Introduction
Membranous nephropathy (MN) occurs when circulating antibodies permeate the glomerular basement membrane and in the subepithelial space, form immune complexes with epitopes on podocyte membranes. One dominant antigen on podocytes is the M-type phospholipase A2 receptor (PLA2R), accounting for autoantibody target in approximately 75%–80% of patients with primary MN (1); other podocyte autoantigens (e.g., thrombospondin type 1 domain–containing 7A) have also been identified (2), accounting for a much smaller fraction of patients with primary MN.
The ability to test for the PLA2R antigen in kidney biopsy specimens and anti-PLA2R antibodies in serum has allowed a more precise nomenclature for most patients with MN. We no longer need to use “idiopathic” or “primary” MN, and instead, we label patients as PLA2R positive or PLA2R negative. These new tests and the knowledge they impart have changed not only nomenclature but also, the way that patients with MN are managed. In this review, I will outline three ways that I use PLA2R testing in patients with MN: (1) to make a diagnosis of MN noninvasively, (2) to distinguish primary MN from secondary MN, and (3) to guide treatment decisions on whether to use immunosuppression.
Illustrative Patients
Patient 1: Using Serum Anti-PLA2R Antibodies to Make a Noninvasive Diagnosis of MN
A 49-year-old white man was noted to have blood and protein in the urine when evaluated for left lower back pain. When he began to notice feet swelling, he presented to a local hospital, where a lower-extremity Doppler ultrasound showed no evidence for deep venous thrombosis, but a computed tomography (CT) scan showed a left kidney vein thrombus. He was started on anticoagulation. His laboratory values were notable for serum creatinine 1.24 mg/dl, serum albumin 2.9 g/dl, total cholesterol 453 mg/dl, urine protein-to-creatinine ratio 5200 mg/g, and otherwise negative or normal serologies, including hepatitis B and C, HIV, antinuclear antibodies, rheumatoid factor, and complement components 3 and 4. He was referred for evaluation of nephrotic syndrome, including discussion of kidney biopsy.
On the basis of his age, race, and kidney vein thrombus, a diagnosis of primary MN was considered the most likely etiology. Before the availability of serum anti-PLA2R antibody testing, a nephrologist would essentially have two options for this patient: perform a high-risk biopsy or treat on the basis of an empirical diagnosis. For this patient, we had a third option of testing for serum anti-PLA2R antibodies, because a positive test could establish the MN diagnosis given the >99% specificity of these antibodies for primary MN (1). The more difficult dilemma would be if his anti-PLA2R antibody test returned negative; in that case, he could still have a 20%–25% chance of having primary MN, because the test’s sensitivity ranges from 75% to 80%, depending on whether testing is done solely on serum samples or combined with immunofluorescence (IF) staining for PLA2R on biopsy specimens (3). We discussed that, if his antibody test returned negative, we would plan for a biopsy.
His serum anti-PLA2R titer returned positive at 1:160, and he started a regimen of steroids and cyclophosphamide in alternating months. After completing this 6-month regimen, his laboratory values showed a complete clinical remission—creatinine 1.01 mg/dl, albumin 4.1 g/dl, total cholesterol 175 mg/dl, and urine protein-to-creatinine ratio 225 mg/g—as well as immunologic remission of disease with negative anti-PLA2R antibody titers.
This case highlights the use of serum anti-PLA2R testing as a noninvasive method of diagnosing MN. I reserve this use of the test only for patients in whom a biopsy is considered high risk or contraindicated, because there is important prognostic information that can be gleaned from a kidney biopsy beyond just establishing a diagnosis. Although some nephrologists might not biopsy patients with preserved kidney function, proteinuria, positive serum anti-PLA2R antibodies, and a negative work-up for secondary causes of nephrotic syndrome, this “diagnostic” use of anti-PLA2R is not yet adequately supported by the literature.
Patient 2: Using PLA2R Testing to Distinguish Primary from Secondary MN
A 71-year-old woman presented with nephrotic syndrome. Her kidney biopsy showed MN. She had a 20 pack-year cigarette history, and a recent CT scan of her chest, ordered to evaluate a chronic cough, showed two small (≤5-mm) lung nodules with a recommendation to reassess in 6 months. Her nephrologist, concerned about malignancy as a secondary cause of MN, coordinated a cancer screening that included a repeat chest CT scan with no interim change in the nodules’ size or appearance, a positron emission tomography scan of her lungs that returned inconclusive, and a colonoscopy revealing multiple polyps. The lung nodules could only be biopsied via open procedure on the basis of their location.
She was referred for a second opinion. Our current protocol for PLA2R testing on kidney biopsy specimens uses indirect IF staining on frozen sections; performing such testing on archived biopsy material was not feasible. A serum test for anti-PLA2R antibodies, however, returned positive at a titer of 1:40. She was, therefore, given a diagnosis of primary MN, and routine follow-up of the lung nodules via imaging was chosen over open lung biopsy.
