Abstract
Glucocorticoids exert anti-inflammatory and immunosuppressive activities by genomic and nongenomic effects. The classic genomic effects are mediated by cytosolic glucocorticoid receptors that can upregulate the expression of anti-inflammatory proteins in the nucleus (transactivation) or repress the translocation of proinflammatory transcription factors from the cytosol into the nucleus (transrepression). The nongenomic effects are probably mediated by membrane glucocorticoid receptors. Glucocorticoid receptors are expressed also in podocytes and experimental data suggest that glucocorticoids may protect from podocyte injury. Glucocorticoids have a low therapeutic index and may exert a number of time-dependent and dose-dependent side effects. Measures to prevent or attenuate side effects include single-morning administration of short-acting glucocorticoids, dietetic counseling, increasing physical activity, frequent monitoring, and adapting the doses to the clinical conditions of the patient. Synthetic glucocorticoids, either given alone or in combination with other immunosuppressive drugs, are still the cornerstone therapy in multiple glomerular disorders. However, glucocorticoids are of little benefit in C3 glomerulopathy and may be potentially deleterious in patients with maladaptive focal glomerulosclerosis. Their efficacy depends not only on the type and severity of glomerular disease, but also on the timeliness of administration, the dosage, and the duration of treatment. Whereas an excessive use of glucocorticoids can be responsible for severe toxicity, too low a dosage and too short duration of glucocorticoid treatment can result in false steroid resistance.
Keywords: primary glomerulonephritis; cortisol; immunosuppression; Humans; Glucocorticoids; Anti-Inflammatory Agents; Counseling; Cytosol; Dietetics; Exercise; Genomics; Glomerulosclerosis, Focal Segmental; Immunosuppressive Agents; Kidney Glomerulus; Podocytes; Receptors, Glucocorticoid; Steroids; Therapeutic Index; transcription factors; Transcriptional Activation
Introduction
Glucocorticoids have been extensively used in glomerular diseases. However, their indications for different subtypes of glomerular diseases are variable and in some instances their use may be inappropriate or even deleterious. This review focuses on important pharmacologic characteristics of glucocorticoids, potential side effects, and possible measures to prevent adverse events. The indications for glucocorticoids in treating different subtypes of glomerular diseases, and the possible flaws with their use, will be discussed.
Pharmacology of Glucocorticoids
The naturally occurring glucocorticoid, cortisol, is released by adrenal glands, following a circadian rhythm regulated by the brain’s central pacemaker. Circulating levels are low at sleep onset, begin to increase between 2 and 4 am, peak a few minutes after awakening, and then decline through the day, reaching a nadir between eleven at night and one in the morning (1). As opposed to cortisol, synthetic glucocorticoids have longer half-lives because their hepatic metabolism is low. Glucocorticoids may be divided into short acting (prednisone, prednisolone, methylprednisolone, and deflazacort, with plasma half-lives of 60–200 minutes), intermediate acting (paramethasone and triamcinolone, with plasma half-lives of about 300 minutes), and long acting (dexamethasone and betamethasone, which suppress adrenocorticotrophic hormone levels for >48 hours).
Glucocorticoids may exert their activities by two main mechanisms of action: the classic genomic effects and secondary nongenomic effects.
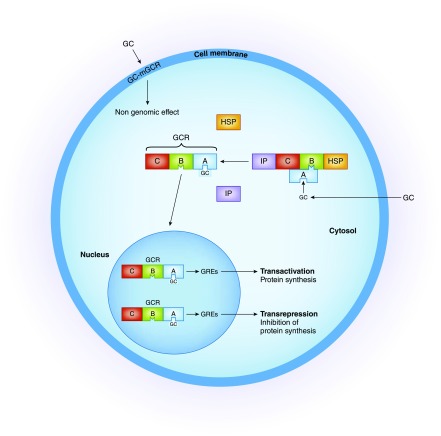
The genomic effects develop with a time lag of 4–24 hours. These effects depend on the doses of synthetic glucocorticoids and on the density, availability, and affinity of glucocorticoid receptors. In the cytoplasm, glucocorticoids bind to a specific glucocorticoid receptor forming a complex that enters the nucleus. This complex binds to specific glucocorticoid response elements in genes and increases the expression of anti-inflammatory proteins (transactivation), or decreases the production of proinflammatory proteins (transrepression) (2,3) (Figure 1). It is believed that the adverse effects of glucocorticoids are induced by transactivation, whereas the beneficial anti-inflammatory effects are mainly due to transrepression. Although the molecular mechanisms of glucocorticoid-induced side effects are not completely understood, it is reasonable to assume that adverse events, such as steroid diabetes, require glucocorticoid receptor–DNA transactivation. In fact, transactivation increases the synthesis of phosphoenolpyruvate carboxykinase and glucose-6-phosphatase that are required for gluconeogenesis induced by glucocorticoids. Other adverse events of glucocorticoids (osteoporosis, skin atrophy, growth retardation, Cushing syndrome) are subject to complex regulation involving at least partially transactivation mechanisms (4). Accordingly, research for compounds that exert only transrepression is critical. However, because transactivation also plays an important anti-inflammatory role these compounds might lose clinical efficacy. Although this transrepression versus transactivation concept may be overly simplistic, it may allow for future development of selective glucocorticoid receptor agonists and modulators that might represent the basis for safer and more effective therapies.
Figure 1.
