Abstract
Background and objectives
Hydroxychloroquine is widely used in patients with rheumatoid arthritis. However, large-scale studies examining the long-term effects of hydroxychloroquine on the development of kidney disease in patients with rheumatoid arthritis are lacking. We aimed to assess the long-term association of hydroxychloroquine use with the risk of developing CKD in this population.
Design, setting, participants, & measurements
We conducted an observational cohort study for patients with newly diagnosed rheumatoid arthritis who were enrolled prospectively in Taiwan’s National Health Insurance Research Database between January 1, 2000 and December 31, 2013. We used multivariable Cox proportional hazard regression to analyze the association of hydroxychloroquine use with incident CKD.
Results
A total of 2619 patients, including 1212 hydroxychloroquine users and 1407 hydroxychloroquine nonusers, were analyzed. Incident CKD was reported in 48 of 1212 hydroxychloroquine users and 121 of 1407 hydroxychloroquine nonusers. The incidence rate of CKD was lower in hydroxychloroquine users than in hydroxychloroquine nonusers (10.3 versus 13.8 per 1000 person-years). After multivariable adjustment, hydroxychloroquine users still had a lower risk of incident CKD (adjusted hazard ratio, 0.64; 95% confidence interval, 0.45 to 0.90; P=0.01) than hydroxychloroquine nonusers. The lower risk of subsequent CKD development was dose dependent and consistent across subgroup analyses.
Conclusions
Hydroxychloroquine use in patients with newly diagnosed rheumatoid arthritis is associated with a significantly lower risk of incident CKD compared with in nonusers.
Keywords: chronic kidney disease; hydroxychloroquine; rheumatoid arthritis; Humans; Hydroxychloroquine; Cohort Studies; Confidence Intervals; Incidence; Taiwan; Risk; Arthritis, Rheumatoid; Proportional Hazards Models; Renal Insufficiency, Chronic; National Health Programs
Introduction
Rheumatoid arthritis is one of the most prevalent and devastating chronic inflammatory diseases. Rheumatoid arthritis predominantly affects the joints; however, it may also affect extra-articular structures, including the skin, eye, heart, lung, and gastrointestinal, neurologic, and vascular systems (1). CKD is another chronic inflammatory disease of great public health concern. Recent studies have found a relationship between rheumatoid arthritis and CKD (2–4). Patients with rheumatoid arthritis have a higher risk of reduced kidney function (2,5). Both diseases could result in progressive decline in physical function, quality of life, and mortality (1,6).
Hydroxychloroquine, originally developed as an antimalaria agent, was found to ameliorate the symptoms of arthritis, and it was recognized as one of the disease-modifying antirheumatic drugs (DMARDs). DMARDs can suppress inflammatory responses and retard disease progression in several autoimmune diseases, like rheumatoid arthritis, SLE, and psoriasis. Little is known about the effect of DMARDs on long-term kidney outcomes (5,7). Rheumatologists have been concerned about the kidney toxicity of DMARDs since 1990s (7,8). Cyclosporin, d-penicillamine, and gold are associated with kidney adverse effects, including increased serum creatinine levels, proteinuria, and hematuria (7). In contrast, methotrexate, azathioprine, sulfasalazine, and hydroxychloroquine have relatively little kidney toxicity (5,7). Pons-Estel et al. (9) reported that hydroxychloroquine could prevent kidney damage in patients with lupus nephritis. Therefore, hydroxychloroquine is currently suggested for treatment of patients with lupus nephritis of any class (10). Additionally, no large-scale study examining whether hydroxychloroquine treatment can reduce the risk of CKD in patients with rheumatoid arthritis is available. The effect of hydroxychloroquine on long-term kidney outcomes in rheumatoid arthritis remains unclear.
In this study, we hypothesized that hydroxychloroquine use may be beneficial for reducing kidney diseases, and we conducted a nationwide observational cohort study to assess the associations between hydroxychloroquine use and long-term kidney outcomes in patients with rheumatoid arthritis.
Materials and Methods
Data Source
Patients’ information was obtained from the National Health Insurance Research Database (NHIRD) of Taiwan, which includes health care data covering 99% of the Taiwanese population (approximate 23 million people) (11). We used the Longitudinal Health Insurance Database 2005 (LHID2005), which contains 1 million patients randomly selected from the NHIRD in 2005, as the research dataset. The LHID2005, longitudinally linked with the NHIRD from 1996 to 2013, includes deidentified data on demographics, outpatient visits, hospital admissions, diagnostic codes on the basis of the International Classification of Diseases Ninth Revision Clinical Modification (ICD-9-CM), drug prescriptions, and medical procedures. This study was approved by the Institutional Review Board of the Changhua Christian Hospital with an exempt informed consent (approval number 170521).
