Abstract
Objectives
Hypertrophic cardiomyopathy (HCM) is a life-threatening genetic cardiovascular disease that often goes undetected in young athletes. Neither history nor physical examination are reliable to identify those at risk. The objective of this study is to determine whether minimally trained medical student volunteers can use ultrasound to screen for HCM.
Methods
This was a prospective enrollment of young athletes performed at 12 area high schools and three area colleges, between May 2012 and August 2013. All participants underwent point-of-care ultrasound performed screening for HCM by trained medical students and reviewed by a pediatric cardiologist. An interventricular septum to left ventricular posterior wall ratio greater than 1.25 was considered to be abnormal (positive screen).
Results
A total of 2332 participants were enrolled. There were 137 (5.8%) with a positive screening for HCM, of which 7 (5.1%) were confirmed to have HCM by a pediatric cardiologist. In a small cohort with positive screen for HCM, there was a 100% sensitivity (95% confidence interval, 59.04 to 100%) and 4.86% (95% confidence interval, 1.98 to 9.76%) positive predictive value of for having HCM.
Conclusions
Volunteer medical students, using point-of-care ultrasound, were able to effectively screen for HCM in young athletes.
Keywords: cardiac screening, echocardiography, hypertrophic cardiomyopathy, ultrasound
Hypertrophic cardiomyopathy (HCM) is an autosomal dominant cardiac disease that results in left ventricular hypertrophy.1 It is the most common genetic cardiovascular disease, and occurs in approximately 1 in 500 of the general population.2,3 Based on its prevalence, at least 600,000 people in the United States are affected by this condition, most of which remain unidentified.4 This is of particular importance among patients under 35 with arrhythmia, as HCM is the principle cause of cardiac arrest, accounting for over 30% of sudden cardiac arrest.5,6 Unfortunately, neither history nor physical examination is effective at identifying those with the disease.7 The only reliable way to diagnosis of HCM is with echocardiography,8 in which a septal thickness measurement greater than 15 mm is considered diagnostic.9 Increased septal thickness increases the risk of cardiac death, with the highest risk among patients having a wall thickness greater than 30 mm.10
Despite the recognized high concern for mortality with unidentified HCM, there is no agreed upon screening modality for this disease process. Currently, the American Heart Association recommends that high school and college athletes undergo a preparticipation physical examination every 2 years,11 which includes a personal and family medical history and a brief physical examination. Further testing is recommended for patients with abnormal findings or high suspicion for cardiovascular disease. The European Society of Cardiology recommends similar screening, but with the addition of an electrocardiogram (ECG).12 In Italy, beginning in 1971, all young athletes are required by law to undergo mandatory screening before participating in competitive sports.13,14 This has led to an 89% decline in sudden cardiac death since the implementation of the program.15 Despite this significant improvement, there are still athletes who experience sudden cardiac death, possibly as a result of HCM. There is also controversy over the optimum screening tools and criteria for testing.
In recent years, use of point-of-care ultrasound (POCUS) has significantly expanded in medicine, and is now routinely taught in many undergraduate medical education programs.16–18 The use of POCUS, performed by medical students with limited training, may be an easy and reliable method to help identify young athletes at increased risk for HCM. Therefore, we sought to evaluate whether POCUS performed by medical students and interpreted by a board-certified pediatric cardiologist was a beneficial and effective screening adjunct in the search to identify young athletes with HCM.
Materials and Methods
Study Design and Population
This was a prospective study of young athletes from 12 area high schools and three area colleges, between May 2012 and August 2013 (Table 1). Young athletes, who were between the ages of 14 and 30 years and involved in any school-sponsored sporting activity, were eligible for participation. The study was approved by our institutional review board. Before the enrollment dates, school personnel informed all student athletes of the research opportunity and distributed institutional review board–approved study information, including basic research information, consent forms, and HIPAA forms. All enrolled subjects provided written, informed consent. For patients who were minors, parents or guardians were required to complete assent and consent forms, respectively.
Table 1.
