Abstract
High plasma levels of fatty acids occur in a variety of metabolic diseases. Cellular effects of fatty acid overload resulting in negative cellular responses (lipotoxicity) are often studied in vitro, in an attempt to understand mechanisms involved in these diseases. Fatty acids are poorly soluble, and thus usually studied when complexed to albumins such as bovine serum albumin (BSA). The conjugation of fatty acids to albumin requires care pertaining to preparation of the solutions, effective free fatty acid concentrations, use of different fatty acid species, types of BSA, appropriate controls and ensuring cellular fatty acid uptake. This review discusses lipotoxicity models, the potential problems encountered when using these cellular models, as well as practical solutions for difficulties encountered.
Keywords: Lipotoxicity, lipids, fatty acid metabolism, fatty acids, cells, tissues
1. Introduction: In vitro lipotoxicity models
High circulating levels of free fatty acids (FAs) are associated with obesity and the development of Type 2 diabetes, among other diseases [1]. The term ‘lipotoxicity’, coined in 1994 [2], has been used for studies in which the negative effects of chronically elevated FAs on cellular function are monitored by adding supraphysiological concentrations of lipid. These in vitro studies enable researchers to investigate lipotoxicity in cell cultures, in a manner that is similar and complimentary to in vivo obesity models. This review covers biological effects of lipotoxicity, distinct lipotoxicity models and discusses characteristics of the FAs and BSA-conjugation systems used that should be taken into account when establishing these protocols. We begin by highlighting selected in vitro biological effects of FA overload in a few different tissues and cell types with which the authors of this review have experience.
1.1. Lipotoxicity in the liver
The liver plays a central role in lipid metabolism. Indeed, hepatic steatosis occurs when high concentrations of circulatory FAs reaching the liver and de novo lipogenesis are not counterbalanced by FA oxidation or lipid export as lipoproteins [3]. Lipid droplets (LD) are formed comprised of neutral lipid depots, surrounded by a monolayer of amphipathic lipids in a stable and controlled emulsion in the cytoplasm [4]. The neutral core in hepatic LDs contains mainly triacylglycerol [3; 5]. Interestingly, the accumulation of LDs in the liver could be a physiological and protective response. Overactivation of triacylglycerol synthesis favors steatosis but does not promote insulin resistance, while inhibition of triacylglycerol synthesis reduces steatosis and increases liver damage [6; 7]. Liver FA buffering is central not only for hepatic, but also whole body homeostatic maintenance [8]. In obesity, nearly 75% of hepatic FAs derive from white adipose tissue lipolysis and diet [9], and thus originate from circulating lipids.
Experimental strategies have been developed to understand how hepatocytes respond to lipid overload. Models in which hepatocyte lipid handling is challenged involve modulating FA concentration, acyl-chain lengths and unsaturation, and dissociated or not from other circulating signals such as hormones and cytokines. These in vitro assays induce FA accumulation in human hepatocyte lines 18–24 h after incubation in the presence of high lipid concentrations [10–18]. Some authors [13; 14] do not observe any differences in the accumulation induced by saturated or unsaturated FAs, while others [15–17] find that monounsaturated FAs promote higher lipid accumulation compared to saturated FAs when alone or as mixtures, in a dose-dependent manner. On the other hand, saturated FAs consistently promote lipotoxic cell death [8; 19]. In the HepG2 cell line, palmitate and stearate, but not monounsaturated palmitoleate or oleate, promote ER stress that promotes JNK pathway activation, leading to Bim-mediated Bax activation and apoptosis [14; 20]. These results were also observed using rodent cell lines and primary cultures [17; 21; 22]. Ceramide production is increased with saturated FA overload and could mediate FA-induced apoptosis. However, studies modulating ceramide production in hepatocytes yield conflicting results [23; 24]. Interestingly, palmitate is able to increase hepatocyte proinflammatory cytokine production. These cytokines may mediate cell death and contribute toward liver injury during steatosis progression [10; 12]. Kupffer cells, the liver resident macrophages, may also participate in liver lipotoxic effects by promoting local inflammation. Pardo and colleagues [25] elegantly used conditioned medium from macrophages pre-treated with palmitate on primary hepatocyte cultures, and found that it promoted ER stress, apoptosis, and insulin resistance. Oleate-conditioned medium did not have the same effects [25]. These results associating lipotoxicity and inflammation are in agreement with in vivo data in which liver steatosis is accompanied by cell death and inflammation, markers for undesirable disease progression [5; 26].
Lipotoxicity in the liver may impair hepatic function, promoting metabolic disease. Saturated, but not monounsaturated, FAs induce insulin resistance in hepatocytes [11; 13; 27; 28], a possible consequence of lipid-induced post-translational modifications [29; 30] and/or palmitate-stimulated proteasomal degradation of insulin cascade proteins [13]. These metabolic effects are accompanied by mTORC1 activation [27], mitochondrial network fragmentation [18], and increased oxidative imbalance [11; 28; 31]. Egnatchik [32] demonstrated that palmitate promotes calcium release from the ER and its subsequent uptake by mitochondria; this disturbs the citric acid cycle and causes oxidative imbalance and cell death. Indeed, the toxic effects of palmitate were suppressed with a calcium chelator [32]. Overall, liver lipotoxicity studies have shown a link between steatosis, inflammation and insulin resistance, caused primarily by saturated FA. In vivo, these effects of high circulating lipids will interact with changes occurring in other key metabolic regulators.
1.2. Lipotoxicity in pancreatic beta cells
Sako et al. [33] first showed time-dependent effects of lipid infusion on isolated perfused pancreatic islet insulin secretion: it was enhanced after a short-term, 3 hour, lipid infusion, while 48 hours of lipid infusion blunted glucose-stimulated insulin secretion. An in vitro rat islet model of lipotoxicity [34] in which the effect of chronic exposure to palmitate, oleate, or octanoate for 48 hours was studied showed increased basal insulin secretion and inhibited secretion stimulated with high glucose levels. In a subsequent study, Bollheimer et al. [35] found similar changes. This study also found that although oleate potentiated preproinsulin mRNA production in islets, there was an inhibitory effect on proinsulin translation regulation by glucose. Grill and Qvigstad [36] discuss early work on effects of FAs on beta cell function and insulin secretion in numerous experimental in vitro and in vivo models. While the effects of FAs are similar on insulin secretion, effects on other parameters such as ER stress and apoptosis are disparate. As discussed above for liver, saturated FAs have been shown to be more detrimental when compared to unsaturated FAs in islets. Indeed, Maedler et al. [37] showed that unsaturated FAs oleate and palmitoleate display a protective role by restricting the apoptotic and anti-proliferative effects of saturated FA palmitate on human islets. Ceramide signaling mediated palmitate-induced apoptosis, as the use of fumosinin B1 abrogated the effects of palmitate. Palmitate downregulated the expression of Bcl2 and increased cytochrome c release, while addition of palmitoleate or oleate (which increased Bcl2 expression), or inhibition of ceramide synthesis, prevented the release of cytochrome c.
Palmitate has also been shown to promote to ER stress. Karaskov et al. [38] examined the consequences of chronic exposure of the insulin-secreting cell line INS1 to palmitate or oleate and determined that palmitate, but not oleate, stimulated ER stress, with increased phosphorylated PERK, nuclear ATF4 protein, XBP-1, and GADD153/CHOP. However, Grp78/94 and PDI ER chaperone protein levels were unchanged as compared to the BSA controls. Palmitate treatment resulted in marked morphological changes in ER, mitochondria and Golgi. This study, however, was performed in a serum free system, which would increase sensitivity of the cells. Consistent with these findings are studies showing that unsaturated FAs and expression of desaturases (SCD1) are protective against palmitate-induced damage of beta cells [39; 40]. Nevertheless, chronic exposure to oleate and palmitate remains detrimental to beta cell function by inducing mitochondrial fragmentation and inhibition of autophagic flux [41; 42].
Oprescu et al. [43] showed the effects of oleate on insulin secretion in vivo, via 48 hour infusion by applying hyperglycemic clamps and ex vivo, in response to glucose at nonstimulatory, basal, and elevated glucose levels. In this study, oleate impairment of glucose-stimulated insulin secretion was abrogated by treatment with the antioxidants N-acetylcysteine, taurine and tempol, indicating a role of redox signalling in this process.
An in vitro model of glucolipotoxicity exposing primary mouse beta cells to palmitate and 20 mM glucose for 48 hours (glucolipotoxicity) found increased mitochondrial fragmentation associated with a reduction in mitochondrial fusion [41, 44]. Further characterization of the relative contributions of palmitate as compared to glucose in INS1 cells revealed that palmitate is primarily contributing toward mitochondrial fragmentation. Glucose displayed a synergistic amplifying effect. Conversely, treatment with glucose alone displayed a minimal effect [41].
Autophagy, which serves as a quality control mechanism for clearance of damaged cellular components, is also affected by lipotoxicity. Beta cell-specific ATG7 deficient mice exhibit impaired autophagy and glucose tolerance, as well as a more severe phenotype under high fat diets when compared to WT mice [45]. Furthermore, loss of ATG7 results in increased oxidative imbalance, mitochondrial dysfunction, reduced insulin content, and reduced beta cell mass [46; 47]. Given that lipotoxicity produces a similar phenotype, an in vitro model of lipotoxicity was used to investigate chronic effects of FAs on autophagic turnover [42]. Primary or clonal beta cells were exposed either to palmitate or oleate complexed to BSA and added in media containing 1% FBS. This resulted in inhibition of autophagic flux via impairment of lysosome acidification along with impaired bioenergetics and mitochondrial fragmentation. Lowering FBS concentrations is likely to stimulate autophagy in some cells, thus determination of changes of autophagosome formation and/or survivial upon reduction of FBS is suggested.