The anti-inflammatory and immunosuppressive activity of glucocorticoids is mediated primarily by genomic effects. In the cytoplasm the glucocorticoid actions are mediated by a specific receptor (glucocorticoid receptor [GCR]) which has two main isoforms, α and β. Isoform α is the main isoform and can mediate the genomic effects, whereas isoform β is unable to bind glucocorticoid. The nonactivated receptor is complexed with immunophilins (IP) and heat shock proteins (HSP). The glucocorticoid receptor has three exposed domains: the ligand-binding domain (domain A), the DNA-binding domain (domain B), and an immunogenic domain (domain C). Binding of glucocorticoid to domain A causes dissociation of immunophilin and HSP from the receptor. The newly formed complex glucocorticoid–glucocorticoid receptor (GC-GCR) undergoes an allosteric change that allows its translocation to the nucleus. Here, the new complex binds to specific glucocorticoid response elements (GREs) and this binding can either increase the production of anti-inflammatory genes (transactivation) or repress the activity of many important proinflammatory genes by binding to and inhibiting key transcription factors, like NF-κB and activator protein 1, that induce expression of many proinflammatory and vasodilating cytokines (transrepression). Nongenomic effects are mediated by plasma membrane glucocorticoid receptors (mGCR) or by nonspecific interaction with membrane-bound glucocorticoid receptors. There are two types of receptors: the classic glucocorticoid receptor α, that localizes in the plasma membrane, and the nonclassic receptor, with different pharmacologic characteristics. Nongenomic effects may prepare the cell for subsequent glucocorticoid-induced genomic changes, may bridge the gap between the early need of change and the delay in the expression of genomic effects, and may induce specific changes that in some instances are opposite to those induced by genomic effects. GC, glucocorticoid; mGCR, membrane glucocorticoid receptor.
It is important to realize that intrinsic sensitivity to glucocorticoids varies among individuals. For instance, functional polymorphisms of the glucocorticoid receptor gene NR3C1 may be associated with either impaired or increased glucocorticoid sensitivity (5). Multidrug resistance–1 gene polymorphisms can also increase the susceptibility or resistance to glucocorticoids (6). The glucocorticoid receptor isoform α is the most abundant isoform and the primary mediator of glucocorticoid action. Instead, the isoform β inhibits the glucocorticoid activity. The unchecked expression of isoform β may lead to the formation of α/β heterodimers that decrease the sensitivity of target tissues to glucocorticoids.
Nongenomic effects do not require protein synthesis and are characterized by rapid onset (seconds to minutes) and short duration of action (60–90 minutes). These effects are mediated by plasma membrane glucocorticoid receptors. There are two types of receptors. The classic glucocorticoid receptors, α, are cell surface receptors which rapidly alter cell signaling via modulation of intracellular signaling cascades (7). The nonclassic membrane glucocorticoid receptors are probably G protein–coupled receptors with poorly defined pharmacologic characteristics.
Podocyte Effects
Experimental studies suggest that glucocorticoids may protect podocytes from injury. Cultured podocytes express key components of the glucocorticoid receptor complex, including heat shock protein 90 and the immunophilins FKBP51 and FKBP52 (8). Either short-term high-dose or long-term low-dose dexamethasone treatment increased podocyte gene expression and induced phosphorylation and downregulation of the glucocorticoid receptor in isolated rat glomeruli (8). In human cultured podocytes, dexamethasone treatment for 24 hours increased the phosphorylation of nephrin through the serum- and glucocorticoid-regulated kinase 1 (9). In a murine model of focal glomerulosclerosis induced by cytotoxic antipodocyte antibody, prednisone reduced podocyte apoptosis and increased the number of podocyte progenitors (10). In rat podocytes and immortalized mouse podocytes stimulated by vasoactive factors, pretreatment with dexamethasone prevented podocyte motility and actin disassembly by modulating the production of cyclic guanosine monophosphate (11). These effects might explain the antiproteinuric effects of steroids.
Glucocorticoid Adverse Events and Measures to Prevent Toxicity
Glucocorticoids have a narrow therapeutic index and are responsible of a number of side effects. (Table 1) Side effects are usually dose-and time-dependent but may also be caused by inappropriate administration. Some measures may reduce the risk of adverse events. Patients on glucocorticoid therapy dosed multiple times a day exhibit a proinflammatory state and weakened immune defense. Switching to a once-daily administration reduces weight gain, normalizes the immune cell profile, and reduces infections (12). Proton pump inhibitors to minimize gastrointestinal side effects should be used with caution because their long-term use may rarely deteriorate kidney function and lead to magnesuria and osteoporosis (13). Psychotropic medications may be helpful in the management of individuals with psychiatric problems caused by glucocorticoids (14) (Table 2).
Table 1.
Some of the most frequent side effects of glucocorticoids
| Side Effects of Glucocorticoids |
|---|
| Infection |
| Diabetes mellitus |
| Arterial hypertension |
| Cardiovascular disease |
| Severe psychiatric reaction |
| Peptic ulcer |
| Hyperlipidemia |
| Obesity-metabolic syndrome |
| Osteoporosis |
| Aseptic necrosis |
| Myopathy |
| Adrenal insufficiency |
| Cataract |
| Hypercoagulability |
| Growth retardation in children |
| Skin changes (atrophy, friableness, acne, hirsutism, striae, Cushingoid facies) |
Table 2.