Number of Scans Performed by Site and Year
| School | 2012 | 2013 | Total |
|---|---|---|---|
| High School A | 148 | 244 | 392 |
| High School B | 188 | 154 | 342 |
| High School C | 158 | 88 | 246 |
| High School D | 121 | 109 | 230 |
| High School E | 136 | 68 | 204 |
| High School F | 173 | 13 | 186 |
| College A | 82 | 84 | 166 |
| College B | 75 | 64 | 139 |
| College C | 0 | 110 | 110 |
| High School G | 109 | 0 | 109 |
| High School H | 53 | 12 | 65 |
| High School I | 0 | 57 | 57 |
| High School J | 0 | 23 | 23 |
| High School K | 0 | 15 | 15 |
| High School L | 0 | 48 | 48 |
| Total | 1243 | 1089 | 2332 |
Study Protocol
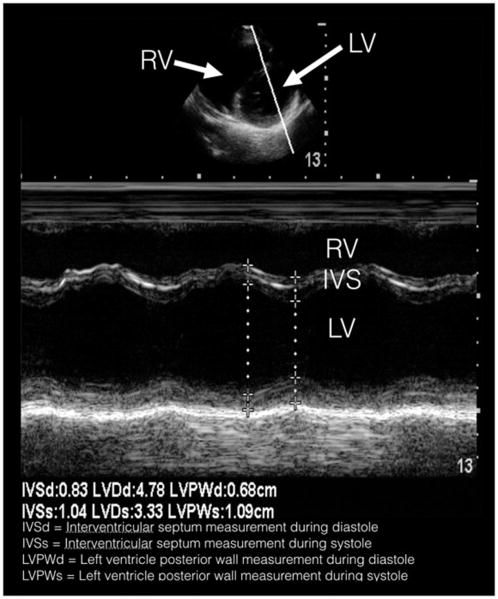
The research team consisted of medical students in their first or second year of medical school. The first-year students had 8 hours of didactic and 8 hours of hands-on training in various normal anatomical findings of POCUS, two of which were focused on the heart. The second-year students had an additional 8 hours of didactic and 8 hours of hands-on training that focused on pathological findings, two of which were also focused on the heart. This standard curriculum included ultrasound physics and instrumentation, cardiac windows, and liver, gallbladder, lungs, aorta, inferior vena cava, musculoskeletal, trauma, and genitourinary systems. In addition to their standard curriculum, the students involved in this study were also required to review a 36-slide PowerPoint (Microsoft Corp, Redmond, WA) presentation detailing basic cardiac anatomy and findings on ultrasound that suggest HCM. Following this, students were then required to attend a 2-hour hands-on training session led by trained cardiac sonographers and supervised by a board certified pediatric cardiologist. Students were required to demonstrate proficiency in obtaining the parasternal long axis view, the parasternal short axis view, and use M-mode to measure the interventricular septum and posterior wall thicknesses (Figure 1). At the conclusion of this training session, each student was required to pass a competency examination, in which students needed to demonstrate their ability to obtain parasternal long-axis and short-axis views, and use M-mode in the parasternal short axis to determine septal thickness. A physician investigator monitored each student’s ability to save these windows.
Figure 1.
Parasternal short-axis still image with M-mode tracing, depicting the cardiac cycle in systole and diastole.
During the enrollment period, 35 different medical students performed all echocardiogram screenings using Sonosite S-FAST ultrasound machines (FUJIFILM SonoSite Inc, Bothell, WA). Working in groups of two, one medical student entered the participant-identifying data into the machine, while the other student positioned the subject on the table. During the ultrasound, one student acquired the necessary images while the other student operated the machine to freeze, measure, and record the values onto a data collection sheet. Using a phased array transducer in the cardiac software setting, 2-second clips were recorded of a parasternal long-axis view. This view included visualization of the interventricular septum, left ventricular posterior wall, mitral valve, and aortic valve. Next, a 2-second parasternal short-axis clip included visualization of the left ventricle and partial visualization of the right ventricle at the level of the apex. In the parasternal short-axis view, M-mode was then used to measure the wall thickness. An M-mode tracing was placed perpendicular to the septum and left ventricular posterior wall. After three consecutive cardiac cycles were visualized, measurements of the interventricular septum width, left ventricle diameter, and left ventricular posterior wall thickness were recorded in both systole and diastole. All digital recordings, including still images and video clips, were exported to a secure central archival system. All images and measurements were then reviewed by a board-certified pediatric cardiologist blinded to the participant’s history and demographics. Participants with an interventricular septum to left ventricular posterior wall ratio greater than 1.25 or left ventricular thickness greater than 12 mm were considered abnormal and underwent a repeat echocardiogram by the pediatric cardiologist.