1.3. Lipotoxicity in the brain
Obesity correlates with saturated FA accumulation in the hypothalamus, the brain area controlling energy balance [48; 49] and hypothalamic lipid overload is accompanied by alterations in neuronal and glial cell function, leading to overfeeding and uncontrolled body weight gain [50]. In vivo studies suggest these changes are related to saturated FA-induced inflammatory and ER stress responses [51]. Indeed, the inhibition pathways leading to these responses alleviates several metabolic impairments caused by saturated FA accumulation [51]. In vitro studies using neuronal cell lines have shown that palmitate, the predominant saturated FA in fat-rich diets, rapidly induces ER stress followed by insulin resistance and loss of energy balance control [52–54]. These effects are prevented by chemical chaperones or oleate pre-treatment, indicating that palmitate-induced ER stress can directly modulate hypothalamic neuronal function [52; 54]. Notably, palmitate treatment is often accompanied by elevated levels of neuronal cell death, that must be considered when analyzing these data [52; 55; 56]. Studies have shown that palmitate can activate inflammatory markers in hypothalamic neurons in parallel with alterations in insulin signaling [53; 57–59]. Conversely, some authors suggest these cells are resistant to palmitate-induced inflammation and insulin resistance [60; 61]. In addition to differences between cell lines, these conflicting results may be a consequence of discrepancies in the lipotoxicity protocol. One relevant difference is the FA:BSA ratio used, that range from 3:1 to more than 6:1 [52; 53; 60]. Since FA:BSA ratios determine lipid availability and hence toxicity, neuronal inflammation could be a consequence of excessive cell death instead of direct activation of this pathway. While palmitate lipotoxicity assays have yielded conflicting results, in vitro experiments involving mono and polyunsaturated FA effects on neuronal inflammation have consistently shown that hypothalamic neurons treated with oleate and docosahexaenoic acid (DHA) present attenuated inflammatory cytokine expression and reduced cell death [53; 61].
Hypothalamic saturated FA accumulation also promotes inflammation by increasing the number of reactive microglia, the macrophages of the brain [51]. Microglial activation is triggered early on after rodent exposure to high fat diets and persists with the establishment of the obese phenotype [62]. In vitro studies have demonstrated that saturated FA treatment rapidly promotes an inflammatory response in these cells [51; 63–65]. Indeed, primary microglia and BV2 cells (a microglial cell line) incubated with palmitate and stearic acid increase inflammatory signaling and cytokine secretion [51; 63; 64]. In contrast, some studies describe palmitate-induced increases in anti-inflammatory marker expression in BV2 cells [66; 67]. This divergence could be a consequence of the use of a different lipotoxicity protocols, as there is a more than five fold difference in the FA:BSA ratio between these studies. To evaluate the contribution of different cells in lipotoxic hypothalamic inflammation, Valdearcos and colleagues [51] treated organotypic hypothalamic slice cultures with oleate and palmitate and found that only palmitate-treated slices presented increases in cytokine release and neuronal stress markers. These effects were absent in microglia-depleted slices, suggesting saturated FA-induced microglial activation triggers hypothalamic inflammation. Collectively, these data indicate that cell culture models are valuable to expand the understanding of pathways promoting hypothalamic dysfunction upon saturated FA overload. However, results in these systems must be compared carefully to in vivo models since saturated FA treatment is often accompanied by pronounced cell death. As such, it is advisible to use a combination of saturated and unsaturated FA to monitor physiologically relevant biological effects. Furthermore, the experimental definition of lipotoxicity remains loose when referring to protocols used to deliver lipids to cells. Different labs employ protocols that vary in characteristics such as FA complexation (usually to BSA), FA species used and concentrations, making comparisons difficult.
2. Fatty acid transport and uptake
Lipids are usually transported primarily by means of association with proteins in the blood forming hydrosoluble lipoprotein particles that allow for controlled recognition and delivery of lipids such as triglycerides and cholesterol to tissues [68]. Nonetheless, nonesterified FAs are transported mainly with serum albumin [69; 70], an abundant protein present in plasma and interstitial fluids able to bind FA and other lipophilic molecules [69; 70]. Albumin-FA interactions maximize the amount of FA transported and cleared from the circulation [70; 71]. Studies conducted using primary hepatocyte cell cultures show that, in the presence of albumin, palmitate uptake by cells is more efficient [72], an effect that is not mediated by receptors [70; 73]. In in vitro systems, albumin-promoted FA delivery follows the “pseudofacilitation-model” [73; 74] in which FAs are delivered as function of their unbound concentration in the medium at a physiological concentration of albumin (~200 µM). At lower concentrations (< 150 µM), delivery will depend on albumin concentration.
FA uptake by cells is proposed to occur through membrane diffusion or facilitated transport by membrane proteins [75; 76]. Both types of transport require the FA movement from an aqueous solution to another, through a lipid bilayer, involving mechanisms that are still under debate [76–82]. Transport rates depend on FA size and number of unsaturations, membrane lipid composition, and protein composition [82; 83]. CD36 is the membrane receptor not consensually suggested to be a fatty acid translocator. The use of CD36 inhibitors AP5055 and AP5258 reduces triacylclygerol accumulation in cultured cells [84]. Some recent findings suggest that transport is probably not dependent on direct mediated FA translocation by CD36, but diffusion would be driven by increased cytosolic demand [81; 85].
Inside the cell, FAs will act as energy sources, signaling molecules, incorporated into membranes or as triacylglycerols [8]. Incorporation of lipids into the cell as well their intracellular fate under lipotoxic conditions may influence metabolic changes observed in different cellular systems - the balance between lipid oxidation and storage may be determinant toward lipotoxicity [86]. Interestingly, fatty acid oxidation may be decreased in cell cultures of muscular origin simply because of the lack of a physical workload in the in vitro system. Indeed, impaired fatty acid oxidation and consequent increment in lipid storage causes lipotoxicity in tissues with limited fat accumulation capacity such as skeletal muscle and the heart [86; 87]. Confirming this hypothesis, inhibition of FA oxidation in macrophages with etomoxir exacerbates palmitate-induced inflammation and ER stress [88]. Etomoxir is an irreversible inhibitor of acyl carnitine formation, preventing FA uptake by mitochondria, in which their oxidation occurs. On the other hand, increases in FA oxidation prevent inflammation and apoptosis in neuronal and muscle cells, respectively [58; 89; 90, 91]. Fatty acid binding proteins (FABPs) are intracellular lipid chaperones that bind to FAs and help redistribute them to exert their biological functions [92]. FABPs have important functions in metabolic control, and pathways sensitive to lipid signaling can be studied by inhibiting them [92; 93].
Overall, BSA is a suitable method to deliver FA to cells since it is similar to the physiological system of nonesterified FA transport. However, care must be taken to adapt concentration of the molecules to avoid discrepancies in delivery kinetics. Since uptake per se cannot be effectively inhibited, modulation of FA fate inside the cell may be an interesting strategy to understand the signaling pathways sensitive to FA. Furthermore, precaution must be taken regarding the type of BSA used and the preparation of FA/BSA solutions, as will be discussed in detail below.
3. Types of BSA
The type of BSA used in cellular lipotoxicity models must be pondered carefully, since differences in isolation methods, purity and contaminants can interfere in the biological effects of lipid preparations. The choice of a specific BSA type and consistent maintenance of this type is thus necessary when planning experiments.
Albumin purity is the main feature to be considered since it directly alters lipid binding and availability. Indeed, different impurities in BSA used are able to modulate added FA binding to this protein [69; 94]. Contaminant bovine FAs, for example, are present in all less purified BSA samples, and should be avoided since they compete with added lipids for the albumin hydrophobic clefts. These endogenous FAs can induce biological effects themselves [69; 94; 95]. It is thus imperative to use FA-free BSA. FA removal is promoted through charcoal treatment or organic solvent precipitation [95]. Indeed, a wide variety of FA-free albumin is commercially available, with different purity levels. Commercial FA-free BSA usually binds lipids more efficiently than Charcoal-purified albumin, resulting in a smaller fraction of unbound FAs after conjugation [94]. This issue should be taken into consideration when comparing the biological effects of varying BSA concentrations.
In addition to endogenous bovine FA, BSA preparations can carry other contaminants depending on the isolation method employed. In fact, different ligands such as hormones, drugs and toxins can remain bound to albumin after isolation [71]. Moreover, globulins and other blood proteins are present in several types of commercial BSA [96]. The biological activity of these contaminants should be considered, since they can modulate the FA effects in different systems. Further albumin purification requires appropriate methods to remove residual contaminants. For example, globulins can be removed by heat treatment and glycoproteins by affinity chromatography [95; 96]. A wide diversity of albumin contaminant combinations can be found in commercially available BSA. The Sigma-Aldrich® catalog, for example, currently offers 48 types of BSA with different contaminant and purity grades, depending on the isolation/purification method applied.
4. Preparing FA/BSA solutions
Lipid and albumin conjugation and solubilization must be verified when preparing FA/BSA solutions. Indeed, differences in these formulations can generate disparate responses in the same system. For instance, microglial cells treated with palmitate at a FA:BSA ratio of 2:1 present an anti-inflammatory profile, while a 10:1 ratio induces a strong pro-inflammatory profile [9; 64; 66]. Therefore, FA/BSA protocols must be carefully controlled to ensure reliable results, and the ratios used must be described.
Long-chain FA solubilization may be difficult due to low solubility in aqueous solutions. The strategies employed to solve this issue usually include the use of organic solvents and heating. In fact, several groups have used ethanol to dissolve FA at concentrations ranging from 12.5 to 95% and heating to 50–70°C [64; 66; 97–100]. The final concentration of organic solvent has to be considered since it can affect cell viability [101]. Ethanol concentrations should not exceed 0.05% in the cell culture medium [102] and this solvent should be avoided when studying FA effects on cells able to oxidize this alcohol [103]. In addition to ethanol, dimethyl sulfoxide (DMSO) has been employed to solubilize long-chain FA [41; 104], although the cytotoxicity of this solvent must also be considered, and concentrations over 1:200 (v:v) should be avoided. Heated aqueous solutions have also been used to dissolve FA [73; 105; 106]. In these protocols, the complete solubilization of the FA is achieved at 70°C, when the solution becomes clear [106]. The addition of NaOH can improve solubility when the acidic form of FA is used instead of the sodium salt [73; 105].
BSA is normally dissolved directly in water or cell culture media due to its high solubility in aqueous solutions [69]. However, concentrated BSA stock solutions can be hard to produce. Concentrated BSA solutions can be produced by heating the preparation to 37°C [106]. However, albumins undergo denaturation when heated to 50°C or above, and form water-insoluble aggregates [96; 107]. Thus, over-heating must be carefully avoided. It is worth highlighting that defatted or FA-free BSA is more sensitive to heat denaturation, whereas presence of FAs in BSA stabilizes BSA from heat denaturation [108].