Suggested measures to reduce the side effects of glucocorticoids
| Variable | Details |
|---|---|
| Type of drugs | For chronic treatment short-acting glucocorticoids should be preferred. |
| Time of administration | The daily dose of glucocorticoid should be given in a single morning administration between 7 and 9 am to mimic the circadian cycle of cortisol. |
| Concomitant drugs | Glucocorticoids are metabolized by cytochrome P450 enzymes. Simultaneous use of drugs that inhibit the activity of cytochrome P450 enzymes increase the blood and tissue levels of glucocorticoids (ketoconazole, itraconazole, clarithromycin). On the other hand, drugs that activate cytochrome P450 (phenobarbital, phenytoin, and rifampin) reduce the blood levels of glucocorticoids. The prolonged use of proton pump inhibitors may cause interstitial nephritis, magnesuria, and osteoporosis. |
| Hygienic measures | Good personal hygiene, low-calorie diet, limited salt intake, physical activity, smoking cessation, mild alcohol intake, and strict control of BP are recommended. |
| Monitoring | Patients should be instructed to promptly report side effects and physicians should not disregard apparently trivial adverse events. |
| Gradual discontinuation | To avoid acute hypoadrenalism episodes glucocorticoids should be withdrawn gradually, tapering the doses over weeks or months to allow the atrophied cortex to regain functional status. |
| Selection of patients | Glucocorticoids should be used with caution in patients with chronic infection, severe hypertension, diabetes, obesity, psychiatric disease, and in those with eGFR<30 ml/min per 1.73 m2. |
Glucocorticoid Resistance
Generalized glucocorticoid resistance is a rare familial or sporadic genetic condition characterized by target-tissue insensitivity to glucocorticoids, with compensatory elevations in circulating cortisol and adrenocorticotropic hormone concentrations that result in adrenal hyperplasia, and increased production of adrenal steroids with mineralocorticoid activity and/or androgenic activity.
Partial steroid resistance may be caused by the hyperacetylation of heat shock protein 90 which is required for proper folding of the glucocorticoid receptor. This causes the glucocorticoid receptor to be defective in ligand binding with impaired nuclear translocation and transcriptional activation (15). Multidrug resistance–1 gene polymorphisms (6), low expression of glucocorticoid receptors (16), or high numbers of glucocorticoid receptor type β (7) may also cause steroid resistance (Table 3).
Table 3.
Gucocorticoid resistance
| Resistance categories |
|---|
| Generalized steroid resistance |
| Generalized genetic or familial glucocorticoid resistance. |
| Partial steroid resistance |
| Hyperacetylation of heat shock proteins causes poor nuclear translocation of glucocorticoid receptor. |
| Mutation or polymorphism of genes encoding multidrug resistance. |
| Low expression or low affinity of glucocorticoid receptors. |
| Polymorphism of glucocorticoid receptors (isoform β). |
| False steroid resistance |
| Insufficient dosage. |
| Short duration of treatment. |
| Poor gastrointestinal absorption due to mucosal edema or use of gastric protectors. |
| Accelerated catabolism caused by drugs that increase the activity of cytochrome P450 (anticonvulsants, rifampin, etc.). |
Importantly, a number of patients are wrongly considered to be steroid resistant. Some steroid-sensitive patients do not respond to glucocorticoids because of an inadequate dosage of the drug. This mistake, which is often related to concerns about side effects, is made more frequently in children and older individuals. In other cases, the duration of steroid treatment is too short. For instance, the response to glucocorticoids may occur after months in adults with minimal change disease and in patients with FSGS. Furthermore, in patients with severe nephrotic syndrome with significant hypoalbuminemia, mucosal edema of the gastrointestinal tract may reduce the absorption of oral drugs. Patients taking anticonvulsants, rifampin, or other drugs that increase the activity of cytochrome P450 have an accelerated catabolism of glucocorticoids and may require higher doses.
Indications for Glucocorticoids in GN
Minimal Change Disease
Glucocorticoids represent the drug of choice for initial treatment (Table 4) (17). Complete remission may be achieved in up to 90% of children and in 80%–85% of adults with prolonged treatment. Most steroid-resistant patients show underlying FSGS at repeated biopsy. In a few cases the poor response to glucocorticoids may be related to multidrug resistance–1 gene polymorphisms, low expression of glucocorticoid receptors, or high numbers of glucocorticoid receptor isoform type β (see above). In patients with frequent relapses or steroid-dependence, if there are no sign of glucocorticoid toxicity patients can be treated with glucocorticoids again. Nevertheless, frequent relapsers and steroid-dependent patients may develop signs of glucocorticoid toxicity and will require different therapies. Glucocorticoid-sparing strategies include a course of 2–3 months of cyclophosphamide, levamisole, mycophenolate (18), cyclosporine (19), or rituximab (20).
Table 4.