Study Design and Participants
After excluding 838 patients with rheumatoid arthritis diagnosis (ICD-9-CM code 714.0 or A code A430) established between 1996 and 1999, we identified 3573 patients with newly diagnosed rheumatoid arthritis after 2000 (Figure 1). We further excluded 954 patients who had unqualified demographic information, experienced confounding conditions, or developed proposed outcomes within 90 days after rheumatoid arthritis diagnosis. The remaining 2619 patients with incident rheumatoid arthritis were selected for further analysis. Among them, those who had received hydroxychloroquine within 90 days after rheumatoid arthritis diagnosis were defined as hydroxychloroquine users, whereas the remaining patients were defined as hydroxychloroquine nonusers (Figure 1, Supplemental Figure 1). Patients were followed up from the 91st day after rheumatoid arthritis diagnosis to the development of death, an investigated event, censoring, or end of follow-up (December 31, 2013), whichever came first. Hydroxychloroquine users were censored at 180 days after their last prescription of hydroxychloroquine was filled. Additionally, hydroxychloroquine no-users were censored if they received a hydroxychloroquine prescription in their follow-up period.
Figure 1.
Flowchart of patient selection. HCQ, hydroxychloroquine; NHIRD, National Health Insurance Research Database; RA, rheumatoid arthritis.
Outcomes and Relevant Variables
The primary outcome was incident CKD defined by at least three ICD-9-CM codes for at least 3 months (Supplemental Table 1) (12–15). Potential relevant confounding variables included demographics, important comorbidities identified by at least three diagnosis codes within 1 year before rheumatoid arthritis diagnosis (Supplemental Table 1), and long-term medications, such as antidiabetic drugs, diuretics, angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers, and nonsteroidal anti-inflammatory drugs (NSAIDs). Charlson comorbidity index score was used to quantify the severity of baseline comorbidity (16).
Statistical Analyses
Continuous variables were presented as mean±SD, and discrete variables were presented as numbers and percentages. Categorical and continuous variables were compared using chi-squared tests and t tests, respectively. We calculated the incidence rate (per 1000 person-years of follow-up) for CKD events. A propensity score, representing the probability of being treated by hydroxychloroquine, was calculated by nonparsimonious multivariable logistic regression using all variables from Table 1 for every patient. The assessed propensity score was used for covariate adjustment and propensity score matching (Supplemental Table 2). The c statistic of the propensity score model was 0.60.
Table 1.
Baseline characteristics of CKD-free patients with rheumatoid arthritis by use of hydroxychloroquine within 90 days after rheumatoid arthritis diagnosis
| Characteristica | Hydroxychloroquine Nonusers, n=1407 | Hydroxychloroquine Users, n=1212 |
|---|---|---|
| Demographics | ||
| Age, yr | 51±14 | 52±14 |
| Men | 342 (24%) | 290 (24%) |
| Monthly income, new Taiwan dollars | 18,838±15,982 | 17,689±14,610 |
| Geographic location | ||
| Northern Taiwanb | 721 (51%) | 554 (46%) |
| Central Taiwanb | 277 (20%) | 320 (26%) |
| Southern Taiwan | 371 (26%) | 311 (26%) |
| Eastern Taiwan and islands | 38 (3%) | 27 (2%) |
| Clinic visit frequency, visits per yearb | 26±16 | 27±15 |
| Comorbidities | ||
| Charlson comorbidity index score | 1.2±1.4 | 1.2±1.4 |
| Hypertension | 305 (22%) | 274 (23%) |
| Hyperlipidemia | 183 (13%) | 146 (12%) |
| Diabetes mellitus | 106 (8%) | 81 (7%) |
| Coronary artery disease | 107 (8%) | 84 (7%) |
| Congestive heart failure | 33 (2%) | 20 (2%) |
| Goutb | 214 (15%) | 131 (11%) |
| Stroke | 51 (4%) | 53 (4%) |
| Chronic obstructive pulmonary disease | 146 (10%) | 111 (9%) |
| Peripheral artery occlusive disease | 11 (0.8%) | 15 (1%) |
| Arrhythmia | 65 (5%) | 41 (3%) |
| Baseline long-term medications | ||
| Antidiabetic drugs | 63 (4%) | 64 (5%) |
| Antihypertensive drugs | 244 (17%) | 220 (18%) |
| Angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers | 103 (7%) | 94 (8%) |
| Diuretics | 71 (5%) | 65 (5%) |
| Nonsteroidal anti-inflammatory drugs | 160 (11%) | 138 (11%) |
| Analgesic drugs other than nonsteroidal anti-inflammatory drugsc | 133 (9%) | 137 (11%) |
| Glucocorticoidsb | 89 (6%) | 149 (12%) |
| Calcineurin inhibitorsd | 1 (0.1%) | 3 (0.3%) |
| TNF-α inhibitorsb | 86 (6%) | 116 (10%) |
| Other immunosuppressantse | 72 (5%) | 66 (5%) |
| Cumulative dose of hydroxychloroquine within 90 d, g | — | 43.2±24.8 |
| Propensity scoreb | 0.45±0.09 | 0.48±0.10 |
—, not applicable.