Statistical Analysis
Because of the small number of positive cases of HCM, age, body mass index, heart rate, and blood pressure for students with and without HCM were compared with the Kruskal-Wallis rank test, and the distribution of categorical variables was compared using the Fisher’s exact test. For both race and sport, multiple responses were allowed, and students selecting each response were compared with all other students with no missing responses using Fisher’s exact test. The data were analyzed using Stata, version 14.1 (StataCorp, College Station, TX).
Results
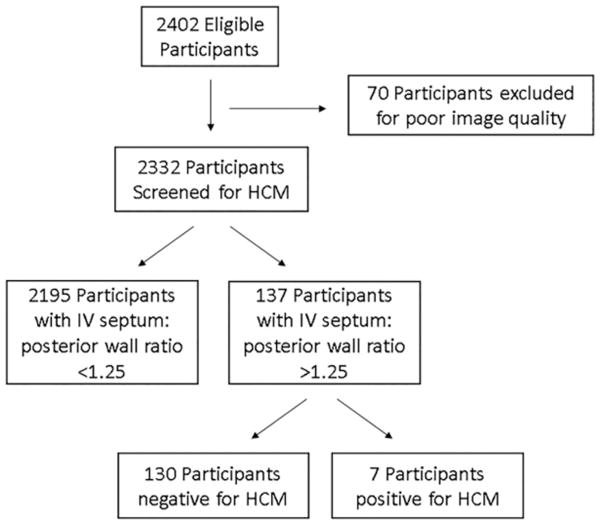
A total of 2402 participants were eligible to participate in the study. Seventy (2.9%) were excluded because of medical student inability to obtain cardiac views or poor image quality (Figure 2). These subjects were referred to their primary care doctor for further testing. Therefore, 2332 participants were enrolled in the study, but demographic information was only present for 2,271 (97.4%). The average age was 15.8 (standard deviation of 2.3) years old, average body mass index was 22.9 (standard deviation of 3.5), and 1455 (64%) were male. Complete and detailed demographic information is listed in Table 2.
Figure 2.
Flow chart illustrating participant characteristics.
Table 2.
Participant Demographics (N = 2271)
| Category | N (%) |
|---|---|
| Age (years) | |
| 13 | 223 (9.8%) |
| 14 | 693 (30.5%) |
| 15 | 372 (16.4%) |
| 16 | 271 (11.9%) |
| 17 | 148 (6.5%) |
| 18 | 161 (7.1%) |
| 19–20 | 264 (11.6%) |
| 21+ | 109 (4.8%) |
| No response | 30 (1.3%) |
| Sex | |
| Male | 1455 (64.1%) |
| Female | 814 (35.8%) |
| No response | 2 (0.1%) |
| Body mass index | |
| <18.5 | 267 (11.8%) |
| 18.5–24.999 | 1467 (64.6%) |
| 25.0–29.999 | 382 (16.8%) |
| >= 30 | 123 (5.4%) |
| No response | 32 (1.4%) |
| Racea | |
| White | 1296 (57%) |
| Latino | 462 (20.3%) |
| Asian | 293 (12.9%) |
| Black | 215 (9.5%) |
| Other | 137 (6.0%) |
| No response | 152 (6.7%) |
Multiple responses allowed.
A total of 137 (5.8%) were found to have an inter-ventricular septum to left ventricular posterior wall ratio greater than 1.25 mm, which triggered follow-up ultrasonography by a pediatric cardiologist. Of these 137 participants with initial concern for HCM, all 137 (100%) underwent repeat scanning by a pediatric cardiologist. Seven (4.86%) were confirmed to have HCM (Table 3). Therefore, in a cohort of 137 out of 2332 participants with a positive screen for potential HCM, there was 100% sensitivity (95% confidence interval, 59.04 to 100.00%) and 4.86% (95% confidence interval, 1.98 to 9.76%) positive predictive value of for having HCM. Complete demographic information for participants with HCM and a comparison with individual categories are presented in Tables 3 and 4.
Table 3.