Indications to glucocorticoids in GN
| Indication | Details |
|---|---|
| Minimal change disease | In children, the first-line therapy rests on prednisone to be given as a single daily dose starting at 60 mg/m2 per day or 2 mg/kg per day to a maximum of 60 mg/d. This dosage should be given for at least 4 wk, followed by alternate-day administration starting at 40 mg/m2 to be continued for 2–5 mo. In adults, the initial dose is 1 mg/kg per day or 2 mg/kg every other day and should be given for at least 16 wk if complete remission is not achieved. In frequently relapsing/steroid-dependent patients mycopenolic acid salts, calcineurin inhibitors, and/or rituximab are frequently used. |
| Idiopathic FSGS | Patients should receive a minimum of 16 wk of glucocorticoid treatment. In patients who remit prednisone should be tapered off over 6 mo. Calcineurin inhibitors may be used in patients with poor tolerance or resistance to glucocorticoids. Focal glomerular sclerosis secondary to genetic, toxic, viral, or maladaptive causes should not be treated with glucocorticoids. |
| IgA nephropathy | For patients with an eGFR>50 ml/min per 1.73 m2 and proteinuria>1 g/d, a 6-mo glucocorticoid therapy can decrease proteinuria, reduce the deterioration of kidney function, and improve kidney survival. Patients with impaired kidney function should not be treated with steroid therapy or should receive lower doses of glucocorticoids. |
| Membranous nephropathy | There is no evidence that glucocorticoids alone may be of benefit. However, a cyclical treatment alternating glucocorticoids with an alkylating agent every other month for 6 mo may increase the number of remissions and protect kidney function in the long term. Low-dose glucocorticoids are also used to reinforce the efficacy of calcineurin inhibitors. Rituximab is also effective and well tolerated. |
| C3 glomerulopathy | Glucocorticoids alone are of little benefit. In combination with mycophenolate mofetil glucocorticoids may induce remission in C3 GN. Eculizumab can improve or stabilize kidney function in some patients. |
| Pauci-immune rapidly progressive GN | In the induction phase, intravenous high-dose glucocorticoids are strongly indicated and should be started as early as possible. Cyclophosphamide, rituximab, and/or plasmapheresis may also be used. Maintenance therapy rests on oral glucocorticoids associated with either mycophenolate or azathioprine. |
| Lupus nephritis | Intravenous methylprednisolone pulses, either alone or associated with rituximab, cyclophosphamide, or mycophenolate, are frequently used as a first therapy and for treating flares. Oral prednisone is used for maintenance therapy. Azathioprine, mycophenolate, or cyclosporine can allow the doses of prednisone to reduce. |
FSGS
This glomerular disease may be caused by an idiopathic disease or by genetic, toxic, viral, or maladaptive forms. Current evidence, mostly derived from retrospective analyses, favors prolonged glucocorticoid therapy (≥4–6 months) to induce remission (17). However, almost all of the monogenic forms of FSGS are steroid unresponsive. Calcineurin inhibitors, often administered with low-dose prednisone, may obtain a good rate of partial or complete remission (21). However, relapses are frequent after interruption of the drug.
Glucocorticoids should not be administered to newborns with nephrotic syndrome or to subjects with maladaptive glomerular sclerosis. In children <1 year of age, the nephrotic syndrome is caused by polymorphisms or mutations of genes encoding podocyte proteins, such as nephrin, podocyn, NPHS3, and others. The only effective treatment for these children is kidney transplantation. Maladaptive glomerular sclerosis can be caused by a loss of nephrons due to kidney or systemic diseases, morbid obesity, or exposure to drugs, such as calcineurin inhibitors, intravenous bisphosphonates, and others. Glucocorticoid treatment in these patients may not be effective and may be deleterious.
IgA Nephropathy
The outcome of patients with IgA nephropathy may be variable, with some patients remaining asymptomatic over time whereas up to 40%–50% of patients may progress to ESKD within 10–30 years. The most reliable factors that may predict progression are represented by proteinuria>1 g/d and hypertension (17). Renin-angiotensin system blockers may reduce proteinuria and BP and are generally considered to be the first therapeutic step in IgA nephropathy. For patients with eGFR>50 ml/min and proteinuria>1 g/d despite 6 months of renin-angiotensin system inhibitors, randomized controlled trials have demonstrated the benefits of a 6-month course of glucocorticoid therapy (22,23). A retrospective analysis of 1147 European patients with IgA nephropathy reported that glucocorticoid treatment was associated with significant reduction in proteinuria, slower rate of renal function decline, and better kidney survival (24). However, two studies warned against glucocorticoid use in IgA nephropathy, because of an increased risk of infections. In the Supportive Versus Immunosuppressive Therapy for the Treatment of Progressive IgA Nephropathy trial, participants with an eGFR≥60 ml/min per 1.73 m2 and proteinuria>0.75 g/d were randomized to supportive care or to treatment with glucocorticoids, whereas participants with eGFR<60 ml/min per 1.73 m2 received glucocorticoids plus cyclophosphamide for 3 months and azathioprine from month 4 to 36. Treated participants obtained a significant reduction of proteinuria in comparison with those given supportive care, but there was no effect on the decline of kidney function. Side effects, including death, were more serious with immunosuppressive therapy (25). The rate of kidney function decline was too low to appreciate a significant difference after a follow-up of 3 years. Moreover, the most severe adverse events were observed in patients with declining kidney function in whom cyclophosphamide and azathioprine were added to glucocorticoids. The Therapeutic Evaluation of Steroids in IgA Nephropathy Global trial assigned patients with eGFR between 20 and 120 ml/min per 1.73 m2 and proteinuria>1 g/d to symptomatic therapy or high-dose methylprednisolone (26). The study was discontinued after a median follow-up of 25 months because of an excess of infections in the methylprednisolone arm. At interruption, the primary end point (death, ESKD, deterioration of kidney function) was reached in 5.9% participants in the glucocorticoid arm versus 15.9% in the placebo arm (P=0.02). The decline in eGFR and the mean levels of daily proteinuria were significantly lower in participants given methylprednisolone. Importantly, participants randomized to treatment were given the same dosage of methylprednisolone independent of their kidney function. Moreover, some participants had been previously treated with immunosuppressive drugs and received no prophylaxis against Pneumocystis jirovecii. Patients with these characteristics are more susceptible to side effects of glucocorticoids and their inclusion in the study may have contributed to the high rate of side effects (27). Budesonide is a nonabsorbable steroid acting locally in the small intestine. It has a low oral bioavailability due to high first pass hepatic metabolism (28). A preliminary trial showed that a 9-month treatment with budesonide is effective in patients with IgA nephropathy (29). However, most patients who discontinued treatment experienced steroid-related adverse events. Long-term follow-up is needed to better appreciate the effectiveness and tolerance of budesonide.
