BW, Kohan LR, Vorenkamp KE: Postoperative oxycodone toxicity in a patient with chronic pain and end-stage renal disease. A A Case Rep 4: 44–46, 2015 [DOI] [PubMed] [Google Scholar]
- 43.Dean M: Opioids in renal failure and dialysis patients. J Pain Symptom Manage 28: 497–504, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Davison SN, Koncicki H, Brennan F: Pain in chronic kidney disease: A scoping review. Semin Dial 27: 188–204, 2014 [DOI] [PubMed] [Google Scholar]
- 45.Conway BR, Fogarty DG, Nelson WE, Doherty CC: Opiate toxicity in patients with renal failure. BMJ 332: 345–346, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koncicki HM, Brennan F, Vinen K, Davison SN: An approach to pain management in end stage renal disease: Considerations for general management and intradialytic symptoms. Semin Dial 28: 384–391, 2015 [DOI] [PubMed] [Google Scholar]
- 47.Koncicki HM, Unruh M, Schell JO: Pain management in CKD: A guide for nephrology providers. Am J Kidney Dis 69: 451–460, 2017 [DOI] [PubMed] [Google Scholar]
- 48.Nagar VR, Birthi P, Salles S, Sloan PA: Opioid use in chronic pain patients with chronic kidney disease: A systematic review. Pain Med 18: 1416–1449, 2017 [DOI] [PubMed] [Google Scholar]
- 49.Lee MA, Leng ME, Tiernan EJ: Retrospective study of the use of hydromorphone in palliative care patients with normal and abnormal urea and creatinine. Palliat Med 15: 26–34, 2001 [DOI] [PubMed] [Google Scholar]
- 50.Shafi T, Sozio SM, Luly J, Bandeen-Roche KJ, St Peter WL, Ephraim PL, McDermott A, Herzog CA, Crews DC, Scialla JJ, Tangri N, Miskulin DC, Michels WM, Jaar BG, Zager PG, Meyer KB, Wu AW, Boulware LE; DEcIDE Network Patient Outcomes in End Stage Renal Disease Study Investigators : Antihypertensive medications and risk of death and hospitalizations in US hemodialysis patients: Evidence from a cohort study to inform hypertension treatment practices. Medicine (Baltimore) 96: e5924, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.