Demographics of Participants With Confirmed HCM
| Patient | Age | Sex | Race | Body Mass Index | PMH or FH | Sport(s) |
|---|---|---|---|---|---|---|
| 1 | 13 | Male | Asian | 24.2 | FH of fainting | Baseball, football |
| 2 | 17 | Male | Asian | 29.5 | None | Football, track & field |
| 3 | 15 | Male | Black | 27.5 | None | Basketball, football, track & field |
| 4 | 16 | Male | Black | 21.3 | None | Wrestling |
| 5 | 16 | Male | Black | 31.1 | None | Football, track & field |
| 6 | 20 | Female | No response | 22.5 | None | Basketball |
| 7 | 22 | Male | Black | 30.3 | None | Football |
FH, family history; PMH, past medical history.
Table 4.
Comparison of Participant’s Characteristics With Confirmed HCM per Individual Category
| Category | HCM | Prevalence (95% CI) | P |
|---|---|---|---|
| All Patients | 7 | 0.31% (0.12–0.63%) | |
| Age (years) | |||
| 13 | 1 | 0.45% (0.01–2.47%) | .19a |
| 14 | 0 | 0.00% (0.00–0.43%) | |
| 15 | 1 | 0.27% (0.01–1.49%) | |
| 16 | 2 | 0.73% (0.01–2.64%) | |
| 17 | 1 | 0.68% (0.02–3.71%) | |
| 18 | 0 | 0.00% (0.00–1.84%) | |
| 19–20 | 1 | 0.38% (0.01–2.09%) | |
| 21+ | 1 | 0.92% (0.02–5.01%) | |
| Sex | |||
| Male | 6 | 0.41% (0.15–0.90%) | .43b |
| Female | 1 | 0.12% (0.01–0.68%) | |
| Body Mass Index | |||
| <18.5 | 0 | 0.00% (0.00–1.12%) | <.01a |
| 18.5–24.999 | 3 | 0.20% (0.04–0.60%) | |
| 25.0–29.999 | 2 | 0.52% (0.06–1.88%) | |
| >= 30 | 2 | 1.63% (0.20–5.75%) | |
| Racec | |||
| White | 0 | 0.00% (0.00–0.23) | <.01a |
| Latino | 0 | 0.00% (0.00–0.65) | .36a |
| Asian | 2 | 0.68% (0.08–2.44%) | .22a |
| Black | 4 | 1.86% (0.51–4.69%) | <.01a |
| Other | 0 | 0.00% (0.00–2.21%) | 1.00a |
| No response | 1 | 0.66% (0.03–3.08%) | .38a |
CI, confidence interval.
By Kruskal-Wallis rank test.
By Fisher’s exact test, comparing categories.
Multiple responses allowed.
Discussion
Hypertrophic cardiomyopathy is a genetic and potentially lethal abnormality that often remains asymptomatic. The classic physical exam finding is a crescendo-decrescendo harsh systolic murmur along the upper-left sternal border that increases with Valsalva.19 However, this finding along with a focused history has been demonstrated in the literature to yield, at best, a sensitivity of less than half (range 2.5 to 40%).20–22 Although HCM has a relatively low prevalence, the high risk of sudden death in an otherwise asymptomatic, young athlete creates a situation in which generalized screening could be of great benefit.
Although ECG and physical examination screening programs such as those in Italy exist, there continues to be debate over the ideal screening modality.23 In young adults, an ECG diagnosis of HCM is challenging because of the lack of specificity.24 Sheikh et al has demonstrated that the ECG patterns of atrial enlargement, axis deviation, and right ventricular hypertrophy are of little diagnostic yield for the identification of cardiac pathology.25 Widespread recommendations including the European Society of Cardiology and the “Seattle Criteria” have been cited for use as screening tools, yet these methods demonstrate false positive rates up to 22%.26–28 This is much higher than in our study, in which we observed a false positive rate of only 5.6%.
For patients with concern for HCM, trans-thoracic echocardiography is considered the gold standard for establishing the diagnosis.1 Findings associated with HCM include asymmetric septal wall thickening, decreased left ventricle chamber size, and decreased diastolic filling.29 Because of logistics such as cost, widespread cardiac point-of-care ultrasound (POCUS) has not been thoroughly evaluated as a screening modality for HCM. Recently, POCUS use has significantly expanded in undergraduate medical education.16–18 With expanding education and training along with increasing portability of machines, there may be a role for minimally trained practitioners to perform cardiac screening for young athletes at high risk. Therefore, we aimed to explore whether minimally trained medical students who are often eager to engage in service learning could screen large numbers of young athletes.