In summary, most studies indicate that glucocorticoids may lower the risk of kidney disease progression for IgA nephropathy. Meta-analyses have supported this conclusion (30,31). The main limiting issues are side effects of glucocorticoids. It is important to point out that administration of the same dosage of glucocorticoids to patients with impaired kidney function as that used in subjects with normal kidney function is a mistake that can increase the risk of severe side effects. In patients with low GFR the doses of glucocorticoids, either given orally or intravenously, should be reduced and patients should be closely monitored. The risk of toxicity may be further aggravated by the long-term administration of glucocorticoids with other immunosuppressive drugs, such as azathioprine that proved to be ineffective (32). Avoiding these pitfalls and following the recommendations reported above can substantially reduce toxicity while preserving effectiveness of glucocorticoids in IgA nephropathy.
Membranous Nephropathy
There is agreement that immunosuppressive treatment of membranous nephropathy should be limited to patients with idiopathic disease and nephrotic syndrome or progressive disease. A systematic review of therapeutic studies in idiopathic membranous nephropathy demonstrated that glucocorticoids did not improve either the probability of entering complete remission of proteinuria or the actuarial kidney survival at 5 years (33). Although the use of glucocorticoids alone is ineffective, glucocorticoids in combination with other immunosuppressive drugs may exert synergistic or additive effects. Two long-term randomized trials showed that alternating glucocorticoids with an alkylating agent every other month for 6 months significantly improved the chances of remission and preserved kidney function at 10 years (34,35). Good results have also been reported with a combination of glucocorticoids with mycophenolate mofetil, but most patients relapsed when treatment was interrupted (36). In summary, treatments on the basis of glucocorticoids alone should be avoided in membranous nephropathy. When using glucocorticoids with an alkylating agent, it is recommended to alternate the two drugs every other month, thus allowing a period of wash-out of either drug. This strategy may reduce side effects, whereas a simultaneous combination of steroids and cytotoxic agents can increase toxicity. Other successful strategies that allow reduction of the doses of steroids and avoidance of alkylating agents include the use of cyclosporine with low-dose glucocorticoids (19), tacrolimus (37), and rituximab (38).
C3 Glomerulopathy and Membranoproliferative GN
Membranoproliferative GN is a pattern of injury, not a specific disease. A new classification of this histologic pattern on the basis of immunofluorescence microscopy has been proposed by Sethi and Fervenza (39). The membranoproliferative lesion may be classified as an immune complex–mediated disorder or complement-mediated disorder. In most cases, immune complex–mediated disorders are secondary to chronic infections, autoimmune diseases, thrombotic microangiopathy, malignancy, or monoclonal gammopathy. A consensus conference proposed to use the term C3 glomerulopathy to describe glomerular lesions in which there is glomerular accumulation of C3 with little or no Ig. On the basis of electron microscopy features, C3 glomerulopathy may be subdivided into dense deposit disease (ribbon-like intramembranous electron-dense deposits) and C3 GN (mesangial and subendothelial, subepithelial, or intramembranous electron-dense deposits, with fraying of lamina densa) (40).
There is no evidence that glucocorticoid monotherapy may be effective in dense deposit disease. In C3 GN a prolonged treatment with glucocorticoids is unlikely to be of benefit and may be associated with serious adverse events.
Better results may be obtained by combining glucocorticoids with mycophenolate mofetil (41). In C3 glomerulopathy encouraging results have been reported with eculizumab, an anti-C5 mAb (42).
Pauci-Immune Rapidly Progressive GN
This is the most common form of crescentic GN, accounting for around 60%–80% of all cases. A consensus conference proposed reclassification of crescentic GN as anti–neutrophil cytoplasmic autoantibody–associated vasculitis (43). However, some patients with pauci-immune crescentic GN lack anti–neutrophil cytoplasmic autoantibody (44). The natural course of crescentic GN is ominous and usually leads to ESKD, whereas appropriate treatment may halt the progression and may even achieve a complete remission. Many practitioners (and a number of nephrologists) wait to start treatment until a firm diagnosis is made. This is a mistake that puts the patient at risk of irreversible kidney lesions and life-threatening complications. Any patient presenting with a nephritic syndrome and a rapidly progressive course should start immediate treatment with intravenous high-dose methylprednisolone pulses (45). In the meantime, immunologic investigations and a kidney biopsy should be done to establish the cause. Once a complete diagnosis has been ascertained, a specific treatment can be initiated. Cyclophosphamide or rituximab, with or without plasmapheresis, may be added unless there are contraindications such as advanced age or severe comorbidity. Eculizumab may be considered if there is evidence of severe activation of the complement cascade (46). Oral prednisone, progressively tapered, together with azathioprine or mycophenolate is used for maintenance.
Lupus Nephritis
Glucocorticoids are used in all forms of lupus nephritis. Whenever an aggressive treatment is needed, as in the case of induction therapy and management of flares, intravenous high-dose methylprednisolone pulses (0.5–1.0 g each) are frequently used, often in association with cyclophosphamide, administered orally or intravenously, or mycophenolate mofetil, or rituximab (47). Infusions of high-dose methylprednisolone may be complicated by infection or cardiac arrhythmias. To prevent infections, many centers do not exceed three pulse administrations, either given every day or every other day. To prevent cardiac events associated with high-dose methylprednisolone, serum potassium should be checked before infusion; methylprednisolone should be infused in a peripheral vein over 30–60 minutes, rather than rapid injection of the drug using a central vein; and patients with cardiac problems should be monitored during infusion. For maintenance therapy for this condition, glucocorticoids remain the cornerstone. Steroid-sparing strategies have been proposed for maintenance therapy in patients with lupus nephritis, including oral or intravenous cyclophosphamide (48), mycophenolate (49), azathioprine (50), and/or calcineurin inhibitors (50,51).
Although glucocorticoids are clearly associated with side effects, their use is critical in a number of glomerular diseases. Avoiding improper indications and inappropriate administration may increase the benefit/risk ratio for these indispensable drugs.