Variables are expressed as mean±SD or n (%).
Statistically significant.
Includes cyclooxygenase-2 inhibitors, aspirin, and acetaminophen.
Includes cyclosporin and tacrolimus.
Includes methotrexate, leflunomide, sulfasalazine, and azathioprine.
The cumulative incidence of CKD over time between hydroxychloroquine users and nonusers was estimated using the Kaplan–Meier method and compared using log rank tests. Cox proportional hazard models were used to examine the association of hydroxychloroquine use and outcomes of interest, and results were reported as crude hazard ratios or adjusted hazard ratios (aHRs) with 95% confidence intervals (95% CIs). Additionally, because patients with rheumatoid arthritis have increased mortality, which would compete with the risk of CKD, we calculated the Fine and Gray subdistribution hazards and extended the Cox proportional hazard model for competing risks adjustment. To determine the dose-response association, we estimated the risk of CKD according to the cumulative defined daily dose during the 90-day exposure period (≤35, 36–70, or >70 defined daily dose) and the prescribed daily dose (≤200, 201–400, or >400 mg) compared with no hydroxychloroquine use. Defined daily dose is the assumed average maintenance dose per day for a drug used for its main indication in adults, and it is 516 mg for hydroxychloroquine defined by the World Health Organization (17). Subgroup analysis was performed to determine the effect modification and compare the association of hydroxychloroquine use with aHRs of incident CKD among different patient groups. Furthermore, we performed sensitivity analyses to ascertain our results. First, all clinical variables (demographics, clinical visit frequency, all comorbidities, and medications) were controlled in multivariable Cox proportional hazard model. Second, we evaluated misclassification bias by defining hydroxychloroquine use at intervals of 120, 150, and 180 days after first rheumatoid arthritis diagnosis. Third, we used a stringent criterion to enroll patients with rheumatoid arthritis according to the Registry for Catastrophic Illness Patients. Moreover, the primary outcome was defined by a stringent criterion (Supplemental Table 1) (18). Fourth, we modeled hydroxychloroquine use as a time-dependent covariate in the extended Cox proportional hazard model. Fifth, we included patients with rheumatoid arthritis and concomitant SLE or psoriasis in the analysis. Sixth, patients who discontinued hydroxychloroquine use were not censored, because this may cause informative censoring. Seventh, we performed a propensity score matching to balance distributions of measured covariates in the two cohorts. Each patient with rheumatoid arthritis from the hydroxychloroquine user cohort was matched to one nonuser control on the basis of the propensity score (±10% SD). All statistical analyses were conducted using SAS 9.4 software (SAS Institute Inc., Cary, NC). Two-sided P values <0.05 were considered statistically significant.
Results
Characteristics of Patients
A total of 2619 patients without previous kidney diseases were diagnosed with rheumatoid arthritis in the LHID2005 between January 1, 2000 and December 31, 2013 (Figure 1). Of them, 1212 patients were hydroxychloroquine users, and 1407 were nonusers. The mean follow-up times for hydroxychloroquine users and nonusers were 4.1±3.7 and 6.5±4.0 years, respectively (P<0.001). Among the users, the median time of using hydroxychloroquine was 4.4 years (interquartile range, 0.8–12.9 years). Table 1 shows that hydroxychloroquine users were more distributed in central Taiwan and less distributed in northern Taiwan, had more frequent clinic visits, had lower proportions of gout, and were more likely to use long-term medications, including glucocorticoids and TNF-α inhibitors.
Long-Term Risk of Incident CKD
During follow-up, 48 (4%) of 1212 hydroxychloroquine users and 121 (9%) of 1407 nonusers developed CKD. The incidence rate of CKD (10.3 versus 13.8 per 1000 person-years) (Table 2) was lower in the hydroxychloroquine users than in the nonusers.
Table 2.