A primary goal of this study was to determine whether minimally trained medical students could successfully screen for HCM. Rather than having a trained cardiologist or sonographer devote time to performing ultrasound screening exams on thousands of young athletes, our data support that the labor-intensive aspect of this screening process can be undertaken by medical students. The cost savings of such a model consisting of volunteers rather than highly trained professional sonographers is potentially significant, especially as POCUS equipment becomes less and less expensive to rent, lease, or purchase. Our model of using minimally trained volunteer medical students may be the first step of a large-scale cost-effective screening effort. From start to finish, the time it took to screen a student athlete for HCM was 5 minutes per participant, on average. With 10 stations, a screening of up to 200 athletes took approximately 4 hours. This time frame matched well with the already implemented student athletes’ physical examination day. Although in our study we had a volunteer board-certified pediatric cardiologist read over each study performed by the medical students, we suspect that with future training a cardiologist may only be necessary in cases with abnormalities detected by POCUS. However, future studies will be needed to determine the minimum amount of training needed for students to reliably screen for HCM.
Although we appreciate the difficulty and potential costs of implementing a national screening program for all adolescents and young adults, medical students are often keen to fill in the gaps where financial interests and the economic realities of medicine in America leave populations without proper medical care. Medical student–run clinics exist all across the country30,31 and have been shown to promote better clinical skills and an interest in helping the underserved.32 If medical schools can start establishing medical school–run clinics to screen for HCM, not only will more cases of HCM be detected, but more data will be acquired to aid in determining the broad utility of such a screening protocol.
There were multiple limitations of this study. First, the study was performed within a single geographic area, so the demographics of our target study population may vary significantly from other regions of the country. Second, the nature of the convenience sampling may have produced a potential selection bias, although we hope this is limited as most of our participants who were eligible for enrollment were able to be enrolled in the study. Third and potentially most significant, is that we were unable to obtain true sensitivity, specificity, positive, and negative likelihood ratios for our HCM screening process, as not all of the participants underwent separate scanning by a pediatric trained cardiologist. It is our hope that this last limitation will be mitigated by using a generous cut-off interventricular septum to left ventricular posterior wall ratio greater than 1.25, which would confer a relatively high sensitivity for possible HCM. Additionally, 2.9% of the participants were excluded as a result of an inability to obtain adequate images. This may indicate that additional training is required. Finally, patients with asymmetric left ventricular hypertrophy may not have been identified with this method. Future study may be able to validate this ratio and identify a smaller ratio with an adequately high sensitivity and specificity for HCM.
In conclusion, we found that in this study, using POCUS, volunteer medical students were able to detect seven cases of HCM with less than a 6% false-positive rate. Our data indicate that this protocol may have the potential to mature into a national screening program to help prevent needless sudden death among our youth. Further large-scale clinical trials are needed to validate these findings and to determine the minimum amount of training required for POCUS identification of HCM.
Acknowledgments
Dr J. Christian Fox receives stock options from Sonosim for consulting. However, no Sono-sim products were used in this research project. The UC Irvine Health Department of Emergency Medicine and the UC Irvine School of Medicine are gratefully acknowledged.