Disclosures
C.P. was a member of the steering committee of the Italian IgA study. F.L. reported consulting on IgA nephropathy for Pharmalink and was a member of the steering committee of the targeted-release budesonide versus placebo in patients with IgA nephropathy study and the Italian IgA study.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Debono M, Ghobadi C, Rostami-Hodjegan A, Huatan H, Campbell MJ, Newell-Price J, Darzy K, Merke DP, Arlt W, Ross RJ: Modified-release hydrocortisone to provide circadian cortisol profiles. J Clin Endocrinol Metab 94: 1548–1554, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kirschke E, Goswami D, Southworth D, Griffin PR, Agard DA: Glucocorticoid receptor function regulated by coordinated action of the Hsp90 and Hsp70 chaperone cycles. Cell 157: 1685–1697, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kadmiel M, Cidlowski JA: Glucocorticoid receptor signaling in health and disease. Trends Pharmacol Sci 34: 518–530, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schäcke H, Schottelius A, Döcke WD, Strehlke P, Jaroch S, Schmees N, Rehwinkel H, Hennekes H, Asadullah K: Dissociation of transactivation from transrepression by a selective glucocorticoid receptor agonist leads to separation of therapeutic effects from side effects. Proc Natl Acad Sci U S A 101: 227–232, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teeninga N, Kist-van Holthe JE, van den Akker EL, Kersten MC, Boersma E, Krabbe HG, Knoers NV, van der Heijden AJ, Koper JW, Nauta J: Genetic and in vivo determinants of glucocorticoid sensitivity in relation to clinical outcome of childhood nephrotic syndrome. Kidney Int 85: 1444–1453, 2014 [DOI] [PubMed] [Google Scholar]
- 6.Dhandapani MC, Venkatesan V, Rengaswamy NB, Gowrishankar K, Nageswaran P, Perumal V: Association of ACE and MDR1 gene polymorphisms with steroid resistance in children with idiopathic nephrotic syndrome. Genet Test Mol Biomarkers 19: 454–456, 2015 [DOI] [PubMed] [Google Scholar]
- 7.Ramamoorthy S, Cidlowski JA: Corticosteroids: Mechanisms of action in health and disease. Rheum Dis Clin North Am 42: 15–31, vii, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guess A, Agrawal S, Wei CC, Ransom RF, Benndorf R, Smoyer WE: Dose- and time-dependent glucocorticoid receptor signaling in podocytes. Am J Physiol Renal Physiol 299: F845–F853, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohashi T, Uchida K, Uchida S, Sasaki S, Nitta K: Dexamethasone increases the phosphorylation of nephrin in cultured podocytes. Clin Exp Nephrol 15: 688–693, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Zhang J, Pippin JW, Krofft RD, Naito S, Liu ZH, Shankland SJ: Podocyte repopulation by renal progenitor cells following glucocorticoids treatment in experimental FSGS. Am J Physiol Renal Physiol 304: F1375–F1389, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lewko B, Waszkiewicz A, Maryn A, Gołos M, Latawiec E, Daca A, Witkowski JM, Angielski S, Stępiński J: Dexamethasone-dependent modulation of cyclic GMP synthesis in podocytes. Mol Cell Biochem 409: 243–253, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isidori AM, Venneri MA, Graziadio C, Simeoli C, Fiore D, Hasenmajer V, Sbardella E, Gianfrilli D, Pozza C, Pasqualetti P, Morrone S, Santoni A, Naro F, Colao A, Pivonello R, Lenzi A: Effect of once-daily, modified-release hydrocortisone versus standard glucocorticoid therapy on metabolism and innate immunity in patients with adrenal insufficiency (DREAM): A single-blind, randomised controlled trial [published online ahead of print December 8, 2017]. Lancet Diabetes Endocrinol 10.1016/S2213-8587(17)30398-4 [DOI] [PubMed] [Google Scholar]
- 13.Lazarus B, Chen Y, Wilson FP, Sang Y, Chang AR, Coresh J, Grams ME: Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med 176: 238–246, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Judd LL, Schettler PJ, Brown ES, Wolkowitz OM, Sternberg EM, Bender BG, Bulloch K, Cidlowski JA, de Kloet ER, Fardet L, Joëls M, Leung DY, McEwen BS, Roozendaal B, Van Rossum EF, Ahn J, Brown DW, Plitt A, Singh G: Adverse consequences of glucocorticoid medication: Psychological, cognitive, and behavioral effects. Am J Psychiatry 171: 1045–1051, 2014 [DOI] [PubMed] [Google Scholar]
- 15.Kovacs JJ, Murphy PJ, Gaillard S, Zhao X, Wu JT, Nicchitta CV, Yoshida M, Toft DO, Pratt WB, Yao TP: HDAC6 regulates Hsp90 acetylation and chaperone-dependent activation of glucocorticoid receptor. Mol Cell 18: 601–607, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Han SH, Park SY, Li JJ, Kwak SJ, Jung DS, Choi HY, Lee JE, Moon SJ, Kim DK, Han DS, Kang SW: Glomerular glucocorticoid receptor expression is reduced in late responders to steroids in adult-onset minimal change disease. Nephrol Dial Transplant 23: 169–175, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Kidney Disease Improving Global Outcomes (KDIGO) : KDIGO clinical practice guideline for glomerulonephritis. Kidney Int 2[Suppl 2]: 1–216, 2012 [Google Scholar]