The first step in managing a patient with newly diagnosed MN is determining whether the lesion is primary or secondary, because this distinction will drive treatment decisions. Specifically, secondary forms of MN (e.g., MN associated with malignancy, lupus, or hepatitis B) are expected to remit if the underlying systemic condition is successfully treated. A secondary MN in this patient would most likely be due to malignancy on the basis of her smoking history and age, because a solid tumor is identified in about 25% of MN cases diagnosed in patients ≥65 years old, with the majority of these malignancies, otherwise occult, detected within 1 year of the MN diagnosis (4). The >99% specificity of the anti-PLA2R antibody testing allows for this kind of differentiation of disease types, because a false positive result could lead to a missed diagnosis of malignancy as well as inappropriate use of immunosuppression with its associated risk of toxicities. In a series of patients with biopsy-proven MN and no clinical suspicion of a secondary cause, the presence of anti-PLA2R antibodies was associated with a 92% reduced risk for malignancy compared with those patients with negative anti-PLA2R testing, of whom 37% (10 of 27) were diagnosed with malignancy after their kidney biopsy (5).
In the majority of patients with MN, prebiopsy serologies (e.g., antinuclear antibodies and hepatitis B and C antibodies) and imaging (e.g., chest x-ray) will have already been performed to rule out secondary forms of disease. There are also morphologic features on the kidney biopsy that favor a secondary MN, including IF staining for C1q antibodies, predominance of IgG1–3 (rather than IgG4) if IgG subtyping is performed, and the presence of mesangial and subendothelial deposits. However, because these features can sometimes appear in patients with positive PLA2R testing, nephrologists and pathologists are increasingly relying on PLA2R status—by IF staining of the biopsy and/or serologic antibody testing—to categorize patients into PLA2R-positive versus -negative forms of disease. Although negative serum anti-PLA2R antibody status can be seen in up to 25% of patients with “primary” MN, combining biopsy antigen testing with serum antibody testing raises the negative predictive value of the assay. The newly diagnosed patient with MN with double-negative PLA2R testing (i.e., biopsy and serum) should be considered more likely to have a secondary MN, and initial management efforts should focus on identifying an underlying etiology (3).
Patient 3: Using Serum Anti-PLA2R Antibody Testing to Guide Treatment Decisions
A 20-year-old woman presented with 6 months of “puffiness” around her eyelids as well as lower extremity swelling and weight gain. She was referred to a nephrologist after urinalysis showed 3+ proteinuria. Her laboratory values revealed serum creatinine 0.4 mg/dl, serum albumin 2.1 g/dl, total cholesterol 263 mg/dl, and 4300 mg/d proteinuria. Tests for lupus, hepatitis, and HIV were negative. A kidney biopsy showed MN with positive IF staining for PLA2R. Given the 6-month duration of periorbital edema and marked hypoalbuminemia, her nephrologist suggested a course of tacrolimus. She sought a second opinion.
We began by checking serum anti-PLA2R antibody titer, which returned at a level of 1:640. In patients with positive IF staining for the PLA2R antigen on kidney biopsy, there is still utility in checking serum antibody levels. First, patients with low or negative serum levels seem to have the greatest chance of achieving spontaneous remission, approaching 40% in those with low-level titers (6) and 80% in those with negative titers (7). In the “kidney as a sink” hypothesis (2), anti-PLA2R antibodies become detectable in the serum only after the buffer capacity of the kidney is exceeded, and therefore, low or negative titers may suggest a low antibody load and relatively mild disease. Second, as with this patient, those with moderate to high levels can be followed with serial anti-PLA2R levels to gauge for evidence of immunologic remission. Because there is no consensus definition on “low” versus “high” anti-PLA2R titers, the most reliable way to use these titers is via serial changes in levels rather than absolute values. Checking serial anti-PLA2R titers early in the disease course, while a patient is being managed on conservative therapy, can replace the traditional method of following such patients with 24-hour or spot urine protein collections (8). Serial anti-PLA2R testing allows the physician to survey for immunologic remission, which on average, occurs 3–6 months before clinical remission, regardless of whether remission is spontaneous or drug induced (9). De Vriese et al. (3) recently suggested a management algorithm for MN using anti-PLA2R titers checked monthly or every other month, depending on the magnitude of initial titer, to guide initiation of immunosuppression (rising titers), modification of immunosuppression (unchanging titers), and cessation of immunosuppression (>90% reduction in titers).