Risk of incident CKD in hydroxychloroquine users compared with nonusers
| Exposure | Events, n/N | Incident Ratea (95% CI) | cHR (95% CI) | P Value | aHRb,c (95% CI) | P Value |
|---|---|---|---|---|---|---|
| Cohorts | ||||||
| Hydroxychloroquine nonusers | 121/1407 | 13.8 (11.3–16.2) | 1.00 | — | 1.00 | — |
| Hydroxychloroquine users | 48/1212 | 10.3 (7.4–13.2) | 0.67 (0.48 to 0.94) | 0.02 | 0.64 (0.45 to 0.90) | 0.01 |
| Cumulative defined daily dosed | ||||||
| Hydroxychloroquine | 121/1407 | 13.8 (11.3–16.2) | 1.00 | — | 1.00 | — |
| Hydroxychloroquine users | ||||||
| ≤35 | 22/521 | 12.1 (7.1–17.2) | 0.79 (0.50 to 1.24) | 0.30 | 0.77 (0.48 to 1.21) | 0.25 |
| 36–70 | 20/470 | 10.6 (6.0–15.3) | 0.70 (0.44 to 1.12) | 0.14 | 0.66 (0.40 to 1.07) | 0.09 |
| >70 | 6/221 | 6.1 (1.2–11.0) | 0.40 (0.18 to 0.90) | 0.03 | 0.37 (0.16 to 0.84) | 0.02 |
| P value for trend | 0.01 | 0.004 | ||||
| Prescribed daily dose | ||||||
| Hydroxychloroquine nonusers | 121/1407 | 13.8 (11.3–16.2) | 1.00 | — | 1.00 | — |
| Hydroxychloroquine users, mg | ||||||
| ≤200 | 17/441 | 11.0 (5.8–16.2) | 0.71 (0.43 to 1.18) | 0.19 | 0.69 (0.42 to 1.16) | 0.16 |
| 201–400 | 24/523 | 11.1 (6.7–15.5) | 0.73 (0.47 to 1.13) | 0.16 | 0.69 (0.44 to 1.08) | 0.11 |
| >400 | 7/248 | 7.3 (1.9–12.6) | 0.47 (0.22 to 1.00) | 0.05 | 0.45 (0.21 to 0.96) | 0.04 |
| P value for trend | 0.02 | 0.01 |
cHR, crude hazard ratio; 95% CI, 95% confidence interval; aHR, adjusted hazard ratio; —, not applicable.
Per 1000 person-years.
Adjusted for propensity scores.
All-cause death was considered a competing risk.
Within 90 days after rheumatoid arthritis diagnosis.
Kaplan–Meier curves showed a lower cumulative incidence of CKD in hydroxychloroquine users (log rank test P=0.03) (Figure 2A). The users of hydroxychloroquine had a lower risk of incident CKD than hydroxychloroquine nonusers (crude hazard ratio, 0.67; 95% CI, 0.48 to 0.94; P=0.02) (Table 2). The association was unchanged after adjustment for confounders and competing risk of death (aHR, 0.64; 95% CI, 0.45 to 0.90; P=0.01) (Table 2).
Figure 2.
Kaplan–Meier curves for cumulative incidences of CKD in hydroxychloroquine users and nonusers. (A) The cumulative incidence of CKD was lower in hydroxychloroquine users. (B) There was an inverse dose-response relationship between incident CKD and hydroxychloroquine use.
An inverse dose-response relationship was found between hydroxychloroquine use and risk of incident CKD. Patients with rheumatoid arthritis and cumulative doses of hydroxychloroquine >70 defined daily dose had a significantly lower risk of CKD (aHR, 0.37; 95% CI, 0.16 to 0.84; P=0.004 for trend [Table 2] and log rank test for trend P=0.01 [Figure 2B]). Similarly, compared with patients not taking hydroxychloroquine, those who used >400 mg/dhydroxychloroquine had the lowest risk of CKD (aHR, 0.45; 95% CI, 0.21 to 0.96; P=0.01 for trend) (Table 2).
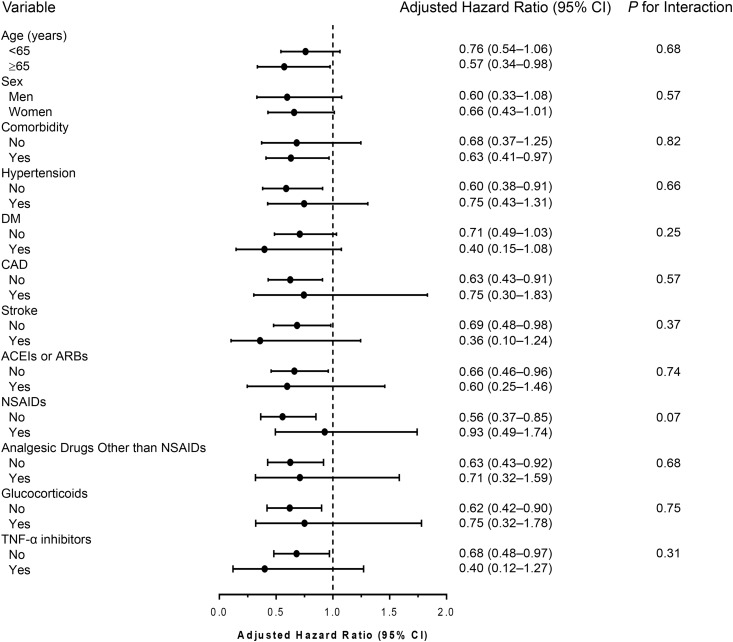
Subgroup analyses revealed similar results to the primary analyses. The trend of lower risk of CKD with hydroxychloroquine use was consistent across clinically relevant subgroups, and none of these subgroups significantly interacted with hydroxychloroquine treatment (all interactions P>0.05) (Figure 3).
Figure 3.
Subgroup analysis of associations of hydroxychloroquine use with the risk of incident CKD in patients with rheumatoid arthritis. ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CAD, coronary artery disease; 95% CI, 95% confidence interval; DM, diabetes mellitus; NSAID, nonsteroidal anti-inflammatory drug.