- 18.Vivarelli M, Massella L, Ruggiero B, Emma F: Minimal change disease. Clin J Am Soc Nephrol 12: 332–345, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cattran DC, Alexopoulos E, Heering P, Hoyer PF, Johnston A, Meyrier A, Ponticelli C, Saito T, Choukroun G, Nachman P, Praga M, Yoshikawa N: Cyclosporin in idiopathic glomerular disease associated with the nephrotic syndrome: Workshop recommendations. Kidney Int 72: 1429–1447, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Ravani P, Bonanni A, Rossi R, Caridi G, Ghiggeri GM: Anti-CD20 antibodies for idiopathic nephrotic syndrome in children. Clin J Am Soc Nephrol 11: 710–720, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laurin LP, Gasim AM, Poulton CJ, Hogan SL, Jennette JC, Falk RJ, Foster BJ, Nachman PH: Treatment with glucocorticoids or calcineurin inhibitors in primary FSGS. Clin J Am Soc Nephrol 11: 386–394, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pozzi C, Andrulli S, Del Vecchio L, Melis P, Fogazzi GB, Altieri P, Ponticelli C, Locatelli F: Corticosteroid effectiveness in IgA nephropathy: Long-term results of a randomized, controlled trial. J Am Soc Nephrol 15: 157–163, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Manno C, Torres DD, Rossini M, Pesce F, Schena FP: Randomized controlled clinical trial of corticosteroids plus ACE-inhibitors with long-term follow-up in proteinuric IgA nephropathy. Nephrol Dial Transplant 24: 3694–3701, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Tesar V, Troyanov S, Bellur S, Verhave JC, Cook HT, Feehally J, Roberts IS, Cattran D, Coppo R; VALIGA study of the ERA-EDTA Immunonephrology Working Group : Corticosteroids in IgA nephropathy: A retrospective analysis from the VALIGA study. J Am Soc Nephrol 26: 2248–2258, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rauen T, Eitner F, Fitzner C, Sommerer C, Zeier M, Otte B, Peters H, Benck U, Mertens PR, Kuhlmann U, Witzke O, Gross O, Vielhauer V, Mann JF, Hilgers RD, Floege J; STOP-IgAN Investigators : Intensive supportive care plus immunosuppression in IgA nephropathy. N Engl J Med 373: 2225–2236, 2015 [DOI] [PubMed] [Google Scholar]
- 26.Lv J, Zhang H, Wong MG, Jardine MJ, Hladunewich M, Jha V, Monaghan H, Zhao M, Barbour S, Reich H, Cattran D, Glassock R, Levin A, Wheeler D, Woodward M, Billot L, Chan TM, Liu ZH, Johnson DW, Cass A, Feehally J, Floege J, Remuzzi G, Wu Y, Agarwal R, Wang HY, Perkovic V; TESTING Study Group : Effect of oral methylprednisolone on clinical outcomes in patients with IgA nephropathy: The TESTING randomized clinical trial. JAMA 318: 432–442, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coppo R: Corticosteroids in IgA nephropathy: Lessons from recent studies. J Am Soc Nephrol 28: 25–33, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abdalla MI, Herfarth H: Budesonide for the treatment of ulcerative colitis. Expert Opin Pharmacother 17: 1549–1559, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fellström BC, Barratt J, Cook H, Coppo R, Feehally J, de Fijter JW, Floege J, Hetzel G, Jardine AG, Locatelli F, Maes BD, Mercer A, Ortiz F, Praga M, Sørensen SS, Tesar V, Del Vecchio L; NEFIGAN Trial Investigators : Targeted-release budesonide versus placebo in patients with IgA nephropathy (NEFIGAN): A double-blind, randomised, placebo-controlled phase 2b trial. Lancet 389: 2117–2127, 2017 [DOI] [PubMed] [Google Scholar]
- 30.Lv J, Xu D, Perkovic V, Ma X, Johnson DW, Woodward M, Levin A, Zhang H, Wang H; TESTING Study Group : Corticosteroid therapy in IgA nephropathy. J Am Soc Nephrol 23: 1108–1116, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vecchio M, Bonerba B, Palmer SC, Craig JC, Ruospo M, Samuels JA, Molony DA, Schena FP, Strippoli GF: Immunosuppressive agents for treating IgA nephropathy. Cochrane Database Syst Rev 8: CD003965, 2015 [DOI] [PubMed] [Google Scholar]
- 32.Pozzi C, Andrulli S, Pani A, Scaini P, Del Vecchio L, Fogazzi G, Vogt B, De Cristofaro V, Allegri L, Cirami L, Procaccini AD, Locatelli F: Addition of azathioprine to corticosteroids does not benefit patients with IgA nephropathy. J Am Soc Nephrol 21: 1783–1790, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hogan SL, Muller KE, Jennette JC, Falk RJ: A review of therapeutic studies of idiopathic membranous glomerulopathy. Am J Kidney Dis 25: 862–875, 1995 [DOI] [PubMed] [Google Scholar]
- 34.Ponticelli C, Zucchelli P, Passerini P, Cesana B, Locatelli F, Pasquali S, Sasdelli M, Redaelli B, Grassi C, Pozzi C: A 10-year follow-up of a randomized study with methylprednisolone and chlorambucil in membranous nephropathy. Kidney Int 48: 1600–1604, 1995 [DOI] [PubMed] [Google Scholar]