The patient was started on lisinopril, atorvastatin, and furosemide, with resolution of her edema. Repeat laboratory tests 3 months later showed stable creatinine (0.3 mg/dl), rising proteinuria (5900 mg/d), and unchanged hypoalbuminemia (2.0 g/dl) and hypercholesterolemia (266 mg/dl). With no evidence of clinical remission and rising proteinuria, now >9 months from the onset of symptoms, the traditional proteinuria-based algorithm for MN would suggest initiation of immunosuppression for this patient (8). However, her serum anti-PLA2R antibody titer returned at 1:320, one half her prior level. She was continued on conservative management alone with the expectation that clinical remission would follow the immunologic remission that she was beginning to manifest. Over the next 6 months, her titer fell further to 1:160 and 1:80, with corresponding improvements in proteinuria (down to <2000 mg/d) and albuminemia (up to 3.1 g/dl).
This patient should continue to have anti-PLA2R titers checked. Achievement of negative anti-PLA2R status in a previously positive patient is an important benchmark in treatment, because antibody status at the end of therapy has been shown to predict long-term outcomes. In one study, for example, 14 of 24 (58%) antibody-negative patients were in persistent remission at 5 years compared with zero of nine (0%) antibody-positive patients (10). In addition, rising anti-PLA2R titers in a patient whose disease seems to be in clinical remission may suggest impending relapse and should heighten the physician’s surveillance for such an event.
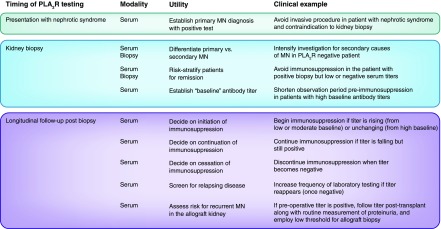
In the relatively short time since PLA2R was reported as the specific podocyte antigen responsible for eliciting immune complex formation with circulating autoantibodies in the majority of patients with primary MN, PLA2R testing has become a standard part of the diagnosis of MN and promises to become an important tool in longitudinal management of patients with MN (Figure 1). The above patients illustrate a paradigm of bench to bedside translational medicine, because the advances from landmark proteomic studies have rapidly transformed the care that nephrologists can provide.
Figure 1.
The use of serum antiphospholipase A2 receptor (anti-PLA2R) antibody testing and biopsy PLA2R antigen staining in the diagnosis and management of patients with membranous nephropathy (MN) is determined by the timing of such tests.
Disclosures
None.
Acknowledgments
A.S.B. is supported by National Institute of Diabetes and Digestive and Kidney Diseases grant UM1-DK100876 and National Institute on Minority Health and Health Disparities grant R01-MD009223.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Beck Jr. LH, Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, Klein JB, Salant DJ: M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 361: 11–21, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beck Jr. LH, Salant DJ: Membranous nephropathy: From models to man. J Clin Invest 124: 2307–2314, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Vriese AS, Glassock RJ, Nath KA, Sethi S, Fervenza FC: A Proposal for a serology-based approach to membranous nephropathy. J Am Soc Nephrol 28: 421–430, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lefaucheur C, Stengel B, Nochy D, Martel P, Hill GS, Jacquot C, Rossert J; GN-PROGRESS Study Group: Membranous nephropathy and cancer: Epidemiologic evidence and determinants of high-risk cancer association. Kidney Int 70: 1510–1517, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Timmermans SA, Ayalon R, van Paassen P, Beck Jr. LH, van Rie H, Wirtz JJ, Verseput GH, Frenken LA, Salant DJ, Cohen Tervaert JW; Limburg Renal Registry: Anti-phospholipase A2 receptor antibodies and malignancy in membranous nephropathy. Am J Kidney Dis 62: 1223–1225, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Hofstra JM, Debiec H, Short CD, Pellé T, Kleta R, Mathieson PW, Ronco P, Brenchley PE, Wetzels JF: Antiphospholipase A2 receptor antibody titer and subclass in idiopathic membranous nephropathy. J Am Soc Nephrol 23: 1735–1743, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoxha E, Harendza S, Pinnschmidt HO, Tomas NM, Helmchen U, Panzer U, Stahl RA: Spontaneous remission of proteinuria is a frequent event in phospholipase A2 receptor antibody-negative patients with membranous nephropathy. Nephrol Dial Transplant 30: 1862–1869, 2015 [DOI] [PubMed] [Google Scholar]
- 8.Cattran D: Management of membranous nephropathy: When and what for treatment. J Am Soc Nephrol 16: 1188–1194, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Ronco P, Debiec H: Anti-phospholipase A2 receptor antibodies and the pathogenesis of membranous nephropathy. Nephron Clin Pract 128: 232–237, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Bech AP, Hofstra JM, Brenchley PE, Wetzels JF: Association of anti-PLA2R antibodies with outcomes after immunosuppressive therapy in idiopathic membranous nephropathy. Clin J Am Soc Nephrol 9: 1386–1392, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]