ABBREVIATIONS
- ECG
electrocardiogram
- HCM
hypertrophic cardiomyopathy
- POCUS
point-of-care ultrasound
References
- 1.Maron BJ. Hypertrophic cardiomyopathy: a systematic review. JAMA. 2002;287:1308–1320. doi: 10.1001/jama.287.10.1308. [DOI] [PubMed] [Google Scholar]
- 2.Maron BJ. Hypertrophic cardiomyopathy: an important global disease. Am J Med. 2004;116:63–66. doi: 10.1016/j.amjmed.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 3.Zou Y, Song L, Wang Z, et al. Prevalence of idiopathic hypertrophic cardiomyopathy in China: a population-based echocardiographic analysis of 8080 adults. Am J Med. 2004;116:14–18. doi: 10.1016/j.amjmed.2003.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Maron BJ, Estes NAM, III, Maron MS, et al. Primary prevention of sudden death as a novel treatment strategy in hypertrophic cardiomyopathy. Circulation. 2003;107:2872–2875. doi: 10.1161/01.CIR.0000072343.81530.75. [DOI] [PubMed] [Google Scholar]
- 5.Burke AP, Farb A, Virmani R, et al. Sports-related and non-sports-related sudden cardiac death in young adults. Am Heart J. 1991;121:568–575. doi: 10.1016/0002-8703(91)90727-y. [DOI] [PubMed] [Google Scholar]
- 6.Van Camp SP, Bloor CM, Mueller FO, et al. Non-traumatic sports death in high school and college athletes. Med Sci Sports. 1995;27:641–647. [PubMed] [Google Scholar]
- 7.Soor GS, Luk A, Ahn E, et al. Hypertrophic cardiomyopathy: current understanding and treatment objectives. J Clin Pathol. 2009;62:226–235. doi: 10.1136/jcp.2008.061655. [DOI] [PubMed] [Google Scholar]
- 8.Ho CY, Sweitzer NK, McDonough B, et al. Assessment of diastolic function with Doppler tissue imaging to predict genotype in preclinical hypertrophic cardiomyopathy. Circulation. 2002;105:2992–2997. doi: 10.1161/01.cir.0000019070.70491.6d. [DOI] [PubMed] [Google Scholar]
- 9.Maron BJ, Casey SA, Hurrell DG, et al. Relation of left ventricular thickness to age and gender in hypertrophic cardiomyopathy. Am J Cardiol. 2003;91:1195–1198. doi: 10.1016/s0002-9149(03)00266-2. [DOI] [PubMed] [Google Scholar]
- 10.Spirito P, Bellone P, Harris KM, et al. Magnitude of left ventricular hypertrophy and risk of sudden death in hypertrophic cardiomyopathy. N Engl J Med. 2000;342:1778–1785. doi: 10.1056/NEJM200006153422403. [DOI] [PubMed] [Google Scholar]
- 11.Maron BJ, Thompson PD, Puffer JC, et al. Cardiovascular preparticipation screening of competitive athletes. Circulation. 1996;94:850–856. doi: 10.1161/01.cir.94.4.850. [DOI] [PubMed] [Google Scholar]
- 12.Corrado D, Pelliccia A, Bjornstad HH, et al. Cardiovascular preparticipation screening of young competitive athletes for prevention of sudden death: a proposal for a common European protocol: consensus statement of the study group of sport cardiology of the Working Group of Cardiac Rehabilitation and Exercise Physiology and the Working Group of Myocardial and Pericardial Diseases of the European Society of Cardiology. Eur Heart J. 2005;26:516–524. doi: 10.1093/eurheartj/ehi108. [DOI] [PubMed] [Google Scholar]
- 13.Gazzetta Ufficiale della Republica Italiana. Dec 23, 1971. Tutela Sanitaria Delle Attivita Sportive; pp. 8162–8164. [Google Scholar]
- 14.Pelliccia A, Maron BJ. Preparticipation cardiovascular evaluation of the competitive athlete: perspectives from the 30 year Italian experience. Am J Cardiol. 1995;75:827–829. doi: 10.1016/s0002-9149(99)80421-4. [DOI] [PubMed] [Google Scholar]
- 15.Corrado D, Basso C, Thiene G. Comparison of United States and Italian experiences with sudden cardiac deaths in young competitive athletes and implications for preparticipation screening strategies. Am J Cardiol. 2010;105:421–422. doi: 10.1016/j.amjcard.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 16.Fox JC, Schlang JR, Maldonado G, Lotfipour S, Clayman RV. Proactive medicine: the “UCI 30,” an ultrasound-based clinical initiative from the University of California, Irvine. Acad Med. 2014;89:984–989. doi: 10.1097/ACM.0000000000000292. [DOI] [PubMed] [Google Scholar]