Lipid conjugation to albumin represents a critical step in the preparation of FA/BSA solutions. Changes in temperature, time and FA:BSA ratios can produce solutions with different FA availability and, consequently, altered biological activity [94]. The temperatures used to complex lipids to albumin vary from 37°C to 70°C [66; 94; 105; 106]. Temperatures higher than 50°C, however, can lead to the formation of albumin aggregates, increasing FA availability in the solution [64; 107]. Lower temperatures, in turn, decrease FA solubility. This is particularly relevant when lipids are dissolved in aqueous or low ethanol solutions.
In respect to conjugation time, FAs can be added to BSA immediately before cell treatment or lipids can be pre-complexed to albumin and stored prior to use [94; 97; 106]. Pre-conjugation is frequently performed under continuous stirring with serial additions of FA to BSA solutions, a process that can last from minutes to hours [94; 106]. Of note, pre-complexed solutions can result in a lower content of unbound FA, allowing the use of reduced albumin concentrations to induce the same effects compared to freshly conjugated preparations [94].
5. FA:BSA ratios versus free FA levels
During lipid/albumin conjugation, FA:BSA ratios should be considered since they determine FA availability. In healthy humans, serum FA:BSA ratios range from 1:1 to 3:1. These ratios can be higher than 5:1 in disease states [109]. Accordingly, the use of high FA:BSA ratios in experiments enhances the biological effects of lipids [8; 9]. A variable to keep in mind under these conditions is cell death, since decreased viability can introduce several experimental biases.
BSA has six (or possibly seven) high affinity binding sites for FAs and is thus an efficient carrier serving to substantially increase the solubility of FAs in aqueous solutions [110]. Because of this characteristic, it is important to report the FA to BSA molar ratio or, ideally, to report the fraction of bound to unbound FA, which may vary in different preparations and create difficulties in reproducibility of biological effects. For example, the amount of unbound FA has been shown to be the main contributor to changes in pancreatic beta cell insulin secretion [111]. While it is possible to estimate the fraction of unbound FA by using previously developed algorithms [69; 112], tools that enable the direct experimental measurement of unbound FA are now available. Measurements of FA fractions can be achieved by several means such as the use of colorimetric Nonesterified Fatty Acid (NEFA) kits consisting of an enzymatically-coupled assay with a detection sensitivity of 15 µM (Cell Biolabs, [113]) to determine the total free FA concentration. This measures non-esterified fatty acids, both bound and unbound to BSA. This colorimetric probe measures total free fatty acids since it uses a reaction that consumes the unbound FA, and so more will be released due to changes in the equilibrium. An alternative is the use of FA fluorescent probes such as Acrylodan-Labeled Intestinal Fatty Acid Binding Protein, which has a detection sensitivity of 1 nM for unbound, non-estereified FA [114; 115]. It will only detect unbound FAs, since it has a lower affinity does not modify these FAs.
The use of FA-free BSA to deliver FA to the cell makes the selection of proper controls challenging. Cell culture sera typically consist of 2% albumin, to which FAs obviously also bind, thus necessitating a reduction in the serum concentration of the media to avoid a change in the FA:BSA ratio. Addition of FA-free BSA to 1% serum has been deemed sufficient to maintain the endogenous BSA of the media low, while still supporting the necessary cellular function that the fetal bovine serum provides [42; 116]. These experiments require controls, such as the presence of BSA without added FA, which may not accurately reflect real biological systems. For example, while studying the effects of lipotoxicity on beta-cell secretory function, we have found that overnight incubation with BSA promotes decreased insulin secretion, in a dose dependent manner (Shirihai et al., unpublished observations). On the other hand, Straub et al. [117] have shown that rat islet incubation in BSA significantly augments insulin secretion. The differences from our findings may be due to time-dependent changes. The second study system is more reflective of acute changes, since islets are incubated in BSA for 4 hours [117], whereas the first study incubates the islets with the FA/BSA solution overnight.
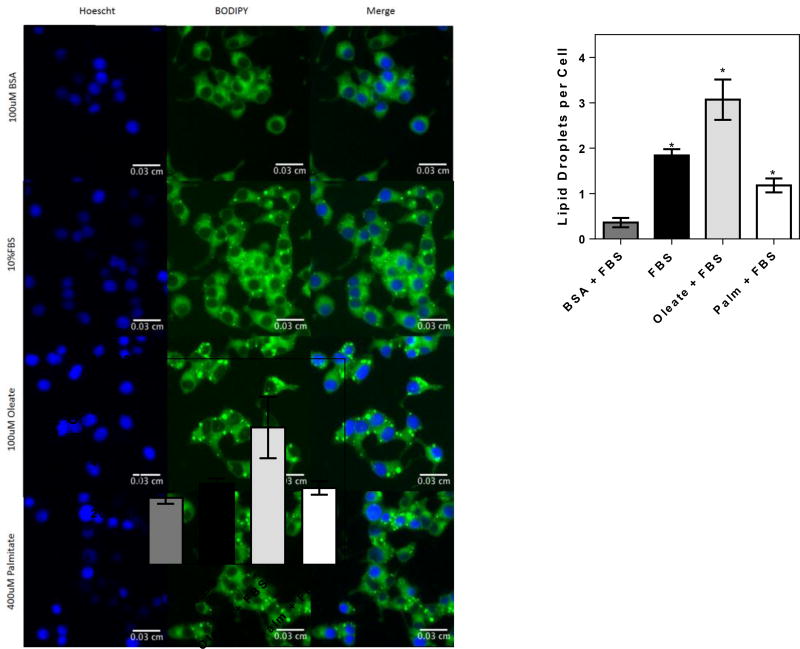
Haber et al. [118] looked into the possibility that FA-free BSA may serve as a sink for cellular lipids. This study measured FA efflux from the beta-cells, leading to the depletion of lipid content and impairment of insulin secretory function. The addition of FA-free BSA in the control media traps and absorbs free FAs, thereby altering secretory function [118]. An experimental example of this effect is shown in Figure 1, in which cellular fatty acid content in fetal bovine serum is clearly higher than in BSA devoid of added FA. It is valuable to mention that BSA has also been shown to have antioxidant properties, which may affect beta-cell function, since oxidants have been implicated as a signal for beta-cell insulin secretion [119; 120].
Figure 1. BSA decreases cellular lipid content.
INS1 cells were grown to 70% confluence in 10% FBS RPMI culturing media, after which they were treated with 100 µM BSA plus 1% FBS or 400 µM palmitate conjugated to BSA/FBS in a 4:1 ratio for 16 h. Oleate (100 µM) was conjugated directly to 10% FBS. Cells were then fixed with 4% PFA for 15 min and stained with Hoechst and BODIPY493 for 15 min. Cells were washed twice with PBS and imaged using the Operetta imager, after which the lipid droplets per cell were quantified. Typical images shown left, quantifications of the number of lipid dropets and lipid droplet area, right. Note the decrease in lipid content with BSA. * p < 0.05 versus BSA plus 1% FBS.
Thus, it seems necessary to reevaluate the use of FA-free BSA as a control in cellular lipotoxicity experiments. Titrating lower concentrations of FA to the BSA in control experiments, avoiding endogenous lipid quenching, may be an adequate control. An alternative solution is to dissolve the FA of interest and add directly to media containing FBS as in [34] or to complex the FA directly to the BSA in FBS, as used by Erion et al. [121]. The advantage of this method is that it minimizes the undesirable effects of FA-free BSA while maintaining the serum concentration at 10% during culture conditions.
Incorporation of lipids into the cell under conditions of lipotoxicity may influence metabolic changes observed in different cellular systems. A relatively straightforward way to examine accumulation of lipid is the use of fluorescence-based methods such as FA uptake kits with dyes dyes such as Nile Red for measurement of lipid droplets or BODIPY® lipid probes to assay different length FAs. Alternatively, the use of radiolabeled 14C FA may also be employed to measure uptake of the FA of interest. Dubikovskaya et al. [91] overview various methods to measure FA uptake.
6. Mixtures of different types of FA
FAs have a gross structural similarity, but have distinct physical and biological features depending on the number of carbons and unsaturations in the acyl chain [122]. The capacity of a FA to bind to BSA relates to hydrophobic and electrostatic interactions between the FA and one of the 6–7 possible binding sites within the protein [110; 13]. Therefore, saturated FA and unsaturated FA will bind to BSA with different affinities at the same sites, and this will lead to competition between these FA [73; 123; 124].
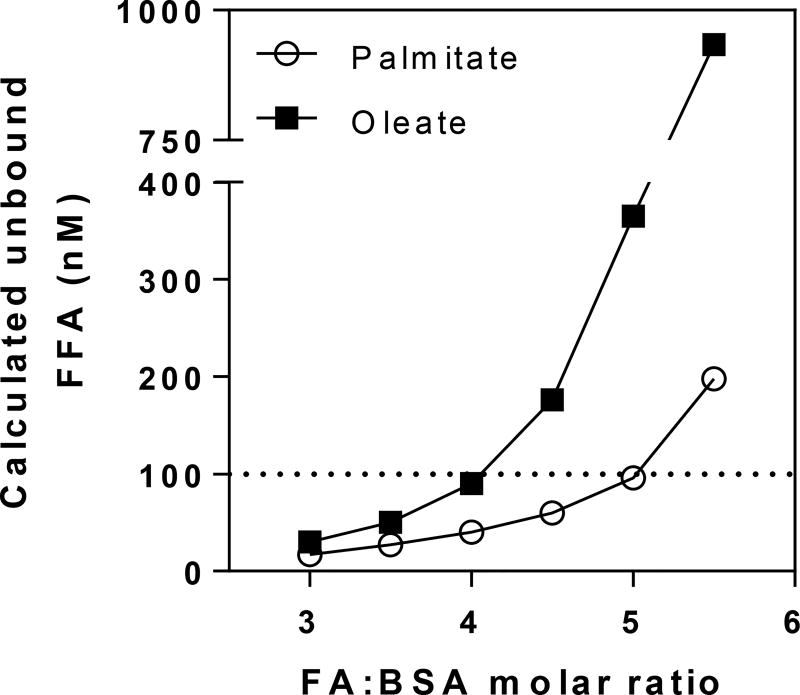
It is important to keep in mind that the biologically active fraction of the FA/BSA preparation is the unbound free FA. Free FA levels depend on binding constants of the FA for each binding site, FA:BSA molar ratios, and FA interactions with other molecules, including other FAs and BSA itself [125; 126]. Figure 2 shows how free FA quantities vary distinctly with different FAs when presented with BSA. Despite the well-known interaction of BSA with FAs, drugs, and some other endogenous ligands such as bilirubin [71], little information is available about the kinetics of in vitro FA binding in the presence of two or more types of FA and how they influence each other´s binding. This scarcity of knowledge is possibly due to the difficulty in developing specific and sensitive methods to deal with the chemical similarities among different FAs in the nanomolar concentration range.