We evaluated the relationship between the duration of hydroxychloroquine exposure and the risk of CKD in all participants. Compared with those exposed for ≤180 days, a constant association was found between the exposure and risk of CKD among those exposed for 181–365, 366–730, and >730 days (Supplemental Figure 2).
Sensitivity Analyses
Six steps of sensitivity analyses were performed to test the reliability of our main results. First, we performed a multivariable Cox regression model adjusted for all covariates in Table 1. The lower risk of CKD in hydroxychloroquine users was similar to that adjusted for propensity scores (aHR, 0.67; 95% CI, 0.47 to 0.95; P=0.03) (Table 3). Second, we conducted a series of analyses defining hydroxychloroquine use at intervals of 120, 150, and 180 days after first rheumatoid arthritis diagnosis to reduce misclassification bias. The risk of CKD did not change despite using different definitions for hydroxychloroquine use (Supplemental Tables 3–5). Third, we reanalyzed the risk of CKD in patients with rheumatoid arthritis defined by a stringent criterion (only patients with a catastrophic illness certificate for rheumatoid arthritis were analyzed). The stringent criterion for patient selection did not affect the results (aHR, 0.51; 95% CI, 0.33 to 0.80; P=0.003) (Table 3). Additionally, incident CKD was identified using a more stringent criterion (Supplemental Table 1). Similarly, more stringent criterion for diagnosing CKD did not affect the results (aHR, 0.64; 95% CI, 0.45 to 0.91; P=0.01) (Table 3). Fourth, we conducted a time-dependent Cox regression model to evaluate the association of hydroxychloroquine treatment with CKD after treating hydroxychloroquine as a time-dependent variable. The analysis showed that the association between hydroxychloroquine use and new-onset CKD was in agreement with our primary analysis (aHR, 0.60; 95% CI, 0.45 to 0.81; P=0.001 in Table 3 versus aHR, 0.64; 95% CI, 0.45 to 0.90; P=0.01 in Table 2). Fifth, we originally excluded patients with rheumatoid arthritis and coexisting SLE or psoriasis from the study, because these patients had ambiguous status with regard to their autoimmune diseases. However, the inclusion of patients with rheumatoid arthritis and these coexisting diseases did not affect the results (aHR, 0.64; 95% CI, 0.46 to 0.89; P=0.01) (Table 3). Sixth, maintaining group assignment despite discontinuation of hydroxychloroquine produced similar results to the primary analysis (aHR, 0.59; 95% CI, 0.45 to 0.79; P<0.001) (Table 3). Finally, we performed a propensity score matching. No significant differences were found between hydroxychloroquine users and their matched cohort, except for a higher proportion of using analgesic drugs other than NSAIDs in hydroxychloroquine users (Supplemental Table 6). The results were consistent with those of our primary analyses (aHR, 0.54; 95% CI, 0.30 to 0.98; P=0.04) (Supplemental Table 7).
Table 3.
Sensitivity analyses for the risk of CKD
| Analysis | Events, n/N | cHR (95% CI) | P Value | aHRa,b (95% CI) | P Value |
|---|---|---|---|---|---|
| Adjusted for all covariates in Table 1 | |||||
| Hydroxychloroquine nonusers | 121/1407 | 1.00 | — | 1.00 | — |
| Hydroxychloroquine users | 48/1212 | 0.67 (0.48 to 0.94) | 0.02 | 0.67 (0.47 to 0.95) | 0.03 |
| Rheumatoid arthritis defined by a stringent criterionc | |||||
| Hydroxychloroquine nonusers | 55/493 | 1.00 | — | 1.00 | — |
| Hydroxychloroquine users | 31/662 | 0.52 (0.33 to 0.80) | 0.003 | 0.51 (0.33 to 0.80) | 0.003 |
| CKD defined by a stringent criteriond | |||||
| Hydroxychloroquine nonusers | 117/1407 | 1.00 | — | 1.00 | — |
| Hydroxychloroquine users | 46/1212 | 0.67 (0.48 to 0.94) | 0.02 | 0.64 (0.45 to 0.91) | 0.01 |
| Hydroxychloroquine use as a time-dependent covariate | |||||
| Hydroxychloroquine nonusers | 121/1407 | 1.00 | — | 1.00 | — |
| Hydroxychloroquine users | 48/1212 | 0.63 (0.48 to 0.84) | 0.002 | 0.60 (0.45 to 0.81) | 0.001 |
| Including patients with SLE and psoriasis | |||||
| Hydroxychloroquine nonusers | 129/1514 | 1.00 | — | 1.00 | — |
| Hydroxychloroquine users | 52/1335 | 0.66 (0.48 to 0.91) | 0.01 | 0.64 (0.46 to 0.89) | 0.01 |
| Maintaining group assignment despite discontinuation of hydroxychloroquine | |||||
| Hydroxychloroquine nonusers | 136/1407 | 1.00 | — | 1.00 | — |
| Hydroxychloroquine users | 86/1212 | 0.63 (0.48 to 0.82) | 0.001 | 0.59 (0.45 to 0.79) | <0.001 |
cHR, crude hazard ratio; 95% CI, 95% confidence interval; aHR, adjusted hazard ratio; —, not applicable.