- 17.Bahner DP, Adkins EJ, Hughes D, et al. Integrated medical school ultrasound: development of an ultrasound vertical curriculum. Crit Ultrasound J. 2013;5:6. doi: 10.1186/2036-7902-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dinh VA, Lakoff D, Hess J, et al. Medical student core clinical ultrasound milestones. J Ultrasound Med. 2016;35:421–434. doi: 10.7863/ultra.15.07080. [DOI] [PubMed] [Google Scholar]
- 19.Maron BJ, Thompson PD, Ackerman MJ, et al. Recommendations and considerations related to preparticipation screening for cardiovascular abnormalities in competitive athletes: 2007 update: a scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2007;115:1643–1655. doi: 10.1161/CIRCULATIONAHA.107.181423. [DOI] [PubMed] [Google Scholar]
- 20.Baggish AL, Hutter AM, Jr, Wang F, et al. Cardiovascular screening in college athletes with and without electrocardiography: a cross-sectional study. Ann Intern Med. 2010;152:269–275. doi: 10.7326/0003-4819-152-5-201003020-00004. [DOI] [PubMed] [Google Scholar]
- 21.Le VV, Wheeler MT, Mandic S, et al. Addition of the electrocardiogram to the preparticipation examination of college athletes. Clin J Sport Med. 2010;20:98–105. doi: 10.1097/JSM.0b013e3181d44705. [DOI] [PubMed] [Google Scholar]
- 22.Maron BJ, Shirani J, Poliac LC, Mathenge R, Roberts WC, Mueller FO. Sudden death in young competitive athletes. Clinical, demographic, and pathological profiles. JAMA. 1996;276:199–204. [PubMed] [Google Scholar]
- 23.Kaltman JR, Thompson PD, Lantos J, et al. Screening for sudden cardiac death in the young. Report from a national heart, lung and blood institute working group. Circulation. 2011;123:1911–1918. doi: 10.1161/CIRCULATIONAHA.110.017228. [DOI] [PubMed] [Google Scholar]
- 24.Sathanandam S, Zimmerman F, Davis J, et al. ECG screening criteria for LVH does not correlate with diagnosis of hypertrophic cardiomyopathy [Abstract 2484] Circulation. 2009:120. [Google Scholar]
- 25.Sheikh N, Papadakis M, Ghani S, et al. Comparison of ECG criteria for the detection of cardiac abnormalities in elite black and white athletes. Circulation. 2014;129:163. doi: 10.1161/CIRCULATIONAHA.113.006179. [DOI] [PubMed] [Google Scholar]
- 26.Corrado D, Pelliccia A, Heidbuchel H, et al. Recommendations for interpretation of 12-lead electrocardiogram in the athlete. Eur Heart J. 2010;31:243–259. doi: 10.1093/eurheartj/ehp473. [DOI] [PubMed] [Google Scholar]
- 27.Drezner JA, Ackerman MJ, Anderson J, et al. Electrocardiographic interpretation in athletes: the ‘Seattle criteria’. Br J Sports Med. 2013;47:122–124. doi: 10.1136/bjsports-2012-092067. [DOI] [PubMed] [Google Scholar]
- 28.Reisdorff EJ, Prodinger RJ. Sudden cardiac death in the athlete. Emerg Med Clin North Am. 1998;16:281–294. doi: 10.1016/s0733-8627(05)70004-3. [DOI] [PubMed] [Google Scholar]
- 29.Futterman LG, Myerburg R. Sudden death in athletes: an update. Sports Med. 1998;26:335–350. doi: 10.2165/00007256-199826050-00004. [DOI] [PubMed] [Google Scholar]
- 30.Ouyang D, Yuan N, Sheu L, Lau G, Chen C, Lai CJ. Community health education at student-run clinics leads to sustained improvement in patients’ hepatitis B knowledge. J Community Health. 2013;38:471–479. doi: 10.1007/s10900-012-9631-3. [DOI] [PubMed] [Google Scholar]
- 31.Smith S, Thomas R, 3rd, Cruz M, Griggs R, Moscato B, Ferrara A. Presence and characteristics of student-run free clinics in medical schools. JAMA. 2014;312:2407–2410. doi: 10.1001/jama.2014.16066. [DOI] [PubMed] [Google Scholar]
- 32.Smith SD, Yoon R, Johnson ML, Natarajan L, Beck E. The effect of involvement in a student-run free clinic project on attitudes toward the underserved and interest in primary care. J Health Care Poor Underserved. 2014;25:877. doi: 10.1353/hpu.2014.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]