Figure 2. The unbound FFA fraction in solution increases with FA:BSA molar ratios.
The unbound fraction was calculated for BSA and palmitate or oleate by multiple stepwise equilibrium analysis as described in [123].
Kleinfeld’s group has described methods to calculate free FA fractions in mixtures using fluorescent probes [126–128]. They demonstrated that human serum is composed of at least 40 different kinds of free FAs. Markedly, the unbound fraction does not represent the total fraction of a given FFA [126]. A similar observation was made when comparing saturated with unsaturated FAs in an in vitro mix. Huber and co-workers [127] found that in a mixture containing arachidonate, linoleate, oleate, palmitate, and stearate, with the total molar fractions of, 10, 15, 24, 21, and 29%, the free FA fractions observed were 23, 21, 20, 2 and 15%, respectively. This outcome is expected, since the solubility and albumin affinity for each type of FA is different. Thus, to obtain the same unbound fraction, more stearate is needed than arachidonate, since the first has higher affinity for albumin than the latter [127].
These discrepancies observed in total versus free FA fractions are important to highlight questions about in vitro assays that use BSA as a vehicle. If the experiment is constructed to keep the initial FA:BSA ratios constant and compare palmitate versus oleate or mixtures containing both, for example, free FA concentrations will be different among these preparations. If the aim is to test the same free FA fraction for each type of FA, however, the total FA fractions will be different and measurements should be conducted to establish these conditions. For both objectives, the “vacancy” of some binding sites in albumin can directly interfere with the cell. As seen in Figure 1, these sites can serve as interaction spots for cellular lipids and decrease their bioavailability. To maintain results produced by different labs comparable, it is critical to acknowledge the exact composition of FA and BSA in the methodology and, preferentially, measure and indicate the initial free FA concentrations, when possible.
7. Immune responses to FA/BSA preparations
Various studies have suggested that high fat diets are direct inducers of obesity-related low grade inflammation [8; 129]. This has led to an increase in the number of studies aiming to uncover mechanisms by which FAs can activate immune cells. FA/BSA preparations have thus been widely employed in assays testing the inflammatory effects of lipids. Nonetheless, some other effects of these protocols should be considered to ensure correct result interpretations.
Lipotoxicity is one of the main side effects which should be analyzed, since cell viability is compromised at elevated FA:BSA ratios [8; 94]. Indeed, cell damage and death are classical inducers of immune cell activation to a pro-inflammatory profile [128–130]. In this sense, the inflammatory response verified in the presence of high FA:BSA proportions can be promoted by cell death instead of a direct activation of inflammatory responses by the FA. Therefore, an accurate control for cell viability is necessary to prevent data misinterpretation when working with FA/BSA solutions and inflammation.
An additional side effect that is usually neglected is the intrinsic immune reactivity of BSA. Evidence suggests that conformational and linear epitopes of BSA are potent inducers of allergic reactions [131]. In fact, BSA epitopes are fully recognized by sensitized IgE from sensitized subjects, and are able to induce T cell proliferation [131–133]. This could be an undesired side-effect when the immunological properties of lipids are tested in in vivo infusion studies. Furthermore, a specific BSA epitope has been related to autoimmune reactions against pancreatic islet cells [134]. However, most of these studies were conducted in human models, and the immune reactivity of BSA in rodents remains to be elucidated.
Contaminants carried by albumin, such as globulins and endotoxins, are also able to activate immune cells and should be avoided to prevent biases in data interpretation [135]. Indeed, we find that BSA itself induces the expression of pro-inflammatory cytokines by innate immune cells (Kowaltowski et al., unpublished observations). Corroborating this, BSA-treated human adipocytes present an increase in the expression of IL-6, IL-8 and TNF-α [136]. Interestingly, methyl-β-cyclodextrin, which can be used to replace BSA as a FA carrier, also promotes increases in the expression of pro-inflammatory cytokines by adipocytes [137]. This suggests that lipid homeostasis plays a central role in the control of inflammatory marker expression.
Altogether, these data highlight the necessity of controls when working with FA/BSA solutions. Within this perspective, BSA must always be present in the control groups. Some of these effects can be prevented by reducing albumin content in the preparations, such as when using pre-complexed FA/BSA solutions [94].
8. Conclusions
When conducting lipotoxicity studies, many factors should be considered, such as those compiled in Table 1, which lists technical issues to be considered in FA:BSA lipotoxicity models, as well as solutions that have been adopted for these problems. We find it important that the effect of BSA be controlled by including an experimental group with identical concentrations of BSA and organic solvent used to dissolve the FA. However, as we have discussed throughout this manuscript, albumin has biological effects itself [136; 138; 139], including lipid quenching, that should be considered specifically for each model. If endogenous lipid quenching occurs, determining an appropriate concentration of serum or titrating low quantities of FA to inhibit lipid quenching by FA-free BSA are possible solutions to prevent this undesired effect. Furthermore, we believe FA and BSA concentrations, as well FA:BSA molar ratios, must be described in the methodology. Since the biologically-active fraction will be free FA [127], this will create awareness of differences between laboratory protocols. It is equally important to consider the amount of albumin inherently present in the serum, if the cells require serum supplementation concomitantly to FA treatment [105]. Finally, FAs can be cytotoxic [8] in a manner dependent on the type of cell, type of FA, FA and BSA concentrations, and exposure time [140–142]. The treatment effect must therefore be separated from the cytotoxic effect by measuring cell viability.
Table 1.
BSA/Lipotoxicity Trouble Shooting Checklist
| Issue | Solution | References |
|---|---|---|
| BSA Purity | Check purification method and source, as well as contaminants described in the product sheet. Consistently use the same BSA type. | [71; 94; 96] |
| FA solubility | Determine most appropriate solvent and solubility method. Check cell viability. | [94; 102; 106] |
| Temperature | Determine and maintain appropriate temperature for FA solution preparations, under 50°C for FA/BSA. | [96; 106; 107] |
| Conjugation time | Maintain consistent time points for serial FA additions. | [94; 106] |
| FA:BSA Ratios | Use realistic healthy vs disease state FA:BSA molar ratios. Check cell viability. | [126; 142] |
| Unbound FA fraction | Measure FFAs. | [114; 123; 126] |
| BSA effects in the control groups | Check BSA purity, inflammatory markers and endogenous lipid quenching. Titration of lower FA concentrations to avoid quenching. | [91; 134; 136] |
| Ensuring that added FAs change intracellular lipid levels | Measure changes in lipid uptake and accumulation. | [15; 91] |
| Choice of FAs (saturated/unsaturated) | Check cell viability, differences in FFAs. Ascertain cells can metabolize FA used and the FA choice matches the experimental goal. | [15; 141] |
Altogether, working with FA and BSA does require some special attention to control all the particularities of the system, as outlined in Table 1, but also provides unique mechanistic insights into the toxic effects of lipids. Our objective in compiling these particularities and discussing solutions, is to contribute toward better interpretation and reproducibility of lipotoxicity findings in the future.
Highlights.
Lipid-treated cells are used as models to study mechanisms of metabolic disease
Lipotoxicity has been monitored in distinct tissues and cells, with varying protocols
Fatty acids are poorly soluble and must be adequately prepared and conjugated
The use and choice of conjugation systems (such as BSA) must be carefully considered
Acknowledgments
The authors thank Profs. Marcus Oliveira and Daniel Dagan for critical reading of the manuscript, Jude Deeney, Barbara Corkey, Karel Erion, Eleni Ritou for valuable discussions and M. Sci. Gustavo Grenfell for help with unbound fraction calculations. Funded by NIH-NIDDK 5-R01DK099618-02, CEPID 2013/07937-8, FAPESP PD 2015/07670-7, FAPESP DD 2015/25862-0 and CNPq. NA is funded by a fellowship from Kuwait University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Paolisso G, Tataranni PA, Foley JE, Bogardus C, Howard BV, Ravussin E. A high concentration of fasting plasma non-esterified fatty acids is a risk factor for the development of NIDDM. Diabetologia. 1995;38:1213–1217. doi: 10.1007/BF00422371. [DOI] [PubMed] [Google Scholar]