Adjusted for propensity scores.
All-cause death was considered a competing risk.
Defined according to the Registry for Catastrophic Illness Patients only.
Defined by the most specific and clinically relevant diagnosis codes for CKD (Supplemental Table 1).
Discussion
This study is the first to show that hydroxychloroquine use was associated with a 36% lower risk of incident CKD in patients with newly diagnosed rheumatoid arthritis. Additionally, an inverse dose-response relationship was found, with the lowest risk seen in patients who received a cumulative dose of hydroxychloroquine >70 defined daily dose within 90 days after rheumatoid arthritis diagnosis or a hydroxychloroquine dose >400 mg/d. The association between hydroxychloroquine use and lower CKD risk was constant over time. Moreover, all subgroup and sensitivity analyses further confirmed our results, indicating that we have minimized potential bias and that our results are robust.
Hydroxychloroquine was first approved in 1955 in the United States for prevention and treatment of malaria. Hydroxychloroquine has various immunomodulatory effects, such as inhibition of chemotaxis and phagocytosis, toll-like receptor signaling, calcium signaling in B and T cells, macrophage-mediated cytokine production, and matrix metalloproteinases (19). It is currently classified as a DMARD, and it is widely used for rheumatoid arthritis, SLE, and porphyria cutanea tarda treatment. Hydroxychloroquine has pleiotropic benefits in addition to immunomodulation. It is associated with lower risks of diabetes mellitus and cardiovascular disease in patients with rheumatoid arthritis (20,21). Our findings expand existing knowledge about hydroxychloroquine use and propose a preferential treatment to prevent kidney diseases in rheumatoid arthritis.
Kidney involvement is common in rheumatoid arthritis, including drug-related kidney disease (the nephrotoxicity of NSAIDs and DMARDs), secondary kidney amyloidosis, GN, and interstitial diseases (5,22). Among them, mesangial proliferative GN is the most common pathologic change (34%–36%) in patients with rheumatoid arthritis (23,24). In addition to patients with rheumatoid arthritis, those with SLE or IgA nephropathy may develop mesangial proliferative GN. Pons-Estel et al. (9) reported that hydroxychloroquine delayed the occurrence of kidney damage in patients with lupus nephritis. Moreover, Gao et al. (25) reported that hydroxychloroquine ameliorated proteinuria in patients with IgA nephropathy. It seems that hydroxychloroquine is effective in these autoimmune diseases. Our results suggest that the beneficial effect of hydroxychloroquine on kidney outcomes can be extended to patients with incident rheumatoid arthritis.
Chloroquine, another derivative of 4-aminoquinoline similar to hydroxychloroquine, is the foremost treatment for malaria, and it is widely used in autoimmune diseases. Chloroquine has an extremely high tissue-to-plasma ratio in the kidneys (26). It would be interesting to know whether there is a similar effect between hydroxychloroquine and chloroquine on reducing CKD risk in patients with rheumatoid arthritis. However, the supply of chloroquine has been discontinued in Taiwan since 1989, earlier than the start of Taiwan’s National Health Insurance. Additional studies are required to elucidate the effect of chloroquine on long-term kidney outcomes in these patients.
The relationship between hydroxychloroquine and long-term kidney outcomes was not well demonstrated in previous studies. A small retrospective study on 118 patients with rheumatoid arthritis reported that 55% of patients receiving chloroquine and 15% of those receiving hydroxychloroquine showed a >10% decrease of creatinine clearance after the start of antimalarial drugs (8). However, later literature suggested that hydroxychloroquine is “not nephrotoxic” (5,7). These studies were small in size, were cross-sectional, or lacked long-term follow-up. On the contrary, our study has a larger sample size, and it was the first to show the independent association of hydroxychloroquine with CKD in patients with rheumatoid arthritis during long-term follow-up. Additionally, the results showed the dose-response and constant association over time. Therefore, hydroxychloroquine use may be beneficial for decreasing CKD risk and can be encouraged in patients with rheumatoid arthritis.
CKD is classically defined by a reduced kidney function or presence of kidney damage (27). However, serum creatinine level and eGFR were not available in the NHIRD. In this study, we used ICD-9-CM codes for identifying incident CKD during follow-up (12,14,18). The positive predictive value of ICD-9-CM codes for diagnosing CKD was approximately 90% (13,28). However, previous studies have also shown that ICD-9-CM codes are not sensitive but are specific for patients with CKD (29). Therefore, we performed analyses using a lenient criterion to improve the sensitivity for CKD diagnosis and a stringent criterion to further confirm our results. Conversely, CKD is usually asymptomatic and insidious in early stages. The detection of incident CKD may be biased by the frequency of clinical follow-up. Thus, in the multivariable models, we considered the frequency of clinical visits to minimize the detection bias. Hydroxychloroquine users were followed up more closely, and they were more likely to use glucocorticoids and TNF-α inhibitors, implying that hydroxychloroquine users might have increased severity of rheumatoid arthritis and need more rheumatoid arthritis medications and closer monitoring. Considering the above-mentioned facts, hydroxychloroquine use is significantly associated with lower risk of CKD.