- 35.Jha V, Ganguli A, Saha TK, Kohli HS, Sud K, Gupta KL, Joshi K, Sakhuja V: A randomized, controlled trial of steroids and cyclophosphamide in adults with nephrotic syndrome caused by idiopathic membranous nephropathy. J Am Soc Nephrol 18: 1899–1904, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Branten AJ, du Buf-Vereijken PW, Vervloet M, Wetzels JF: Mycophenolate mofetil in idiopathic membranous nephropathy: A clinical trial with comparison to a historic control group treated with cyclophosphamide. Am J Kidney Dis 50: 248–256, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Caro J, Gutiérrez-Solís E, Rojas-Rivera J, Agraz I, Ramos N, Rabasco C, Espinosa M, Valera A, Martín M, Frutos MÁ, Perea L, Juárez GF, Ocaña J, Arroyo D, Goicoechea M, Fernández L, Oliet A, Hernández Y, Romera A, Segarra A, Praga M; Grupo de Estudio de las Enfermedades Glomerulares de la Sociedad Española de Nefrología (GLOSEN) : Predictors of response and relapse in patients with idiopathic membranous nephropathy treated with tacrolimus. Nephrol Dial Transplant 30: 467–474, 2015 [DOI] [PubMed] [Google Scholar]
- 38.Ruggenenti P, Cravedi P, Chianca A, Perna A, Ruggiero B, Gaspari F, Rambaldi A, Marasà M, Remuzzi G: Rituximab in idiopathic membranous nephropathy. J Am Soc Nephrol 23: 1416–1425, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sethi S, Fervenza FC: Membranoproliferative glomerulonephritis—A new look at an old entity. N Engl J Med 366: 1119–1131, 2012 [DOI] [PubMed] [Google Scholar]
- 40.Pickering MC, D’Agati VD, Nester CM, Smith RJ, Haas M, Appel GB, Alpers CE, Bajema IM, Bedrosian C, Braun M, Doyle M, Fakhouri F, Fervenza FC, Fogo AB, Frémeaux-Bacchi V, Gale DP, Goicoechea de Jorge E, Griffin G, Harris CL, Holers VM, Johnson S, Lavin PJ, Medjeral-Thomas N, Paul Morgan B, Nast CC, Noel LH, Peters DK, Rodríguez de Córdoba S, Servais A, Sethi S, Song WC, Tamburini P, Thurman JM, Zavros M, Cook HT: C3 glomerulopathy: Consensus report. Kidney Int 84: 1079–1089, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rabasco C, Cavero T, Román E, Rojas-Rivera J, Olea T, Espinosa M, Cabello V, Fernández-Juarez G, González F, Ávila A, Baltar JM, Díaz M, Alegre R, Elías S, Antón M, Frutos MA, Pobes A, Blasco M, Martín F, Bernis C, Macías M, Barroso S, de Lorenzo A, Ariceta G, López-Mendoza M, Rivas B, López-Revuelta K, Campistol JM, Mendizábal S, de Córdoba SR, Praga M; Spanish Group for the Study of Glomerular Diseases (GLOSEN) : Effectiveness of mycophenolate mofetil in C3 glomerulonephritis. Kidney Int 88: 1153–1160, 2015 [DOI] [PubMed] [Google Scholar]
- 42.Nester CM, Smith RJ: Complement inhibition in C3 glomerulopathy. Semin Immunol 28: 241–249, 2016 [DOI] [PubMed] [Google Scholar]
- 43.Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, Flores-Suarez LF, Gross WL, Guillevin L, Hagen EC, Hoffman GS, Jayne DR, Kallenberg CG, Lamprecht P, Langford CA, Luqmani RA, Mahr AD, Matteson EL, Merkel PA, Ozen S, Pusey CD, Rasmussen N, Rees AJ, Scott DG, Specks U, Stone JH, Takahashi K, Watts RA: 2012 revised international chapel hill consensus conference nomenclature of vasculitides. Arthritis Rheum 65: 1–11, 2013 [DOI] [PubMed] [Google Scholar]
- 44.Chen M, Kallenberg CG, Zhao MH: ANCA-negative pauci-immune crescentic glomerulonephritis. Nat Rev Nephrol 5: 313–318, 2009 [DOI] [PubMed] [Google Scholar]
- 45.Moroni G, Ponticelli C: Rapidly progressive crescentic glomerulonephritis: Early treatment is a must. Autoimmun Rev 13: 723–729, 2014 [DOI] [PubMed] [Google Scholar]
- 46.Manenti L, Urban ML, Maritati F, Galetti M, Vaglio A: Complement blockade in ANCA-associated vasculitis: An index case, current concepts and future perspectives. Intern Emerg Med 12: 727–731, 2017 [DOI] [PubMed] [Google Scholar]
- 47.Ponticelli C, Glassock RJ, Moroni G: Induction and maintenance therapy in proliferative lupus nephritis. J Nephrol 23: 9–16, 2010 [PubMed] [Google Scholar]
- 48.Illei GG, Austin HA, Crane M, Collins L, Gourley MF, Yarboro CH, Vaughan EM, Kuroiwa T, Danning CL, Steinberg AD, Klippel JH, Balow JE, Boumpas DT: Combination therapy with pulse cyclophosphamide plus pulse methylprednisolone improves long-term renal outcome without adding toxicity in patients with lupus nephritis. Ann Intern Med 135: 248–257, 2001 [DOI] [PubMed] [Google Scholar]
- 49.Dooley MA, Jayne D, Ginzler EM, Isenberg D, Olsen NJ, Wofsy D, Eitner F, Appel GB, Contreras G, Lisk L, Solomons N; ALMS Group : Mycophenolate versus azathioprine as maintenance therapy for lupus nephritis. N Engl J Med 365: 1886–1895, 2011 [DOI] [PubMed] [Google Scholar]
- 50.Moroni G, Doria A, Mosca M, Alberighi OD, Ferraccioli G, Todesco S, Manno C, Altieri P, Ferrara R, Greco S, Ponticelli C: A randomized pilot trial comparing cyclosporine and azathioprine for maintenance therapy in diffuse lupus nephritis over four years. Clin J Am Soc Nephrol 1: 925–932, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Mok CC: Calcineurin inhibitors in systemic lupus erythematosus. Best Pract Res Clin Rheumatol 31: 429–438, 2017 [DOI] [PubMed] [Google Scholar]