- 2.Lee Y, Hirose H, Ohneda M, Johnson JH, McGarry JD, Unger RH. Beta-cell lipotoxicity in the pathogenesis of non-insulin-dependent diabetes mellitus of obese rats: impairment in adipocyte-beta-cell relationships. Proc Natl. Acad. Sci. USA. 1994;91:10878–10882. doi: 10.1073/pnas.91.23.10878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haas JT, Francque SM, Staels B. Pathophysiology and mechanisms of nonalcoholic fatty liver disease. Annu. Rev. Physiol. 2016;78:18.1–18.25. doi: 10.1146/annurev-physiol-021115-105331. [DOI] [PubMed] [Google Scholar]
- 4.Walther TC, Farese RV. Lipid droplets and cellular lipid metabolism. Annu. Rev. Biochem. 2012;81:687–714. doi: 10.1146/annurev-biochem-061009-102430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hardy T, Oakley F, Anstee QM, Day CP. Nonalcoholic fatty liver disease: Pathogenesis and disease spectrum. Annu. Rev. Pathol. Mech. Dis. 2016;11:451–496. doi: 10.1146/annurev-pathol-012615-044224. [DOI] [PubMed] [Google Scholar]
- 6.Monetti M, Levin MC, Watt MJ, Sajan MP, Marmor S, Hubbard BK, Stevens RD, Bain JR, Newgard CB, Farese RV, Hevener AL, Farese RV. Dissociation of hepatic steatosis and insulin resistance in mice overexpressing DGAT in the liver. Cell Metab. 2007;6:69–78. doi: 10.1016/j.cmet.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Yamaguchi K, Yang L, McCall S, Huang J, Xing XY, Pandey SK, Bhanot S, Monia BP, Li YX, Diehl AM. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology. 2007;45:1366–1374. doi: 10.1002/hep.21655. [DOI] [PubMed] [Google Scholar]
- 8.Ertunc ME, Hotamisligil GS. Lipid signaling and lipotoxicity in metabolic inflammation: indications for metabolic disease pathogenesis and treatment. J. Lipid Res. 2016:1–56. doi: 10.1194/jlr.R066514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Invest. 2005;115:1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feldstein AE, Werneburg NW, Canbay A, Guicciardi ME, Bronk SF, Rydzewski R, Burgart LJ, Gores GJ. Free fatty acids promote hepatic lipotoxicity by stimulating TNF-α expression via a lysosomal pathway. Hepatology. 2004;40:185–194. doi: 10.1002/hep.20283. [DOI] [PubMed] [Google Scholar]
- 11.Gao D, Nong S, Huang X, Lu Y, Zhao H, Lin Y, Man Y, Wang S, Yang J, Li J. The effects of palmitate on hepatic insulin resistance are mediated by NADPH oxidase 3-derived reactive oxygen species through JNK and p38 MAPK pathways. J. Biol. Chem. 2010;285:29965–29973. doi: 10.1074/jbc.M110.128694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joshi-Barve S, Barve SS, Amancherla K, Gobejishvili L, Hill D, Cave M, Hote P, McClain CJ. Palmitic acid induces production of proinflammatory cytokine interleukin-8 from hepatocytes. Hepatology. 2007;46:823–830. doi: 10.1002/hep.21752. [DOI] [PubMed] [Google Scholar]
- 13.Ishii M, Maeda A, Tani S, Akagawa M. Palmitate induces insulin resistance in human HepG2 hepatocytes by enhancing ubiquitination and proteasomal degradation of key insulin signaling molecules. Arch. Biochem. Biophys. 2015;566:26–35. doi: 10.1016/j.abb.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 14.Malhi H, Bronk SF, Werneburg NW, Gores GJ. Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. J. Biol. Chem. 2006;281:12093–12101. doi: 10.1074/jbc.M510660200. [DOI] [PubMed] [Google Scholar]
- 15.Ricchi M, Odoardi MR, Carulli L, Anzivino C, Ballestri S, Pinetti A, Fantoni LI, Marra F, Bertolotti M, Banni S, Lonardo A, Carulli N, Loria P. Differential effect of oleic and palmitic acid on lipid accumulation and apoptosis in cultured hepatocytes. J. Gastroenterol. Hepatol. 2009;24:830–840. doi: 10.1111/j.1440-1746.2008.05733.x. [DOI] [PubMed] [Google Scholar]
- 16.Gómez-Lechón MJ, Donato MT, Martínez-Romero A, Jiménez N, Castell JV, O’Connor JE. A human hepatocellular in vitro model to investigate steatosis. Chem. Biol. Interact. 2007;165:106–116. doi: 10.1016/j.cbi.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Akazawa Y, Cazanave S, Mott JL, Elmi N, Bronk SF, Kohno S, Charlton MR, Gores GJ. Palmitoleate attenuates palmitate-induced Bim and PUMA up-regulation and hepatocyte lipoapoptosis. J. Hepatol. 2010;52:586–593. doi: 10.1016/j.jhep.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, Jiang L, Hu W, Zheng Q, Xiang W. Mitochondrial dysfunction during in vitro hepatocyte steatosis is reversed by omega-3 fatty acid-induced up-regulation of mitofusin 2. Metabolism. 2011;60:767–775. doi: 10.1016/j.metabol.2010.07.026. [DOI] [PubMed] [Google Scholar]
- 19.Hirsova P, Ibrahim SH, Gores GJ, Malhi H. Lipotoxic Lethal and Sublethal Stress Signaling in Hepatocytes: Relevance to NASH Pathogenesis. J. Lipid Res. 2016;57:1758–1770. doi: 10.1194/jlr.R066357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jung TW, Lee YJ, Lee MW, Kim SM. Full-length adiponectin protects hepatocytes from palmitate-induced apoptosis via inhibition of c-Jun NH2 terminal kinase. FEBS J. 2009;276:2278–2284. doi: 10.1111/j.1742-4658.2009.06955.x. [DOI] [PubMed] [Google Scholar]
- 21.Pfaffenbach KT, Gentile CL, Nivala AM, Wang D, Wei Y, Pagliassotti MJ. Linking endoplasmic reticulum stress to cell death in hepatocytes: roles of C / EBP homologous protein and chemical chaperones in palmitate-mediated cell death. Am. J. Physiol. Endocrinol. Metab. 2010;298:1027–1035. doi: 10.1152/ajpendo.00642.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cazanave SC, Mott JL, Bronk SF, Werneburg NW, Fingas CD, Meng XW, Finnberg N, El-Deiry WS, Kaufmann SH, Gores GJ. Death receptor 5 signaling promotes hepatocyte lipoapoptosis. J. Biol. Chem. 2011;286:39336–39348. doi: 10.1074/jbc.M111.280420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martínez L, Torres S, Baulies A, Alarcón-Vila C, Elena M, Fabriàs G, Casas J, Caballeria J, Fernandez-Checa JC, García-Ruiz C. Myristic acid potentiates palmitic acid-induced lipotoxicity and steatohepatitis associated with lipodystrophy by sustaning de novo ceramide synthesis. Oncotarget. 2015;6:41479–96. doi: 10.18632/oncotarget.6286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei Y, Wang D, Topczewski F, Pagliassotti MJ. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am. J. Physiol. Endocrinol. Metab. 2006;291:275–281. doi: 10.1152/ajpendo.00644.2005. [DOI] [PubMed] [Google Scholar]
- 25.Pardo V, González-Rodríguez Á, Guijas C, Balsinde J, Valverde ÁM. Opposite cross-talk by oleate and palmitate on insulin signaling in hepatocytes through macrophage activation. J. Biol. Chem. 2015;290:11663–11677. doi: 10.1074/jbc.M115.649483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Than NN, Newsome PN. A concise review of non-alcoholic fatty liver disease. Atherosclerosis. 2015;239:192–202. doi: 10.1016/j.atherosclerosis.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 27.Mordier S, Iynedjian PB. Activation of mammalian target of rapamycin complex 1 and insulin resistance induced by palmitate in hepatocytes. Biochem. Biophys. Res. Commun. 2007;362:206–211. doi: 10.1016/j.bbrc.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura S, Takamura T, Matsuzawa-Nagata N, Takayama H, Misu H, Noda H, Nabemoto S, Kurita S, Ota T, Ando H, Miyamoto KI, Kaneko S. Palmitate induces insulin resistance in H4IIEC3 hepatocytes through reactive oxygen species produced by mitochondria. J. Biol. Chem. 2009;284:14809–14818. doi: 10.1074/jbc.M901488200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petersen MC, Madiraju AK, Gassaway BM, Marcel M, Nasiri AR, Butrico G, Marcucci MJ, Zhang D, Abulizi A, Zhang X, Philbrick W, Hubbard SR, Jurczak MJ, Samuel VT, Rinehart J, Shulman GI. Insulin receptor Thr 1160 phosphorylation mediates lipid-induced hepatic insulin resistance. J. Clin. Invest. 2016;126:1–11. doi: 10.1172/JCI86013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Charbonneau A, Marette A. Inducible nitric oxide synthase induction underlies lipid-induced hepatic insulin resistance in mice: potential role of tyrosine nitration of insulin signaling proteins. Diabetes. 2010;59:861–871. doi: 10.2337/db09-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Egnatchik RA, Leamy AK, Noguchi Y, Shiota M, Young JD. Palmitate-induced activation of mitochondrial metabolism promotes oxidative stress and apoptosis in H4IIEC3 rat hepatocytes. Metabolism. 2014;63:283–295. doi: 10.1016/j.metabol.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Egnatchik RA, Leamy AK, Jacobson DA, Shiota M, Young JD. ER calcium release promotes mitochondrial dysfunction and hepatic cell lipotoxicity in response to palmitate overload. Mol. Metab. 2014;3:544–553. doi: 10.1016/j.molmet.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sako Y, Grill VE. A 48-hour lipid infusion in the rat time-dependently inhibits glucose-induced insulin secretion and B cell oxidation through a process likely coupled to fatty acid oxidation. Endocrinology. 1990;127:1580–1589. doi: 10.1210/endo-127-4-1580. [DOI] [PubMed] [Google Scholar]
- 34.Zhou YP, Grill VE. Long-term exposure of rat pancreatic islets to fatty acids inhibits glucose-induced insulin secretion and biosynthesis through a glucose fatty acid cycle. J. Clin. Invest. 1994;93:870–876. doi: 10.1172/JCI117042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bollheimer LC, Skelly RH, Chester MW, McGarry JD, Rhodes CJ. Chronic exposure to free fatty acid reduces pancreatic beta cell insulin content by increasing basal insulin secretion that is not compensated for by a corresponding increase in proinsulin biosynthesis translation. J. Clin. Invest. 1998;101:1094–1101. doi: 10.1172/JCI420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grill V, Qvigstad E. Fatty acids and insulin secretion. Br. J. Nutr. 2000;83:S79–84. doi: 10.1017/s0007114500000994. [DOI] [PubMed] [Google Scholar]