Our study has several advantages. The first is its larger sample size and national representation. Also, this cohort study followed up patients with rheumatoid arthritis up to 13 years to evaluate long-term kidney outcomes. Finally, our findings were robust across subgroup and sensitivity analyses. However, our study has limitations. First, as mentioned earlier, detailed information on laboratory data, such as eGFR or albuminuria, was not available in the NHIRD. Thus, we identified CKD events using different definitions. Similar results were found between the two different CKD definitions. Second, information on over-the-counter (OTC) drugs, which may contain nephrotoxic NSAIDs, was not available in the NHIRD. However, the cost of OTC NSAIDs is usually higher than an outpatient visit copayment in Taiwan. Thus, most patients would obtain OTC NSAIDs only under an urgent condition. Third, other potential confounding data, such as family history of kidney diseases, smoking habits, body mass index, BP, lipid profile, or uric acid levels, were not available in the NHIRD. Fourth, the underlying mechanism of how hydroxychloroquine reduces CKD risk remains unknown, and further studies are warranted.
In conclusion, in a nationwide registry of patients with newly diagnosed rheumatoid arthritis, hydroxychloroquine users had a significantly lower risk of incident CKD compared with nonusers. The lower risk of CKD was dose dependent and consistent across patient subgroups. Our findings expand the existing evidence of hydroxychloroquine use to prevent subsequent CKD development in patients with rheumatoid arthritis. Whether hydroxychloroquine treatment reduces the transition of CKD to ESKD remains unclear and needs further studies for clarification.
Disclosures
None.
Supplementary Material
Acknowledgments
Our study was in part on the basis of data from the National Health Insurance Research Database provided by the Bureau of National Health Insurance (NHI), Department of Health and managed by Taiwan’s National Health Research Institutes (NHRI).
This study was funded by grants 105-CCH-IRP-011 and 105-CCH-IRP-093 from the Changhua Christian Hospital Research Foundation.
The interpretation and conclusions contained herein do not represent those of the Bureau of NHI, the Department of Health, or the NHRI.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Antimalarial Drugs for the Prevention of Chronic Kidney Disease in Patients with Rheumatoid Arthritis: The Importance of Controlling Chronic Inflammation?,” on pages 679–680.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.11781017/-/DCSupplemental.
References
- 1.Smolen JS, Aletaha D, McInnes IB: Rheumatoid arthritis. Lancet 388: 2023–2038, 2016 [DOI] [PubMed] [Google Scholar]
- 2.Hickson LJ, Crowson CS, Gabriel SE, McCarthy JT, Matteson EL: Development of reduced kidney function in rheumatoid arthritis. Am J Kidney Dis 63: 206–213, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kochi M, Kohagura K, Shiohira Y, Iseki K, Ohya Y: Inflammation as a risk of developing chronic kidney disease in rheumatoid arthritis. PLoS One 11: e0160225, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Icardi A, Araghi P, Ciabattoni M, Romano U, Lazzarini P, Bianchi G: [Kidney involvement in rheumatoid arthritis]. Reumatismo 55: 76–85, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Karie S, Gandjbakhch F, Janus N, Launay-Vacher V, Rozenberg S, Mai Ba CU, Bourgeois P, Deray G: Kidney disease in RA patients: Prevalence and implication on RA-related drugs management: The MATRIX study. Rheumatology (Oxford) 47: 350–354, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Webster AC, Nagler EV, Morton RL, Masson P: Chronic kidney disease. Lancet 389: 1238–1252, 2017 [DOI] [PubMed] [Google Scholar]
- 7.Schiff MH, Whelton A: Renal toxicity associated with disease-modifying antirheumatic drugs used for the treatment of rheumatoid arthritis. Semin Arthritis Rheum 30: 196–208, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Landewé RB, Vergouwen MS, Goeei The SG, Van Rijthoven AW, Breedveld FC, Dijkmans BA: Antimalarial drug induced decrease in creatinine clearance. J Rheumatol 22: 34–37, 1995 [PubMed] [Google Scholar]