- 37.Maedler K, Oberholzer J, Bucher P, Spinas GA, Donath MY. Monounsaturated fatty acids prevent the deleterious effects of palmitate and high glucose on human pancreatic beta-cell turnover and function. Diabetes. 2003;52:726–733. doi: 10.2337/diabetes.52.3.726. [DOI] [PubMed] [Google Scholar]
- 38.Karaskov E, Scott C, Zhang L, Teodoro T, Ravazzola M, Volchuk A. Chronic palmitate but not oleate exposure induces endoplasmic reticulum stress, which may contribute to INS-1 pancreatic beta-cell apoptosis. Endocrinology. 2006;147:3398–3407. doi: 10.1210/en.2005-1494. [DOI] [PubMed] [Google Scholar]
- 39.Busch AK, Gurisik E, Cordery DV, Sudlow M, Denyer GS, Laybutt DR, Hughes WE, Biden TJ. Increased fatty acid desaturation and enhanced expression of stearoyl coenzyme A desaturase protects pancreatic beta-cells from lipoapoptosis. Diabetes. 2005;54:2917–2924. doi: 10.2337/diabetes.54.10.2917. [DOI] [PubMed] [Google Scholar]
- 40.Thörn K, Hovsepyan M, Bergsten P. Reduced levels of SCD1 accentuate palmitate-induced stress in insulin-producing β-cells. Lipids Health Dis. 2010;9:108. doi: 10.1186/1476-511X-9-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Molina AJA, Wikstrom JD, Stiles L, Las G, Mohamed H, Elorza A, Walzer G, Twig G, Katz S, Corkey BE, Shirihai OS. Mitochondrial networking protects beta-cells from nutrient-induced apoptosis. Diabetes. 2009;58:2303–2315. doi: 10.2337/db07-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Las G, Serada SB, Wikstrom JD, Twig G, Shirihai OS. Fatty acids suppress autophagic turnover in β-cells. J. Biol. Chem. 2011;286:42534–42544. doi: 10.1074/jbc.M111.242412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oprescu AI, Bikopoulos G, Naassan A, Allister EM, Tang C, Park E, Uchino H, Lewis GF, Fantus IG, Rozakis-Adcock M, Wheeler MB, Giacca A. Free fatty acid-induced reduction in glucose-stimulated insulin secretion: evidence for a role of oxidative stress in vitro and in vivo. Diabetes. 2007;56:2927–2937. doi: 10.2337/db07-0075. [DOI] [PubMed] [Google Scholar]
- 44.Maechler P. Mitochondria as the conductor of metabolic signals for insulin exocytosis in pancreatic beta-cells. Cell. Mol. Life Sci. 2002;59:1803–1818. doi: 10.1007/PL00012507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ebato C, Uchida T, Arakawa M, Komatsu M, Ueno T, Komiya K, Azuma K, Hirose T, Tanaka K, Kominami E, Kawamori R, Fujitani Y, Watada H. Autophagy is important in islet homeostasis and compensatory increase of beta cell mass in response to high-fat diet. Cell Metabolism. 2008;8:325–332. doi: 10.1016/j.cmet.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 46.Wu JJ, Quijano C, Chen E, Liu H, Cao L, Fergusson MM, Rovira II, Gutkind S, Daniels MP, Komatsu M, Finkel T. Mitochondrial dysfunction and oxidative stress mediate the physiological impairment induced by the disruption of autophagy. Aging (Albany NY) 2009;1:425–437. doi: 10.18632/aging.100038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jung HS, Chung KW, Won Kim J, Kim J, Komatsu M, Tanaka K, Nguyen YH, Kang TM, Yoon KH, Kim JW, Jeong YT, Han MS, Lee MK, Kim KW, Shin J, Lee MS. Loss of autophagy diminishes pancreatic beta cell mass and function with resultant hyperglycemia. Cell Metabolism. 2008;8:318–324. doi: 10.1016/j.cmet.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 48.Posey KA, Clegg DJ, Printz RL, Byun J, Morton GJ, Vivekanandan-giri A, Pennathur S, Baskin DG, Heinecke JW, Woods SC, Schwartz MW, Niswender KD. Hypothalamic proinflammatory lipid accumulation, inflammation, and insulin resistance in rats fed a high-fat diet. Am. J. Physiol. Endocrinol. Metab. 2009;296:1003–1012. doi: 10.1152/ajpendo.90377.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Karmi A, Iozzo P, Viljanen A, Hirvonen J, Fielding BA, Virtanen K, Oikonen V, Kemppainen J, Viljanen T, Guiducci L, Haaparanta-solin M, Någren K, Solin O, Nuutila P. Increased brain fatty acid uptake in metabolic syndrome. Diabetes. 2010;59:2171–2177. doi: 10.2337/db09-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tran DQ, Tse EK, Kim MH, Belsham DD. Diet-induced cellular neuroinflammation in the hypothalamus: Mechanistic insights from investigation of neurons and microglia. Mol. Cell. Endocrinol. 2016;438:18–26. doi: 10.1016/j.mce.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 51.Valdearcos M, Robblee MM, Xu AW, Koliwad SK, Valdearcos M, Robblee MM, Benjamin DI, Nomura DK, Xu AW, Koliwad SK. Microglia dictate the impact of saturated fat consumption on hypothalamic inflammation and neuronal function. Cell Reports. 2014;9:2124–2138. doi: 10.1016/j.celrep.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mayer CM, Belsham DD. Palmitate attenuates insulin signaling and induces endoplasmic reticulum stress and apoptosis in hypothalamic neurons: rescue of monophosphate-activated protein kinase activation. Endocrinology. 2010;151:576–585. doi: 10.1210/en.2009-1122. [DOI] [PubMed] [Google Scholar]
- 53.Kwon B, Lee HK, Querfurth HW. Oleate prevents palmitate-induced mitochondrial dysfunction, insulin resistance and inflammatory signaling in neuronal cells. Biochim. Biophys. Acta. 2014;1843:1402–1413. doi: 10.1016/j.bbamcr.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 54.Cragle FK, Baldini G. Mild lipid stress induces profound loss of mc4r protein abundance and function. Mol. Endocrinol. 2014;28:357–367. doi: 10.1210/me.2013-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hsiao Y, Lin C, Liao H, Chen Y, Lin S. Palmitic acid-induced neuron cell cycle G2/M arrest and endoplasmic reticular stress through protein palmitoylation. Int. J. Mol. Sci. 2014;15:20876–20899. doi: 10.3390/ijms151120876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Park HR, Kim JY, Park KY, Lee J. Lipotoxicity of palmitic Acid on neural progenitor cells and hippocampal neurogenesis. Toxicol. Res. 2011;27:103–110. doi: 10.5487/TR.2011.27.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morari J, Anhe GF, Nascimento LF, De Moura RF, Razolli D, Solon C, Guadagnini D, Souza G, Mattos AH, Tobar N, Ramos CD, Pascoal VD, Saad MJ, Lopes-cendes I, Moraes JC, Velloso LA. Fractalkine (CX3CL1) is involved in the early activation of hypothalamic inflammation in experimental obesity. Diabetes. 2014;63:3770–3784. doi: 10.2337/db13-1495. [DOI] [PubMed] [Google Scholar]
- 58.Mcfadden JW, Aja S, Li Q, Bandaru VVR, Kim E, Haughey NJ, Kuhajda FP, Ronnett GV. Increasing fatty acid oxidation remodels the hypothalamic neurometabolome to mitigate stress and inflammation. PLoS One. 2014;9:e115642. doi: 10.1371/journal.pone.0115642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ye W, Ramos EH, Wong BC, Belsham DD. Beneficial effects of metformin and/or salicylate on palmitate- or TNFα-induced neuroinflammatory marker and neuropeptide gene regulation in immortalized NPY/AgRP neurons. PLoS One. 2016;11:e0166973. doi: 10.1371/journal.pone.0166973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Choi SJ, Kim F, Schwartz MW, Wisse BE. Cultured hypothalamic neurons are resistant to inflammation and insulin resistance induced by saturated fatty acids. Am. J. Physiol. Endocrinol. Metab. 2010;298:1122–1130. doi: 10.1152/ajpendo.00006.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Greco JA, Oosterman JE, Belsham DD. Differential effects of omega-3 fatty acid docosahexaenoic acid and palmitate on the circadian transcriptional profile of clock genes in immortalized hypothalamic neurons. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014;307:1049–1060. doi: 10.1152/ajpregu.00100.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thaler JP, Yi C, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, Zhao X, Sarruf DA, Izgur V, Maravilla KR, Nguyen HT, Fischer JD, Matsen ME, Wisse BE, Morton GJ, Horvath TL, Baskin DG, Tschöp MH, Schwartz MW. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Invest. 2012;122:153–162. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Button EB, Mitchell AS, Domingos MM, Chung JH, Bradley RM, Hashemi A, Marvyn PM, Patterson AC, Stark KD, Quadrilatero J, Duncan RE. Microglial cell activation increases saturated and decreases monounsaturated fatty acid content, but both lipid species are proinflammatory. Lipids. 2014;49:305–316. doi: 10.1007/s11745-014-3882-y. [DOI] [PubMed] [Google Scholar]
- 64.Wang Z, Liu D, Wang F, Liu S, Zhao S, Ling EA, Hao A. Saturated fatty acids activate microglia via Tolllike receptor 4/NFκB signalling. Br. J. Nutr. 2012;107:229–241. doi: 10.1017/S0007114511002868. [DOI] [PubMed] [Google Scholar]
- 65.Valdearcos M, Douglass JD, Robblee MM, Barres BA, Thaler JP, Koliwad SK. Microglial inflammatory signaling orchestrates the hypothalamic immune response to dietary excess and mediates obesity susceptibility. Cell Metab. 2017;26:185–197. doi: 10.1016/j.cmet.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tracy LM, Bergqvist F, Ivanova EV, Jacobsen KT, Iverfeldt K, Sjöholm Å. Exposure to the saturated free fatty acid palmitate alters BV-2 microglia inflammatory response. J. Mol. Neurosci. 2013;51:805–812. doi: 10.1007/s12031-013-0068-7. [DOI] [PubMed] [Google Scholar]
- 67.Kappe C, Tracy LM, Patrone C, Iverfeldt K, Sjöholm Å. GLP-1 secretion by microglial cells and decreased CNS expression in obesity. J. Neuroinflammation. 2012;9:276. doi: 10.1186/1742-2094-9-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Smith LC, Pownall HJ, Gotto AM. The Plasma Lipoproteins: Structure and Metabolism. Ann. Rev. Biochem. 1978;47:751–77. doi: 10.1146/annurev.bi.47.070178.003535. [DOI] [PubMed] [Google Scholar]
- 69.Spector AA. Fatty acid binding to plasma albumin. J. Lipid Res. 1975;16:165–179. [PubMed] [Google Scholar]
- 70.van der Vusse GJ. Albumin as fatty acid transporter. Drug Metab. Pharmacokinet. 2009;24:300–307. doi: 10.2133/dmpk.24.300. [DOI] [PubMed] [Google Scholar]
- 71.Fujiwara S, Amisaki T. Fatty acid binding to serum albumin: molecular simulation approaches. Biochim. Biophys. Acta. 2013;1830:5427–5434. doi: 10.1016/j.bbagen.2013.03.032. [DOI] [PubMed] [Google Scholar]
- 72.Fleischer AB, Shurmantine WO, Luxon BA, Forker EL. Palmitate uptake by hepatocyte monolayers: Effect of albumin binding. J. Clin. Invest. 1986;77:964–970. doi: 10.1172/JCI112397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sorrentino D, Stump DD, Van Ness K, Simard A, Schwab AJ, Zhou SL, Goresky CA, Berk PD. Oleate uptake by isolated hepatocytes and the perfused rat liver is competitively inhibited by palmitate. Am. J. Physiol. Gastrointest. Liver Physiol. 1996;270:385–392. doi: 10.1152/ajpgi.1996.270.2.G385. [DOI] [PubMed] [Google Scholar]
- 74.Sorrentino D, Robinson RB, Kiang CL, Berk PD. At physiologic albumin/oleate concentrations oleate uptake by isolated hepatocytes, cardiac myocytes, and adipocytes is a saturable function of the unbound oleate concentration: Uptake kinetics are consistent with the conventional theory. J. Clin. Invest. 1989;84:1325–1333. doi: 10.1172/JCI114301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Su X, Abumrad NA. Cellular fatty acid uptake: a pathway under construction. Trends Endocrinol. Metab. 2009;20:72–77. doi: 10.1016/j.tem.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schwenk RW, Holloway JJFP, Luiken GP, Bonen A, Glatz JFC. Fatty acid transport across the cell membrane: regulation by fatty acid transporters. Prostaglandins. Leukot. Essent. Fatty Acids. 2011;82:149–54. doi: 10.1016/j.plefa.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 77.Kleinfeld AM. Lipid phase fatty acid flip-flop, is it fast enough for cellular transport? J. Membr. Biol. 2000;175:79–86. doi: 10.1007/s002320001056. [DOI] [PubMed] [Google Scholar]
- 78.Wei C, Pohorille A. Flip-Flop of Oleic Acid in a Phospholipid Membrane: Rate and Mechanism. J. Phys. Chem. B. 2014;118:12919–12926. doi: 10.1021/jp508163e. [DOI] [PubMed] [Google Scholar]
- 79.Pownall H, Moore K. Commentary on fatty acid wars: The diffusionists versus the translocatists. Arterioscler. Thromb. Vasc. Biol. 2014;34:8–10. doi: 10.1161/ATVBAHA.114.303380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hamilton JA. New insights into the roles of proteins and lipids in membrane transport of fatty acids. Prostaglandins Leukot. Essent. Fat. Acids. 2007;77:355–361. doi: 10.1016/j.plefa.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 81.Xu S, Jay A, Brunaldi K, Huang N, Hamilton JA. CD36 enhances fatty acid uptake by increasing the rate of intracellular esterification but not transport across the plasma membrane. Biochemistry. 2013;52:7254–7261. doi: 10.1021/bi400914c. [DOI] [PubMed] [Google Scholar]
- 82.Kampf JP, Cupp D, Kleinfeld AM. Different mechanisms of free fatty acid flip-flop and dissociation revealed by temperature and molecular species dependence of transport across lipid vesicles. J. Biol. Chem. 2006;281:21566–21574. doi: 10.1074/jbc.M602067200. [DOI] [PubMed] [Google Scholar]
- 83.Lebarron J, London E. Effect of lipid composition and amino acid sequence upon transmembrane peptide-accelerated lipid transleaflet diffusion (flip-flop) Biochim. Biophys. Acta - Biomembr. 2016;1858:1812–1820. doi: 10.1016/j.bbamem.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 84.Geloen A, Helin L, Geeraert B, Malaud E, Holvoet P, Marguerie G. CD36 inhibitors reduce postprandial hypertriglyceridemia and protect against diabetic dyslipidemia and atherosclerosis. PLoS One. 2012;7:1–12. doi: 10.1371/journal.pone.0037633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jay AG, Hamilton JA. The enigmatic membrane fatty acid transporter CD36: New insights into fatty acid binding and their effects on uptake of oxidized LDL. Prostaglandins Leukot. Essent. Fat. Acids. 2016:1–7. doi: 10.1016/j.plefa.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 86.Lelliott C, Vidal-Puig AJ. Lipotoxicity, an imbalance between lipogenesis de novo and fatty acid oxidation. Int. J. Obes. (Lond) 2004;28:22–28. doi: 10.1038/sj.ijo.0802854. [DOI] [PubMed] [Google Scholar]
- 87.Haffar T, Bérubé-Simard F, Bousette N. Impaired fatty acid oxidation as a cause for lipotoxicity in cardiomyocytes. Biochem Biophys Res Commun. 2015;468:73–78. doi: 10.1016/j.bbrc.2015.10.162. [DOI] [PubMed] [Google Scholar]
- 88.Namgaladze D, Lips S, Leiker TJ, Murphy RC, Ekroos K, Ferreiros N, Geisslinger G, Brüne B. Inhibition of macrophage fatty acid β-oxidation exacerbates palmitate-induced inflammatory and endoplasmic reticulum stress responses. Diabetologia. 2014;55:1067–1077. doi: 10.1007/s00125-014-3173-4. [DOI] [PubMed] [Google Scholar]
- 89.Henique C, Mansouri A, Fumey G, Lenoir V, Girard J, Bouillaud F, Prip-buus C, Cohen I. Increased mitochondrial fatty acid oxidation is sufficient to protect skeletal muscle cells from palmitate-induced apoptosis. J. Biol. Chem. 2010;285:36818–36827. doi: 10.1074/jbc.M110.170431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Laurens C, Bou V, Mairal A, Louche K, Badin P, Mouisel E, Montagner A, Marette A, Tremblay A, Weisnagel JS, Guillou H, Langin D, Joanisse DR, Moro C. Perilipin 5 fine-tunes lipid oxidation to metabolic demand and protects against lipotoxicity in skeletal muscle. Sci Rep. 2016;6:38310. doi: 10.1038/srep38310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dubikovskaya E, Chudnovskiy R, Karateev G, Park HM, Stahl A. Measurement of long-chain fatty acid uptake into adipocytes. Meth. Enzymol. 2014;538:107–134. doi: 10.1016/B978-0-12-800280-3.00007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hotamisligil GS, Bernlohr DA. Metabolic functions of FABPs - mechanisms and therapeutic implications. Nat. Rev. Endocrinol. 2015;11:592–605. doi: 10.1038/nrendo.2015.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Storch J, Thumser AE. Tissue-specific functions in the fatty acid-binding protein family. J. Biol. Chem. 2010;285:32679–32683. doi: 10.1074/jbc.R110.135210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Oliveira AF, Cunha DA, Ladriere L, Bugliani M, Marchetti P, Cnop M. In vitro use of free fatty acids bound to albumin: A comparison of protocols. Biotechniques. 2015;58:228–233. doi: 10.2144/000114285. [DOI] [PubMed] [Google Scholar]
- 95.Saifer A, Goldman L. The free fatty acids bound to human serum albumin. J. Lipid Res. 1961;2:268–270. [Google Scholar]
- 96.Sigma Guidelines: Albumin from bovine serum. Retrieved from: www.sigmaaldrich.com/catalog/substance/bovineserumalbumin12345904846811?lang=pt®ion=BR.
- 97.Lee JY, Sohn KH, Rhee SH, Hwang D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J. Biol. Chem. 2001;276:16683–16689. doi: 10.1074/jbc.M011695200. [DOI] [PubMed] [Google Scholar]
- 98.Håversen L, Danielsson KN, Fogelstrand L, Wiklund O. Induction of proinflammatory cytokines by long-chain saturated fatty acids in human macrophages. Atherosclerosis. 2009;202:382–393. doi: 10.1016/j.atherosclerosis.2008.05.033. [DOI] [PubMed] [Google Scholar]
- 99.Prieur X, Mok CY, Velagapudi VR, Núñez V, Fuentes L, Montaner D, Ishikawa K, Camacho A, Barbarroja N, O'Rahilly S, Sethi JK, Dopazo J, Orešič M, Ricote M, Vidal-Puig A. Differential lipid partioning between adipocytes and tissue macrophages modulates macrophage lipotoxicity and M2/M1 polarization in obese mice. Diabetes. 2011;60:797–809. doi: 10.2337/db10-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, Brickey WJ, Ting JP. Fatty acid-induced NLRP3 ASC inflammasome activation interferes with insulin signaling. Nat. Immunol. 2011;12:408–415. doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Baker RC, Kramer RE. Cytotoxicity of short-chain alcohols. Annu. Rev. Pharmacol. Toxicol. 1999;39:127–150. doi: 10.1146/annurev.pharmtox.39.1.127. [DOI] [PubMed] [Google Scholar]
- 102.Siddiqui RA, Jenski LJ, Neff K, Harvey K, Kovacs RJ, Stillwell W. Doco sahexaenoic acid induces apoptosis in Jurkat cells by a protein phosphatase-mediated process. Biochim. Biophys. Acta. 2001;1499:265–275. doi: 10.1016/s0167-4889(00)00128-2. [DOI] [PubMed] [Google Scholar]
- 103.Crow KE, Cornell NW, Veech RL. The rate of ethanol metabolism in isolated rat hepatocytes. Alcohol Clin. Exp. Res. 1997;1:43–50. doi: 10.1111/j.1530-0277.1977.tb05765.x. [DOI] [PubMed] [Google Scholar]
- 104.Cerqueira FM, Chausse B, Baranovski BM, Liesa M, Lewis EC, Shirihai OS, Kowaltowski AJ. Diluted serum from calorie-restricted animals promotes mitochondrial cell adaptations and protect against glucolipotoxicity. FEBS J. 2016;283:822–833. doi: 10.1111/febs.13632. [DOI] [PubMed] [Google Scholar]
- 105.Listenberger LL, Ory DS, Schaffer JE. Palmitate-induced apoptosis can occur through a ceramide-independent pathway. J. Biol. Chem. 2001;276:14890–14895. doi: 10.1074/jbc.M010286200. [DOI] [PubMed] [Google Scholar]
- 106.Seahorse Protocol: Preparation of Bovine Serum Albumin (BSA)-Conjugated Palmitate. Retrieved from: http://www.agilent.com/en-us/products/cell-analysis-(seahorse)/seahorse-xfconsumables/kits-reagents-media/seahorse-xf-palmitate-bsa-fao-substrate.