- 9.Pons-Estel GJ, Alarcón GS, McGwin G Jr, Danila MI, Zhang J, Bastian HM, Reveille JD, Vilá LM; Lumina Study Group : Protective effect of hydroxychloroquine on renal damage in patients with lupus nephritis: LXV, data from a multiethnic US cohort. Arthritis Rheum 61: 830–839, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beck L, Bomback AS, Choi MJ, Holzman LB, Langford C, Mariani LH, Somers MJ, Trachtman H, Waldman M: KDOQI US commentary on the 2012 KDIGO clinical practice guideline for glomerulonephritis. Am J Kidney Dis 62: 403–441, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Cheng TM: Taiwan’s new national health insurance program: Genesis and experience so far. Health Aff (Millwood) 22: 61–76, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Yu TM, Lin CL, Chang SN, Sung FC, Kao CH: Increased risk of stroke in patients with chronic kidney disease after recurrent hypoglycemia. Neurology 83: 686–694, 2014 [DOI] [PubMed] [Google Scholar]
- 13.Lin MY, Chiu YW, Chang JS, Lin HL, Lee CT, Chiu GF, Kuo MC, Wu MT, Chen HC, Hwang SJ: Association of prescribed Chinese herbal medicine use with risk of end-stage renal disease in patients with chronic kidney disease. Kidney Int 88: 1365–1373, 2015 [DOI] [PubMed] [Google Scholar]
- 14.Wu CL, Tsai CC, Kor CT, Tarng DC, Lian IB, Yang TH, Chiu PF, Chang CC: Stroke and risks of development and progression of kidney diseases and end-stage renal disease: A nationwide population-based cohort study. PLoS One 11: e0158533, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu CL, Kor CT, Chiu PF, Tsai CC, Lian IB, Yang TH, Tarng DC, Chang CC: Long-term renal outcomes in patients with traumatic brain injury: A nationwide population-based cohort study. PLoS One 12: e0171999, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deyo RA, Cherkin DC, Ciol MA: Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 45: 613–619, 1992 [DOI] [PubMed] [Google Scholar]
- 17.WHO Collaborating Centre for Drugs Statistics Methodology: Definition and General Considerations. DDD, 2016. Available at: https://www.whocc.no/ddd/definition_and_general_considera/. Accessed June 15, 2017
- 18.Hsu CC, Wang H, Hsu YH, Chuang SY, Huang YW, Chang YK, Liu JS, Hsiung CA, Tsai HJ: Use of nonsteroidal anti-inflammatory drugs and risk of chronic kidney disease in subjects with hypertension: Nationwide longitudinal cohort study. Hypertension 66: 524–533, 2015 [DOI] [PubMed] [Google Scholar]
- 19.Ben-Zvi I, Kivity S, Langevitz P, Shoenfeld Y: Hydroxychloroquine: From malaria to autoimmunity. Clin Rev Allergy Immunol 42: 145–153, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharma TS, Wasko MC, Tang X, Vedamurthy D, Yan X, Cote J, Bili A: Hydroxychloroquine use is associated with decreased incident cardiovascular events in rheumatoid arthritis patients. J Am Heart Assoc 5: e002867, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wasko MC, Hubert HB, Lingala VB, Elliott JR, Luggen ME, Fries JF, Ward MM: Hydroxychloroquine and risk of diabetes in patients with rheumatoid arthritis. JAMA 298: 187–193, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Braun A, Zeier M: Rheumatoid arthritis and the kidney: Uneasy companions. Nephron Clin Pract 96: c105–c106, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Nakano M, Ueno M, Nishi S, Shimada H, Hasegawa H, Watanabe T, Kuroda T, Sato T, Maruyama Y, Arakawa M: Analysis of renal pathology and drug history in 158 Japanese patients with rheumatoid arthritis. Clin Nephrol 50: 154–160, 1998 [PubMed] [Google Scholar]
- 24.Helin HJ, Korpela MM, Mustonen JT, Pasternack AI: Renal biopsy findings and clinicopathologic correlations in rheumatoid arthritis. Arthritis Rheum 38: 242–247, 1995 [DOI] [PubMed] [Google Scholar]
- 25.Gao R, Wu W, Wen Y, Li X: Hydroxychloroquine alleviates persistent proteinuria in IgA nephropathy. Int Urol Nephrol 49: 1233–1241, 2017 [DOI] [PubMed] [Google Scholar]
- 26.Berliner RW, Earle DP, Taggart JV, Zubrod CG, Welch WJ, Conan NJ, Bauman E, Scudder ST, Shannon JA: Studies on the chemotherapy of the human malarias. VI. The physiological disposition, antimalarial activity, and toxicity of several derivatives of 4-aminoquinoline. J Clin Invest 27: 98–107, 1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andrassy KM: Comments on ‘KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease.’ Kidney Int 84: 622–623, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Winkelmayer WC, Schneeweiss S, Mogun H, Patrick AR, Avorn J, Solomon DH: Identification of individuals with CKD from Medicare claims data: A validation study. Am J Kidney Dis 46: 225–232, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Kern EF, Maney M, Miller DR, Tseng CL, Tiwari A, Rajan M, Aron D, Pogach L: Failure of ICD-9-CM codes to identify patients with comorbid chronic kidney disease in diabetes. Health Serv Res 41: 564–580, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.