- 107.Werfel SJ, Cooke SK, Sampson HA. Clinical reactivity to beef in children allergic to cow's milk. J. Allergy Clin. Immunol. 1997;99:293–300. doi: 10.1016/s0091-6749(97)70045-9. [DOI] [PubMed] [Google Scholar]
- 108.Brandt J, Andersson LO. Heat denaturation of human serum albumin. Migration of bound fatty acids. Int. J. Pept. Protein Res. 1976;8:33–37. doi: 10.1111/j.1399-3011.1976.tb02478.x. [DOI] [PubMed] [Google Scholar]
- 109.Kleinfeld AM, Prothro D, Brown DL, Davis RC, Richieri GV, DeMaria A. Increases in serum unbound free fatty acid levels following coronary angioplasty. Am. J. Cardiol. 1996;78:1350–1354. doi: 10.1016/s0002-9149(96)00651-0. [DOI] [PubMed] [Google Scholar]
- 110.Spector AA, John K, Fletcher JE. Binding of long-chain fatty acids to bovine serum albumin. J. Lipid Res. 1969;10:56–67. [PubMed] [Google Scholar]
- 111.Warnotte C, Nenquin M, Henquin JC. Unbound rather than total concentration and saturation rather than unsaturation determine the potency of fatty acids on insulin secretion. Mol. Cell Endocrinol. 1999;153:147–153. doi: 10.1016/s0303-7207(99)00069-6. [DOI] [PubMed] [Google Scholar]
- 112.Abumrad NA, Perkins RC, Park JH, Park CR. Mechanism of long chain fatty acid permeation in the isolated adipocyte. J. Biol. Chem. 1981;256:9183–9191. [PubMed] [Google Scholar]
- 113.Rotondo F, Romero MD, Ho-Palma AC, Remesar X, Fernández-López JA, Alemany M. Quantitative analysis of rat adipose tissue cell recovery, and non-fat cell volume, in primary cell cultures. PeerJ. 2016;4:e2725. doi: 10.7717/peerj.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Simard JR, Kamp F, Hamilton JA. Acrylodan-labeled intestinal fatty acid-binding protein to measure concentrations of unbound fatty acids. Methods Mol. Biol. 2007;400:27–43. doi: 10.1007/978-1-59745-519-0_3. [DOI] [PubMed] [Google Scholar]
- 115.Carley AN, Kleinfeld AM. Fatty acid (FFA) transport in cardiomyocytes revealed by imaging unbound FFA is mediated by an FFA pump modulated by the CD36 protein. J. Biol. Chem. 2011;286:4589–4597. doi: 10.1074/jbc.M110.182162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.El-Assaad W, Buteau J, Peyot ML, Nolan C, Roduit R, Hardy S, Joly E, Dbaibo G, Rosenberg L, Prentki M. Saturated fatty acids synergize with elevated glucose to cause pancreatic beta-cell death. Endocrinology. 2003;144:4154–4163. doi: 10.1210/en.2003-0410. [DOI] [PubMed] [Google Scholar]
- 117.Straub SG, Sharp GW. Massive augmentation of stimulated insulin secretion induced by fatty acid-free BSA in rat pancreatic islets. Diabetes. 2004;53:3152–3158. doi: 10.2337/diabetes.53.12.3152. [DOI] [PubMed] [Google Scholar]
- 118.Haber EP, Ximenes HMA, Procopio J, Carvalho CRO, Curi R, Carpinelli AR. Pleiotropic effects of fatty acids on pancreatic B-cells. J. Cell Physiol. 2003;194:1–12. doi: 10.1002/jcp.10187. [DOI] [PubMed] [Google Scholar]
- 119.Francis GL. Albumin and mammalian cell culture: implications for biotechnology applications. Cytotechnology. 2010;62:1–16. doi: 10.1007/s10616-010-9263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pi J, Bai Y, Zhang Q, Wong V, Floering LM, Daniel K, Reece JM, Deeney JT, Andersen ME, Corkey BE, Collins S. Reactive oxygen species as a signal in glucose-stimulated insulin secretion. Diabetes. 2007;56:1783–1791. doi: 10.2337/db06-1601. [DOI] [PubMed] [Google Scholar]
- 121.Erion KA, Berdan CA, Burritt NE, Corkey BE, Deeney JT. Chronic exposure to excess nutrients left-shifts the concentration dependence of glucose-stimulated insulin secretion in pancreatic β-cells. J. Biol. Chem. 2015;290:16191–16201. doi: 10.1074/jbc.M114.620351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rustan AC, Drevon CA. Fatty acids: structures and properties. In Encyclopedia of Life Sciences. 2005:1–7. [Google Scholar]
- 123.Richieri GV, Anel A, Kleinfeld AM. Interactions of long-chain fatty acids and albumin: determination of free fatty acid levels using the fluorescent probe ADIFAB. Biochemistry. 1993;32:7574–7580. doi: 10.1021/bi00080a032. [DOI] [PubMed] [Google Scholar]
- 124.Choi JK, Ho J, Curry S, Qin D, Bittman R, Hamilton J. Interactions of very long-chain saturated fatty acids with serum albumin. J. Lipid Res. 2002;43:1000–1010. doi: 10.1194/jlr.m200041-jlr200. [DOI] [PubMed] [Google Scholar]
- 125.Hajri T, Abumrad NA. Fatty acid transport across membranes: relevance to nutrition and metabolic pathology. Annu. Rev. Nutr. 2002;22:383–415. doi: 10.1146/annurev.nutr.22.020402.130846. [DOI] [PubMed] [Google Scholar]
- 126.Huber AH, Kleinfeld AM. Unbound free fatty acid profiles in human plasma and the unexpected absence of unbound palmitoleate. J. Lipid Res. 2017;58:578–585. doi: 10.1194/jlr.M074260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Huber AH, Kampf JP, Kwan T, Zhu B, Kleinfeld AM. Fatty acid-specific fluorescent probes and their use in resolving mixtures of unbound free fatty acids in equilibrium with albumin. Biochemistry. 2006;45:14263–14274. doi: 10.1021/bi060703e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gao HM, Zhou H, Zhang F, Wilson BC, Kam W, Hong JS. HMGB1 acts on microglia Mac1 to mediate chronic neuroinflammation that drives progressive neurodegeneration. J. Neurosci. 2011;31:1081–1092. doi: 10.1523/JNEUROSCI.3732-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bordt EA, Polster BM. NADPH oxidase- and mitochondria-derived reactive oxygen species in proinflammatory microglialactivation: a bipartisan affair? Free Radic. Biol. Med. 2014;76:34–46. doi: 10.1016/j.freeradbiomed.2014.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Velloso LA, Schwartz MW. Altered hypothalamic function in diet-induced obesity. Int. J. Obes. (Lond) 2011;35:1455–1465. doi: 10.1038/ijo.2011.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Restani P, Ballabio C, Cattaneo A, Isoardi P, Terracciano L, Fiocchi A. Characterization of bovine serum albumin epitopes and their role in allergic reactions. Allergy. 2004;78:21–24. doi: 10.1111/j.1398-9995.2004.00568.x. [DOI] [PubMed] [Google Scholar]
- 132.Tanabe S, Kobayashi Y, Takahata Y, Morimatsu F, Shibata R, Nishimura T. Some human B and T cell epitopes of bovine serum albumin, the major beef allergen. Biochim. Biophys. Acta. 2002;293:1348–1353. doi: 10.1016/S0006-291X(02)00381-9. [DOI] [PubMed] [Google Scholar]
- 133.Chruszcz M, Mikolajczak K, Mank N, Majorek KA, Porebski PJ, Minor W. Serum albumins - unusual allergens. Biochim. Biophys. Acta. 2013;1830:5375–5381. doi: 10.1016/j.bbagen.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Karjalainen J, Martin JM, Knip M, Ilonen J, Robinson BH, Savilahti E, Akerblom HK, Dosch HM. A bovine albumin peptide as a possible trigger of insulin-dependent diabetes mellitus. N. Engl. J. Med. 1992;327:302–307. doi: 10.1056/NEJM199207303270502. [DOI] [PubMed] [Google Scholar]
- 135.Gordan S, Biburger M, Nimmerjahn F. bIgG time for large eaters: monocytes and macrophages as effector and target cells of antibody-mediated immune activation and repression. Immunol. Rev. 2015;268:52–65. doi: 10.1111/imr.12347. [DOI] [PubMed] [Google Scholar]
- 136.Schlesinger JB, van Harmelen V, Alberti-Huber CE, Hauner H. Albumin inhibits adipogenesis and stimulates cytokine release from human adipocytes. Am. J. Physiol. Cell Physiol. 2006;291:C27–33. doi: 10.1152/ajpcell.00172.2005. [DOI] [PubMed] [Google Scholar]
- 137.Ramsay TG, Blomberg L, Caperna TJ. Methyl-β-cyclodextrin alters adipokine gene expression and glucose metabolism in swine adipose tissue. Animal. 2013;7:1690–1696. doi: 10.1017/S1751731113001250. [DOI] [PubMed] [Google Scholar]
- 138.Garcia-Martinez R, Andreola F, Mehta G, Poulton K, Oria M, Jover M, Soeda J, MacNaughtan J, De Chiara F, Habtesion A, Mookerjee RP, Davies N, Jalan R. Immunomodulatory and antioxidant function of albumin stabilises the endothelium and improves survival in a rodent model of chronic liver failure. J. Hepatol. 2015;62:799–806. doi: 10.1016/j.jhep.2014.10.031. [DOI] [PubMed] [Google Scholar]
- 139.Kim YL, Im YJ, Lee YK, Ha NC, Bae YS, Lim SM, Okajima F, Im DS. Albumin functions as an inhibitor of T cell adhesion in vitro. Biochem. Biophys. Res. Commun. 2006;351:953–957. doi: 10.1016/j.bbrc.2006.10.143. [DOI] [PubMed] [Google Scholar]
- 140.Cnop M, Hannaert JC, Hoorens A, Eizirik DL, Pipeleers DG. Inverse relationship between cytotoxicity of free fatty acids in pancreatic islet cells and cellular triglyceride accumulation. Diabetes. 2001;50:1771–1777. doi: 10.2337/diabetes.50.8.1771. [DOI] [PubMed] [Google Scholar]
- 141.Miller TA, LeBrasseur NK, Cote GM, Trucillo MP, Pimentel DR, Ido Y, Ruderman NB, Sawyer DB. Oleate prevents palmitate-induced cytotoxic stress in cardiac myocytes. Biochem. Biophys. Res. Commun. 2005;336:309–315. doi: 10.1016/j.bbrc.2005.08.088. [DOI] [PubMed] [Google Scholar]
- 142.Gómez-Lechón MJ, Donato MT, Martíez-Romero A, Jiménez N, Castell JV, O’Connor JE. A human hepatocellular in vitro model to investigate steatosis. Chem. Biol. Interact. 2007;165:106–116. doi: 10.1016/j.cbi.2006.11.004. [DOI] [PubMed] [Google Scholar]